Abstract
Introduction
Focal segmental glomerulosclerosis (FSGS), a histologic lesion in the kidney caused by varied pathophysiological processes, leads to end-stage kidney disease in a large proportion of patients. Sparsentan is a first-in-class orally active compound combining endothelin type A (ETA) receptor blockade with angiotensin II type 1 (AT1) receptor antagonism in a single molecule. A Randomized, Multicenter, Double-Blind, Parallel, Active-Control Study of the Effects of Sparsentan, a Dual Endothelin Receptor and Angiotensin Receptor Blocker, on Renal Outcomes in Patients With Primary FSGS (DUPLEX) study evaluates the long-term antiproteinuric efficacy, nephroprotective potential, and safety profile of sparsentan compared with an AT1 receptor blocker alone in patients with FSGS.
Methods
DUPLEX is a multicenter, international, phase 3, randomized, double-blind, active-controlled study of sparsentan in patients with FSGS. Approximately 300 patients aged 8 to 75 years, inclusive (United States), and 18 to 75 years, inclusive (outside United States) will be randomized 1:1 to daily treatment with sparsentan or irbesartan. After renin-angiotensin-aldosterone system inhibitor washout, treatment will be administered for 108 weeks, with the final assessment at week 112, four weeks after withdrawal of study drug.
Results
The primary endpoint will be the slope of estimated glomerular filtration rate from week 6 to week 108. A novel surrogate efficacy endpoint, the proportion of patients achieving urinary protein-to-creatinine (UP/C) ratio of ≤1.5 g/g and >40% reduction from baseline in UP/C (FSGS partial remission endpoint: FPRE), will be evaluated at a planned interim analysis at week 36. Safety and tolerability of sparsentan will also be assessed.
Conclusion
The phase 3 DUPLEX study will characterize the long-term antiproteinuric efficacy and nephroprotective potential of dual ETA and AT1 receptor blockade with sparsentan in patients with FSGS.
Keywords: angiotensin II type 1 receptor blockade, endothelin type A receptor blockade, focal segmental glomerulosclerosis, irbesartan, proteinuria, sparsentan
FSGS is a histologic lesion characterized by segmental accumulation of glomerular extracellular matrix, resulting in capillary obliteration and glomerular scarring. Patients with FSGS typically present with a variable degree of proteinuria and often nephrotic syndrome.1 A variety of heterogeneous clinical conditions may lead to FSGS-type lesions, which can be classified as primary, genetic, and secondary forms. Primary FSGS has no identifiable cause,1 but is presumed to be a consequence of actions of putative circulating permeability factors that cause podocyte injury.2, 3, 4 Genetic causes include mutations in genes encoding proteins required for normal podocyte structure and/or function.5 In contrast, secondary forms of FSGS are caused by loss of renal parenchyma, metabolic derangements, other antecedent diseases, drugs, or infections.1,4 In the United States, at least 50% of patients with primary FSGS and nephrotic-range proteinuria are resistant to treatments and will eventually require renal replacement therapy within 5 to 10 years of diagnosis.6 FSGS accounts for 5% of adult and 12% of pediatric cases of end-stage kidney disease (ESKD).7,8
In responsive patients, treatment with corticosteroids or other immunosuppressive drugs decreases proteinuria,9 an independent predictor of long-term kidney survival in patients with FSGS.10,11 These agents are routinely combined with renin-angiotensin-aldosterone system inhibitors (RAASIs).9,12, 13, 14 However, a spectrum of serious side effects limits the use of immunomodulating drugs.8,9 As a result, the availability of effective, safe, and well-tolerated drugs that protect kidney function or slow the rate of progressive decline in glomerular filtration rate (GFR) is a high unmet need in patients with FSGS.15
Comparable with RAASIs, ETA receptor antagonists (ERAs) have shown a wide range of beneficial hemodynamic, anti-inflammatory, antifibrotic, and podocyte-protective effects in various models of glomerular diseases.16 Importantly, the actions of RAASIs and ERAs in combination have demonstrated additional benefits in experimental models of kidney disease17, 18, 19 and in patients with both diabetic and nondiabetic nephropathies.20, 21, 22, 23
Sparsentan is a first-in-class orally active compound that combines ETA receptor and AT1 receptor inhibitory activities in a single molecule.24 The ongoing phase 2 randomized, double-blind DUET study is assessing the antiproteinuric effect of sparsentan in patients with FSGS.25 During an 8-week, double-blind treatment period, sparsentan-treated patients demonstrated significantly greater reductions in proteinuria, with a similar safety profile compared with those treated with the active control irbesartan.26 In addition, a significantly larger percentage of patients receiving sparsentan compared with irbesartan achieved the FPRE, defined as UP/C ≤1.5 g/g in conjunction with a >40% reduction in UP/C from baseline.11,26 The DUET trial has entered its open-label extension (OLE) phase, during which all patients are treated with sparsentan.26 The OLE provides additional information about safety and efficacy, including monitoring of kidney function,25 and 84-week interim analyses of the OLE have supported sustained decrease in proteinuria and blood pressure (BP) and stable estimated GFR (eGFR).27 However, further studies are needed to assess the long-term nephroprotective potential of sparsentan systematically, with a focus on preservation of kidney function in a controlled, double-blind setting.
On the basis of the positive findings from the DUET study, the phase 3 randomized, double-blind, active-controlled DUPLEX (A Randomized, Multicenter, Double-Blind, Parallel, Active-Control Study of the Effects of Sparsentan, a Dual Endothelin Receptor and Angiotensin Receptor Blocker, on Renal Outcomes in Patients With Primary FSGS; NCT0349368528) trial has been initiated to address knowledge gaps directly and provide extended surveillance for safety signals associated with combined inhibition of ETA and AT1 over a 2-year period. This paper describes the study design and methodology of the DUPLEX trial, including a novel approach of using FPRE as a surrogate endpoint.11
Methods
Study Participants and Sites
Patients aged 8 to 75 years, inclusive (United States) and 18 to 75 years, inclusive (outside the United States), weighing ≥20 kg at screening, with biopsy-proven FSGS, or those with documented genetic mutations in a podocyte protein associated with FSGS, will be eligible for the study after exclusion of secondary causes. Inclusion and exclusion criteria are summarized in Tables 1 and 2. Approximately 300 patients will be enrolled from an estimated 240 investigational study centers globally (Supplementary Table S1). Approval of the study design and procedures by the institutional review board/independent ethics committee at each site will be required before enrollment.
Table 1.
DUPLEX key inclusion criteria
| 1. | The patient or parent/legal guardian (as appropriate) is willing and able to provide signed informed consent, and, when required, the patient is willing to provide assent before any screening procedures. |
| 2. | The patient has biopsy-proven FSGS or documentation of a genetic mutation in a podocyte protein associated with FSGS. |
| 3. | Sites within the United States: The patient is male or female aged 8 to 75 yr, inclusive, weighing ≥20 kg at screening. Sites outside the United States: The patient is male or female aged 18 to 75 yr, inclusive, weighing ≥20 kg at screening. |
| 4. | The patient has a UP/C ≥1.5 g/g at screening. |
| 5. | The patient has an eGFR ≥30 ml/min per 1.73 m2 at screening. |
| 6. | WOCBP must agree to the use of 1 highly reliable method of contraception from 7 d before the first dose of study medication until 90 d after the last dose of study medication and simultaneous use of 1 additional barrier method of contraception during sexual activity from day 1/randomization until 90 d after the last dose of study medication.a |
DUPLEX, A Randomized, Multicenter, Double-Blind, Parallel, Active-Control Study of the Effects of Sparsentan, a Dual Endothelin Receptor and Angiotensin Receptor Blocker, on Renal Outcomes in Patients With Primary FSGS; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; ETA, endothelin type A; ETB, endothelin type B; UP/C, urinary protein-to-creatinine ratio; WOCBP, women of childbearing potential.
Contraception requirements are based on the fetal harm effects associated with selective and nonselective ETA/ETB receptor antagonists and angiotensin receptor blockers.
Table 2.
DUPLEX key exclusion criteriaa
| 1. | The patient has FSGS secondary to another condition. |
| 2. | The patient is ≥18 yr of age and has positive findings on serological tests that, in the investigator’s opinion, are diagnostic of another primary or secondary glomerular disease.b |
| 3. | The patient has a history of type 1 diabetes mellitus or uncontrolled type 2 diabetes mellitus or nonfasting blood glucose >180 mg/dl at screening. |
| 4. | The patient has undergone any organ transplantation, with the exception of corneal transplants, or has received certain immunosuppressive medications. |
| 5. | The patient has a documented history of heart failure, coronary artery disease, or cerebrovascular disease. |
| 6. | The patient has significant liver disease. |
| 7. | The patient is positive at screening for HIV or markers indicating acute or chronic hepatitis B infection or hepatitis C infection. |
| 8. | The patient has a history of malignancy other than adequately treated basal cell or squamous cell skin cancer or cervical carcinoma within the past 2 yr. |
| 9. | The patient has disqualifying laboratory abnormalities during a screening, including hematocrit value <27%, hemoglobin value <9 g/dl, potassium value >5.5 mEq/l, or alanine aminotransferase and/or aspartate aminotransferase >2 times the upper limit of the normal range. |
| 10. | The patient is extremely obese (i.e., ≥18 yr of age with a BMI >40 kg/m2 or is <18 yr of age with a BMI in the 99th percentile plus 5 units at screening), in whom, in the investigator’s opinion, there is a causal relationship between obesity and development of the FSGS lesion. |
| 11. | The patient has a history of alcohol or illicit drug-use disorder. |
| 12. | The patient has a history of serious side effect or allergic response to any angiotensin II antagonist or endothelin receptor antagonist. |
| 13. | The female patient is pregnant, plans to become pregnant during the course of the study, or is breastfeeding.c |
| 14. | The patient, in the opinion of the investigator, is unable to adhere to the requirements of the study, including the ability to swallow the study medication capsules whole. |
BMI, body mass index; DUPLEX, A Randomized, Multicenter, Double-Blind, Parallel, Active-Control Study of the Effects of Sparsentan, a Dual Endothelin Receptor and Angiotensin Receptor Blocker, on Renal Outcomes in Patients With Primary FSGS; ETA, endothelin type A; ETB, endothelin type B; FSGS, focal segmental glomerulosclerosis.
The exclusion criteria are intended to limit inclusion of patients with known etiology of FSGS (serologies, obesity, viral infections, and diabetic nephropathy); to secure patient safety at baseline (exclusion based on hyperkalemia, liver tests, heart failure and conditions frequently associated with heart failure, worsening of anemia); and other conditions that may interfere with a patient’s appropriate adherence to the study protocol (such as, psychiatric disorders and drug abuse).
Includes antinuclear antibody, antidouble-stranded deoxyribonucleic acid antibodies, complement C3 and C4, antineutrophil cytoplasmic antibody, rheumatoid factor, antiglomerular basement membrane antibodies, any clinically significant abnormalities identified by serum and urine protein electrophoresis, or κ and λ chains.
Contraception requirements are based on the fetal harm effects associated with selective and nonselective ETA/ETB receptor antagonists and angiotensin receptor blockers.
Study Design and Treatment
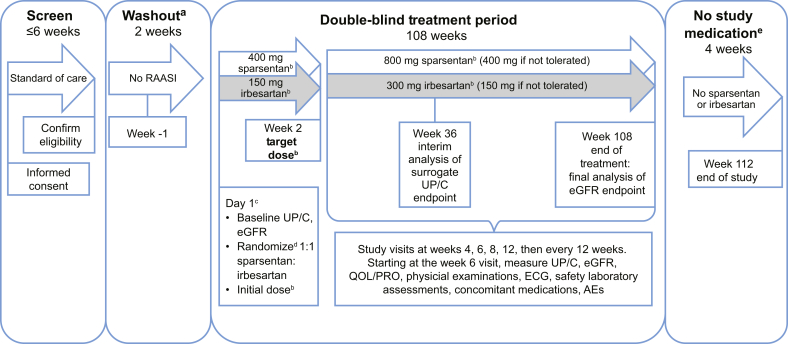
The study design is illustrated in Figure 1. Patients will provide informed consent before entering an eligibility screening period. Successfully screened patients will undergo baseline evaluations and laboratory tests (preceded by a 2-week washout period from RAASIs, when applicable). They will then be randomly assigned 1:1 via an interactive web response system (IWRS) to receive sparsentan or the active control, irbesartan. Randomization will be stratified by eGFR (≥30 to <60 ml/min per 1.73 m2 or ≥60 ml/min per 1.73 m2) and UP/C (≤3.5 g/g or >3.5 g/g for patients ≥18 years of age; ≤2 g/g or >2 g/g for patients <18 years of age) at screening. After the 108-week double-blind treatment period, treatment with study medications will be discontinued, and patients will return for a final study visit 4 weeks later. At week 108, following the end of study treatment, the investigator will resume standard-of-care treatment, including RAASIs (with the exception of irbesartan) if there are no contraindications for their use, and additional adjustments in antihypertensive medications to adequately control blood pressure can be made as clinically indicated.
Figure 1.
A Randomized, Multicenter, Double-Blind, Parallel, Active-Control Study of the Effects of Sparsentan, a Dual Endothelin Receptor and Angiotensin Receptor Blocker, on Renal Outcomes in Patients With Primary FSGS study design. aFor patients who are undergoing washout from renin-angiotensin-aldosterone system inhibitor (RAASI). bPatients whose body weight is ≤50 kg at screening will receive half the otherwise specified doses of sparsentan or irbesartan (active control). Weight will be measured at each visit and the dose increased at the investigator’s discretion if the patient’s weight reaches >50 kg. cDay 1 events shown will occur in the order in which they are listed. dRandomization will be stratified by estimated glomerular filtration rate (eGFR) value (≥30 to <60 ml/min per 1.73 m2 and ≥60 ml/min per 1.73 m2 for all patients) and urinary protein-to-creatinine ratio (UP/C) (≤3.5 g/g and >3.5 g/g [patients ≥18 yr of age] or ≤2 g/g and >2 g/g [patients <18 yr of age]) at screening. eFollowing the 108-week blinded treatment period, treatment with study medication will be discontinued. At this time, the investigator should resume standard-of-care treatment, including treatment with RAASI (with the exception of irbesartan) provided there are no contraindications for their use. The investigator may make additional adjustments in antihypertensive medications as clinically indicated to adequately control the patient’s blood pressure. AEs, adverse events; ECG, electrocardiogram; PRO, patient-reported outcome; QOL, quality of life.
The treatment period will include dose titration in the first 2 weeks. During the dose titration period, patients weighing >50 kg at screening will receive sparsentan 400 mg or irbesartan 150 mg daily. At the week 2 visit, patients who are able to tolerate the initial dose, as assessed by the investigator, will be prescribed the target dose (i.e., sparsentan 800 mg or irbesartan 300 mg daily). Criteria for tolerability will include blood pressure (BP) >100/60 mm Hg (patients ≥18 years of age) or BP above the 5th percentile for sex and height (patients <18 years of age) after 2 weeks and the absence of adverse events (AEs) or laboratory values that do not allow continuation of treatment with study drugs. Patients with asymptomatic BP ≤100/60 mm Hg (patients ≥18 years of age) or ≤90/50 mm Hg or below the 5th percentile for sex and height (whichever is lower in patients <18 years of age), or clinical symptoms of orthostatic hypotension but with otherwise acceptable tolerance of medication, will continue treatment without titration up to the target dose. Patients who do not titrate to the target dose at week 2 may titrate up to that dose at any time, based on evaluation and agreement of the investigator and the medical monitor. Patients who weigh ≤50 kg at screening will receive 50% of the initial or target dose; if the patient’s weight increases to >50 kg during the study, the patient may be prescribed an increased dose at the discretion of the investigator. Dose reductions from the target dose back to the initial dose are permitted at any time after titration up to the target dose, based on safety concerns. Patients weighing >50 kg at screening who do not tolerate the initial dose may continue at one-half the initial dose (i.e., 200 mg sparsentan or 75 mg irbesartan daily). Patients who weigh ≤50 kg at screening cannot have further dose reductions beyond their 50% of the initial dose and may have to discontinue study medication if they do not tolerate the initial dose.
Sparsentan will be dispensed as 200-mg tablets overencapsulated (blinded) with size 00 capsules, and the active control irbesartan will be dispensed as 75-mg tablets overencapsulated (blinded) with size 00 capsules. Patients will be advised to take the prescribed daily dose before the morning meal, except on study-visit days, when they will take the medication during the clinic visit. To enable assessment of treatment adherence, patients will be asked to return all unused study medication and used or unused packaging at each visit.
Medications prohibited during the study will include RAASIs (e.g., angiotensin-converting enzyme inhibitors, aldosterone blockers, angiotensin receptor blockers [ARBs], aliskiren, spironolactone), ERAs (e.g., bosentan, macitentan, ambrisentan), potassium-sparing diuretics, selected antidiabetic drugs (i.e., thiazolidinediones, sodium-glucose cotransporter-2 inhibitors), selected antiarrhythmics (e.g., digoxin, amiodarone), amphetamines and amphetamine derivatives, St. John’s wort or other hypericum-derived products, and strong CYP3A inhibitors (Supplementary Table S2). Other antidiabetic drugs (e.g., metformin, glyburide) should be used in accordance with guidelines for use in patients with impaired kidney function. Medications to be used with caution, with potential dosage adjustments, include strong inhibitors of P-glycoprotein such as cyclosporine A, cytochrome P450 2B6 substrates, statins, nonsteroidal anti-inflammatory drugs used for >1 week (aspirin at a dose >325 mg/d is not allowed), lithium, and warfarin. Steroids, calcineurin inhibitors, mycophenolate mofetil, and azathioprine doses must be stable for at least 30 days before screening and during the screening period.
Clinic visits during the double-blind treatment period will occur at weeks 2, 4, 6, 8, 12, 24, 36, 48, 60, 72, 84, 96, and 108 (end of treatment), with a final assessment at week 112 (end of study). Patients who discontinue study medication prematurely will complete the early-termination visit assessments and will be encouraged to continue through the remainder of the study for efficacy and safety assessments unless they withdraw consent. Patients who elect to withdraw from the study will complete the early-termination visit assessments. The reason(s) for the discontinuation of study medication and/or study withdrawal will be documented.
The eGFR for each visit will be calculated for adults using the Chronic Kidney Disease Epidemiology Collaboration equation,29 and for children aged <18 years, the Modified Schwartz Formula will be used.30 UP/C values will be obtained as an average of 3 first-morning void urine samples collected within 5 days before each visit.
Blood samples for genotyping of FSGS and nephrotic syndrome-associated genes and apolipoprotein A1 gene polymorphisms will be taken at Day 1/Randomization. Test results may be provided to investigators after study completion. Samples will be analyzed by the Clinical Laboratory Improvement Amendments (CLIA)–certified central laboratory.
Endpoints
Efficacy assessments are presented in Table 3. The primary efficacy endpoint will be the slope of eGFR assessed from week 6 to week 108. Evaluation of the slope from week 6 has been applied to account for possible acute hemodynamic effects of the study drugs and their impact on eGFR slope determination. The surrogate efficacy endpoint is the proportion of patients in each treatment group achieving a UP/C ≤1.5 g/g and a >40% reduction from baseline in UP/C (i.e., FPRE11,26) at week 36, assessed at the interim analysis. FPRE was chosen as the proteinuric outcome for the early surrogate endpoint as it has been recently described as a strong predictor of renal survival in primary FSGS.11 Secondary efficacy endpoints will be percent change in eGFR from week 6 to week 108 and percent change in eGFR from baseline to 4 weeks postcessation of randomized treatment at week 112.
Table 3.
Efficacy endpoints
| Primary efficacy endpoint | The slope of eGFR, assessed from week 6 to week 108 at the final analysis |
| Surrogate efficacy endpoint | The proportion of patients achieving a UP/C ≤1.5 g/g and a >40% reduction from baseline in UP/C (i.e., FPRE) at week 36 (interim analysis) |
| Secondary efficacy endpoints | The percent change in eGFR from week 6 to week 108 |
| The percent change in eGFR from baseline to 4 wk postcessation of randomized treatment at week 112 | |
| Exploratory endpoints | Slope of eGFR assessed from week 6 to week 60 |
| Absolute and percent change in eGFR from baseline at each visit | |
| Percent change in eGFR from week 6 at each visit | |
| Proportion of patients achieving FPRE at each visit | |
| Percent change in UP/C from baseline at each visit | |
| Time to achieve FPRE | |
| Proportion of patients reaching a confirmed 40% reduction in eGFR, ESKD (defined as initiation of renal replacement therapy, kidney transplantation, or sustained eGFR <15 ml/min per 1.73 m2), or death | |
| Change from baseline in blood pressure at each visit | |
| Proportion of patients requiring initiation of or intensification in immunosuppressive medication | |
| Proportion of patients undergoing reduction in immunosuppressive medication | |
| Change from baseline in quality of life, assessed using patient-reported outcome measures at each visit beginning at week 12 | |
| Frequency and duration of hospitalizations (for any reason and for reasons related to the kidney) | |
| Trough plasma pharacokinetic concentrations |
eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; FPRE, FSGS partial remission endpoint; UP/C, urinary protein-to-creatinine ratio.
Exploratory endpoints will include the slope of eGFR assessed from week 6 to week 60, the absolute and percent change in eGFR from baseline at each visit, and percent change in eGFR from week 6 at each visit. UP/C exploratory endpoints will include the proportion of patients achieving FPRE at each visit, percent change in UP/C from baseline at each visit, and the time to achieve FPRE. Additional exploratory endpoints will include the proportion of patients reaching a confirmed 40% reduction in eGFR, ESKD (defined as initiation of renal replacement therapy, kidney transplantation, or sustained eGFR <15 ml/min per 1.73 m2), or death; change from baseline in BP at each visit; proportion of patients requiring initiation of or intensification in immunosuppressive medication; proportion of patients undergoing reduction in immunosuppressive medication; change from baseline in quality of life (QOL), assessed using patient-reported outcome measures at each visit beginning at week 12; frequency and duration of hospitalizations (for any reason and for reasons related to the kidney); and trough plasma pharmacokinetics (PK) concentrations.
Safety endpoints include changes from baseline in body weight, vital signs, physical examinations, peripheral edema, 12-lead electrocardiograms, and clinical laboratory parameters (chemistry, hematology) with a focus on known adverse events that have been associated with treatment with ARBs or ERAs such as acute kidney injury, hyperkalemia, hypotension, or markers of fluid retention; changes from baseline in lipid profile; changes from baseline in serum albumin and serum potassium (at each visit); and incidence of treatment-emergent AEs. The safety and tolerability of sparsentan will be assessed by double-blind monitoring of these safety endpoints. Patients will be evaluated for new AEs and the status of existing AEs at each study visit, including screening or washout periods, and at any time contact is made with a patient outside of a scheduled visit. The severity of AEs will be assessed (mild, moderate, severe), as well as their relationship to study treatment (not related, unlikely, possibly, related). Serious AEs will be followed until resolution, until stabilization, or until the investigator and sponsor agree that follow-up is no longer necessary.
Data Monitoring Committee
An independent data-monitoring committee (DMC) will meet regularly and advise the study sponsor on the validity and scientific merit of continuing the study through the review of safety data at regularly scheduled and ad hoc meetings. All DMC sessions will be documented through written minutes. The minutes of closed sessions will be kept confidential during the study and released to the sponsor only after the database is locked and all data are unblinded.
Statistical Analysis
All efficacy analyses will be based on the full analysis set (FAS), which will consist of all randomized patients who take ≥1 dose of double-blind study medication. A sensitivity analysis of the primary endpoint will be conducted using the per-protocol (PP) analysis set, which will include all FAS patients without major protocol violations that could affect the validity of the efficacy assessments. The safety analysis set will include all randomized patients who take ≥1 dose of double-blind study medication. Overall type-1 error for this study at 2-sided α = 0.05 is controlled using a prespecified multiple-testing procedure.
The primary efficacy endpoint analysis will compare sparsentan with irbesartan based on the difference between the treatment groups in eGFR slopes from week 6 to week 108. The primary analysis will use a mixed-effects model that includes fixed effects for treatment, stratification factors, baseline eGFR, time, and time-by-treatment interaction. Random coefficients (i.e., intercept and slopes) will be included for each patient.
The surrogate efficacy endpoint analysis will evaluate the proportion of patients achieving FPRE at week 36, at the planned unblinded interim analysis, using a Cochran-Mantel-Haenszel (CMH) test with adjustment for the stratification factors.
Mixed model repeated measures (MMRM) will be employed to analyze the secondary efficacy endpoint of percent change in eGFR from week 6 to week 108. The model will include fixed effects for treatment, stratification factors, baseline values, visit, and visit-by-treatment interaction, and patient will be included as a random effect. Analysis of covariance will be used to analyze the secondary efficacy endpoint of percent change in eGFR from baseline to 4 weeks postcessation of randomized treatment at week 112. Treatment and baseline values will be included as fixed effects, and the analysis will be stratified by the randomization strata.
MMRM will be employed to analyze the continuous exploratory efficacy endpoints. Responder-type exploratory efficacy endpoints will be analyzed using a CMH approach. Time-to-event will be analyzed for the exploratory efficacy outcome of time to achieve FPRE using Kaplan-Meier product limit survival estimates, with a comparison between treatment groups using the log-rank test, stratified by the randomization stratification.
Select efficacy endpoints will be analyzed by baseline subgroups—for example, sex, geographic region, and genetic test results at both the interim and final analyses—if there is a sufficient number of patients in each subgroup.
Blinding and Unblinding Considerations
Randomized treatment assignment and individual patient information will remain blinded until after the database lock for the final analysis performed at the end of the study with the following exceptions: at the request of the DMC; by an investigator for a medical emergency; or if necessary to satisfy regulatory reporting requirements for a suspected, unexpected serious adverse reaction. The interim analysis for the surrogate endpoint after 36 weeks will be conducted by an independent statistical team (with controlled disclosure of analysis results), and the study team will remain blinded to the interim data.
Sample Size and Power Calculations
The study has appropriate power to test the surrogate FPRE endpoint at the interim analysis and the primary endpoint at the final analysis. Approximately 300 patients will be randomized and allocated 1:1 to receive sparsentan or irbesartan. This sample size takes into consideration enrollment feasibility in this rare disease and was evaluated using simulations. A planned sample-size reassessment procedure will be conducted and overseen by the DMC.
For the surrogate endpoint assessed at the interim analysis with approximately 190 patients, the power to detect a difference in response proportions between treatment groups is >90%, with a 2-sided α of 0.05. The power calculation was based on the χ2 test, assuming that the treatment difference in FPRE response proportions at week 36 is consistent with the observed difference at week 8 (end of the double-blind treatment; 28.1% for sparsentan and 9.4% for irbesartan) from the sparsentan phase 2 DUET study.26
For the primary endpoint assessed at the final analysis, approximately 300 patients provide ≥90% power to detect a difference in eGFR slopes between treatments at a 2-sided α of 0.05. The projected treatment difference in eGFR slopes was obtained by modeling the relationship between eGFR and the surrogate endpoint FPRE using available data from the DUET and disease registry studies. Notably, even what appears to be small differences in eGFR slope (e.g., ranging from 0.80 to 1.52 ml/min per year) have been considered clinically meaningful and associated with hard renal endpoints (ESKD, renal replacement therapy, doubling of serum creatinine, or death) in patients with diabetic nephropathy (e.g., Wanner et al. 2016;31 Brenner et al. 200132; Perkovic et al. 201933 [Supplementary Table S3]).
Discussion
There is a high unmet need for new therapies for patients with FSGS.6,15 Analysis of the double-blind period in the ongoing DUET study has demonstrated that treatment with sparsentan, a dual ETA and AT1 receptor blocker, for 8 weeks results in a significantly greater reduction in proteinuria compared with an ARB (i.e., irbesartan).26 Moreover, data from the ongoing OLE of the DUET study have demonstrated further and sustained reduction in proteinuria with increasing duration of follow-up.27,34,35 However, DUET will not provide information on whether this extended antiproteinuric effect, when compared with standard of care RAASI, can be translated into long-term nephroprotection in these patients. The DUPLEX study is designed to address this crucial question.
To assess the effect of sparsentan on preservation of kidney function, DUPLEX will compare the slope of eGFR between the sparsentan- and irbesartan-treated patients. Clinical outcomes (hard endpoints)—such as doubling of serum creatinine, reaching ESKD or death, or a prespecified percent decline in eGFR— traditionally have been used and recognized as primary endpoints in studies evaluating the long-term nephroprotective potential of new treatments in chronic kidney diseases.9 However, in the context of a rare disease that progresses slowly, achieving a sufficient number of events during a study of reasonable duration and size makes the use of standard hard endpoints impractical and unfeasible for development of new drugs.9 Analysis of differences in slopes of eGFR between the study groups offers an achievable solution to this problem.36 Similar approaches have been evaluated in other kidney diseases.37, 38, 39, 40 To our knowledge, there are no interventional studies in primary FSGS focusing on long-term preservation of eGFR. Available interventional studies have so far evaluated proteinuric responses such as complete and partial remission in response to a variety of treatments.41, 42, 43, 44, 45 Assessment of potential long-term efficacy of these treatments has been based on the widely held assumption that treatment-associated reduction in proteinuria is a strong predictor of long-term outcomes. This notion is supported by several retrospective analyses of the long-term clinical course of FSGS.11,46,47 In this context, the DUPLEX trial will provide an important contribution to this discussion by linking periodic monitoring of treatment-associated changes in proteinuria with long-term trajectories of decline in kidney function.
In addition to the assessment of eGFR slope, DUPLEX will include interim evaluation of the proportion of patients reaching FPRE at 36 weeks. As recently published,11 FPRE is strongly predictive of renal survival in primary FSGS. In the DUET study, 3 times more sparsentan-treated patients reached FPRE compared with their irbesartan-treated counterparts after 2 months of double-blind treatment.26 The proportion of responders on sparsentan increased to approximately 50% during the first year of the open-label extension.34 The novel approach of using FPRE as an early indicator of treatment benefit to be confirmed by eGFR slope in clinical trials for FSGS is the result of meaningful interactions with regulatory agencies.
Fluid retention as a consequence of ETA receptor blockade remains one of the potential safety concerns in patients treated with ETA receptor antagonists,23,48 and diuretics can be used to help effectively manage edema. In the DUET study, edema-related, treatment-related AEs were reported more frequently with sparsentan than with irbesartan (12.3% vs. 2.8% of patients, respectively) during the 8-week double-blind treatment period, but there were no significant changes in severity of edema. Laboratory indicators of hemodilution were also noted. However, no patient withdrew because of edema or fluid overload during the double-blind period. Nonetheless, careful monitoring of various indicators of fluid retention has been incorporated into the DUPLEX design.
Kidney Disease: Improving Global Outcomes guidelines9 assert that endpoints pertaining to patients’ perceptions of QOL related to their disease and treatment are important but often undermeasured and/or underappreciated. Further, these guidelines identify QOL as a substantial data gap in reports of interventional studies in glomerular diseases. FSGS and nephrotic syndrome, as well as the current immunosuppressive treatments for these conditions, have a substantially negative impact on QOL.9,49 Therefore, in addition to evaluation of the safety and efficacy of sparsentan, DUPLEX will provide an opportunity to assess QOL parameters in a long-term double-blind study setting.
In conclusion, the DUPLEX study will, for the first time, enable the evaluation of the long-term nephroprotective effects and safety of a novel agent, sparsentan, in patients with FSGS, potentially offering a new treatment option for this rare disorder.
Disclosure
This study is supported by Retrophin, Inc., San Diego, CA. Retrophin, Inc., was involved in the trial design and protocol development. RK, UD, AL, and WER are employees of Retrophin, Inc., and may have equity or other financial interest in Retrophin, Inc. JKI is an employee of IQVIA, Inc. and was hired by Retrophin, Inc., to conduct the research. She did not receive any payment or honoraria directly from Retrophin, Inc., for services rendered. HT has received consultancy fees from Kaneka Inc., Otsuka, and ChemoCentryx and was previously a consultant to Genzyme and Optherion. He is an unpaid consultant to Retrophin, Inc., and has an agreement with Goldfinch Biopharma through New York University.
Acknowledgments
Editorial support was provided by Kristen W. Quinn, PhD, of Peloton Advantage, LLC, and Lynanne McGuire, PhD, CMPP, of MedVal Scientific Information Services, LLC, and was funded by Retrophin, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP3 Guidelines. Trial registrations: EudraCT number: 2016-005141-23; US ClinicalTrials.gov identifier: NCT03493685.
Author Contributions
UD, JKI, RK, WER, and HT designed the study; HT was the study investigator and managed patient enrollment; UD and HT collected, assembled, and analyzed data; all authors interpreted data; UD, WER, RK, HT, and JKI prepared the manuscript; all authors reviewed, revised, and provided final approval of the manuscript. The authors were involved in the decision to submit this manuscript and will take public responsibility for all aspects of the publication.
Footnotes
Table S1. Countries participating in DUPLEX.
Table S2. Rationale for avoidance of SGLT2 Inhibitors, potassium-sparing diuretics, and CYP3A4 inhibitors.
Table S3. Treatment effects on eGFR slope reported in previous studies.
Supplementary Material
References
- 1.D'Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 2.Reiser J., Nast C.C., Alachkar N. Permeability factors in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis. 2014;21:417–421. doi: 10.1053/j.ackd.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kronbichler A., Saleem M.A., Meijers B., Shin J.I. Soluble urokinase receptors in focal segmental glomerulosclerosis: a review on the scientific point of view. J Immunol Res. 2016;2016:2068691. doi: 10.1155/2016/2068691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadowski C.E., Lovric S., Ashraf S. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26:1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korbet S.M. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23:1769–1776. doi: 10.1681/ASN.2012040389. [DOI] [PubMed] [Google Scholar]
- 7.United States Renal Data System . United States Renal Data System; Ann Arbor, MI: 2015. 2015 USRDS Annual Data Report Volume 2: ESRD in the United States. [Google Scholar]
- 8.Spino C., Jahnke J.S., Selewski D.T., Massengill S., Troost J., Gipson D.S. Changing the paradigm for the treatment and development of new therapies for FSGS. Front Pediatr. 2016;4:25. doi: 10.3389/fped.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 10.Kee Y.K., Yoon C.Y., Kim S.J. Determination of the optimal target level of proteinuria in the management of patients with glomerular diseases by using different definitions of proteinuria. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000008154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troost J.P., Trachtman H., Nachman P.H. An outcomes-based definition of proteinuria remission in focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2018;13:414–421. doi: 10.2215/CJN.04780517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattran D.C., Rao P. Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis. 1998;32:72–79. doi: 10.1053/ajkd.1998.v32.pm9669427. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulos E., Stangou M., Papagianni A., Pantzaki A., Papadimitriou M. Factors influencing the course and the response to treatment in primary focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15:1348–1356. doi: 10.1093/ndt/15.9.1348. [DOI] [PubMed] [Google Scholar]
- 14.Sethna C.B., Gipson D.S. Treatment of FSGS in children. Adv Chronic Kidney Dis. 2014;21:194–199. doi: 10.1053/j.ackd.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Pullen N., Fornoni A. Drug discovery in focal and segmental glomerulosclerosis. Kidney Int. 2016;89:1211–1220. doi: 10.1016/j.kint.2015.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohan D.E., Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014;86:896–904. doi: 10.1038/ki.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagliardini E., Corna D., Zoja C. Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol. 2009;297:F1448–F1456. doi: 10.1152/ajprenal.00340.2009. [DOI] [PubMed] [Google Scholar]
- 18.Zoja C., Cattaneo S., Fiordaliso F. Distinct cardiac and renal effects of ETA receptor antagonist and ACE inhibitor in experimental type 2 diabetes. Am J Physiol Renal Physiol. 2011;301:F1114–F1123. doi: 10.1152/ajprenal.00122.2011. [DOI] [PubMed] [Google Scholar]
- 19.Buelli S., Rosano L., Gagliardini E. b-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J Am Soc Nephrol. 2014;25:523–533. doi: 10.1681/ASN.2013040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann J.F., Green D., Jamerson K. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhaun N., MacIntyre I.M., Kerr D. Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension. 2011;57:772–779. doi: 10.1161/HYPERTENSIONAHA.110.167486. [DOI] [PubMed] [Google Scholar]
- 22.Kohan D.E., Pritchett Y., Molitch M. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2011;22:763–772. doi: 10.1681/ASN.2010080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Zeeuw D., Coll B., Andress D. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol. 2014;25:1083–1093. doi: 10.1681/ASN.2013080830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komers R., Plotkin H. Dual inhibition of renin-angiotensin-aldosterone system and endothelin-1 in treatment of chronic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2016;310:R877–R884. doi: 10.1152/ajpregu.00425.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komers R., Gipson D.S., Nelson P. Efficacy and safety of sparsentan compared with irbesartan in patients with primary focal segmental glomerulosclerosis: randomized, controlled trial design (DUET) Kidney Int Rep. 2017;2:654–664. doi: 10.1016/j.ekir.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trachtman H., Nelson P., Adler S. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J Am Soc Nephrol. 2018;29:2745–2754. doi: 10.1681/ASN.2018010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan J, Derebail VK, Murphy E, et al. Long-term effects of sparsentan, a dual angiotensin and endothelin receptor antagonist in primary focal segmental glomerulosclerosis (FSGS): interim 84-week analysis of the DUET trial [oral presentation]. Annual Meeting of the American Society of Nephrology, October 23–28, 2018, San Diego, CA.
- 28.ClinicalTrials.gov. Study of sparsentan in FSGS (DUPLEX) 2019. https://clinicaltrials.gov/ct2/show/NCT03493685?term=NCT03493685&rank=1
- 29.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz G.J., Work D.F. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 31.Wanner C., Inzucchi S.E., Lachin J.M. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 32.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 33.Perkovic V., Jardine M.J., Neal B. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 34.Trachtman H, Rychlik I, Haws RM, Nester CM, Fornoni A, Komers R. Long-term effect of sparsentan (SPAR), a dual angiotensin and endothelin receptor antagonist, on proteinuria in patients with primary FSGS: interim analysis of the DUET trial [oral presentation]. Annual Meeting of the American Society of Nephrology, October 31–November 5, 2017, New Orleans, LA.
- 35.Trachtman H., Rychlik I., Haws R., Nester C., Fornoni A., Komers R. Newly administered immunosuppressive therapy (IST) has no impact on long-term antiproteinuric effect of sparsentan (SPAR), a dual angiotensin and endothelin receptor antagonist, in patients with primary focal segmental glomerulosclerosis (FSGS): interim analysis of the DUET trial [abstract] Nephrol Dial Transplant. 2018;33(suppl 1):i20. [Google Scholar]
- 36.Levey A.S., Gansevoort R.T., Coresh J. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75:84–104. doi: 10.1053/j.ajkd.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Coresh J., Turin T.C., Matsushita K. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inker L.A., Heerspink H.L. Evaluation of surrogate end points for progression to ESKD: necessary and challenging. Am J Kidney Dis. 2018;72:771–773. doi: 10.1053/j.ajkd.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Weldegiorgis M., de Zeeuw D., Li L. Longitudinal estimated GFR trajectories in patients with and without type 2 diabetes and nephropathy. Am J Kidney Dis. 2018;71:91–101. doi: 10.1053/j.ajkd.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Torres V.E., Chapman A.B., Devuyst O. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gipson D.S., Trachtman H., Kaskel F.J. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868–878. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segarra Medrano A., Vila Presas J., Pou Clave L., Majo Masferrer J., Camps Domenech J. Efficacy and safety of combined cyclosporin A and mycophenolate mofetil therapy in patients with cyclosporin-resistant focal segmental glomerulosclerosis. Nefrologia. 2011;31:286–291. doi: 10.3265/Nefrologia.pre2011.Feb.10870. [DOI] [PubMed] [Google Scholar]
- 43.Hogan J., Bomback A.S., Mehta K. Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin J Am Soc Nephrol. 2013;8:2072–2081. doi: 10.2215/CJN.02840313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogg R.J., Friedman A., Greene T. Renal function and proteinuria after successful immunosuppressive therapies in patients with FSGS. Clin J Am Soc Nephrol. 2013;8:211–218. doi: 10.2215/CJN.08330812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beer A., Mayer G., Kronbichler A. Treatment strategies of adult primary focal segmental glomerulosclerosis: a systematic review focusing on the last two decades. BioMed Res Int. 2016;2016:4192578. doi: 10.1155/2016/4192578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Troyanov S., Wall C.A., Miller J.A., Scholey J.W., Cattran D.C. Toronto Glomerulonephritis Registry Group. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 47.Gipson D.S., Chin H., Presler T.P. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21:344–349. doi: 10.1007/s00467-005-2097-0. [DOI] [PubMed] [Google Scholar]
- 48.Heerspink H.J.L., Parving H.H., Andress D.L. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 49.Gipson D.S., Trachtman H., Kaskel F.J. Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int. 2011;79:678–685. doi: 10.1038/ki.2010.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.