Abstract
Introduction
Online postdilution hemodiafiltration (HDF) is associated with a lower all-cause and cardiovascular mortality than hemodialysis (HD). This may depend on a superior peridialytic (pre- and postdialysis, and the difference between these 2 parameters) hemodynamic profile.
Methods
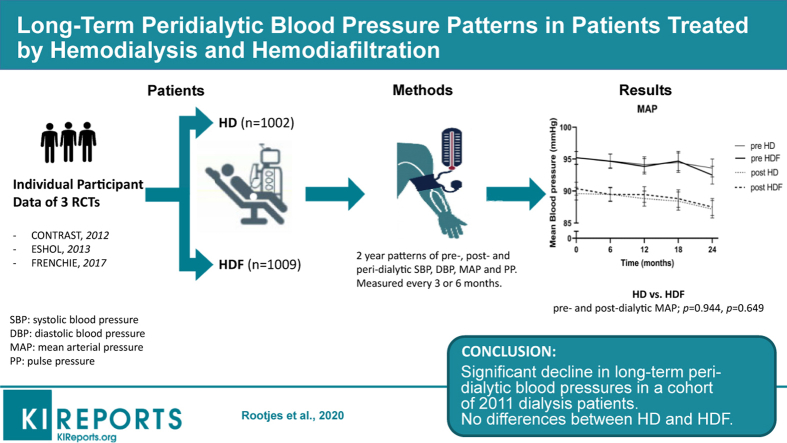
In this retrospective cohort analysis of individual participant data (IPD) from 3 randomized controlled trials (RCTs) (n = 2011), the effect of HDF and HD on 2-year peridialytic blood pressure (BP) patterns was assessed. Long-term peridialytic systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and pulse pressure (PP), as well as the deltas (post- minus predialytic) were assessed in the total group of patients. Thereafter, these variables were compared between patients on HD and HDF, and in the latter group between quartiles of convection volume.
Results
Mean pre- and postdialysis SBP, DBP, and MAP declined significantly during follow-up (predialytic: SBP −2.16 mm Hg, DBP −2.88 mm Hg, MAP −2.64 mm Hg), PP increased (predialytic 0.96 mm Hg). Peridialytic deltas remained unaltered. Differences between the 2 modalities, or between quartiles of convection volume were not observed. BP changes were independent of various baseline characteristics, including the decline in body weight over time.
Conclusion
We speculate that the combination of a decreasing SBP and an increasing PP may be the clinical sequelae of a worsening cardiovascular system. Because especially HDF with a high convection volume has been associated with a beneficial effect on survival, our study does not support the view that superior peridialytic BP control contributes to this effect.
Keywords: blood pressure, hemodiafiltration, hemodialysis, individual participant data, peridialytic
Graphical abstract
Previously, we reported a beneficial effect on overall and cardiovascular survival in a meta-analysis based on IPD of 4 RCTs, comparing online postdilution HDF with HD.1,2
While in HD, uremic retention products are removed by diffusion, in HDF, accumulated uremic toxins are removed by both diffusion and convection. Current efforts to improve the prospects of chronic dialysis patients are not only directed toward a restoration of the milieu interieur, but also toward a reduction of hemodynamic instability during (intradialytic) and around (peridialytic) the dialysis procedure itself.
Clinical studies not only showed varying BP drops during HD,3 but also a decrease in the perfusion of the brain,4 the heart,5 and the kidneys.6 Contradictory findings were found as to whether the prevalence of BP drops and perfusion deficits are interrelated.7,8 In a study using echocardiography, cardiac dysfunction occurred without simultaneous changes in ultrafiltration.5 BP stability could be improved by using cooled dialysate as well as by treatment with HDF.9, 10, 11 Refined research showed that both treatment modalities resulted in a comparable loss of thermal energy, which may have contributed to a superior BP control during ultrafiltration.12
Because HDF may induce less hemodynamic stress than standard HD, and hence less tissue hypoperfusion and organ damage, it is interesting to know whether the long-term course of peridialytic (pre- and postdialysis) BP values differ between HD and HDF. Although several studies analyzed the relation between a single or multiple peridialytic BP measurements at entry and clinical outcome in the years thereafter,13,14 hardly any study analyzed the long-term course of peridialytic BP itself.15 In fact, it is currently uncertain whether the pre- and postdialytic SBP, DBP, MAP, and PP change over time and whether these changes are different between patients treated with HD and HDF.
Our goals were 3-fold. First, to investigate the patterns of the pre- and postdialytic SBP, DBP, MAP, and PP and the delta (post- minus predialytic BP) over time. Second, because HDF has a beneficial effect on overall and cardiovascular survival and the mechanisms of this effect are unknown, it seems relevant to know if HD and HDF have a dissimilar influence on the long-term peridialytic BP values. Third, as recurrent hemodynamic instability is associated with a poor clinical outcome, an analysis of the determinants of long-term peridialytic BP changes was performed.
Methods
Study Design
For this retrospective cohort analysis, the combined IPD base of 4 multicenter RCTs, comparing HD with HDF in adult patients, was used.2 A detailed description of the study designs, patient eligibility criteria, and treatment procedures of each of the individual RCTs has been provided elsewhere.11,16, 17, 18 Because peridialytic BP measurements were not available in one study,18 the data of 3 RCTs were used for the present analysis. Because of limited follow-up time (mean, 21.3 ± 5.9 months) of the French RCT (see later in this article), we used only 2-year data for the main analysis of this study.
Summary of Study Populations
Convective Transport Study (CONTRAST) included 714 patients treated by HD for >2 months in dialysis centers in the Netherlands, Canada, and Norway.16 Online HDF was performed with a recommended target convection volume of 6 l/hour (i.e., generally 24 l/session). The Spanish On-Line Haemodiafiltration Survival Study (ESHOL) cohort included 906 patients treated by HD for >3 months, with a minimum of 18 l/session of convection volume for HDF treatments.11 French Convective versus Hemodialysis in Elderly (FRENCHIE) consisted of 391 individuals with a minimum age of 65 years treated for at least 1 month, with no target HDF convection volume specified.17 In all 3 studies, patients were randomized 1:1 to either continuation of HD or to HDF, for the great majority in a thrice-weekly treatment schedule. Although the patients in the control group of the CONTRAST study were dialyzed with low-flux membranes, in the other 2 studies, mainly high-flux membranes were used.
Peridialytic BP Measurements
In all patients, SBP and DBP were measured immediately before (predialytic) and after (postdialytic) dialysis by means of automatically inflated cuffs using a digital monitor attached to each HD machine according to the standard dialysis unit protocols. MAP was calculated as [1/3∗SBP + 2/3∗DBP] and PP as [SBP – DBP]. Peridialytic hemodynamical changes (deltas) were calculated by subtracting post-HD from pre-HD values. All measurements were averaged at 3 (CONTRAST and ESHOL) or 6 months (FRENCHIE) intervals from the last 3 dialysis sessions before a control visit.
Clinical Measurements
At baseline, information on demographics, history of diabetes, cardiovascular disease (CVD) and duration of dialysis (vintage) was assessed. Body mass index (BMI; kg/m2) was calculated as post-dialysis weight (kg)/height2 (m). History of CVD was defined as myocardial infarction, angina pectoris, therapeutic coronary procedure (percutaneous transluminal coronary angioplasty or stenting), transient ischemic attack, stroke, therapeutic carotid procedure (endarterectomy or stenting), percutaneous transluminal angioplasty, and vascular intervention (revascularization, percutaneous transluminal angioplasty, or stenting), or amputation.2
Statistical Analysis
Descriptive statistics were calculated as mean (SD), median (interquartile range), or number (percentage [%]) when appropriate. Follow-up was limited to 24 months, as these BP values were available in all 3 studies. First, the course over time of all BP values was visualized. Next, the rate of change of the 12 described BP values was calculated using generalized linear mixed models with a random intercept, random slope, or both, depending on the lowest Aikaike Information Criterion. Hereafter, a possible difference in rate of change in various subgroups was investigated using an interaction term in a linear mixed model between the specific subgroup and time. For example, a potential difference in rate of change between patients treated with HD or HDF was investigated using the interaction term dialysis modality × time. The influence of the magnitude of convection volume in patients treated with HDF was analyzed by dividing the convection volume in quartiles (Q) (Q1 <19 L, Q2 19–23 L, Q3 23–26 L and Q4 >26 L). The influence of the change in predialytic, postdialytic, and delta (post- minus predialytic) body weight during the follow-up period on the course of BP over time was analyzed as well. The baseline conditions that were investigated included dialysis modality, the 3 individual RCTs, gender, diabetes, history of CVD (dichotomous), SBP >140 mm Hg (dichotomous), and quartiles of the following variables: age, BMI, dialysis vintage, calcium, and phosphate. To minimize the possibility of a type 1 statistical error as a result of multiple testing, we applied the Holm-Bonferroni Method.19
Sensitivity Analyses
To increase the robustness of our findings, the previously mentioned analyses were repeated by extending the follow-up time to 3 years using only the CONTRAST and ESHOL studies in which these data are available. Furthermore, besides an analysis in quartiles, convection volume was investigated in tertiles and as a continuous variable as well.
Results
Patient Characteristics
In Table 1 and Supplementary Table S1, the baseline demographic and clinical patient characteristics, laboratory data, and treatment-related parameters are summarized. For the pooled cohort (n = 2011), mean age was 67.0 ± 13.7, 1287 (64.0%) were men, 542 (27.0%) had diabetes, and 807 (40.1%) had a history of CVD. As shown in Supplementary Table S1, patients in the French study were older than in the other 2 RCTs and had a longer dialysis vintage. In patients from the CONTRAST study, both the SBP and DBP at baseline were noticeably higher as compared with the other study cohorts. The kidney transplantation rate between HD and HDF patients was more or less equal for the entire follow-up period (n = 156 [15.7%] for HD; n = 182 [18.0%] for HDF patients). The kidney transplantation rate of patients in the French study was considerably lower as compared with CONTRAST and ESHOL (FRENCHIE n = 7 [1.8%]; CONTRAST n = 151 [21.1%]; ESHOL n = 180 [19.9%]).
Table 1.
Baseline patient characteristics
| n | Pooled |
HD |
HDF |
|---|---|---|---|
| 2011 | 1002 | 1009 | |
| Age (yr) | 67.0 ± 13.7 | 67.4 ± 13.6 | 66.7 ± 13.9 |
| Male (%) | 1287 (64.0) | 637 (63.6) | 650 (64.4) |
| Diabetes (%) | 542 (27.0) | 272 (27.1) | 270 (26.8) |
| SBP >140 mm Hg at baselinea (%) | 988 (49.1) | 489 (48.8) | 499 (49.5) |
| CVD history (%) | 807 (40.1) | 413 (41.2) | 394 (39.0) |
| BMI (kg/m2) | 25.3 ± 4.7 | 25.4 ± 4.6 | 25.3 ± 4.8 |
| Cholesterol (mmol/l) | 3.8 ± 1.3 | 3.9 ± 1.3 | 3.8 ± 1.2 |
| Kt/Vureab | 1.5 ± 0.3 | 1.5 ± 0.3 | 1.6 ± 0.3 |
| Dialysis duration (min) | 232.1 ± 22.2 | 232.1 ± 21.7 | 232.0 ± 22.6 |
| Dialysis vintage (yr) | 2.3 (1.1–4.7) | 2.3 (1.1–4.7) | 2.3 (1.1–4.8) |
| Systolic blood pressure (mm Hg)c | 140.8 ± 23.5 | 140.9 ± 23.8 | 140.7 ± 23.1 |
| Diastolic blood pressure (mm Hg)c | 72.1 ± 14.5 | 71.9 ± 14.6 | 72.3 ± 14.4 |
BMI, body mass index in (kg/m2); CVD, cardiovascular disease; HD, hemodialysis; HDF, hemodiafiltration.
Values are mean ± SD or median (Q1–Q3) for continuous variables, and number (%) for categorical variables.
Systolic blood pressure before dialysis at baseline >140 mm Hg.
Kt/Vurea = dialysis clearance of urea.
Before dialysis.
Longitudinal Pre- and Postdialytic Hemodynamic Changes
Overall Cohort
As shown in Table 2 and depicted in Figure 1, both mean pre- and postdialytic SBP, DBP, and MAP declined significantly over a time span of 2 years, in all 3 cohorts (pre- and postdialytic SBP: −2.16 mm Hg [P < 0.001], −1.68 mm Hg [P = 0.005]; pre- and postdialytic DBP: −2.88 mm Hg [P < 0.001], −3.12 mm Hg [P < 0.001]; pre- and postdialytic MAP: −2.64 mm Hg [P < 0.001], −2.64 mm Hg [P < 0.001]). By contrast, mean PP increased (pre- and postdialytic PP: 0.96 mm Hg [P = 0.064], 1.44 mm Hg [P = 0.004]). Even greater declines in SBP, DBP, and MAP were obtained in the IPD analysis of 2 RCTs with a follow-up of 3 years (Supplementary Table S2).
Table 2.
Blood pressure changes in 2 years of follow-up
| Blood pressure | Time point | Change per year (mm Hg) | P value |
|---|---|---|---|
| SBP | Predialytic | −1.08 (−1.56 to −0.48) | <0.001 |
| Postdialytic | −0.84 (−1.44 to −0.24) | 0.005 | |
| Deltaa | −0.00 (−0.60 to 0.60) | 0.964 | |
| DBP | Predialytic | −1.44 (−1.80 to −1.08) | <0.001 |
| Postdialytic | −1.56 (−1.80 to −1.20) | <0.001 | |
| Deltaa | −0.24 (−0.60 to 0.12) | 0.205 | |
| PP | Predialytic | 0.48 (−0.00 to 0.84) | 0.064 |
| Postdialytic | 0.72 (0.24–1.20) | 0.004 | |
| Deltaa | 0.12 (−0.36 to 0.72) | 0.534 | |
| MAP | Predialytic | −1.32 (−1.68 to −0.96) | <0.001 |
| Postdialytic | −1.32 (−1.68 to −0.96) | <0.001 | |
| Deltaa | −0.12 (−0.48 to 0.24) | 0.477 |
DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
Mean yearly blood pressure changes in mm Hg in 2 years of follow-up with 95% confidence intervals.
Delta = postdialytic minus predialytic blood pressure.
Figure 1.
Course of pre- and postdialytic blood pressure over time in hemodialysis (HD) and hemodiafiltration (HDF) patients. (a) Mean pre- and postdialytic systolic blood pressure (SBP). (b) Mean pre- and postdialytic diastolic blood pressure (DBP). (c) Mean pre- and postdialytic pulse pressure (PP). (d) Mean pre- and postdialytic mean arterial pressure (MAP). Error bars indicate 95% confidence intervals of the mean.
HDF Versus HD
As depicted in Figure 1, mean pre- and postdialytic SBP, DBP, MAP, and PP did not differ significantly between HDF and HD patients in a follow-up period of 2 years (difference HDF vs. HD: pre- and postdialytic SBP: P = 0.589, P = 0.692; pre- and postdialytic DBP: P = 0.537, P = 0.656; pre- and postdialytic MAP: P = 0.944, P = 0.649; pre- and postdialytic PP: P = 0.301, P = 0.822).
Rate of Change of Peridialytic Deltas
Overall Cohort
As shown in Table 2, the mean deltas (the difference between post- and predialytic values) of the SBP, DBP, MAP, and PP did not alter over 2 years. As for the mean pre- and postdialytic SBP, DBP, MAP, and PP, similar results were obtained in the sensitivity analysis of 2 RCTs with a follow-up of 3 years (Supplementary Table S2).
HDF Versus HD
As depicted in Supplementary Figure S1, neither the mean deltas of SBP, DBP, MAP, nor PP differed significantly between HDF and HD over a time span of 2 years (difference HDF vs. HD: delta SBP: P = 0.246; delta DBP: P = 0.738; delta MAP: P = 0.613; delta PP: P = 0.146). More or less similar results were obtained in the sensitivity analysis of 2 RCTs with a follow-up of 3 years (data not shown).
Influence of the Magnitude of the Convection Volume on Long-term BP in HDF Patients
For this analysis, the convection volume was divided in quartiles (Q) depending on its magnitude in liters (Q1 <19 L, Q2 19–23 L, Q3 23–26 L, and Q4 >26 L). As shown in Supplementary Table S3, neither the mean pre- and postdialytic SBP, DBP, MAP, and PP were different among the 4 quartiles of the convection volume, nor the mean deltas of these parameters. A sensitivity analysis of 2 RCTs with a follow-up of 3 years yielded similar results (data not shown).
Influence of Baseline Conditions on the Course of BP Over Time
None of the long-term hemodynamic patterns was influenced by the baseline characteristics of age, gender, history of CVD, diabetes, calcium and phosphate level, BMI, dialysis vintage, SBP >140 mm Hg, and study cohort (data not shown).
Influence of the Change in Body Weight on the Course of BP Over Time
For this analysis, first the changes in pre-, postdialytic, and delta (post- minus predialytic) bodyweight during follow-up were analyzed. Both pre- and postdialytic body weights declined significantly (predialytic: −0.971 kg [P < 0.001]; postdialytic: −0.983 kg [P < 0.001]), whereas the delta did not change (0.033 kg [P = 0.359]). Second, the influence of the changes in pre-, postdialytic, and delta bodyweight on the course of BP over time was analyzed. With the exception of PP, none of the other hemodynamic patterns was influenced by the changes in body weight (Supplementary Table S4).
Discussion
As far as we know, this is the first meta-analysis investigating longitudinal peridialytic BP patterns by using an IPD approach. From this retrospective analysis it appears first, that, although both mean pre- and postdialytic SBP, DBP, and MAP decreased over time, mean PP increased. The deltas (post- minus predialytic BP values) remained unaltered. Second, differences between patients treated with HD and HDF were not observed, neither as solitary pre- and postdialytic BP values, nor as deltas over time. Differences between quartiles of the convection volume were not found in the latter group of patients. Third, the long-term course of BP was neither influenced by age, gender, history of CVD, diabetes, calcium and phosphate level, BMI, dialysis vintage, SBP >140 mm Hg at baseline and study cohort (CONTRAST, ESHOL, or FRENCHIE), nor by changes in body weight over time. These findings were observed in all 3 distinct cohorts, although separate patient groups were treated in different countries, by local doctors and nursing staffs, according to regional protocols and local traditions. Therefore, our data seem robust and not determined by an accidental concurrence of circumstances, which support the external generalizability of this study.
Our overall results are remarkably similar to the data of an observational study on BP patterns in almost 10,000 HD patients stratified over various age groups. From this study, it appeared that both peridialytic SBP and DBP decline among elderly HD patients (>50 years of age), whereas MAP remains stable and PP tends to increase.15 As in our study, the latter phenomenon was caused predominantly by a more pronounced drop in DBP.
The (patho)physiological background behind these changes is not readily apparent from our study. The long-term courses of the various BP patterns were neither influenced by age, gender, history of CVD, diabetes, calcium and phosphate level, BMI, dialysis vintage, SBP >140 mm Hg, and study cohort at baseline, nor by changes in body weight over time. Because most patients who start HD are fluid overloaded,20 it is conceivable that a gradual removal of isosmotic body water by ultrafiltration underlies the observed decrease in BP. Yet, correction for change in body weight, which declined over time in this patient group, did not alter the results. Therefore, other factors, such as a worsening ratio between lean body mass and body water, a decreasing cardiac performance,21,22 advanced large vessel disease, and microcirculatory dysfunction23 may cause these changes. Finally, it is possible that a growing atrophy of the kidneys leads to a decreased activity of the renin-angiotensin-aldosterone system and a reduction in sympathic nerve activity, which contribute to a declining peripheral vascular resistance over time in these patients.21,24
Considering the highly significant but, admittedly, small decreases in SBP and DBP, it is questionable whether these findings (mean predialytic: −2.16 mm Hg and −2.88 mm Hg in 2 years, respectively) have any impact on the clinical outcome and cardiac death.1 However, as extensively discussed elsewhere,15 in the general population, SBP tends to rise steadily during the entire life, whereas DBP flattens in midlife and decreases in the years thereafter. Whereas especially a high SBP has been recognized as a risk factor for mortality in all age groups,25 an isolated low DBP has been linked to an inferior clinical outcome in older individuals.26,27 Because SBP rises continuously and DBP declines after midlife, mean PP, which is considered a risk factor for mortality,28 rises in most older people. Translating these data into the elderly HD patients in the present study (mean age, 67.0 ± 13.7 years), the picture seems to emerge of a modest reduction in the mortality risk resulting from a lower SBP on the one hand, and a slight increase resulting from a lower DBP and a greater PP on the other. In fact, one might argue that the harms and benefits of the longitudinal hemodynamic changes in HD patients may neutralize each other. Yet, we wonder whether this idea holds true in these people as the relationships between BP and age differ between dialysis patients and the general population.15 Although in the non-renal population, atherosclerotic large vessel disease generally precedes the occurrence of heart failure, many chronic HD patients suffer from both heart failure and large vessel disease.29 Moreover, because CVD in these individuals is greatly accelerated, its symptoms and signs occur at a much younger age and worsen during their lifetimes. Given the high burden of traditional risk factors for CVD, such as hypertension and diabetes, and nontraditional risk factors, including mineral-bone disease and chronic fluid overload,20 the drop in SBP over time may be the result of premature structural and functional cardiac abnormalities.30 Indeed, in the CONTRAST study, it was previously found that SBP declined most in patients with a decreasing cardiac ejection fraction.22
Notably, the long-term course of the various BP measurements and the peridialytic changes over time were similar in HD and HDF patients. In the latter group, different quartiles of the convection volume yielded analogous results. Because especially high-volume HDF has been related to an improved survival,2 it is therefore unlikely that a superior peridialytic BP control explains these findings.
The strengths of this study include its large sample size, the meticulous data collection, the dedicated composition of the IPD base,2 and the careful extension of the original IPD set with the peridialytic BP values from 3 of the 4 original RCTs. For this purpose, data were transported and united into the IPD base from a diversity of languages and formats, a varying duration of follow-up (minimum 21.1 and maximum 36.5 months) and dissimilar intervals of Riva-Rocci measurements (3–6 months). Important limitations include the use of multiple testing, which was partially averted by application of the Holm-Bonferroni Method,19 the lack of measuring BP in a standardized way among all RCTs, and the lack of information on the use of antihypertensive medication in 2 of the 3 RCTs. Interestingly, in CONTRAST it was previously found that both SBP and DBP decline over the years, despite a significant reduction in antihypertensive medication.31 Other limitations include the limited generalizability, as the study population is mainly European, and the restricted follow-up, which, however, was chosen because of the limited duration of FRENCHIE. In addition, no correction was made for transplanted patients, because BP measurements after transplantation were not available.32
In summary, from this retrospective cohort analysis of IPD from 3 RCTs, it appears that mean pre- and postdialytic SBP, DBP, and MAP declined significantly over time, whereas PP increased (predialytic SBP −2.16 mm Hg, DBP −2.88 mm Hg, MAP −2.64 mm Hg, and PP 0.96 mm Hg in 2 years). Peridialytic BP differences remained unaltered. No differences between HD and HDF, or between quartiles of convection volume were observed. Changes in BP were independent of age, gender, history of CVD, diabetes, calcium and phosphate level, BMI, dialysis vintage, SBP >140 mm Hg at baseline and study cohort, as well as from the change in body weight over time. Although the reductions in the pre- and postdialytic SBP seem rather small, in elderly subjects without kidney disease, SBP tends to rise over the years. Therefore, we speculate that the decrease in SBP, together with the increase in PP, over a time span of only 2 years may be the clinical sequelae of a structural and functional worsening cardiovascular system and embody a poor prognostic sign. At this point, it should be noted, however, that the BP targets for an optimal CVD outcome in HD patients are largely unknown because of the lack of long-term studies.33 Because especially high-volume-HDF has been associated with a beneficial effect on overall and cardiovascular survival, our study does not support the view that a superior peridialytic BP control is an important mechanism behind this effect. Whether the intradialytic hemodynamic profile of (high-volume) HDF compares favorably with HD is an intriguing and fascinating question that deserves further study.
Appendix
HDF Pooling Project Investigators
The following persons participated in the HDF Pooling Project:
Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht the Netherlands: Michiel L. Bots; Department of Nephrology & Hypertension, University Medical Center Utrecht, Utrecht, the Netherlands: Peter J. Blankestijn; Center of Excellence Medical, Fresenius Medical Care GmbH, Bad Homburg, Germany and University of Montpellier, Research and Training Unit Medicine, Montpellier, France: Bernard Canaud; Royal Free Hospital, University College London Medical School, London, United Kingdom: Andrew Davenport; Department of Nephrology and Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, VU University, Amsterdam, the Netherlands: Muriel P.C. Grooteman and Menso J. Nubé; The George Institute for Global Health, University of Oxford, Oxford, United Kingdom: Sanne A.E. Peters; PhyMedExp, National Institute of Health and Medical Research (INSERM), French National Centre for Scientific Research (CNRS), University of Montpellier, Department of Biochemistry and Endocrinology, Central University Hospital (CHU) Montpellier, Montpellier, France: Marion Morena; Department of Nephrology, Hospital Clinic, Barcelona, Spain: Francisco Maduell and Ferran Torres; Division of Nephrology, Ege University School of Medicine, Izmir, Turkey: Ercan Ok and Gulay Asci; and Department of Nephrology, Alessandro Manzoni Hospital, Lecco, Italy: Francesco Locatelli.
Disclosure
PAR, MPCG, and MJN report grant support from Niercentrum aan de Amstel, Elyse Klinieken, and B. Braun Avitum AG. MPCG and MJN report receiving grant support from the Dutch Kidney Foundation, Fresenius Medical Care Netherlands BV, Gambro Sweden, Twiss Fund, and ZonMw during the conduct of the study. BC reports being a part-time employee of Fresenius Medical Care acting as scientific consultant. PJB reports grant support from Fresenius Medical Care, ZonMw, Baxter, Gambro, and the Dutch Kidney Foundation during the conduct of the study. FJvI reports grant support from Elyse Klinieken and B. Braun Avitum AG. He received personal fees from Shire. FM reports receiving personal fees from Baxter, Fresenius Medical Care, Medtronic, and Nipro, all outside this submitted work. SAEP reports grant support by receiving a UK Medical Research Council Skills Development Fellowship grant. AD reports grant support by receiving a UK NIHR grant. All the other authors declared no competing interests.
Acknowledgment
For this particular study there was no funding nor grant support. However, this study used the individual participant data (IPD) from 3 randomized controlled trials: the Convective Transport Study (CONTRAST), the French Convective versus Hemodialysis in Elderly (FRENCHIE) study, and the On-Line Haemodiafiltration Survival Study (ESHOL). These studies did receive support as follows. CONTRAST was supported by a grant from the Dutch Kidney Foundation (Nierstichting Nederland Grant C02.2019) and unrestricted grants from Fresenius Medical Care, Netherlands, and Gambro Lundia AB, Sweden. Additional support was received from the Dr. E.E. Twiss Fund, Roche Netherlands, the International Society of Nephrology/Baxter Extramural Grant Program, and the Netherlands Organization for Health Research and Development (ZONMw Grant 170882802). The FRENCHIE study was supported by a grant from the French Ministry of Health (Programme Hospitaller de Recherche Clinique PHRC national chez les sujets âgés-UF 7753). ESHOL was partly supported by grants from Fresenius Medical Care and Gambro through the Catalan Society of Nephrology.
Footnotes
Table S1. Baseline patient characteristics pooled and stratified by study.
Table S2. Blood pressure change per month (2- and 3-year analysis).
Table S3. P values of the interaction term time × convection volume in quartiles (2-year follow-up).
Table S4. Influence of body weight on blood pressure changes in 2-year follow-up.
Figure S1. Mean delta (post- minus predialytic) blood pressure over time in hemodialysis (HD) and hemodiafiltration (HDF) patients.
STROBE Statement.
Contributor Information
Muriel P.C. Grooteman, Email: mpc.grooteman@amsterdamumc.nl.
HDF Pooling Project Investigators:
Michiel L. Bots, Peter J. Blankestijn, Bernard Canaud, Andrew Davenport, Muriel P.C. Grooteman, Menso J. Nubé, Sanne A.E. Peters, Marion Morena, Francisco Maduell, Ferran Torres, Ercan Ok, Gulay Asci, and Francesco Locatelli
Supplementary Material
References
- 1.Nube M.J., Peters S.A.E., Blankestijn P.J. Mortality reduction by post-dilution online-haemodiafiltration:a cause-specific analysis. Nephrol Dial Transplant. 2017;32:548–555. doi: 10.1093/ndt/gfw381. [DOI] [PubMed] [Google Scholar]
- 2.Peters S.A., Bots M.L., Canaud B. Haemodiafiltration and mortality in end-stage kidney disease patients:a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant. 2016;31:978–984. doi: 10.1093/ndt/gfv349. [DOI] [PubMed] [Google Scholar]
- 3.Kuipers J., Usvyat L.A., Oosterhuis J.K. Variability of predialytic, intradialytic, and postdialytic blood pressures in the course of a week: a study of Dutch and US maintenance hemodialysis patients. Am J Kidney Dis. 2013;62:779–788. doi: 10.1053/j.ajkd.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 4.MacEwen C., Sutherland S., Daly J. Relationship between hypotension and cerebral ischemia during hemodialysis. J Am Soc Nephrol. 2017;28:2511–2520. doi: 10.1681/ASN.2016060704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assa S., Hummel Y.M., Voors A.A. Changes in left ventricular diastolic function during hemodialysis sessions. Am J Kidney Dis. 2013;62:549–556. doi: 10.1053/j.ajkd.2013.02.356. [DOI] [PubMed] [Google Scholar]
- 6.Marants R., Qirjazi E., Grant C.J. Renal perfusion during hemodialysis: intradialytic blood flow decline and effects of dialysate cooling. J Am Soc Nephrol. 2019;30:1086–1095. doi: 10.1681/ASN.2018121194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchanan C., Mohammed A., Cox E. Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J Am Soc Nephrol. 2017;28:1269–1277. doi: 10.1681/ASN.2016060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasselaar J.J., Slart R.H., Knip M. Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant. 2009;24:604–610. doi: 10.1093/ndt/gfn501. [DOI] [PubMed] [Google Scholar]
- 9.Donauer J., Schweiger C., Rumberger B. Reduction of hypotensive side effects during online-haemodiafiltration and low temperature haemodialysis. Nephrol Dial Transplant. 2003;18:1616–1622. doi: 10.1093/ndt/gfg206. [DOI] [PubMed] [Google Scholar]
- 10.Locatelli F., Altieri P., Andrulli S. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J Am Soc Nephrol. 2010;21:1798–1807. doi: 10.1681/ASN.2010030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maduell F., Moreso F., Pons M. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–497. doi: 10.1681/ASN.2012080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Sande F.M., Dekker M.J., Leunissen K.M.L. Novel insights into the pathogenesis and prevention of intradialytic hypotension. Blood Purif. 2018;45:230–235. doi: 10.1159/000485160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lertdumrongluk P., Streja E., Rhee C.M. Changes in pulse pressure during hemodialysis treatment and survival in maintenance dialysis patients. Clin J Am Soc Nephrol. 2015;10:1179–1191. doi: 10.2215/CJN.09000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoji T., Tsubakihara Y., Fujii M. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 15.Rohrscheib M.R., Myers O.B., Servilla K.S. Age-related blood pressure patterns and blood pressure variability among hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1407–1414. doi: 10.2215/CJN.00110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grooteman M.P., van den Dorpel M.A., Bots M.L. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23:1087–1096. doi: 10.1681/ASN.2011121140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morena M., Jaussent A., Chalabi L. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 2017;91:1495–1509. doi: 10.1016/j.kint.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Ok E., Asci G., Toz H. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis:results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28:192–202. doi: 10.1093/ndt/gfs407. [DOI] [PubMed] [Google Scholar]
- 19.Aickin M., Gensler H. Adjusting for multiple testing when reporting research results:the Bonferroni vs Holm methods. Am J Public Health. 1996;86:726–728. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoccali C., Moissl U., Chazot C. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol. 2017;28:2491–2497. doi: 10.1681/ASN.2016121341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin N.W., de Abreu M., Borges L.E. Hemodynamic response to fluid removal during hemodialysis: categorization of causes of intradialytic hypotension. Nephrol Dial Transplant. 2018;33:1643–1649. doi: 10.1093/ndt/gfy048. [DOI] [PubMed] [Google Scholar]
- 22.Mostovaya I.M., Bots M.L., van den Dorpel M.A. Left ventricular mass in dialysis patients, determinants and relation with outcome. Results from the COnvective TRansport STudy (CONTRAST) PLoS One. 2014;9 doi: 10.1371/journal.pone.0084587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thang O.H., Serne E.H., Grooteman M.P. Capillary rarefaction in advanced chronic kidney disease is associated with high phosphorus and bicarbonate levels. Nephrol Dial Transplant. 2011;26:3529–3536. doi: 10.1093/ndt/gfr089. [DOI] [PubMed] [Google Scholar]
- 24.Mostovaya I.M. University Medical Center Utrecht; Enschede, Netherlands: 2014. Chapter 7: Peripheral resistance, cardiac output and blood pressure over time in end stage kidney disease. Results from the CONvective TRAnsport STudy (CONTRAST) [dissertation] [Google Scholar]
- 25.Lewington S., Clarke R., Qizilbash N. Age-specific relevance of usual blood pressure to vascular mortality:a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 26.Post Hospers G., Smulders Y.M., Maier A.B. Relation between blood pressure and mortality risk in an older population: role of chronological and biological age. J Intern Med. 2015;277:488–497. doi: 10.1111/joim.12284. [DOI] [PubMed] [Google Scholar]
- 27.Taylor B.C., Wilt T.J., Welch H.G. Impact of diastolic and systolic blood pressure on mortality: implications for the definition of “normal.”. J Gen Intern Med. 2011;26:685–690. doi: 10.1007/s11606-011-1660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss A., Boaz M., Beloosesky Y. Pulse pressure predicts mortality in elderly patients. J Gen Intern Med. 2009;24:893–896. doi: 10.1007/s11606-009-1008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allon M. Evidence-based cardiology in hemodialysis patients. J Am Soc Nephrol. 2013;24:1934–1943. doi: 10.1681/ASN.2013060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wanner C., Amann K., Shoji T. The heart and vascular system in dialysis. Lancet. 2016;388:276–284. doi: 10.1016/S0140-6736(16)30508-6. [DOI] [PubMed] [Google Scholar]
- 31.Mostovaya I.M. University Medical Center Utrecht; Enschede, Netherlands: 2014. Chapter 6: Blood pressure and antihypertensive use over time in end stage kidney disease patients. Results from the CONvective TRAnsport STudy (CONTRAST) [dissertation] [Google Scholar]
- 32.Hazelbag C.M., Peters S.A.E., Blankestijn P.J. The importance of considering competing treatment affecting prognosis in the evaluation of therapy in trials:the example of renal transplantation in hemodialysis trials. Nephrol Dial Transplant. 2017;32:ii31–ii39. doi: 10.1093/ndt/gfw458. [DOI] [PubMed] [Google Scholar]
- 33.Miskulin D.C., Weiner D.E. Blood pressure management in hemodialysis patients:what we know and what questions remain. Semin Dial. 2017;30:203–212. doi: 10.1111/sdi.12586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.