Abstract
Background
Chemotherapy-induced cardiotoxicity constitutes subclinical myocardial dysfunction, arrhythmias, pericarditis, coronary vasospasm, and significant symptomatic heart failure. Anthracyclines pose higher risk for long-term cardiac dysfunction, with increased incidences of morbidity and mortality. Hence, early detection of chemotherapy-induced cardiac dysfunction may prompt an earlier treatment modification.
Aim
To evaluate global, longitudinal, radial, and circumferential strain changes in adult patients undergoing anthracycline chemotherapy along with the usefulness of three-dimensional (3D) echocardiography as the new modality over two-dimensional (2D) echocardiography.
Methods
This was a single centre, prospective, observational study that included asymptomatic patients free from any cardiac signs and symptoms attributable to heart failure, who underwent potentially cardiotoxic chemotherapy for malignancy from December 2017 to November 2018 at a tertiary care centre in India. Baseline demographics were recorded, and 2D and 3D echocardiography was performed at baseline and after completion of four cycles of chemotherapy.
Results
All the 55 patients received a cumulative dose of doxorubicin of less than 550 mg/m2. Follow-up period from the beginning of doxorubicin therapy was 108 ± 14 days. 9 patients were excluded from the study due to poor 3D images, so data analysis was done only for 46 patients. In 2D echocardiography, only global longitudinal strain (GLS) was observed to be significantly reduced (Δ18.33%; P < 0.001). 2D ejection fraction (EF) did not show significant change (Δ0.67%; P = 0.176), while by 3D echo, EF reduced significantly (Δ3.55%; P < 0.001). 3D global longitudinal (Δ29.19%; P < 0.001), circumferential (Δ30.65%; P < 0.001), area (Δ21.61%; P < 0.001), and radial (Δ29.66%; P < 0.001) strains were observed to be significantly reduced at follow-up.
Conclusion
Myocardial dysfunction induced by cardiotoxic chemotherapy can be detected earlier by using 2D GLS, 3D volumetric analysis, and 3D strain analysis by calculating global, longitudinal, radial, and circumferential strain changes. 3D echocardiographic assessment seems to be more accurate in picking out small changes in left ventricular functions, but at the cost of slightly poor image quality as compared to the 2D echocardiography. These newer techniques could potentially improve the ability for early detection of subclinical abnormalities of LV function in patients undergoing cardiotoxic chemotherapy and thus early initiation of treatment could be possible.
Keywords: Chemotherapy, Cardiotoxicity, Doxorubicin, Echocardiography, Strain imaging
1. Introduction
With advancing research and technology, treatment strategies for various cancers are also getting advanced. Classically, anthracyclines, antimetabolites, alkylating, and anti-microtubule agents were used in chemotherapy and with recent discoveries of new classes such as monoclonal antibodies and tyrosine kinase inhibitors have also been utilized.1 However, every boon comes with a bane, that is, the chemotherapy has been associated with fatal effects on various vital organs of body. Cardiotoxicity is one such major byproduct of chemotherapy. Chemotherapy-induced cardiotoxicity constitutes subclinical myocardial dysfunction, arrhythmias, pericarditis, coronary vasospasm, significant symptomatic heart failure, etc. The extent of cardiotoxicity depends on the type of treatment utilized and the mechanisms of cardiac damage involved. Basically, there are two types of cardiotoxicity; type 1 cardiotoxicity is caused by inhibiting topoisomerase IIβ in cardiomyocytes and inducing deoxyribonucleic acid double strand breaks and transcription changes, which is permanent and irreversible; whereas, type 2 cardiotoxicity is dose-dependent and reversible, caused by blocking of human epidermal growth factor receptor 2, which is expressed on cardiomyocytes in addition to tumour cells. Anthracyclines such as doxorubicin and epirubicin cause type 1 cardiotoxicity and type 2 is caused by trastuzumab.2
Anthracyclines pose higher risk for long-term cardiac dysfunction, with increased incidences of morbidity and mortality. The anthracycline monotherapy results to a 5–10% incidence of cardiomyopathy or heart failure.3 This mandates proper diagnosis and management of cardiotoxicity induced by anthracyclines. In previous decades, two-dimensional (2D) and Doppler echocardiography were the chief modes of diagnosis of cardiac function. However, these presented with some limitations such as lower sensitivity and high variability. The three-dimensional echocardiography (3D)-derived left ventricular ejection fraction (LVEF) and speckle-tracking echocardiography-derived global longitudinal strain (GLS) and other related strain measurements are promising indices that could overcome the limitations of 2D-derived LVEF, by posing high sensitivity, and detectability even at early stages of cardiotoxicity, and better accuracy.2 Thus, the aim of this study was to evaluate the global, longitudinal, radial and circumferential strain changes assessed through 2D and 3D strain echocardiography in adult patients undergoing anthracycline chemotherapy along with usefulness of 3D over 2D echocardiography.
2. Methods
2.1. Study design and population
This was a single centre, prospective, observational study that consisted of asymptomatic patients free from any cardiac signs and symptoms attributable to heart failure, diagnosed with different malignancies who underwent potentially cardiotoxic chemotherapy from December 2017 to November 2018 at a tertiary care centre in India. A synopsis of the study protocol was submitted to the Institutional Ethics Committee and approval was obtained. The study protocol was explained in detail to all subjects. Informed written consent was taken from all subjects willing to participate in the study.
Patients of all ages, free from any cardiac signs and symptoms of heart failure, who underwent cardiotoxic chemotherapy for treatment of cancer were included in the study. Exclusion criteria were: previous history of treatment by cardiotoxic therapy; non-sinus cardiac rhythm and post-pacemaker patients; history of myocardial infarction or symptomatic heart failure, or diagnosed coronary artery disease; previous cardiac surgery, previous thoracic radiotherapy; significant valvular stenosis or regurgitation, prosthetic valves, implanted cardiac devices, 2D echocardiographic wall motion abnormalities, or LVEF <35% in the baseline echocardiography.
Baseline echocardiographic examination was done before the initiation of chemotherapy. Standard 2D echocardiography was done for baseline LVEF and speckle tracking for evaluation of baseline 2D strain. In addition, baseline 3D volumetric analysis and 3D strain analysis were performed. Further follow-up of 2D and 3D echocardiographic assessment including LV strain analysis was performed after completion of four cycles of anthracycline-based chemotherapy. Other associated cardiac risk factors included age, gender, smoking, diabetes, and hypertension.
2.2. Echocardiographic methods
All patients underwent standard Doppler echocardiographic examination using a 3.3 MHz M5Sc 2D transducer with harmonic capability and a 3D volumetric transducer (4 V) for real-time echocardiographic dataset acquisition of the left ventricle by a Vivid E9 XDclear ultrasound machine (GE Healthcare, Norway). All 2D and 3D echocardiography was done by the single operator on the same machine to avoid inter-observer and inter-device variability and discrepancies in the techniques. Intra-observer variability has been shown to be <6% for global longitudinal and circumferential strain, and <9% for global transverse and radial strain in 2D strain imaging4 and relative mean errors of 4.9%–7.3% for 3D.5 In our lab, the intra-observer variation is 5.3%. For each patient, three readings were taken for all the variables by a single operator and an average of them were incorporated into the database to minimize the variability which was 4.1%.
A standard 2D echocardiographic examination was done, and the quantitative analysis of left ventricle was performed according to the recommendations.6 2D EF was derived from LV end-diastolic volume (EDV) and end-systolic volume (ESV) which was calculated according to the modified Simpson rule in apical four- and two-chamber views.
2D and 3D speckle-tracking echocardiography (STE) were performed on the same machine and were analysed as per standard protocols.7 All the datasets were digitally stored in raw data in the same ultrasound machine. It was well equipped with the commercially available software (4D Auto LVQ software, GE Healthcare) for quantification of 2D-derived GLS, 3D volumetric, and 3D STE analysis. We calculated 2D GLS as the average of 18 myocardial segments which were recorded in three apical views. According to ASE/EACVI Expert Consensus,8 a reduction of GLS between pre- and post-chemotherapy examination of more than 15% was considered to be an indicative evidence of subclinical cardiotoxicity. By 3D echocardiography, LV EDV and ESV and EF (%, EDV-ESV/EDV × 100) were obtained. 3D GLS, global circumferential strain (GCS), global area strain (GAS), and global radial strain (GRS) were calculated as weighted averages of the regional values from the 18 myocardial segments. In our study, those patients were excluded in which there was ≥3 rejected segments while calculating the strain, and for them global strain values were not calculated.
2.3. Statistical analysis
Statistical analysis was done by SPSS package version 16 (SPSS Inc, Chicago, IL, USA). Continuous data were presented as mean values ± standard deviation, and categorical data were represented as counts and percentages. Pre- and post-chemotherapy results were compared by Student paired t-test.
3. Results
In our study, 55 patients were enrolled, out of which 9 patients were excluded due to poor 3D echocardiographic image quality. A total of 46 patient were included in the study with complete pre-chemotherapy 2D standard echocardiographic, 3D volumetric, 2D strain, and 3D strain analysis. All these patients were having adequate echocardiographic images at baseline. None of the patients had cardiac symptoms or were on cardiac medication at the time of baseline evaluation and at follow-up examination. All the patients received a cumulative dose of doxorubicin of less than 550 mg/m2. Follow-up period from the beginning of doxorubicin therapy was 108 ± 14 days.
Of all the patients included in the study, the mean age was 44.17 years. Most of the patients were in the age group of 36–50 years (50%), 30.4% were in 51–65 years age group, 15.2% in 18–35 years age group, and rest 4.3% were of age below 18 years.
Majority of the patients included in study were female (82.6%). Most of the patients were having breast cancer (40 patients). No patient in the study group had diabetes mellitus and dyslipidemia. None of the patients were smoker. Moreover, 5 out of 46 patients were hypertensive. Baseline demographics are detailed in Table 1.
Table 1.
Baseline demographic characteristics.
| Variable | n = 46 patients |
|---|---|
| Age (mean ± SD, years) | 44.17 ± 10.95 |
| Female, n (%) | 40 (82.67%) |
| Heart rate (mean ± SD, bpm) | 77.52 ± 7.95 |
| Body mass index (mean ± SD, kg/m2) | 19.92 ± 2.48 |
| Systolic blood pressure (mean ± SD, mmHg) | 118.78 ± 15.89 |
| Diastolic blood pressure (mean ± SD, mmHg) | 74.87 ± 8.34 |
| Hypertension, n (%) | 5 (10.9%) |
| Type of cancer | |
| Breast cancer, n (%) | 40 (82.67%) |
| Osteosarcoma, n (%) | 3 (6.52%) |
| Squamous cell carcinoma, n (%) | 2 (4.34%) |
| Ewings sarcoma, n (%) | 1 (2.17%) |
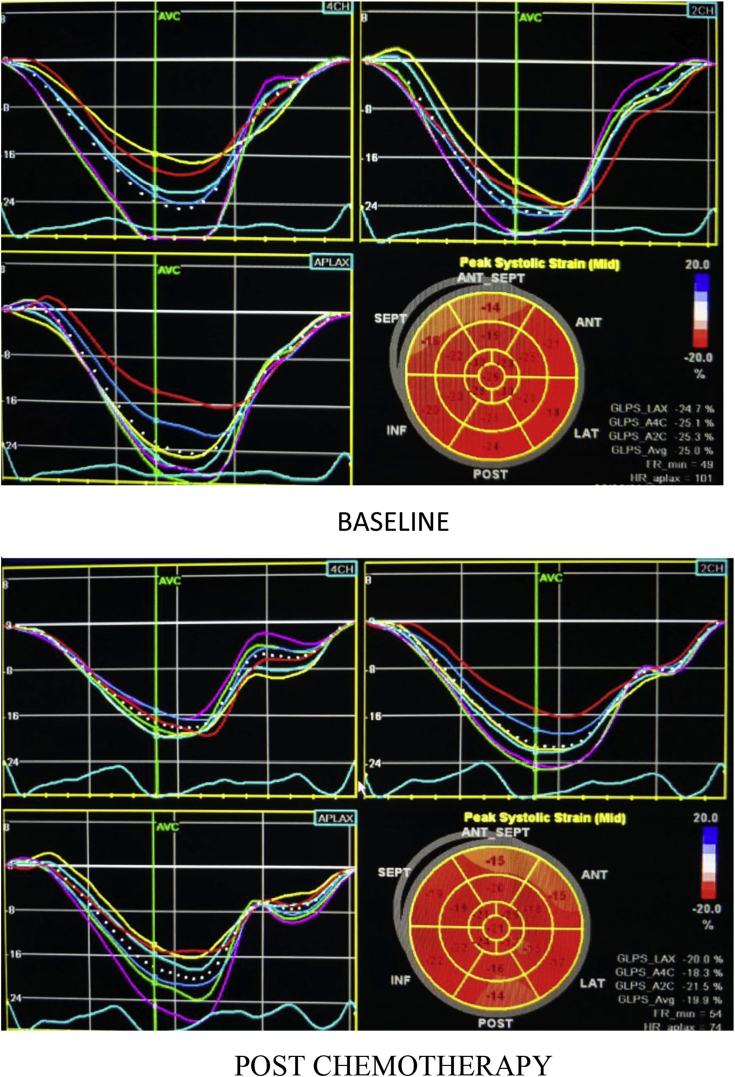
According to the standard 2D echocardiography evaluation, no significant difference was observed in pre- and post-chemotherapy patients, in terms of EF, EDV, and ESV (Table 2). However, there was significant difference observed in 2D-derived average GLS of LV in pre- and post-chemotherapy state with a mean percent change of 18.33% and a P-value of <0.001 (Fig. 1) (Table 3).
Table 2.
Comparison of 2D echocardiographic parameters during chemotherapy.
| Variable | Post-chemotherapy (n = 46) | Baseline (n = 46) | Mean Change | Mean % Change | P-value |
|---|---|---|---|---|---|
| 2D EF (%) | 62.41 ± 5.28 | 62.83 ± 5.23 | −0.42 | −0.67% | 0.176 |
| 2D EDV (ml) | 79.09 ± 13.09 | 79.59 ± 13.50 | −0.50 | −0.63% | 0.531 |
| 2D ESV (ml) | 30.30 ± 8.66 | 30.28 ± 8.94 | −0.02 | −0.07% | 0.966 |
| 2D GLS (%) | −13.90 ± 3.02 | −17.02 ± 2.46 | −3.12 | −18.33% | <0.001 |
EF – ejection fraction; EDV – end-diastolic volume; ESV – end-systolic volume; GLS – global longitudinal strain.
Fig. 1.
2D-derived global longitudinal strain at baseline and after doxorubicin therapy, showing significant reduction in GLS post-chemotherapy.
Table 3.
Comparison of 3D echocardiographic parameters during chemotherapy.
| Variable | Post-chemotherapy (n = 46) | Baseline (n = 46) | Mean Change | Mean % Change | P-value |
|---|---|---|---|---|---|
| 3D EF (%) | 58.48 ± 4.89 | 60.63 ± 4.71 | −2.15 | −3.55% | <0.001 |
| 3D EDV (ml) | 88.02 ± 15.24 | 85.44 ± 14.97 | 2.58 | 3.02% | 0.010 |
| 3D ESV (ml) | 35.82 ± 9.80 | 33.83 ± 9.70 | 1.99 | 5.88% | 0.005 |
| 3D GLS (%) | −9.85 ± 2.14 | −13.91 ± 2.16 | −4.06 | −29.19% | <0.001 |
| 3D GCS (%) | −9.98 ± 3.64 | −14.39 ± 4.28 | −4.41 | −30.65% | <0.001 |
| 3D GAS (%) | −15.78 ± 2.91 | −20.13 ± 2.73 | −4.35 | −21.61% | <0.001 |
| 3D GRS (%) | 20.80 ± 6.97 | 29.57 ± 5.00 | −8.77 | −29.66% | <0.001 |
EF – ejection fraction; EDV – end-diastolic volume; ESV – end-systolic volume; GLS – global longitudinal strain; GCS – global circumferential strain; GAS – global area strain; GRS – global radial strain.
On 3D volumetric analysis, we found that there was significant difference in EF, EDV, and ESV in pre- and post-chemotherapy state. Mean percent change in 3D EF was a decrease by 3.55% with a P-value of <0.001 which was significant. Mean percent change in EDV was an increase by 3.02% with a P-value of 0.010 which was significant. Mean percent change in ESV was an increase by 5.88% with a P-value of 0.005 (Table 3).
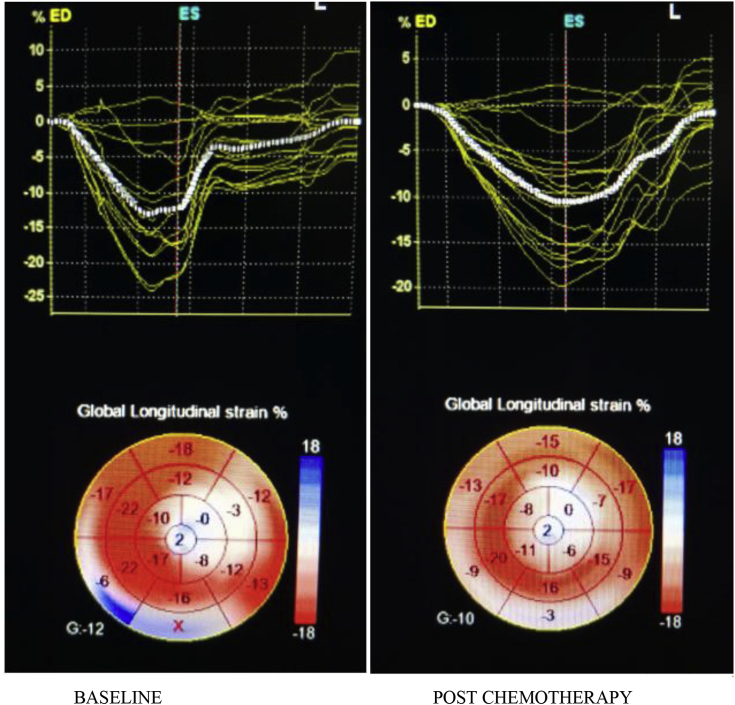
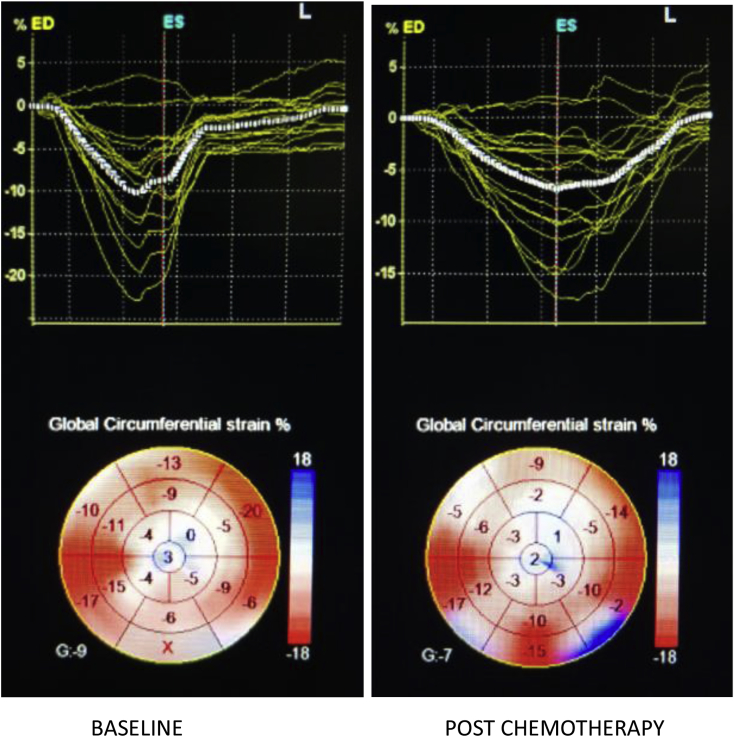
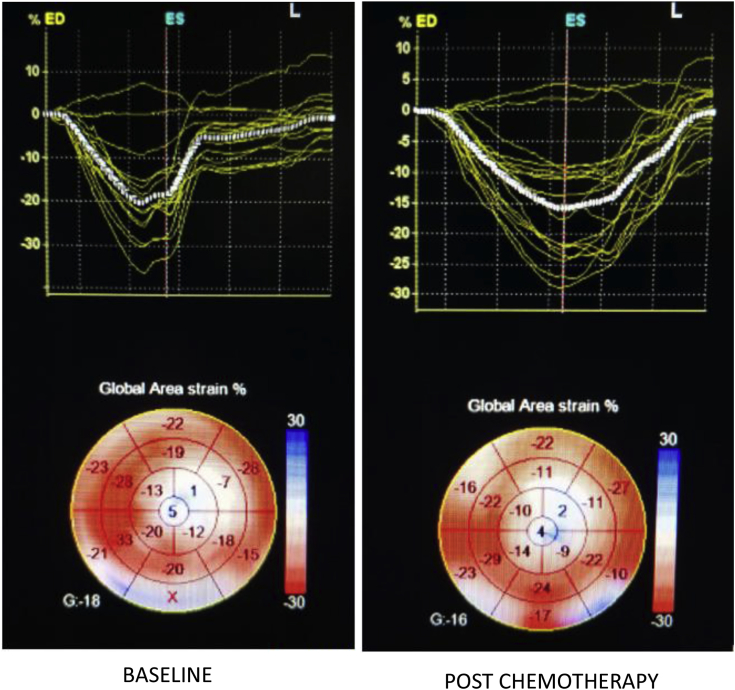
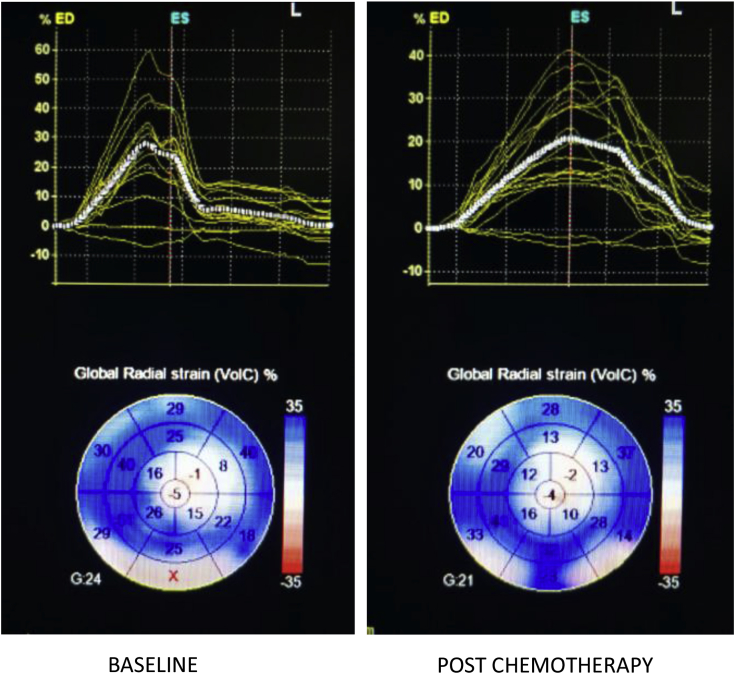
On 3D STE analysis, it was found that all 3D strain parameters were significantly reduced after chemotherapy. 3D GLS reduced by a mean of 29.19% with a P-value of <0.001(Fig. 2). 3D GCS reduced by a mean of 30.65% and a P-value of <0.001 (Fig. 3). 3D GAS reduced by a mean of 21.61% and a P-value of <0.001 (Fig. 4). 3D GRS changed by a mean of 8.77 with a mean percent change of 29.66% and a P-value of <0.001 (Fig. 5).
Fig. 2.
3D-derived global longitudinal strain at baseline and post-chemotherapy showing significant reduction post-chemotherapy.
Fig. 3.
3D-derived global circumferential strain at baseline and post-chemotherapy showing significant reduction post-chemotherapy.
Fig. 4.
3D-derived global area strain at baseline and post-chemotherapy showing significant reduction post-chemotherapy.
Fig. 5.
3D-derived global radial strain at baseline and post-chemotherapy showing significant reduction post-chemotherapy.
Of note, no patient had a fall of EF to less than 50% both by standard 2D echocardiography as well as 3D volumetric analysis after chemotherapy.
4. Discussion
In this study, the diagnostic value of conventional standard 2D echocardiography, 2D-derived global longitudinal strain, 3D volumetric analysis, and 3D-derived strain parameters were tested for early detection of LV function in malignancy patients who underwent doxorubicin-based chemotherapy. Major findings of our study are as follows: on standard 2D echocardiographic examination, 2D-derived EF is not adequately useful in detecting the early changes in LV function; by 2D-derived GLS parameters, subclinical alteration of LV longitudinal function can be detected early in the course; 3D volumetric assessment for ejection fraction helps in identifying the EF alterations not identified upon standard 2D EF quantification techniques; 3D-derived STE clearly demonstrates the alteration of LV myocardial mechanics including all the strain components.
Cardiotoxicity as an adverse event of chemotherapy includes a range of cardiac problems, including heart failure, myocardial ischemia or infarction, hypertension, thromboembolism, and arrhythmias.9 Extent of cardiotoxicity depends upon many factors relating to the chemotherapy used as well as patients, such as type of drug, dose administered during each cycle, cumulative dose, schedule of administration, route of administration, combination of other cardiotoxic drugs, or association with radiotherapy, pre-existing heart disease, history of hypertension, and age >65 years.10 Therefore, timely diagnosis of chemotherapy-induced cardiotoxicity becomes helpful as it allows to modify dose, administration rate, time, or use of analogous drugs which may reduce the extent of cardiotoxicity without any need for discontinuation of chemotherapy cycles.11 Thus, in this study, we examined the presence of any cardiotoxicity after completion of four cycles of chemotherapy, which would help in further management of chemotherapy patients accordingly. We measured strains from various perspectives and observed significant changes in all parameters of strains through 3D echocardiography. The literature states that in addition to longitudinal strain, the reductions in radial strain, circumferential strain, or area strain are also found to get reduced after chemotherapy; however, these depict higher variability and lower reproducibility as compared to GLS. Parallel to this, a meta-analysis also stated that a relative reduction of 10–15% of GLS may be applied to predict subsequent cardiotoxicity in chemotherapy patients.12 Similarly, in this study, we have found a change of 29.19% in GLS through 3D echocardiography, which predicts the prognostic cardiotoxic effects of chemotherapy. Previously, Baratta et al studied 36 breast cancer patients who had received trastuzumab or doxorubicin with echocardiography done at baseline, 2, 3, 4 and 6 months after initiation of therapy and reported a GLS decline of ≥15% at 3 months.13 In another study, Sawaya and colleagues included 43 patients with breast cancer under the treatment of anthracycline or trastuzumab. On performing echocardiography at baseline and at 3 and 6 months, a fall in GLS >10% at 3 months was observed.14 Hence, it can be taken into consideration that GLS by STE is the most useful parameter for predicting subsequent global LV chamber dysfunction and EF reduction.
In addition to early diagnosis using strain imaging, modification of treatment regimen or combining chemotherapeutic treatment with prophylactic drugs can be applied. Emerging evidence, like a study by Cardinale et al15 suggests that certain medications, such as the initiation of enalapril or carvedilol early during the chemotherapy, may slow the progression of LV dysfunction and thereby lower the incidence of heart failure and mortality.13 The SUCCOUR trial is an ongoing randomized control trial which will define the place of GLS measurement for surveillance of patients receiving cardiotoxic chemotherapy.16
4.1. Study limitations
The major limitations of our study were a short follow-up duration and relatively small number of study subjects. This led to obvious reflections on the absence of clinical events as well as lack of development of overt cardiotoxicity and low rate of reduction in LV systolic function which could be revealed by 2D and 3D EF evaluation. A longer follow-up period with a greater number of patients would point out even a small change in strain or EF as shown in this study will be of more clinical importance. Due to this, it was not possible to establish the predictive value of the identified 2D or 3D signs of subclinical cardiac damage. However, in this study, we intentionally studied the effect of doxorubicin cycles which have a well-defined time duration and can predispose to subsequent cardiotoxicity due to trastuzumab. 3D echocardiography was able to detect small changes in ejection fraction which was not detectable by 2D echocardiography. In addition, the image quality as obtained by 3D echo was not of that quality as expected which led to the baseline and post-chemotherapy discrepancies in 3D strain parameters, due to which out of 55 patients enrolled for study complete data of only 46 patients were analysed.
5. Conclusion
Myocardial dysfunction induced by cardiotoxic chemotherapy can be detected earlier by using 2D GLS, 3D volumetric analysis, and 3D strain analysis by calculating GLS, GCS, GRS, and GAS. These newer techniques could potentially improve the ability for early detection of subclinical abnormalities of LV function in patients undergoing cardiotoxic chemotherapy. Overall, our findings confirm the ability of advanced echocardiography techniques in detecting subclinical cardiotoxicity, a possible advantage for prevention of overt LV dysfunction through timely initiation of cardioprotective therapy along with 3D echocardiography standing out to be at advantage in detecting small changes in left ventricular functions over 2D echocardiography at the cost of slightly poorer image quality acquisition.
Conflicts of interest
There is no conflict of interest.
References
- 1.Tan T.C., Scherrer-Crosbie M. Assessing the cardiac toxicity of chemotherapeutic agents: role of echocardiography. Curr Cardiovasc Imaging Rep. 2012;5(6):403–409. doi: 10.1007/s12410-012-9163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C.-L., Chu P.-H. Echocardiography for evaluation of oncology therapy-related cardiotoxicity. Acta Cardiol Sin. 2016;32(5):560. doi: 10.6515/ACS20151024A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tadic M., Cuspidi C. The role of echocardiography in detection of chemotherapy-induced cardiotoxicity in breast cancer patients. Int J Cancer Manag. 2017;10(5) [Google Scholar]
- 4.Cheng S., Larson M.G., McCabe E.L. Reproducibility of speckle-tracking-based strain measures of left ventricular function in a community-based study. J Am Soc Echocardiogr. 2013;26(11):1258–1266. doi: 10.1016/j.echo.2013.07.002. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baysan O., Ocaklı E.P., Saglam Y., Altuner T.K. Advances in echocardiography: global longitudinal strain, intra-cardiac multidirectional flow imaging and automated 3d volume analysis. Heart Vessel Transplant. 2018;2(4):113–122. [Google Scholar]
- 6.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 7.Galderisi M., Nistri S., Mondillo S. Methodological approach for the assessment of ultrasound reproducibility of cardiac structure and function: a proposal of the study group of Echocardiography of the Italian Society of Cardiology (Ultra Cardia SIC) part I. Cardiovasc Ultrasound. 2011;9(1):26. doi: 10.1186/1476-7120-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15(10):1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh E.T., Bickford C.L. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 10.Von Hoff D.D., Layard M.W., Basa P. Risk factors for doxorubicin-lnduced congestive heart failure. Ann Intern Med. 1979;91(5):710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 11.Slamon D., Eiermann W., Robert N. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thavendiranathan P., Poulin F., Lim K.-D., Plana J.C., Woo A., Marwick T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25 Part A):2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 13.Baratta S., Damiano M.A., Marchese M.L. Serum markers, conventional Doppler echocardiography and two-dimensional systolic strain in the diagnosis of chemotherapy-induced myocardial toxicity. Argent J Cardiol. 2013;81(2):133–138. [Google Scholar]
- 14.Sawaya H., Sebag I.A., Plana J.C. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107(9):1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardinale D., Colombo A., Lamantia G. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 16.Negishi T., Thavendiranathan P., Negishi K. Rationale and design of the strain surveillance of chemotherapy for improving cardiovascular outcomes: the SUCCOUR trial. JACC (J Am Coll Cardiol): Cardiovasc Imaging. 2018;11(8):1098–1105. doi: 10.1016/j.jcmg.2018.03.019. [DOI] [PubMed] [Google Scholar]