Abstract
Fabry disease (FD) is an X-linked lysosomal storage disease caused by a deficiency in the lysosomal enzyme α-galactosidase (α-GAL). This in turn leads to the buildup of globotriaosylceramide, resulting classically in progressive kidney disease, peripheral neuropathy, early-onset cerebrovascular disease, gastrointestinal symptoms, hypertrophic cardiomyopathy, arrhythmias, corneal whorls, and angiokeratomas. The diagnosis of FD relies on identification of a low α-GAL enzyme activity, identification of a genetic mutation, or histologic evidence of disease. With more than 900 mutations identified, there is phenotypic variability deriving from both mutational effects as well as the effect of skewed X-inactivation in females. Treatment of this disease has relied on intravenous replacement of the deficient enzyme with agalsidase α or agalsidase β. However, treatment options for some patients with FD have recently expanded, with the approval of migalastat, an oral molecular chaperone. In addition to chaperone-based therapies, there are several additional therapies under development that could substantially reshape treatment options for patients with FD. Four approaches to gene therapy, through both ex vivo and in vivo methods, are under development. Another approach is through the administration of α-GAL mRNA to help stimulate production of α-GAL, which is another unique form of therapy. Finally, substrate reduction therapies act as inhibitors of glucosylceramide synthase, thus inhibiting the production of GB-3, promise another oral option to treat FD. This article will review the literature around current therapies as well as these newer therapeutics agents in the pipeline for FD.
Keywords: antidrug antibodies, chaperone therapy, enzyme replacement therapy, Fabry disease, gene therapy, substrate reduction therapy
Fabry disease (FD) is an X-linked genetic disorder that stems from one of over 900 identified mutations in the α-galactosidase gene leading to a deficiency in the lysosomal enzyme, α-galactosidase (α-GAL).1 The incidence of classical FD is reported at 1:40,000 and 1:117,000 live births.2 However, newborn screening studies have shown that the incidence of FD is much more common, and has been reported at 1:3200 with the inclusion of late-onset variants.3,4 In dialysis-dependent males, for example, it is found between 0.22% and 1.17% of patients.5,6
Under normal circumstances, α-GAL catalyzes the degradation of globotriaosylceramide (GB-3) to galactosylceramide. In FD, deficient α-GAL enzyme activity results in the accumulation of lysosomal GB-3 throughout the body. The accumulation of GB-3 and possibly associated inflammation lead to the characteristic findings of FD. Histologically, this manifests itself as inclusions termed zebra bodies or whorls. Classically, patients present with peripheral neuropathy, pain crisis, gastrointestinal symptoms, progressive renal dysfunction, cerebral white matter disease, early-onset stroke, hypertrophic cardiomyopathy, corneal whorls, and angiokeratomas. However, phenotype can vary due to differences in the varying mutations described as classic and late-onset variants. Females, previously thought of as carriers, have been shown to have significant and even severe disease manifestations. Phenotypic variation is the rule among females, even those with the same mutation owing to variable X-inactivation patterns.7,8
The average age of diagnosis of FD is 23 in male and 32 in female individuals.9 Unfortunately, this delay can lead to prolonged neuropathic pain, as well as distrust in the medical system. Therapy for FD currently consists of replacement of the deficient enzyme with lifelong intravenous infusions every other week. Although enzyme replacement therapy (ERT) has improved multiple aspects of FD, there is room for further improvement. Furthermore, the administration of recurrent i.v. infusions in the pediatric population can be challenging. Lifelong bimonthly infusions can lead to poor venous access, potentially requiring subcutaneous ports, which can carry the risk of bacterial infection and complicate future hemodialysis access should it become necessary.10
Enzyme replacement therapy, like many protein-based infusions, carries the risk of infusion-related reactions with rigors and chills, which are much more severe in male than in female individuals.11 Infusion reactions can be life threatening, and are usually mediated by anti-drug antibody (ADA) responses.12 Neutralizing anti-drug antibodies directed against the active site of ERT has been shown to have an inhibitory effect on the ERT and can correlate with outcomes.13,14
Recent work with pegunigalsidase α, a pegylated dimerized version of agalsidase α, provides important insights into the immunoreactivity of ERT preparations.15 This preparation has an 80-fold increase in half-life following infusion when compared to currently available ERT preparations, which have a very short (<2-hour) half-life. The pegylated enzyme appears to be less immunogenic and to induce tolerance in ERT-naive male FD patients who initially develop ADA to ERT.15 The reduced immunogenicity has been attributed to epitope-masking resulting from pegylation.16 Another explanation could involve tolerance induced by interactions between the terminal mannose units on the glycosylation chains with mannose receptors on T cells.17
Once ADA reactivity is established, there is not any consensus about further treatment approaches or the use of immune-modulating approaches in FD. Retrospective analyses suggest that immunosuppression used in FD patients following renal transplantation may be associated with reduced ADA titers.18 It is clear that ADA cannot be ignored in classic male FD patients, and that the effective delivered dose of currently available ERT preparations with already-noted short plasma half-lives is diminished. Alternative ERT dosing strategies in the presence of ADA need to be examined in the context of each individual patient, and ideally would be examined with clinical trials, to determine their effectiveness in patients with inhibitory ADA.
Therapeutic Approaches
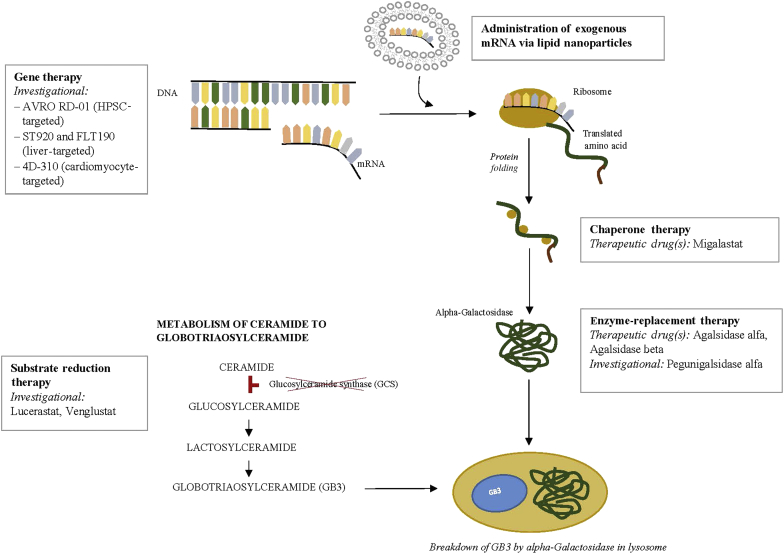
ERT was the earliest therapeutic option for patients with FD. The therapy has been successful at improving patient quality of life and stabilizing kidney function, but there remain unmet clinical needs. Investigational strategies targeting enzyme delivery or production include modification of the enzyme to increase the duration of therapeutic plasma concentrations, mRNA administration, and gene therapy through both ex vivo and in vivo approaches. Non−enzyme-replacement strategies are also being studied and include decreasing the amount of GB-3 production through inhibition of glucosylceramide synthase. Chaperone therapy, now approved, stabilizes the endogenous enzyme in patients with amenable mutations to increase enzyme activity (Figure 1).
Figure 1.
Current and investigational therapeutic agents for Fabry disease are depicted at each potential point of therapeutic intervention. As shown, therapies are directed at either replacing or generating deficient enzyme, or blocking the accumulation of substrate. Clinical trials with gene therapy approaches to the treatment of Fabry disease are ongoing. Chaperone therapy is now available for amenable mutation with migalastat. Enzyme replacement therapy remains the mainstay of treatment for most patients with Fabry disease, with agalsidase α and agalsidase β, while pegunigalsidase is in clinical trials. Substrate reduction therapy (SRT) consists of glucosylceramide synthase inhibitors and aims to reduce the accumulation of toxic substrate; SRT agents are currently in clinical trials.
Exogenous Enzyme Administration
The earliest targeted therapy for FD replaced the deficient enzyme, α-GAL. There are 2 drugs in this class. Agalsidase α (Replagal, Shire Human Genetic Therapies, Lexington, MA) is produced in a lineage of human fibroblasts, and is given at a dose of 0.2 mg/kg as an i.v. infusion and dosed every 2 weeks. Agalsidase β (Fabrazyme, Sanofi Genzyme, Cambridge, MA) is produced in Chinese hamster ovary cells and is administered at a dose of 1 mg/kg as an intravenous infusion every 2 weeks. Results of clinical trials have shown a clearance of GB-3 deposits as it relates to mesangial and glomerular endothelial cells.19 Furthermore, subsequent analyses have shown improvements in the number of severe clinical events even as patients age.20 Pain seems to improve as well for patients who go on therapy. (For a full literature review on enzyme replacement therapies, please refer to a recent review article on the topic.21)
Although it is generally tolerated well, ERT is not without its drawbacks. The enzyme must be delivered i.v., which can be challenging, as it requires repeated cannulations. Ports have been used in patients who have poor venous access as well as in children, but these carry the risk of infection and in children can limit participation in sporting activities, thus affecting quality of life. Furthermore, biweekly i.v. administration, particularly in younger patients, can prove difficult. Infusion reactions can occur and are manifested by hyperpyrexia, dyspnea, and rash. Premedications such as diphenhydramine and steroids can minimize these symptoms. The rate of infusion is an important factor, as faster rates are associated with a higher likelihood of intolerance. Furthermore, ERT leaves some unmet clinical concerns, including continued progression of cardiac fibrosis as shown by cardiac magnetic resonance imaging22 and progression of white matter disease on ERT. Finally, the short- and long-term impacts of ADA titers cannot be ignored (vide supra). For all of these reasons, improvements in ERT therapy for FD are clearly needed.
Investigational Exogenous Enzyme Replacement Therapies
Pegunigalsidase alpha is a novel pegylated form of α-GAL produced in a PlantCell Ex system. The growing use of plant-based biologics is a burgeoning area of biomedicine, and now is being used to help with the expression of different proteins, antibodies, and vaccines that can be used commercially. In contrast to animal-based cell production of exogenous proteins, the PlantCell Ex system could allow for more efficient production at a lower cost.23 Preclinical data demonstrated that the circulatory half-life of this compound was much longer than that of existing ERT. Increased uptake by heart and kidney and decreased hepatic uptake when compared to currently available ERT preparations that have terminal mannose-6-phosphate glycosylation residues has been reported.16 Recently, the results of an open-label, 3-month pharmacokinetics study followed by 9 months of follow-up was published, revealing an average half-life of 80 hours (range, 53−121 hours) as compared with a half-life of 2 hours for existing therapies.15 The drug was well tolerated, with most adverse events reported being either mild or moderate. Kidney biopsy samples were assessed at baseline and then at 6 months. During this time frame, 11 in 13 patients assessed had a 50% reduction in GB-3, with higher clearance being seen in those with classic mutations. Clinical trials to evaluate the potential of pegunigalsidase as compared to agalsidase β dosed at 1 mg/kg biweekly are ongoing. Based on the extended half-life, a clinical trial evaluating the efficacy of 2 mg/kg once-monthly dosing is also currently enrolling FD patients (NCT03180840).
Gene Therapy
Gene therapies carry the promise of a cure for many rare genetic diseases. Recently, the potential for gene therapy in FD has come into focus. Gene editing can occur ex vivo or in vivo. With the ex vivo approach, hematopoietic stem cells are harvested from the patient. These cells undergo gene editing and are then infused back into the patient for engraftment after myeloablative therapy is administered. With the in vivo approach, a vector with gene editing is infused directly into the patient, and then cells within the patient, such as liver cells, directly undergo gene editing to express the missing protein (Figure 1).
Ex Vivo Gene Therapy
Currently, there are a number of gene therapy approaches that are under development for the treatment of FD. Huang et al. demonstrated that CD34-positive hematopoietic stem cells could be harvested and modified through recombinant lentivirus-mediated gene transfer of the α-GAL gene.24 Furthermore, these cells could be infused into autologous recipients with good engraftment and persistent α-GAL production at 1 and 2 years year (Medin JA, Khan A, Huang J, et al. FACTs Fabry Gene Therapy Clinical Trial: two-year data [abstract]. Nephron. 2019;143:193–194). A press release from Avrobio, currently using this gene therapy in a Phase II testing showed persistent elevation in α-GAL activity in 2 patients (2.6 nmol/h per mol and 3.7 nmol/hr per ml) has demonstrated early safety of the protocol.25 The first patient has now discontinued enzyme replacement and is currently undergoing long-term follow-up study.
In Vivo Gene Therapy
Another approach to genetic therapy uses the infusion of adeno-associated virus (AAV)−mediated gene transfer to increase enzyme activity levels. Pre-clinical data using the liver targeted AAV-mediated gene transfer (ST920) has shown in α-GAL A knockout (GLAKO) mouse model in which, after a single injection, α-GAL is produced by the liver and secreted into the bloodstream. α-GAL levels rise in a dose-dependent fashion and have achieved levels more than 300 times those of α-GAL−deficient mice. Furthermore, lyso-GL3 levels were undetectable, and GL-33 levels were reduced to less than 10% of those in the untreated GLAKO mouse model.26 Using a similar approach with a different vector (Freeline Therapeutics, Stevenage, UK) have described marked increases of α-GAL levels after administration of a single-stranded AAV8 vector that was liver targeted (FLT190), with GB-3 and lyso-GB-3 levels reduced close to those of wild-type mice.27 As FD is a multisystemic disease, nontargeted gene therapies that raise the plasma concentrations of α-GAL may be reasonable strategies, and indeed measurements of plasma and/or leukocyte α-GAL may provide important long-term assessments of efficacy, as well as shorter-term endpoints for pivotal clinical trials. Kidney transplantation and liver transplantation for patients with FD have not been successful at alleviating the effects of the disease, probably because the endogenous α-GAL is not optimized for export from the transplanted wild-type engrafted cells. Furthermore, in females, there appears to be little or no cross-correction, in that normal neighboring cells that produce α-GAL do not prevent neighboring enzyme-deficient cells from developing GB-3 inclusions. As such, therapies targeted in cell-specific manners may be necessary to have the largest impact, specifically in mutations that primarily affect a single organ, such as renal or cardiac variants. To this end, 4D Molecular Therapeutics (Emeryville, CA) has described the use of a novel cardiac-tropic vector that demonstrated increased transduction in cardiomyocytes as compared to AAV8 and AAV9 (Whittlesey K, Brooks G, Croze R., et al. A novel cardiotropic AAV variant 4D-C102 demonstrates superior gene delivery and reduced immunogenicity in cardiac tissues versus wildtype AAV in non-human primates, and transduces functional GLA in cardiomyocytes and Fabry fibroblasts [abstract]. Nephron. 2019;143:193).
mRNA Encapsulated in Lipid Nanoparticles
Yet another therapeutic approach that is currently under testing is that of the administration of α-GAL mRNA to stimulate production of α-GAL without the need for either myeloablative therapy or administration of viral vectors for gene transduction (Figure 1). De Rosa et al.28 showed that mRNA for hGLA encapsulated with lipid nanoparticles could increase α-GAL levels expressed in liver, cardiac, and kidney tissues, resulting in improved GB-3 clearance.
Limitations of Gene Therapy Approaches
The common feature of all of these approaches is the increase or introduction of α-GAL enzyme activity. Antibody generation can occur in response to ERT and, in some individuals (i.e., male individuals with classic FD), can inhibit enzyme activity, which has been shown to correlate with worsened clinical outcomes.29 It is currently unknown whether the introduction of sustained enzyme production will lead to increased immunogenicity with associated complications. Alternatively, tolerance may be induced with sustained presence of the neoantigen. Data regarding pegunigalsidase have demonstrated that ADA titers may develop in a limited number of patients but decrease after 1 year.15 It is unclear what the long-term effect or immunogenicity of gene therapy approaches will be, and, as such, non−enzyme-based strategies are still needed for genetic diseases. The current vectors generate antibodies to the viral capsid, precluding administration of more than 1 treatment with a particular vector. Whether or not the current gene therapy approaches will achieve stable viral copy numbers and sufficient α-GAL A activity compared to currently available or developing long-lived ERT preparations is a fundamental question to be answered before gene therapy approaches can be adopted as therapeutic interventions for FD.
Non−Enzyme-Based Therapies
Chaperone Therapy
Chemical chaperone therapy is another therapeutic alternative in the management of FD. Chemical chaperones can bind to defective enzymes and help with correct folding, maturation, and trafficking of the enzyme to the appropriate functional site. Chemical chaperone therapy has been used in muliple lysosomal storage diseases, such as Gaucher and Pompe disease, as well as in FD (Figure 1).30 Migalastat (Galafold; Amicus Therapeutics, Cranbury, NJ; European Commission granted full approval in May 2016, the US Food and Drug Administration granted accelerated approval in August 2018) is an iminosugar that functions as a pharmacologic chaperone. Migalastat is orally available and has a large volume of distribution, including crossing into the central nervous system. By binding to the defective α-GAL in the endoplasmic reticulum, migalastat improves protein folding and promotes trafficking of the protein to the lysosome, the site of enzymatic activity. Enzymatic action in the lysosome exceeds the action of the chaperone itself, allowing for every-other-day dosing. The AT1001 Therapy Compared to Enzyme Replacement in Fabry Patients With AT1001-Responsive Mutations (ATTRACT) study was a randomized controlled trial designed to investigate migalastat in patients previously treated with enzyme replacement therapy. After 18 months of migalastat alone, plasma globotriaosylsphingosine remained low, and left ventricular mass index decreased significantly compared to no decrease in the ERT group.31 There were no significant differences based on renal function. The most common side effects have been urinary tract infection, headaches, nasopharyngitis, nausea, and pyrexia. In August 2018, the Food and Drug Administration approved migalastat based on results from the Study to Evaluate the Efficacy, Safety and Pharmacodynamics of AT1001 in Patients With Fabry Disease and AT1001-Responsive GLA Mutations (FACETS), a phase III, randomized, double-blind, placebo-controlled study that contained an open-label extension in which the placebo group was then treated with active compound.20 Only amenable mutations of GLA can be treated with migalastat, and this number has been estimated to be 35% to 50% of FD mutations. Recently, discrepancies between the effectiveness of migalastat in the in vitro amenability assays for some mutations, and outcomes observed in FD patients with those mutations treated with migalostat, have come into question.32 The current amenability assays use a cell line with low levels of endogenous α-GAL activity, which may suggest that further refinements are needed to ensure appropriate identification of amenable mutations.32
Substrate Reduction Therapies
Lucerastat, or N-butyldeoxygalactonojirimycin (Idorsia Pharmaceutical Ltd, Allschwil, Switzerland) is an iminosugar. It functions as a glucosylceramide synthase inhibitor that functions as a substrate reduction inhibitor, preventing accumulation of GB-3 by limiting the amount of ceramide that is converted to glycosphingolipid (Figure 1).33 A recent study showed that, at doses of 1000 mg twice daily, the circulating levels of globotriacylceramide and other sphingolipids were reduced.34 Lucerastat is currently under investigation in a phase 3 study of FD with neuropathic pain as the primary end point (MODIFY study: ClinicalTrials.gov Identifier: NCT03425539). Lucerastat is being tested as a monotherapy for FD. However, it is possible that substrate reduction therapy in addition to ERT may provide a new form of combination therapy that could be beneficial to this population.35
Another substrate reduction therapy, Venglustat, which is being developed by Sanofi Genzyme (Cambridge, MA), is currently in a long-term, phase 2 clinical trial (NCT02489344) to evaluate its effectiveness in men with FD who completed a previous phase 2 trial (NCT02228460).
Adjunctive Therapies
The need for adjunctive therapies to improve the outcomes in patients with FD cannot be overlooked. These patients should receive all the standards of care for patients with chronic kidney disease or hypertrophic cardiomyopathy who do not have FD. The use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers has been shown to decrease the progression of kidney disease in FD, as it has in other proteinuric kidney disease. High-sodium diets have been shown to decrease the effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers,36 and are associated with higher risks of progression to end-stage renal disease in patients with proteinuria.37 As such, FD patients with proteinuria must be counseled on the importance of adhering to a low-sodium diet. Vitamin D deficiency is common in FD and other forms of chronic kidney disease. Furthermore, it has been associated with left ventricular hypertrophy and with proteinuria.38 Given the low risk of vitamin D therapy, it is recommended that vitamin D levels be checked and repleted as needed with nutritional vitamin D. Fabry disease is a risk factor for stroke, as is the development of chronic kidney disease, and, as such, statin therapy should be considered. Further supportive therapies are discussed in a recent comprehensive review.21
Conclusion
In conclusion, therapeutic options for patients with FD will expand in the foreseeable future. Investigational agents such as modified ERT, glucosyl synthase inhibitors, and gene therapy all hold potential promise for FD patients. Future studies should investigate not only the efficacy of these drugs used as solo agents but also whether the use of combination therapy may better improve outcomes. Even with the advent of potential alternative therapeutic agents, treatment of organ-specific sequelae of FD is necessary through attention to diet and standard-of-care adjunctive therapies. Finally, the overriding question about the optimal time to start specific therapy for FD will need to be revisited as newer therapies become available in the context of sex, family history, and classical versus late-onset FD variants. The latter issue has become very topical in light of the increased prevalence of FD described with newborn screening efforts.
Disclosure
DGW is a consultant for 4D Molecular Therapeutics, Inc., Amicus Therapeutics, AVROBIO Inc, Chiesi USA, Inc., Freeline Therapeutics; IDORSIA PHARMACEUTICALS LTD, NDA Partners, LLC, and Protalix Biotherapeutics, and has an equity position in REATA Pharmaceuticals. EW has received grant support and travel support and is an advisor to Sanofi-Genzyme, is also a primary investigator for Protalix and has received honoraria and travel support from Protalix, and is also a primary investigator for Idorsia. All the other authors declared no competing interests.
References
- 1.Schiffmann R., Hughes D.A., Linthorst G.E. Screening, diagnosis, and management of patients with Fabry disease: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int. 2017;91:284–293. doi: 10.1016/j.kint.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Meikle P.J., Hopwood J.J., Clague A.E. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins P.V., Klug T., Vermette L. Incidence of 4 lysosomal storage disorders from 4 years of newborn screening. JAMA Pediatr. 2018;172:696–697. doi: 10.1001/jamapediatrics.2018.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spada M., Pagliardini S., Yasuda M. High incidence of later-onset Fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakao S., Kodama C., Takenaka T. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a "renal variant" phenotype. Kidney Int. 2003;64:801–807. doi: 10.1046/j.1523-1755.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 6.Linthorst G.E., Hollack C., Korevaar J.C. alpha-Galactosidase A deficiency in Dutch patients on dialysis: a critical appraisal of screening for Fabry disease. Nephrol Dial Transplant. 2003;18:1581–1584. doi: 10.1093/ndt/gfg194. [DOI] [PubMed] [Google Scholar]
- 7.Echevarria L., Benistan K., Toussaint A. X-chromosome inactivation in female patients with Fabry disease. Clin Genet. 2016;89:44–54. doi: 10.1111/cge.12613. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz A., Cianciaruso B., Cizmarik M. End-stage renal disease in patients with Fabry disease: natural history data from the Fabry Registry. Nephrol Dial Transplant. 2010;25:769–775. doi: 10.1093/ndt/gfp554. [DOI] [PubMed] [Google Scholar]
- 9.Eng C.M., Fletcher J., Wilcox W.R. Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis. 2007;30:184–192. doi: 10.1007/s10545-007-0521-2. [DOI] [PubMed] [Google Scholar]
- 10.Kuizon D., Gordon S.M., Dolmatch B.L. Single-lumen subcutaneous ports inserted by interventional radiologists in patients undergoing chemotherapy: incidence of infection and outcome of attempted catheter salvage. Arch Intern Med. 2001;161:406–410. doi: 10.1001/archinte.161.3.406. [DOI] [PubMed] [Google Scholar]
- 11.Mauhin W., Lidove O., Masat E. Innate and adaptive immune response in Fabry disease. JIMD Rep. 2015;22:1–10. doi: 10.1007/8904_2014_371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholls K., Bleasel K., Becker G. Severe infusion reactions to Fabry enzyme replacement therapy: rechallenge after tracheostomy. JIMD Rep. 2012;5:109–112. doi: 10.1007/8904_2011_106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lenders M., Stypmann J., Duning T. Serum-mediated inhibition of enzyme replacement therapy in Fabry disease. J Am Soc Nephrol. 2016;27:256–264. doi: 10.1681/ASN.2014121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenders M., Schmitz B., Brand S.M. Characterization of drug-neutralizing antibodies in patients with Fabry disease during infusion. J Allergy Clin Immunol. 2018;141:2289–2292. doi: 10.1016/j.jaci.2017.12.1001. [DOI] [PubMed] [Google Scholar]
- 15.Schiffmann R., Goker-Alpan O., Holida M. Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: a 1-year phase 1/2 clinical trial. J Inherit Metab Dis. 2019;42:534–544. doi: 10.1002/jimd.12080. [DOI] [PubMed] [Google Scholar]
- 16.Kizhner T., Azulay Y., Hainrichson M. Characterization of a chemically modified plant cell culture expressed human alpha-galactosidase-A enzyme for treatment of Fabry disease. Mol Genet Metab. 2015;114:259–267. doi: 10.1016/j.ymgme.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Schuette V., Embgenbroich M., Ulas T. Mannose receptor induces T-cell tolerance via inhibition of CD45 and up-regulation of CTLA-4. Proc Natl Acad Sci U S A. 2016;113:10649–10654. doi: 10.1073/pnas.1605885113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenders M., Oder D., Nowak A. Impact of immunosuppressive therapy on therapy-neutralizing antibodies in transplanted patients with Fabry disease. J Intern Med. 2017;282:241–253. doi: 10.1111/joim.12647. [DOI] [PubMed] [Google Scholar]
- 19.Eng C.M., Guffon N., Wilcox W.R. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz A., Abiose A., Bichet D.G. Time to treatment benefit for adult patients with Fabry disease receiving agalsidase beta: data from the Fabry Registry. J Med Genet. 2016;53:495–502. doi: 10.1136/jmedgenet-2015-103486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz A., Germain D.P., Desnick R.J. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. 2018;123:416–427. doi: 10.1016/j.ymgme.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Hanneman K., Karur G.R., Wasim S. Prognostic significance of cardiac magnetic resonance imaging late gadolinium enhancement in Fabry disease. Circulation. 2018;138:2579–2581. doi: 10.1161/CIRCULATIONAHA.118.037103. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q., Davis K.R. The potential of plants as a system for the development and production of human biologics. F1000Res. 2016;5:912. doi: 10.12688/f1000research.8010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J., Khan A., Au B.C. Lentivector iterations and pre-clinical scale-up/toxicity testing: targeting mobilized CD34(+) cells for correction of Fabry disease. Mol Ther Methods Clin Dev. 2017;5:241–258. doi: 10.1016/j.omtm.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.AVROBIO announces updated clinical data for AVR-RD-01 gene therapy in Fabry disease. 2018. https://www.globenewswire.com/news-release/2018/10/01/1587610/0/en/AVROBIO-Announces-Updated-Clinical-Data-for-AVR-RD-01-Gene-Therapy-in-Fabry-Disease.html Available at:
- 26.Huston M., Yasuda M., Silvere P. Liver-targeted AAV gene therapy vectors produced by a clinical scale manufacturing process result in high, continuous therapeutic levels of enzyme ativity and effective substrate reduction in mouse model of Fabry disease. Mol Genet Metab. 2019;126:S77. [Google Scholar]
- 27.Jeyakumar J., Kia A., McIntosh J. Liver-directed gene therapy corrects Fabry disease in mice. Mol Genet Metab. 2019;126:S80. [Google Scholar]
- 28.DeRosa F., Smith L., Shen Y. Improved efficacy in a Fabry disease model using a systemic mRNA liver depot system as compared to enzyme replacement therapy. Mol Ther. 2019;27:878–889. doi: 10.1016/j.ymthe.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenders M., Neußer L.P., Rudnicki M. Dose-dependent effect of enzyme replacement therapy on neutralizing antidrug antibody titers and clinical outcome in patients with Fabry disease. J Am Soc Nephrol. 2018;29:2879–2889. doi: 10.1681/ASN.2018070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motabar O., Sidransky E., Goldin E., Zheng W. Fabry disease–current treatment and new drug development. curr chem genomics. 2010;4:50–56. doi: 10.2174/1875397301004010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes D.A., Nicholls K., Shankar S.P. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet. 2017;54:288–296. doi: 10.1136/jmedgenet-2016-104178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenders M., Stappers F., Niemietz C. Mutation-specific Fabry disease patient-derived cell model to evaluate the amenability to chaperone therapy. J Med Genet. 2019;56:548–556. doi: 10.1136/jmedgenet-2019-106005. [DOI] [PubMed] [Google Scholar]
- 33.Welford R.W.D., Mühlemann A., Garzotti M. Glucosylceramide synthase inhibition with lucerastat lowers globotriaosylceramide and lysosome staining in cultured fibroblasts from Fabry patients with different mutation types. Hum Mol Genet. 2018;27:3392–3403. doi: 10.1093/hmg/ddy248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerard N., Oder D., Nordbeck P. Lucerastat, an iminosugar for substrate reduction therapy: tolerability, pharmacodynamics, and pharmacokinetics in patients with Fabry disease on enzyme replacement. Clin Pharmacol Ther. 2018;103:703–711. doi: 10.1002/cpt.790. [DOI] [PubMed] [Google Scholar]
- 35.Brady R.O., Schiffmann R. Possible future therapies for Fabry disease. In: Mehta A., Beck M., Sunder-Plassmann G., editors. Fabry Disease: Perspectives from 5 Years of FOS. Oxford PharmaGenesis; Oxford, UK: 2006. [PubMed] [Google Scholar]
- 36.De'Oliveira J.M., Price D.A., Fisher N.D. Autonomy of the renin system in type II diabetes mellitus: dietary sodium and renal hemodynamic responses to ACE inhibition. Kidney Int. 1997;52:771–777. doi: 10.1038/ki.1997.394. [DOI] [PubMed] [Google Scholar]
- 37.Vegter S., Perna A., Postma M.J. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012;23:165–173. doi: 10.1681/ASN.2011040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilz S., Tomaschitz A., Drechsler C. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res. 2010;54:1103–1113. doi: 10.1002/mnfr.200900474. [DOI] [PubMed] [Google Scholar]