Introduction
Antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) is a small vessel necrotizing vasculitis with a predilection for the respiratory tract and kidneys.1,2 Prompt initiation of remission induction immunosuppression is paramount to prevent irreversible organ damage.3 Standard initial therapy for severe AAV consists of cyclophosphamide or rituximab in combination with high-dose glucocorticoids.4,5 In cases of severe pulmonary hemorrhage or rapidly progressive glomerulonephritis, plasma exchange is often added to facilitate rapid removal of ANCA.6
Improved understanding of disease pathogenesis has provided the rationale for targeting the complement pathway in AAV.7 In particular, the anaphylatoxin complement component 5a (C5a) has been identified as a key pathogenic mediator of AAV because of its ability to prime and recruit neutrophils.8 Inhibitors of C5a and the C5a receptor are being evaluated in randomized trials, but are not currently available for clinical use.9 Here, we report the use of eculizumab, a monoclonal antibody against C5, in 2 cases of aggressive AAV with the intention of rapidly inducing remission by inhibiting C5a generation. In both patients, religious beliefs prohibiting the receipt of blood products precluded the use of plasma exchange and cyclophosphamide.
Case Presentation
Case 1
A 61-year-old woman with a history of hypothyroidism presented to the hospital for evaluation of 3 weeks of progressive dyspnea. On presentation, she was tachypneic and had an oxygen saturation of 85% while breathing ambient air. Her hemoglobin concentration, which was previously normal, had fallen to 6.7 g/dl. There was no history of bleeding, and stool guaiac test results were negative. The serum creatinine (SCr) level was 1.1 mg/dl (unknown baseline), and urinalysis was significant for blood (2+) and protein (2+). Examination of the urine sediment revealed the presence of dysmorphic red blood cells and red blood cell casts. Chest computed tomography demonstrated diffuse ground-glass and consolidative opacities in a distribution consistent with pulmonary hemorrhage. The patient’s hypoxemia rapidly worsened, requiring high-flow nasal cannula with a fraction of inspired oxygen of 70%. Pulse i.v. methylprednisolone was initiated for a suspected pulmonary-renal syndrome, and the patient was admitted to the intensive care unit.
On the second hospital day, testing for myeloperoxidase ANCA returned positive at a titer of 1024 U (negative, <2.8 U) and the hemoglobin concentration fell to 5.7 g/dl. Testing for anti–glomerular basement membrane antibodies was negative. Levels of C3 and haptoglobin were normal. The lactate dehydrogenase level was mildly elevated at 246 U/l (normal range, 110–210 U/l). No schistocytes were observed on the peripheral blood smear. The patient was a practicing Jehovah’s Witness and declined all blood products including fresh frozen plasma. Severe anemia with the inability to transfuse red blood cells and ongoing pulmonary hemorrhage with the inability to administer fresh frozen plasma precluded the use of cyclophosphamide and plasma exchange, respectively. Pulse methylprednisolone was continued, and rituximab 1000 mg i.v. was administered (Figure 1a). However, the patient’s respiratory status remained tenuous, and invasive mechanical ventilation was considered.
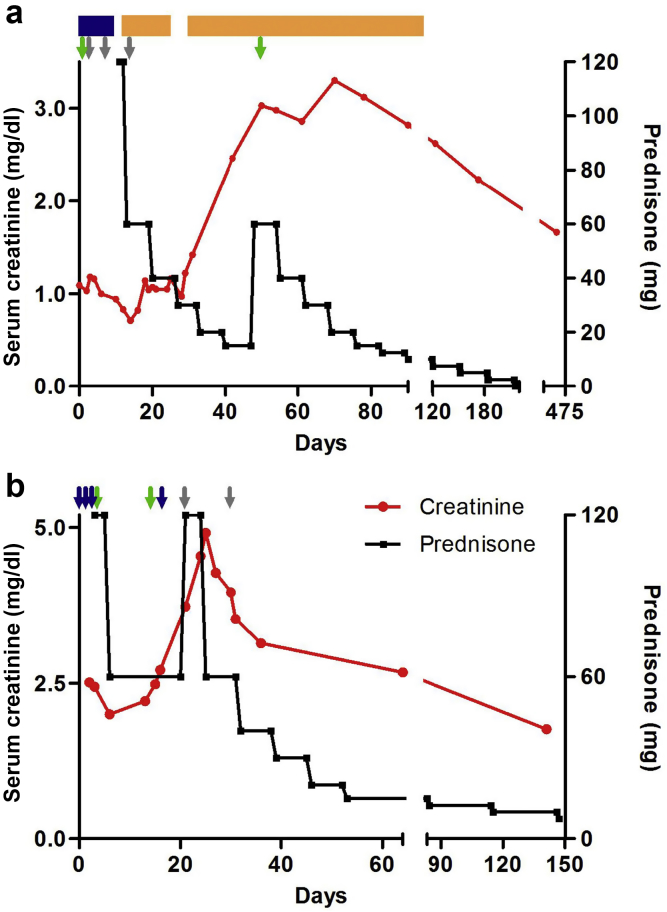
Figure 1.
Clinical course of patients treated with eculizumab. Shown is the treatment regimen and clinical response for patient 1 (a) and patient 2 (b). Therapy for both patients included pulse methylprednisolone (blue arrows and blue rectangle), prednisone (black line), rituximab (green arrows), and eculizumab (gray arrows). Patient 1 also received low-dose oral cyclophosphamide (orange rectangle). The second rituximab infusion in patient 1 was slightly delayed, but flow cytometry confirmed that the patient had complete B-cell depletion immediately before this dose.
Eculizumab 900 mg i.v. was administered on days 3, 10, and 17 (Figure 1a). After the second dose, the respiratory status rapidly improved, allowing weaning of supplemental oxygen to 4 l nasal cannula and tapering of glucocorticoids. However, ∼2.5 weeks after the final eculizumab dose, the patient’s renal function started to decline and the SCr level peaked at 3.3 mg/dl (Figure 1a). Given improvement in the patient’s anemia with high-dose epoetin alfa, oral cyclophosphamide was initiated. The patient’s SCr level ultimately improved to a new baseline of 1.6 mg/dl.
Case 2
An 83-year-old woman with hypothyroidism and coronary artery disease was transferred to our hospital for evaluation of fatigue, weight loss, small-volume hemoptysis, and acute kidney injury. The SCr level on presentation was 2.5 mg/dl, increased from a baseline of 0.7 mg/dl two months prior. Review of the urine sediment revealed abundant dysmorphic red blood cells, and a spot urine protein-to-creatinine ratio was elevated at 1.8 g/g. Urinalysis was significant for blood (3+) and protein (2+). Computed tomography of the chest revealed bilateral ground-glass opacities, but oxygen saturation remained normal while breathing ambient air. The patient was severely anemic on presentation (hemoglobin concentration, 6.1 g/dl), which was attributed to a combination of pulmonary hemorrhage, inflammation, and renal disease. The patient was a practicing Jehovah’s Witness and declined blood transfusion. Workup was significant for myeloperoxidase ANCA at a titer of 1515 U (normal <2.8 U). Additional laboratory tests including anti–glomerular basement membrane antibodies, C3, lactate dehydrogenase, haptoglobin, and peripheral blood smear were unremarkable.
The patient was initiated with rituximab and pulse i.v. methylprednisolone followed by high-dose oral glucocorticoids for the treatment of AAV (Figure 1b). Despite the continuation of high-dose glucocorticoids, renal function continued to deteriorate over the ensuing weeks, reaching a peak SCr level of 4.9 mg/dl. The patient’s religious beliefs coupled with severe anemia prevented the use of plasma exchange or cyclophosphamide. Eculizumab 900 mg i.v. was administered on days 24 and 31. The patient’s renal function rapidly improved, and the SCr level reached a nadir of 1.8 mg/dl on the last check (Figure 1b).
Discussion
AAV typically presents with normal levels of serum complement, and minimal complement deposition is found on renal biopsy in cases of pauci-immune necrotizing glomerulonephritis.1 Historically, these observations have led to the belief that complement was not a significant mediator of the inflammatory cascade in AAV. However, recent investigation has identified activation of the alternative complement pathway as necessary for disease activity (Table 1). Here, we provide 2 cases of aggressive AAV in which blockade of C5 cleavage yielded demonstrable clinical benefit.
Table 1.
Teaching points
|
|
|
|
AAV, antineutrophil cytoplasmic antibody–associated vasculitis; C5a, complement component 5a.
Xiao et al. initially demonstrated that mice deficient in complement factor B, but not those deficient in C4, were protected from the induction of necrotizing and crescentic glomerulonephritis in a murine anti-myeloperoxidase IgG transfer model.7 This suggests that the alternative pathway, rather than the classical or lectin pathways (both of which require C4), is needed for disease activity. Additional experiments revealed that mice deficient in C6 were not protected from disease, indicating that the anaphylatoxin C5a, and not the membrane attack complex, is the key pathogenic mediator.S1
Indeed, treatment with a C5a receptor antagonist significantly abrogated disease activity in a mouse model.S1 Given its role in the terminal inflammatory cascade in AAV, C5a blockade is an attractive therapeutic option to rapidly control disease activity (Figure 2). Currently, eculizumab and ravulizumab are the only available drugs in clinical use to target the complement cascade. Antagonism of C5 precludes the generation of C5a, and treatment with an anti-C5 monoclonal antibody has been shown to attenuate AAV disease activity in murine models.S2 This was the rationale for the administration of eculizumab in the 2 patients reported herein, both of whom had an aggressive and progressive disease with limited alternative therapeutic options due to religious considerations.
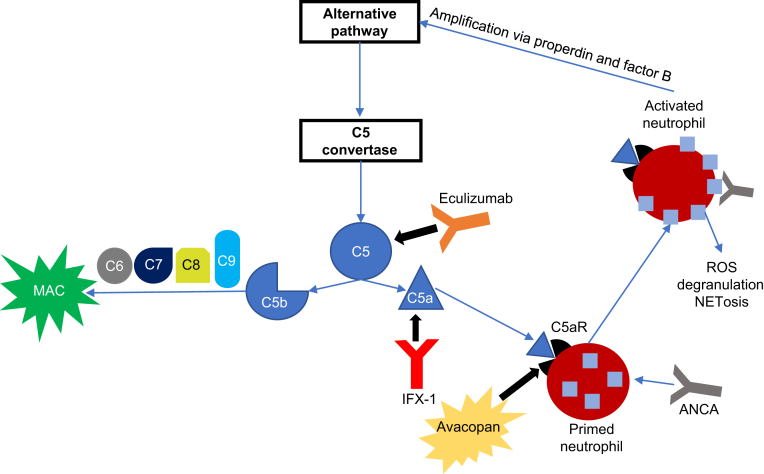
Figure 2.
Targeting of the complement cascade in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis. ANCA activation of primed neutrophils stimulates degranulation and release of reactive oxygen species (ROS), leading to tissue damage. In addition, ANCA activation leads to the production of factor B and properdin, which serve to activate the alternative complement pathway. Complement component 5a (C5a) enhances the inflammatory response by recruiting and priming additional neutrophils for ANCA activation. Disease activity can be controlled by targeting C5 (eculizumab), C5a (IFX-1), or the C5a receptor (C5aR; avacopan). Notably, targeting of C5 also prevents activation of the membrane attack complex (MAC). NETosis, cell death through release of neutrophil extracellular traps.
In patient 1, pulmonary hemorrhage rapidly improved after eculizumab. Moreover, renal function rapidly deteriorated after eculizumab was discontinued. This suggests the possibility that blockade of C5 was preventing the development of necrotizing and crescentic glomerulonephritis in this patient, akin to what has been observed in animal models. Likewise, patient 2 had rapid improvement in renal function after the administration of eculizumab. It should be noted that in both cases, renal biopsy was not performed because of the presence of anemia and the inability to transfuse should a bleeding complication occur. However, a markedly positive ANCA level in the setting of pulmonary hemorrhage and a clinical syndrome of rapidly progressive glomerulonephritis has a positive predictive value that approaches 100%.S3 Our report adds to 2 prior patients with AAV described in the literature who appear to have responded to eculizumab.S4,S5
Despite its potential efficacy in treating AAV, blockade of C5 provides more immunosuppression than is required to control disease activity. Inactivation of C5 prevents formation of the membrane attack complex, which does not appear to play a significant role in AAV (Figure 2). Therefore, C5 blockade unnecessarily exposes patients with AAV to a higher risk of infection with Neisseria meningitidis even with the use of appropriate vaccination protocols and prophylactic antibiotics.S6 Fortunately, therapeutic strategies to directly target C5a are currently being tested in clinical trials.
In the phase II CLEAR trial, treatment with avacopan, a small molecule inhibitor of the C5a receptor, was noninferior to that with high-dose glucocorticoids in patients with new or relapsing AAV receiving cyclophosphamide or rituximab for remission induction therapy.9 Patients receiving avacopan also had more rapid resolution of albuminuria. The majority of data implicating C5a in the pathogenesis of AAV have been derived predominantly from animal models, and a role of other complement components, including the membrane attack complex, cannot be completely excluded in human disease. However, the results of the CLEAR trial provide compelling evidence that C5a is also the key complement-derived mediator of AAV activity in humans.
The preliminary success of C5a blockade in the CLEAR trial provided the impetus for the ongoing ADVOCATE trial (clinicaltrials.gov identifier: NCT02994927), a pivotal phase III trial testing whether avacopan can replace glucocorticoids during induction therapy. In addition, phase II studies are ongoing to test the safety and efficacy of IFX-1 (clinicaltrials.gov identifiers: NCT03712345 and NCT03895801), a monoclonal antibody against the C5a molecule itself (Figure 2).
Blockade of C5a has the potential to significantly advance the treatment of AAV by obviating the need for glucocorticoids. Moreover, enhanced understanding of disease pathogenesis has revealed an important role of complement across a large spectrum of glomerulonephritides, including IgA nephropathy, C3 glomerulopathy, lupus nephritis, and anti–glomerular basement membrane disease.S7–S9 New agents with the ability to selectively manipulate the complement cascade are poised to revolutionize the treatment of glomerulonephritis by rapidly controlling disease activity while minimizing treatment-related toxicity.
Disclosure
All authors are currently working on the ADVOCATE trial with ChemoCentryx and the phase II trial of IFX-1 with InflaRx (clinicaltrials.gov identifiers: NCT02994927 and NCT03895801). Our site previously participated in ChemoCentryx’s Phase II CLASSIC trial of avacopan (clinicaltrials.gov identifier: NCT02222155). FBC and JLN have served as consultants for ChemoCentryx.
Acknowledgments
The authors thank the outstanding staff at the vasculitis and glomerulonephritis center for their work in patient care and data collection.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Zonozi R., Niles J.L., Cortazar F.B. Renal involvement in antineutrophil cytoplasmic antibody-associated vasculitis. Rheum Dis Clin North Am. 2018;44:525–543. doi: 10.1016/j.rdc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Jennette J.C., Falk R.J., Gasim A.H. Pathogenesis of antineutrophil cytoplasmic autoantibody vasculitis. Curr Opin Nephrol Hypertens. 2011;20:263–270. doi: 10.1097/MNH.0b013e3283456731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman G.S., Kerr G.S., Leavitt R.Y. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 4.Stone J.H., Merkel P.A., Spiera R. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Groot K., Harper L., Jayne D.R. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–680. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 6.Jayne D.R., Gaskin G., Rasmussen N. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–2188. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 7.Xiao H., Schreiber A., Heeringa P. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber A., Xiao H., Jennette J.C. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20:289–298. doi: 10.1681/ASN.2008050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayne D.R.W., Bruchfeld A.N., Harper L. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28:2756–2767. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.