Membranous nephropathy (MN) is a common cause of nephrotic syndrome in adults and can be primary or secondary. Primary MN is most commonly associated with anti−M-type phospholipase A2 receptor (PLA2R) antibodies and is usually IgG4 dominant, whereas secondary MN can be seen in the setting of malignancies, infections, autoimmune diseases, or as a side effect of certain medications or toxins, and is most commonly IgG1 dominant.1,2 The occurrence of crescents in MN is extremely rare, with a prevalence estimated between 0.39%3 and 0.26%.4 Crescents can be seen in both primary and secondary MN3, 4, 5 or in association with a superimposed disease process, such as anti−neutrophil cytoplasmic antibody (ANCA)−associated glomerulonephritis or anti−glomerular basement membrane (GBM) disease. Previous series in the United States have either focused on cases of MN with crescents in the absence of ANCAs or anti-GBM antibodies,3 or in association with ANCAs.5 In light of the recently published series of MN with crescents from the United Kingdom,4 and given the rarity of this entity, we analyzed its prevalence and its clinical and pathological characteristics in biopsy findings reviewed at our institution.
A total of 14,800 native and transplant renal biopsy specimens were received at the Ohio State University from 2010 to 2019. Of these, 15 cases (0.1%) showed MN with crescents (fibrous crescents only, 3; diffuse crescents [in >50% of glomeruli], 3; focal crescents [in <50% of glomeruli], 9). In addition, 3 cases had only segmental subepithelial deposits (segmental MN). Cases of lupus nephritis were excluded. PLA2R staining was performed retrospectively in 7 cases.
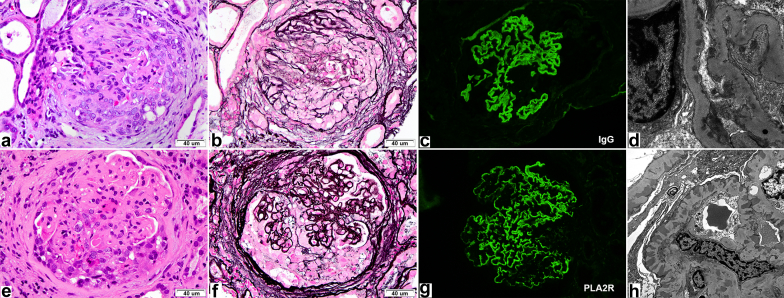
There were 8 female and 7 male patients. Two had anti-GBM antibodies (Figure 1a–d), 9 had ANCAs (8 MPO, 2 both MPO and PR3), and 4 showed anti-PLA2R−positive deposits in the biopsy specimen (Figure 1e–g), including 1 with positive serum anti-PLA2R. Of note, not all of these antibodies were checked in all patients. Clinical features are summarized in Table 1 and pathological features in Table 2. One patient had prior ANCA-associated cutaneous vasculitis, and 1 patient had prior biopsy-proven infection-related glomerulonephritis; both had a history of cocaine use. Potential secondary causes of MN included solid organ malignancies (n =3), hepatitis C (n = 2), and rheumatoid arthritis (n = 1). Three patients had a monoclonal gammopathy of undetermined significance, and 5 patients had positive autoantibodies (4 anti−nuclear antibodies, 2 anticardiolipin, and 2 anti-SSB). Eight patients had pulmonary symptoms, including hemoptysis in 3 and documented diffuse alveolar hemorrhage in 2. Twelve patients had hematuria, which was usually significant, and 14 had proteinuria (1 patient was anuric). Proteinuria was measured by spot urine protein-to-creatinine ratio or 24-hour collection and was >3 g in 7 of the 14 patients. Median creatinine at presentation was 2.9 mg/dl (1.07−18.6). Two patients had normal renal function on presentation (1 patient had a single fibrous and the other a single cellular crescent). The remaining 13 patients had estimated glomerular filtration rate of <60 ml/min per 1.73 m2. Eleven patients presented with severe kidney dysfunction (eGFR ≤30 ml/min per 1.73 m2), 4 of whom required dialysis (2 with anti-GBM and 2 with MPO). Twelve patients received immunosuppression, 2 did not (1 had end-stage renal disease [ESRD]), and the other had a single fibrous crescent on biopsy); treatment data were not available for 1 patient. The most common drugs used were corticosteroids (n = 11) and cyclophosphamide (n = 10), followed by mycophenolate mofetil (MMF) (n = 4), azathioprine (n = 1), and rituximab (n = 1). Plasmapheresis was performed in 3 patients: 2 with anti-GBM and 1 with myeloperoxidase (MPO) antibodies and hemoptysis. Follow-up data were available for 13 patients, with a median follow-up of 38 months (4−98 months). Of these 13 patients, 5 reached ESRD (3 MPO, 2 anti-GBM), of whom 3 received a kidney transplant. Of the patients who reached ESRD, 2 had >50% crescents on biopsy, and 1 patient had >90% interstitial fibrosis and tubular atrophy (IFTA); the other 2 patients had up to 25% crescents and 25% to 35% IFTA (Table 2). Five patients developed chronic kidney disease, 2 died (both had solid organ malignancies), and only 3 (20%) had eGFR >60 ml/min 1.73 m2 (all of these patients had only focal crescents and mild IFTA on biopsy). Conversely, renal survival in MN without crescents is estimated to be between 70% and 90%.6
Figure 1.
Top row (a–d): a case of anti–glomerular basement membrane (GBM) disease (case 12) with segmental membranous nephropathy (MN). (a) Glomerulus with a cellular crescent (hematoxylin and eosin [H&E] stain, original magnification ×400). (b) Silver stain shows a compressed glomerular tuft with no obvious spikes or lucencies along the GBM (original magnification ×4000). (c) Linear IgG staining (immunofluorescence, original magnification ×400). (d) Electron microscopic image showing segmental subepithelial deposits. Bottom row (e−h): a case of PLA2R-positive MN with concomitant p-ANCA (case 8). (e) Glomerulus with a cellular crescent (H&E stain, original magnification ×400). (f) Silver stain highlights capillary wall irregularities (fine lucencies and spikes) in the same glomerulus that contains a cellular crescent (original magnification ×400). (g) An anti-PLA2R antibody shows diffuse granular positivity along the GBM (immunofluorescence, original magnification ×400). (h) Electron microscopy confirms diffuse subepithelial deposits.
Table 1.
Clinical characteristics
| Case | Sex | Age (yr) | Ethnicity | Baseline Cr (mg/dl) | Cr at biopsy (mg/dl) | GFR at diagnosis (ml/min per 1.73 m2) | Hematuria | Proteinuria at biopsy | Follow-up proteinuria | Albumin (g/dl) | Edema | Lung symptoms | Medical comorbidities | Antibodies | Other relevant labs | Treatment | Dialysis | Transplant | Cr at last follow-up (mg/dl) | GFR at follow-up (ml/min per 1.73 m2) | Follow-up duration (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 68 | White | 1.6 | 9 | 4 | 3+ | 4 g/24 h | NA | 2.5 | Yes | SOB | Rheumatoid arthritis, NSAID use | MPO | NA | Cyclophosphamide, prednisone complicated by aspergillus pneumonia | Yes | Yes | NA | NA | NA |
| 2 | F | 21 | White | NA | 15 | 3 | Anuric | Anuric | ESRD | NA | NA | DAH, acute respiratory failure | NA | Anti-GBM | NA | PLEX, prednisone, and cyclophosphamide | Yes | No | ESRD | ESRD | 47 |
| 3 | M | 65 | White | 0.9 | 2.2 | 30 | >100 RBCs/HPF | 2.89g/24h | ESRD | 3.7 | No | Hemoptysis | Raynaud syndrome | pANCA, MPO | ANA 1:80 | prednisone, MMF switched to cyclophosphamide, PLEX, then azathioprine and prednisone | Yes | Yes | ESRD (5.38) after transplant nephrectomy | ESRD | 98 |
| 4 | F | 64 | AA | 2 | 2.5 | 24 | Moderate, 20−29 RBCs/HPF | 1.6 g/24h | 1.1 g UPC | 2.2 | Yes | Hemoptysis, DAH | Diabetes, hep C cirrhosis, HCC | pANCA, MPO, PR3 | ANA 1:320, +anticardiolipin IgM, c3 77, c4 14 | Prednisone and cyclophosphamide then MMF | No | None | 4.33 Deceased | 12 | 13 |
| 5 | F | 62 | Asian | NA | 1.09 | 51 | NA | 16.9g/24h | NA | 0.6 | Yes | NA | Liver dysfunction | NA | NA | None | NA | NA | 1.14 | 49 | 79 |
| 6 | F | 61 | AA | 0.8 | 9 | 5 | Few RBCs | 8 g UPC | 1.6 g/ 24 h | 1.4 | Yes | Pulmonary nodule | HTN, CKD, heavy smoker | NA | NA | Prednisone and cyclophosphamide | No | No | 1.32 | 49 | 10 |
| 7 | F | 66 | AA | 1.3 | 2.9 | 20 | NA | 3.5 g UPC | NA | NA | NA | NA | Bladder cancer, MGUS | pANCA | NA | NA | NA | NA | NA | NA | NA |
| 8 | M | 70 | White | 1.3 | 2.35 | 28 | >20 RBCs/HPF | 2 g/24 h | 1.3 g UPC | 3.4 | No | Cough | Previous MN with crescents, MGUS, resected pancreatic neoplasm, metastatic sarcomatoid carcinoma, HTN, DM | pANCA, MPO, PR3 | Minute IgG K | Prednisone and cyclophosphamide | No | No | 1.99 Deceased | 33 | 14 |
| 9 | M | 79 | White | 1.5 | 3 | 21 | 21−30 RBCs/HPF | 1 g UPC | 0.2 g UPC | 4.5 | No | None | HTN, MGUS, diabetes | pANCA, MPO | None | Cyclophosphamide, prednisone, then MMF | No | No | 2.02 | 32 | 46 mo |
| 10 | M | 56 | AA | 1.07 | 1.07 | >60 | Large | 4.7 g/24 h | 0.7 g/ 24 h | 2.7 | Yes | None | Diabetes, HTN | NA | None | Cyclophosphamide | No | No | 0.83 | >60 | 49 mo |
| 11 | F | 42 | NA | 1 | 1.8 | NA | >60 RBCs/HPF | 1.8 g UPC | 0.2 g UPC | 1.7 | NA | NA | Prior MPO-ANCA cutaneous vasculitis, history of cocaine abuse, positive MRSA skin culture | MPO | Positive ANA and anti-SSB | Prednisone, MMF | NA | NA | 0.8 | >60 | 41 mo |
| 12 | M | 26 | White | NA | 18.6 | 3 | Large | 2.8 g/24 h | 0.07 g UPC | 3.6 | None | Hemoptysis | Obesity | Anti-GBM >8 | None | PLEX, steroids, cyclophosphamide | Yes | Yes | 2.01 | 39 | 38 mo |
| 13 | M | 34 | AA | 1.03 | 1.18 | 93 | 45 RBCs/HPF | 1.8 g UPC | 1.2 g UPC | 3.8 | NA | NA | HTN, obesity | pANCA, MPO | ANA 1:40 | Prednisone, rituximab | No | No | 0.98 | 113 | 28 mo |
| 14 | M | 41 | White | 1.8 | 10.9 | 5 | Large | 9.5 g UPC | ESRD | 2.5 | None | NA | Prior infection-related GN, i.v. drug use (including cocaine), hep C, endocarditis, seizure | pANCA, MPO | Positive anticardiolipin and anti-SSB | None | Yes | No | ESRD (10.2) | ESRD | 4 mo |
| 15 | F | 20 | AA | 0.83 | 3 | 24 | 3−5 RBCs/HPF | 24.8 g/24 h | 3.4 g UPC | 1.4 | Yes | SOB | Schizophrenia, bipolar disorder, Bell palsy, obesity | Anti-PLA2R antibody | Low C4 (17), normal C3 | Prednisone, cyclophosphamide | No | No | 1.51 | 52 | 17 mo |
AA, African American; ANA, anti−nuclear antibodies; ANCA, anti−neutrophil cytoplasmic antibodies; Cr, creatinine; C3, complement factor 3; C4, complement factor 4; CKD, chronic kidney disease; DAH, diffuse alveolar hemorrhage; DM, diabetes mellitus; ESRD, end-stage renal disease; F, female; GBM, glomerular basement membrane; GFR, glomerular filtration rate; GN, glomerulonephritis; HCC, hepatocellular carcinoma; hep C, hepatitis C; HPF, high-power field; HTN, hypertension; labs, laboratory investigations; M, male; MGUS, monoclonal gammopathy of undetermined significance; MMF, mycophenolate mofetil; MN, membranous nephropathy; MPO, myeloperoxidase; MRSA, methicillin-resistant Staphylococcus aureus; NA, not available; NSAIDs, nonsteroidal anti-inflammatory drugs; PLA2R, M-type phospholipase A2 receptor; PLEX, plasma exchange; PR3, proteinase 3; RBCs, red blood cells; SOB, shortness of breath; SSB, anti-Sjögren's syndrome type B; UPC, urine protein-to-creatinine ratio.
Table 2.
Pathological characteristics
| Case | Final diagnosis | Total glomeruli | Crescents/FN | GS | % Active lesionsa | IFTA% | Immunofluorescenceb | IgG1 | IgG2 | IgG3 | IgG4 | PLA2R | EM stage | Mesangial deposits | TRIs | Extraglomerular deposits |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ANCA-associated crescentic and necrotizing GN; membranous GN | 16 | 4 | 8 | 25 | 25 | IgG, IgA, C3, kappa, lambda GBM | 1 | 0 | 1.5 | 0.5 | Negative | NA | NA | NA | 0 |
| 2 | Diffuse crescentic and necrotizing anti-GBM disease; segmental MN | 21 | 18 | 0 | 85 | 0 | Linear GBM IgG, kappa and lambda; linear TBM IgG (focal) | 2.5 Linear | 1.5 Linear | 0 | 3 Linear | Negative | 1 | 0 | 0 | 0 |
| 3 | Focal crescentic GN with MN | 30 | 1 | 10 | 3 | 35 | IgG, IgA, C3, kappa, lambda GBM and mesangial; IgG, kappa, lambda TBM | 1.5 | 1 | 0 | 0.5 | Negative | 1 to 2 | 1 | 0 | TBM deposits by IF |
| 4 | MN with fibrous crescents; underlying diabetic glomerulosclerosis | 24 | 4 (Fibrous) | 10 | 0 | 60-70 | IgG, IgM, C1q, C3, kappa and lambda GBM, IgG TBM (focal) | 1.5 | 0.5 | 0.5 | 2 | Positive | 3 to 4 | 0 | 0 | TBM deposits by IF |
| 5 | MN with fibrous crescents | 34 | 1 (Fibrous) | 9 | 0 | 20 | IgG, C3, kappa, lambda GBM | 3 | 2 | 0 | 2 | Negative | 2 | 0 | 0 | 0 |
| 6 | MN with focal crescents | 13 | 3 | 0 | 23 | 20-25 | IgG, IgA, IgM, C1q, C3, Kappa and lambda GBM | 2 | 1 | 2 | 3 | Weak and segmental | 2 to 3 | 0 | 0 | 0 |
| 7 | ANCA-associated crescentic and necrotizing GN with MN | 33 | 8 | 12 | 24 | 60 | IgG, IgM, kappa, lambda GBM | 2 | 1 | 0.5 | 3 | Positive | 2 to 3 | 0 | 0 | 0 |
| 8 | ANCA-associated crescentic and necrotizing GN; membranous GN | 12 | 6 | 0 | 50 | 30 | IgG, C3, kappa, lambda GBM | 1.5 | 0.5 | 1 | 0 | Negative | 1 to 4 | 0 | 0 | 0 |
| 9 | ANCA-associated necrotizing and crescentic GN with MN and TBM deposits | 5 | 1 | 0 | 20 | 35 | IgG, kappa, lambda GBM; IgG, C1q, kappa, lambda TBM (focal) | 1 | 0.5 | 0 | 2 | Negative | 1 to 3 | 0 | 0 | TBM deposits by IF and EM |
| 10 | MN with focal crescents | 12 | 1 | 2 | 8 | 5 | IgG, C3, kappa and lambda | 3 | 1 | 2 | 3 | Positive | 2 | 0 | 0 | 0 |
| 11 | Immune-complex GN with segmental MN and focal necrotizing lesions | 13 | 2 | 0 | 15 | 10 | IgG, IgM, C3, kappa and lambda GBM and segmental mesangial | 2 | 0.5 | 0.5 | 0 | Negative | 2 | Rare | 1 | 0 |
| 12 | Diffuse crescentic and necrotizing anti-GBM disease; segmental MN | 23 | 18 | 0 | 78 | 25 | Linear IgG, IgA, C3, kappa and lambda | 2 Linear | 1 Linear | 1 Linear | 2 Linear | Negative | 1 to 2 | 0 | 0 | 0 |
| 13 | MN with focal crescents | 42 | 1 | 7 | 2 | 20 | IgG, C3, kappa and lambda GBM | 2 | 1 | 0.5 | 2 | Negative | 1 to 2 | 0 | 0 | 0 |
| 14 | Advanced chronic renal injury with underlying MN; acute TMA | 32 | 5 (Fibrous) | 29 | 0 | 90 | IgG, IgM, C3, kappa and lambda GBM and mesangial | 1 | 0.5 | 0.5 | 0 | Negative | 2 | 1 | 0 | 0 |
| 15 | MN with focal crescents | 9 | 3 | 0 | 33 | 20 | IgG, C3, kappa and lambda GBM and mesangial | 3 | 1 | 2 | 3 | Positive | 2 to 4 | 1 | 1 | 1 |
ANCA, anti−neutrophil cytoplasmic antibodies; C3, complement factor 3; C4, complement factor 4; Ig, immunoglobulin; EM, electron microscopy; FN, fibrinoid necrosis; GBM, glomerular basement membrane; GN, glomerulonephritis; GS, globally sclerotic glomeruli; IFTA, interstitial fibrosis and tubular atrophy; MN, membranous nephropathy; PLA2R, M-type phospholipase A2 receptor; NA, not available; TBM, tubular basement membrane; TMA, thrombotic microangiopathy; TRIs, tubuloreticular inclusions.
Active lesions include cellular or fibrous−cellular crescents and areas of glomerular segmental fibrinoid necrosis.
Deposits are granular unless otherwise specified.
Overall, our series findings are similar to those recently reported by Nikolopoulou et al.,4 with approximately 40% of patients reaching ESRD. We had a greater number of anti-PLA2R−positive cases (26% vs. 13%), whereas they had a greater number of anti-GBM−positive patients (33% vs. 13%). Interestingly, however, outcomes were similar, which may be due to the fact that the prognosis of anti-GBM disease tends to be better in cases with associated MN,7 with close to 40% of patients recovering renal function8 in contrast to only 15% in pure anti-GBM disease.S1 Potential explanations for that could be overall lower levels of anti-GBM antibodies in patients with concomitant MN as well as a narrower antigen reactivity spectrum of the anti-GBM antibodies present.7
In contrast to MN without crescents, in which case >90% of patients have normal renal function at presentation1 and hematuria is generally microscopic and low-grade,S2 most of our patients had significant hematuria, and approximately 75% presented with severe kidney dysfunction. In addition, although approximately 80% of cases of MN without crescents are considered primary, in our series only 26% of cases were PLA2R positive, and 40% were IgG4 dominant/co-dominant. Therefore, it appears that crescents occur more often in cases of secondary MN.
In conclusion, MN with crescents is a rare and heterogeneous entity that can be associated with ANCA, anti-GBM, PLA2R, and potentially other autoantibodies. It presents more often with significant hematuria and renal dysfunction than MN without crescents and progresses more often to ESRD. Whether these cases represent a coincidental occurrence of 2 separate disease entities or whether they are pathogenically related remains to be determined,8 although the latter is conceivable. For example, subepithelial deposits may facilitate GBM damage, leading to anti-GBM antibody production. Conversely, GBM damage caused by anti-GBM (or other) antibodies could expose epitopes that lead to immune-complex deposition along the subepithelial aspect of the GBM.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary References.
Contributor Information
Brad Rovin, Email: rovin.1@osu.edu.
Clarissa A. Cassol, Email: Clarissa.Cassol@osumc.edu.
Supplementary Material
References
- 1.Couser W.G. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemminger J., Nadasdy G., Satoskar A. IgG subclass staining in routine renal biopsy material. Am J Surg Pathol. 2016;40:617–626. doi: 10.1097/PAS.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez E.F., Nasr S.H., Larsen C.P. Membranous nephropathy with crescents: a series of 19 cases. Am J Kidney Dis. 2014;64:66–73. doi: 10.1053/j.ajkd.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Nikolopoulou A., Huang-Doran I., McAdoo S.P. Membranous glomerulonephritis with crescents. Kidney Int Rep. 2019;4:1577–1584. doi: 10.1016/j.ekir.2019.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasr S.H., Said S.M., Valeri A.M. Membranous glomerulonephritis with ANCA-associated necrotizing and crescentic glomerulonephritis. Clin J Am Soc Nephrol. 2009;4:299–308. doi: 10.2215/CJN.04060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cattran D.C., Pei Y., Greenwood C. Predicting progression in membranous glomerulonephritis. Nephrol Dial Transplant. 1992;7(suppl 1):48–52. [PubMed] [Google Scholar]
- 7.Jia X.Y., Hu S.Y., Chen J.L. The clinical and immunological features of patients with combined anti-glomerular basement membrane disease and membranous nephropathy. Kidney Int. 2014;85:945–952. doi: 10.1038/ki.2013.364. [DOI] [PubMed] [Google Scholar]
- 8.Basford A.W., Lewis J., Dwyer J.P., Fogo A.B. Membranous nephropathy with crescents. J Am Soc Nephrol. 2011;22:1804–1808. doi: 10.1681/ASN.2010090923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.