Abstract
Background
Frontal QRS-T angle (FQRST) has previously been correlated with mortality in patients with stable coronary artery disease, but its role as survival predictor after ST-elevation myocardial infarction (STEMI) remains unknown.
Methods
We evaluated 267 consecutive patients with STEMI undergoing reperfusion or coronary artery bypass grafting. Data assessed included demographics, clinical presentation, electrocardiograms, medical therapy, and one-year mortality.
Results
Of 267 patients, 187 (70%) were males and most (49.4%) patients were Caucasian. All-cause mortality was significantly higher among patients with the highest (101–180°) FQRST [28% vs. 15%, p = 0.02]. Patients with FQRST 1–50° had higher survival (85.6%) compared with FQRST = 51–100° (72.3%) and FQRST = 101–180° (67.9%), [log rank, p = 0.01]. Adjusting for significant variables identified during univariate analysis, FQRST (OR = 2.04 [95% CI: 1.31–13.50]) remained an independent predictor of one-year mortality. FQRST-based risk score (1–50° = 0 points, 51–100° = 2 points, 101–180° = 5 points) had excellent discriminatory ability for one-year mortality when combined with Mayo Clinic Risk Score (C statistic = 0.875 [95%CI: 0.813–0.937]. A high (>4 points) FQRST risk score was associated with greater mortality (32% vs. 19%, p = 0.02) and longer length of stay (6 vs. 2 days, p < 0.001).
Conclusion
FQRST represents a novel independent predictor of one-year mortality in patients with STEMI undergoing reperfusion. A high FQRST-based risk score was associated with greater mortality and longer length of stay and, after combining with Mayo Clinic Risk Score, improved discriminatory ability for one-year mortality.
Keywords: ST-elevation myocardial infarction, Risk score, Predictors of mortality, Frontal QRS-T angle, Percutaneous coronary revascularization, Central valley risk score
Abbreviations: STEMI, ST-Elevation Myocardial Infarction; MACE, Major Adverse Cardiac Events; ECG, Electrocardiogram; FQRST, Frontal QRS-T angle; LOS, Length of stay; ER, Emergency Room; ACS, Acute Coronary Syndrome; PCI, Percutaneous Coronary Syndrome; CABG, Coronary Artery Bypass Grafting; MCRS, Mayo Clinic Risk Score; CHF, Congestive Heart Failure; TIMI, Thrombolysis in Myocardial Infarction; SCD, Sudden Cardiac Death
1. Introduction
In patients presenting with an ST-elevation myocardial infarction (STEMI), predicting risk of major adverse cardiovascular events (MACE) and risk of mortality helps to determine both immediate and short-term treatment strategies. Various electrocardiographic (ECG) criteria have been used as prognostic indicators for mortality in patients presenting with STEMI. Population-based studies have shown that certain ECG variables can be used for clinical risk stratification for MACE/mortality. For example, the QRS duration has emerged as a predictor of mortality in patients with left ventricular dysfunction.1,2 Other studies have shown that T-wave loop dispersion and R-to-T total cosine (repolarization measurement) can also be used for risk stratification after myocardial infarction.3
Recent studies have identified the frontal QRS-T angle (FQRST) as a useful ECG measure of the dispersion between depolarization and repolarization.4 In addition, an increased spatial QRS-T angle has been shown to be associated with higher mortality in the general population.4 Although spatial QRS-T angle is not routinely measured on 12-lead ECGs, specialized software can easily be incorporated into ECG machines to calculate it. Alternatively, FQRST can easily be calculated from a routine 12-lead ECG and does not require specialized software. Studies have shown that frontal and spatial QRS-T angles are comparable and have a strong correlation.5 The prognostic role of FQRST in the prediction of MACE after acute STEMI has hitherto not been studied.
This study sought to determine in patients presenting with an STEMI: (1) if FQRST could be used as an independent predictor of all-cause mortality, (2) if FQRST can be incorporated as a variable in a point-of-care risk assessment score for predicting one-year all-cause mortality, (3) if FQRST can be used for assessment of hospital length of stay (LOS) and identify patients suitable for potential early discharge after an STEMI.
2. Methods
2.1. Study population
We retrospectively analyzed 267 consecutive patients who presented to the emergency room of a tertiary-care hospital from January 2014 to January 2015 with a presumed diagnosis of acute coronary syndrome (ACS) and met ECG criteria for STEMI. We calculated that a sample size of 230 patients would be required to show a significant difference with an α value of 0.05 and beta of 0.2 to achieve a power of 80%. Patients who were found to have either pre-existing left bundle branch block, paced rhythm on ECG, or left ventricular hypertrophy by ECG criteria were excluded. In addition, those subjects in whom 12-lead ECG data could not be obtained or interpreted were excluded from the study. Diagnosis of STEMI was based on ST segment elevations measuring 0.2 mV in leads V1 to V3 or 0.1 mV in all other leads with these changes being present in at least two contiguous leads. Myocardial infarction was diagnosed by symptoms of ischemia and elevation in cardiac biomarkers, as listed by the Joint European Society of Cardiology and the American College of Cardiology.6,7
2.2. Frontal QRS-T angle measurement
A 12-lead ECG was obtained with the patient in the supine resting position using a Philips Page Writer Touch Interpretive ECG Machine (Philips Medical Systems, Andover, MA, USA). The ECGs were obtained at a paper speed of 25 mm, a gain of 10 mV, and a paper format of 3 × 4. For every patient, the first ECG recording was used to collect variables including heart rate, PR interval, QRS duration, QT interval, corrected QT interval, QRS axis, and T-wave axis. FQRST was measured as the absolute difference between the frontal QRS and frontal T-wave axis calculated as T-wave axis – QRS axis; and if greater than 180°, the difference was subtracted from 360° to obtain a continuous variable ranging from 0° to 180°.8 Based on the relative risk associated with increasing values of FQRST, they were classified into three groups of 1–50°, 51–100°, and 100–180°.
2.3. Clinical characteristics
A 12-lead ECG as well as basic laboratory and radiographic investigations were obtained at the time of presentation to the emergency room. All subjects underwent emergent coronary angiography and based on the findings underwent either Percutaneous Coronary Intervention (PCI) or Coronary Artery Bypass Grafting (CABG). Concurrent treatment with unfractionated Heparin/low molecular weight heparin and periprocedural intravenous glycoprotein IIb/IIIa inhibitors or bivalirudin was utilized as per the discretion of the operator. After stent placement, patients received adjunctive dual antiplatelet therapy with Aspirin and a thienopyridine antagonist, mostly clopidogrel.
2.4. Outcomes
Mortality data during current admission were obtained from hospital records. Subsequently, one-year mortality data were obtained either from the hospital, California Department of Public Health, or Social Security Death Index records. The standardized risk score [Mayo Clinic Risk Score (MCRS)] was calculated for all patients on presentation.9 The MCRS was developed to predict MACE using the following multivariates: cardiogenic shock (5 points), left main coronary artery disease (5 points), serum creatinine level >265 μmol/L (3 points), urgent or emergent procedure (2 points), New York Heart Association ≥ III (2 points), presence of thrombus (2 points), multivessel disease (2 points), and age/number of decades after 30 years (1 point). MCRS has been externally validated in 3264 PCI patients from the National Heart, Lung, and Blood Institute Dynamic registries between 1997 and 1999 (C statistic 0.76; Hosmer–Lemeshow statistic p = 0.28).10 The study was approved by the local IRB of our institution.
3. Statistical analysis
The data collected from medical charts and laboratory reports were tabulated using Microsoft Excel spreadsheets, whereas, statistical analysis was performed using SPSS Software (PASW for Windows, Rel. 18.0.0.2009; SPSS Inc., Chicago, IL, USA). Continuous variables were summarized as either mean ± SD or median (interquartile range) and compared across groups using a t test, Mann–Whitney U test, or Kruskal–Wallis test as appropriate. Categorical variables were summarized as percentages of the group total and comparisons between groups were analyzed using either Fisher exact test or chi-square test, where deemed appropriate. Assessment of the bivariate relationship between mortality and each risk factor was carried out using data from the 267 patients included in our study. Variables that were identified as significant (p-value less than 0.1) during univariate analysis were then fitted in a logistic regression model by a backward elimination method to adjust for confounders and to determine if variables of interest were associated with an increased risk of mortality. The cumulative occurrence of all-cause mortality as a function over time was obtained by the Kaplan–Meier method after ensuring that all the assumptions of Kaplan–Meier analysis were met. The event curves were created to compare survival based on FQRST (1) Group 1: 1–50°, (2) Group 2: 51–100°, and (3) Group 3: 100–180°. Comparisons between curves were made using a log-rank test. Taking the risk probabilities as test variable and the actual outcome as state variable, receiver operating characteristic (ROC) analysis was performed to get the C statistic which is the area under the ROC curve. Statistical significance was determined by a p-value less than 0.05.
4. Results
4.1. Study population
Of 267 patients, 187 (70%) were males and most (49.4%) patients were Caucasian. Patients with the highest FQRST (100-180°) were more likely to have a history of diabetes, hypertension, congestive heart failure (CHF), and myocardial infarction as shown in Table 1. Admission and peak troponin levels did not differ significantly between the three groups of the FQRST. Heart rate, QRS duration, and incidence of atrial fibrillation were greater among patients with highest FQRST, whereas ST segment resolution did not differ between the three groups. Likewise, Thrombolysis in Myocardial Infarction (TIMI) Grade 3 flow after reperfusion and time to reperfusion did not differ between the three groups. Medical therapy did not differ significantly between the three groups; however, patients with higher FQRST were less likely to have received glycoprotein IIb/IIIa inhibitors during the procedure, as shown in Table 1.
Table 1.
Clinical characteristics.
| Variable | FQRST 1-50° (n = 118) | FQRST 51-100° (n = 65) | FQRST 100-180° (n = 84) | p- value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 59 ± 14 | 61 ± 14 | 68 ± 14 | <0.001 |
| Male Gender | 85 (31.8%) | 44 (16.5%) | 58 (21.7%) | 0.81 |
| Body Mass Index (kg/m2) | 28.67 ± 6.50 | 29.35 ± 5.84 | 26.94 ± 6.03 | 0.05 |
| Clinical characteristics | ||||
| Diabetes type 2 | 25 (9.4%) | 17 (6.4%) | 32 (12.1%) | 0.03 |
| Smoking | 55 (20.6%) | 30 (11.2%) | 34 (44.6%) | 0.66 |
| Hypertension | 75 (28.1%) | 48 (18%) | 70 (26.2) | 0.01 |
| Hyperlipidemia | 65 (24.3%) | 35 (13.1%) | 54 (20.2%) | 0.09 |
| Congestive heart failure | 17 (6.4%) | 17 (6.4%) | 43 (16.1%) | <0.001 |
| Previous myocardial infarction | 27 (10.1%) | 23 (8.6%) | 43 (16.1) | <0.001 |
| Laboratory data | ||||
| Admission troponin (ng/ml) | 26.95 ± 113 | 22.23 ± 73.93 | 35.04 ± 152.37 | 0.82 |
| Peak troponin (ng/ml) | 54.15 ± 94.39 | 111.6 ± 380.11 | 43.28 ± 75.88 | 0.15 |
| Hematocrit (%) | 41.60 ± 6.44 | 40.11 ± 70 | 40.21 ± 5.85 | 0.35 |
| Electrocardiographic data | ||||
| Heart rate (beats/min) | 77.92 ± 23.82 | 81.65 ± 26.25 | 93.46 ± 27.21 | <0.001 |
| PR Interval (ms) | 160.78 ± 66.79 | 148.41 ± 52.42 | 148.11 ± 59.20 | 0.27 |
| QRS duration (ms) | 90.46 ± 15.95 | 91.43 ± 1 9.03 | 114.60 ± 33.90 | <0.001 |
| Corrected QT interval (ms) | 412.92 ± 34.07 | 425.15 ± 34.63 | 451.74 ± 45.21 | <0.001 |
| Atrial fibrillation | 15 (5.6%) | 8 (3%) | 27 (10.1%) | 0.002 |
| ST-elevation resolution | 87 (73.7%) | 45 (69.2%) | 48 (57.8%) | 0.06 |
| Angiographic data | ||||
| Multivessel (≥2 vessels) disease | 42 (43.8%) | 30 (55.6%) | 36 (69.2%) | 0.011 |
| TIMIa Grade 3 flow achieved | 87 (73.8%) | 48 (73.8%) | 54 (64.3%) | 0.14 |
| Time to reperfusion (minutes) | 29.63 ± 79.10 | 36.44 ± 93.87 | 33.60 ± 68.55 | 0.86 |
| Medical therapy | ||||
| Aspirin use | 37 (39.8%) | 23 (24.7%) | 33 (35.5%) | 0.43 |
| Beta-blocker use | 31 (37.3%) | 25 (30.1%) | 27 (32.5%) | 0.19 |
| Statin use | 37 (41.6%) | 23 (25.8%) | 29 (32.6%) | 0.77 |
| ACEI use | 37 (37%) | 19 (19%) | 44 (44%) | 0.002 |
| Heparin use | 26 (41.9%) | 21 (33.9%) | 15 (24.2%) | 0.10 |
| Enoxaparin use | 17 (37%) | 10 (21.7%) | 19 (41.3%) | 0.27 |
| Glycoprotein IIb/IIIa use | 64 (24.2%) | 34 (12.8%) | 29 (10.9%) | 0.02 |
Thrombolysis in Myocardial Infarction.
4.2. Mortality
At one year, 62 (23.3%) patients died due to all causes. 36 (13.5%) patients died due to cardiac-related causes, whereas 43 (16.1%) died during index admission and 11 (4.1%) died within six months of hospitalization. Stratification of endpoints by FQRST is shown in Table 2. All-cause mortality was significantly higher among patients with the highest (101–180°) FQRST [28% vs. 15%, p = 0.02]. When stratified by gender, survival was significantly worse among females for all groups of FQRST (Fig. 1). Variables that were strongly associated with one-year mortality as per univariate analysis are outlined in Table 3. After adjusting for significant variables identified during univariate analysis (age, female gender, brain natriuretic peptide levels, cardiac arrest), a stepwise backward model showed that FQRST remained a significant predictor of mortality (OR = 2.04 [95% CI: 1.31–13.50], p = 0.04).
Table 2.
Outcomes based by frontal QRS-T angle measured on admission ECG in ST-elevation myocardial infarction patients.
| Outcomes | FQRST 1-50° (n = 118) | FQRST 51-100° (n = 65) | FQRST 100-180° (n = 84) | p- value |
|---|---|---|---|---|
| Cardiac death | 9 (19.1%) | 11 (23.4%) | 16 (34%) | 0.15 |
| Index admission mortality | 14 (32.6%) | 14 (32.6%) | 15 (34.9%) | 0.20 |
| 1-year mortality | 17 (27.4%) | 18 (29%) | 27 (32.1%) | 0.01 |
| Length of stay (days) | 5.47 ± 7.92 | 5.63 ± 8.43 | 9.64 ± 14.00 | 0.01 |
Fig. 1.
Kaplan–Meir survival curves among patients presenting with ST-elevation myocardial infarction based on frontal QRS-T angle measured during admission, and stratified by gender. Survival among females was significantly worse for all three groups of frontal QRS-T angles.
Table 3.
Univariate analysis of variables used to predict survival at one year.
| Variable | Mortality in 1 Year (n = 62/267) |
|
|---|---|---|
| (95% Confidence Intervals) | p - value | |
| Gender (male) | 3.83 (2.11–6.94) | <0.001 |
| Race (Caucasian) | 0.83 (0.40–1.69) | 0.60 |
| Age | 1.06 (1.04–1.08) | <0.001 |
| Basic metabolic index | 0.91 (086–0.96) | <0.001 |
| Systolic blood pressure | 0.99 (0.98–1.00) | 0.10 |
| Diastolic blood pressure | 0.98 (0.97–0.99) | 0.03 |
| Diabetes type 2 | 0.76 (0.41–1.41) | 0.45 |
| Smoker | 2.90 (1.54–5.46) | 0.001 |
| Hypertension | 1.36 (0.70–2.65) | 0.37 |
| Hyperlipidemia | 1.47 (0.83–2.61) | 0.19 |
| Congestive heart failure | 3.15 (1.74–5.71) | <0.001 |
| Atrial fibrillation | 1.40 (0.70–2.81) | 0.35 |
| Previous myocardial infarction | 1.28 (071–2.31) | 0.41 |
| Cerebrovascular accident | 3.83 (1.77–8.31) | 0.001 |
| Killip Class 2 | 5.11 (2.56–10.21) | <0.001 |
| Killip Class 3 | 5.31 (1.34–21.26) | 0.02 |
| Killip Class 4 | 7.33 (2.81–19.11) | <0.001 |
| Troponin at admit | 1.00 (0.99–1.00) | 0.64 |
| WBC count | 1.09 (1.04–1.14) | <0.001 |
| Neutrophil/lymphocyte ratio | 1.05 (1.01–1.09) | 0.01 |
| Hematocrit | 0.90 (0.84–0.96) | 0.001 |
| Glomerular filtration rate | 0.98 (0.97–0.99) | <0.001 |
| Chronic kidney disease stage | 12.27 (3.29–45.72) | <0.001 |
| Admit troponin | 1.00 (0.99–1.00) | 0.64 |
| Brain natriuretic peptide | 1.000 (1.10–1.20) | 0.003 |
| Left ventricular hypertrophy | 1.28 (0.67–2.56) | 0.51 |
| Heart rate | 1.01 (1.00–1.02) | 0.05 |
| PR interval | 1.000 (0.99–1.00) | 0.85 |
| QRS duration | 1.00 (099–1.02) | 0.17 |
| Corrected QT interval | 1.00 (0.99–1.01) | 0.33 |
| QRS-T admit (51–100) | 2.28 (1.08–4.81) | 0.03 |
| QRS-T admit (101–180) | 2.81 (1.41–5.60) | 0.003 |
| T-wave changes | 2.81 (1.21–6.55) | 0.02 |
| ST segment elevation | 1.58 (0.88–2.86) | 0.13 |
| Cardiac arrest on presentation | 16.36 (8.10–33.07) | <0.001 |
| TIMI flow preprocedure | 1.008 (0.634–1.604) | 0.97 |
| TIMI flow postprocedure | 1.068 (0.482–2.369) | 0.87 |
4.3. Risk stratification
A risk score was also developed to predict mortality at one year, giving 0 points for FQRST of 1–50°, 2 points for FQRST between 51 and 100°, and 5 points for FQRST between 101 and 180°. The points were assigned based on estimated regression coefficients using the method described by Sullivan et al11 Patients with a low risk score (0–3 points) had significantly lower mortality than those with a high (≥4 points) risk score (19% vs. 32%, p = 0.02). Fig. 2A represents the ROC curve for MCRS, where C statistic for the predicted risk is 0.67 (standard error = 0.04) with a 95% confidence interval (0.59, 0.76). Subsequently, we combined the FQRST risk score with the standardized MCRS, which improved the discriminatory power of the MCRS risk score with a C statistic of 0.71 (standard error = 0.04), 95% confidence interval (0.63, 0.79) [p < 0.001], as shown in Fig. 2B.
Fig. 2.
Receiver operating characteristic (ROC) curve analysis comparing the risk score developed and actual outcome of one-year all-cause mortality among (A) Mayo Clinic Risk Score and (B) Mayo Clinic Risk Score combined with the FQRST score. Addition of frontal QRS-T angle–based risk score to the standardized Mayo Clinic Risk Score increased the C statistic of the area under curve (AUC) to 0.71 (p < 0.001).
4.4. Length of stay
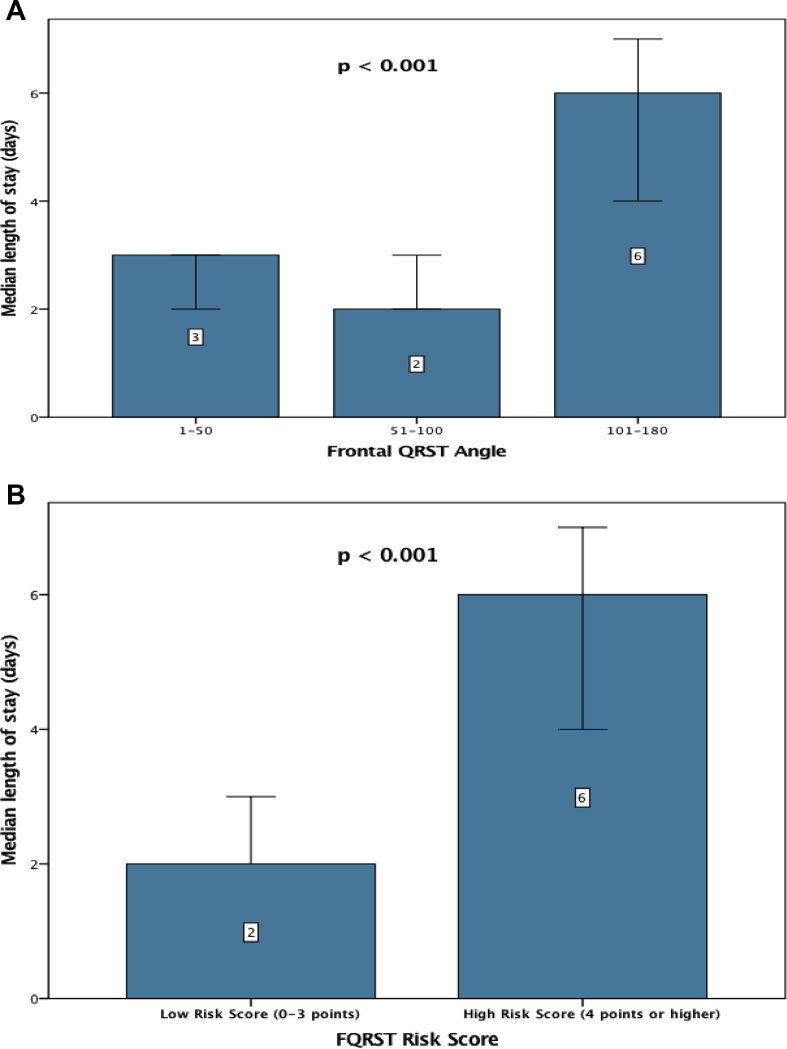
The median LOS among all patients was three days (interquartile range [IQR]: 2, 8 days). As shown in Fig. 3A, the median LOS was significantly higher among patients with FQRST ≥100° than those with FQRST 0–50° and 50–100°, respectively (p < 0.001). In addition, patients with high-risk score (≥4 points) had significantly longer LOS (6 [IQR:2, 12] days vs. 2 [IQR:2, 7] days, p < 0.001) than those who had a low (0–3 points) risk score [Fig. 3B]. Thus, the risk score was not only useful for mortality risk stratification but, also to potentially identify patients suitable for early discharge.
Fig. 3.
Length of stay stratified by (A) frontal QRS-T angle 1–50, 51–100, and 101–180°; (B) Frontal QRST risk score. Length of stay was significantly higher among patients with (A) frontal QRS-T angle >100° compared with those ≤ 100° and those with a (B) frontal QRST risk score ≥ 4 points.
5. Discussion
Our study evaluated the utility of FQRST obtained from a point-of-care 12-lead ECG for risk assessment in patients presenting with STEMI and undergoing revascularization. The study has the following key findings: (1) Higher FQRST was associated with a greater risk of one-year mortality, both during unadjusted and adjusted analysis after adjusting for risk factors; (2) FQRST-based risk score can be used for risk stratification with a higher score associated with a greater one-year mortality; (3) FQRST-based risk score when incorporated with an established standardized risk score (MCRS) helps to improve the discriminatory power of MCRS; (4) FQRST-based risk score can be used for identifying patients suitable for early discharge from the hospital.
5.1. FQRST and mortality
Several studies have shown that ECG measures of depolarization and repolarization can be used to determine short- and long-term prognosis after ACS.12,13 FQRST calculates the vector dispersion between myocardial depolarization and repolarization. In other words, a wide QRS-T angle reflects structural aberrancies affecting the regional pathophysiological changes in ionic channels which affect the sequence of repolarization, in turn, increasing arrhythmogenicity and susceptibility for sudden cardiac death (SCD) due to malignant ventricular arrhythmias.14 This correlation between FQRST and SCD is noted across a wide range of cardiac dysfunction, including, CHF, ACS, stable coronary artery disease, hypertrophic cardiomyopathy, as well as in seemingly normal cardiac structure and function.15 Lingman et al demonstrated that QRS-T angle improved prediction of SCD after ACS in addition to traditional cardiac risk factors.16 Hence, FQRST serves as a useful surrogate for damaged/jeopardized myocardium at risk, thereby, predicting STEMI mortality, as confirmed in our study.
5.2. FQRST and gender
Gender differences in QRS-T angle have been demonstrated in several studies.17,18 The variability in heart size, physiology, and hormonal influences may likely contribute to differences in ventricular depolarization between men and women. Females have been shown to have greater divergence of the L-type calcium current among different layers of the myocardium and a lower density of the repolarizing Kr and Ks currents.19 National Health and Nutritional Examination Survey (NHANES III) data showed that the distribution of QRS-T angle differs between males and females with abnormal spatial QRS-T angle being associated with increased all-cause mortality among both genders.20 The Woman's Health Initiative showed that a wide QRS-T angle was associated with a four-fold increase in the incidence of CHF.21 In our study, survival was significantly lower among females than males, across differing distributions of QRS-T angles. Among females, smaller body mass, coronary artery anatomy, and hormonal influences may render the myocardium more susceptible to damage and less durable with respect to recovery. However, these findings will need further validation in larger cohorts.
5.3. FQRST risk score
FQRST widening can be used to create a simple risk score based on FQRST subdivided into three groups: (1) Group 1: 1–50°, (2) Group 2: 51–100°, and (3) Group 3: 100–180°. The strength of the FQRST-based risk score lies in its accuracy, simplicity, and use of routinely measured objective variables. In addition, the risk score eliminates the need for interpreting normograms and use of specialized computerized applications or diagnostic hand-held devices, further improving its user-friendliness. This study has, therefore, proven the first of its kind to exemplify the clinical utility and cost-effectiveness of the proposed risk score entailing FQRST to predict mortality after STEMI. Numerous studies have previously validated the MCRS which combines eight variables to predict cardiovascular complications after PCI.9,10 Our study is unprecedented in conceptualizing and then methodically demonstrating that combining FQRST risk score with the MCRS significantly augments the latter's discriminatory power.
5.4. FQRST and length of stay
De Luca et al demonstrated that patients with very low Zwolle risk scores could be discharged sooner from the hospital, thus, reducing LOS and increasing cost savings.22 Similarly, another cost-effective utility of FQRST-based risk score could be early discharge of low-risk patients leading to reduced LOS. In our study, patients with low FQRST-based risk score (0–3 points) had significantly shorter LOS than those with high (>4 points) score. Identification of this “low-risk” population early on during the hospitalization can further augment resource utilization by facilitating early discharge while still providing safe and efficient care after STEMI.
6. Limitations
Our study outcomes are derived from a single tertiary health care center and may not be representative of other populations. However, our data set is unique due to its biodiversity illustrated by a high percentage of minority representation, including Hispanics and Asians. Owing to the retrospective nature of the study, there is always a potential for selection bias in-spite of adjusting for potential confounders. Normal ranges for FQRST are well known and may vary by age and gender; however, an abnormal QRS-T angle is found in up to 2% of the general population.23, 24 We recognize that nonmyocardial factors unrelated directly to the genesis of ACS such as autonomic tone, electrolyte, and hormonal derangements could all alter FQRST. Owing to time constraints of meeting door-to-balloon time of 90 min, detailed chemistry including potassium levels are not obtained. Hence, we were unable to evaluate the role of electrolyte imbalances on FQRST values in our study. Additional causes of MACE could not be reliably obtained during follow-up and hence were not evaluated in the study.
7. Conclusion
The study has provided several important results in an ethnically and gender diverse contemporary PCI- or CABG-treated STEMI population. FQRST widening is an independent predictor of mortality in patients presenting with STEMI and can be used for creating a simple but robust “point-of-care” prognostic risk score, which predicts all-cause mortality at one year. The risk score, when combined with a standardized risk score such as MCRS, improved the discriminatory power for risk assessment after STEMI. Finally, this score can potentially be used to identify low-risk patients suitable for early discharge yielding significant health care cost savings.
Conflict of interests
All authors have none to declare.
Acknowledgments
The authors gratefully acknowledge the help of Dr. Mark T. LownBEng, PhD, MBBS, and Dr. Chris P. Gale BSc, MBBS, PhD, Med, from Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, United Kingdom, for their guidance and insight in performing this study.
References
- 1.Desai A.D., Yaw T.S., Yamazaki T. Prognostic significance of quantitative QRS duration. Am J Med. 2006;119(7):600–606. doi: 10.1016/j.amjmed.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Brilakis E.S., Mavogiorgos N.C., Kopecky S.L. Usefulness of QRS duration in the absence of bundle branch block as an early predictor of survival in non-ST elevation acute myocardial infarction. Am J Cardiol. 2002;89:1013–1018. doi: 10.1016/s0002-9149(02)02267-1. [DOI] [PubMed] [Google Scholar]
- 3.Zabel M., Acar B., Klingenheben T. Analysis of 12-lead T-wave morphology for risk stratification after myocardial infarction. Circulation. 2000;102(11):1252–1257. doi: 10.1161/01.cir.102.11.1252. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki T., Froelicher V.F., Myers J. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Zhu-ming Z., Prineas R.J., Case D. Comparison of the prognostic significance of electrocardiographic QRS/T Angles in predicting incident coronary heart disease and total mortality(from the atherosclerosis risk in communities study) Am J Cardiol. 2007;100(5):844–849. doi: 10.1016/j.amjcard.2007.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myocardial infarction redefined–a consensus document of the Joint european society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21(18):1502–1513. doi: 10.1053/euhj.2000.2305. [DOI] [PubMed] [Google Scholar]
- 7.Thygesen K., Alpert J.S., White H.D. Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 8.Lown M.T., Munyombwe T., Harrison W. Association of frontal QRS-T angle--age risk score on admission electrocardiogram with mortality in patients admitted with an acute coronary syndrome. Am J Cardiol. 2012;109(3):307–313. doi: 10.1016/j.amjcard.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Singh M., Lennon R.J., Holmes D.R., Jr. Correlates of procedural complications and a simple integer risk score for percutaneous coronary intervention. J Am Coll Cardiol. 2002;40:387–393. doi: 10.1016/s0735-1097(02)01980-0. [DOI] [PubMed] [Google Scholar]
- 10.Singh M., Rihal C.S., Selzer F. Validation of mayo clinic risk adjustment model for in-hospital complications after percutaneous coronary interventions, using the national heart, lung, and blood institute dynamic registry. J Am Coll Cardiol. 2003;42:1722–1728. doi: 10.1016/j.jacc.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan L.M., Massaro J.M., D'Agostino R.B. Presentation of multivariate data for clinical use: the Framingham study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 12.Kors J.A., van Herpen G., van Bemmel J.H. QT dispersion as an attribute of T-loop morphology. Circulation. 1999;99:1458–1463. doi: 10.1161/01.cir.99.11.1458. [DOI] [PubMed] [Google Scholar]
- 13.Okin P.M., Devereux R.B., Howard B.V. Principal component analysis of the T wave and prediction of cardiovascular mortality in American Indians: the Strong Heart Study. Circulation. 2002;105:714–719. doi: 10.1161/hc0602.103585. [DOI] [PubMed] [Google Scholar]
- 14.Kors J.A., de Bruyne M.C., Hoes A.W. T axis as an indicator of risk of cardiac events in elderly people. Lancet. 1998;352(9128):601–605. doi: 10.1016/S0140-6736(97)10190-8. [DOI] [PubMed] [Google Scholar]
- 15.Aro A.L., Huikuri H.V., Tikkanen J.T. QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace. 2012;14(6):872–876. doi: 10.1093/europace/eur393. [DOI] [PubMed] [Google Scholar]
- 16.Gleeson S., Liao Y.W., Dugo C. ECG-derived spatial QRS-T angle is associated with ICD implantation, mortality and heart failure admissions in patients with LV systolic dysfunction. PLoS One. 2017 Mar 30;12(3) doi: 10.1371/journal.pone.0171069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingman M., Hartford M., Karlsson T. Value of the QRS-T area angle in improving the prediction of sudden cardiac death after acute coronary syndromes. Int J Cardiol. 2016;218:1–11. doi: 10.1016/j.ijcard.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Sur S., Han L., Tereshchenko L.G. Comparison of sum absolute QRST integral, and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057175. Epub 2013 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann M.H., Yang H. Sexual dimorphism in the electrocardiographic dynamics of human ventricular repolarization: characterization in true time domain. Circulation. 2001;104(1) doi: 10.1161/hc2601.091738. [DOI] [PubMed] [Google Scholar]
- 20.Surawicz B., Parikh S.R. Differences between ventricular repolarization in men and women: description, mechanism and implications. Ann Noninvasive Electrocardiol. 2003;8(4):333–340. doi: 10.1046/j.1542-474X.2003.08411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whang W., Shimbo D., Levitan E.B. Relations between QRS|T angle, cardiac risk factors, and mortality in the third National Health and Nutrition Examination Survey (NHANES III) Am J Cardiol. 2012;109(7):981–987. doi: 10.1016/j.amjcard.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rautaharju P.M., Kooperberg C., Larson J.C. Electrocardiographic predictors of incident congestive heart failure and all-cause mortality in postmenopausal women: the Women's Health Initiative. Circulation. 2006;113(4):481–489. doi: 10.1161/CIRCULATIONAHA.105.537415. [DOI] [PubMed] [Google Scholar]
- 23.De Luca G., Suryapranata H., van ‘tHof A.W. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation. 2004;109(22):2737–2743. doi: 10.1161/01.CIR.0000131765.73959.87. [DOI] [PubMed] [Google Scholar]
- 24.Macfarlane P.W. The frontal plane QRS-T angle. Europace. 2012;14(6):773–775. doi: 10.1093/europace/eus057. [DOI] [PubMed] [Google Scholar]