Abstract
Introduction
With longer duration and progression of type 2 diabetes (T2D), β-cell function deteriorates and insulin therapy often becomes necessary. Glucagon-like peptide-1 receptor agonists such as lixisenatide that do not rely only on β-cell function and glucagon suppression primarily, but also lower glucose by other (insulin-independent) mechanisms such as delayed gastric emptying, may be appropriate adjuvant therapy to basal insulin in patients with longstanding T2D.
Methods
We assessed the efficacy and safety of insulin glargine (iGlar) versus iGlarLixi, a fixed-ratio combination of iGlar and lixisenatide, stratified by quartiles (Q) of T2D duration (≤ 7.305 [Q1], > 7.305 to ≤ 10.75 [Q2], > 10.75 to ≤ 15.67 [Q3], and > 15.67 years [Q4]) in the LixiLan-L trial (N = 736).
Results
Across all quartiles, the reduction in glycated haemoglobin was greater with iGlarLixi versus iGlar, and the difference was most pronounced in patients with the longest duration (Q4; least squares mean difference [standard error] − 0.62 [0.13], P < 0.0001). Additionally, hypoglycaemia rates were significantly lower with iGlarLixi versus iGlar in patients in Q4 (3.3 vs. 6.9 events/patient-year, P < 0.0001).
Conclusion
iGlarLixi lowered glycated haemoglobin more versus iGlar regardless of T2D duration, with benefit retained even among patients with the longest T2D duration.
Electronic Supplementary Material
The online version of this article (10.1007/s13300-020-00797-y) contains supplementary material, which is available to authorized users.
Keywords: Glucagon-like peptide-1 analogue, Insulin therapy, Type 2 diabetes
Key Summary Points
| Why carry out this study? |
| Patients with type 2 diabetes (T2D) face deteriorating β-cell function with longer duration of their disease. |
| This leads to decreased insulin production and often necessitates additional therapy. |
| This post hoc study was conducted to assess the efficacy and safety of iGlarLixi, a fixed-ratio combination of insulin glargine (iGlar) and lixisenatide, across patients with different durations of T2D in the LixiLan-L study. |
| What was learned from the study? |
| At the end of the study (week 30), glycated haemoglobin (HbA1c) reduction was significantly greater with iGlarLixi compared with iGlar, and proportions achieving the composite endpoint (HbA1c < 7% [< 53 mmol/mol] with no weight gain at week 30 and no clinically important hypoglycaemia over 30 weeks) were significantly higher in the iGlarLixi arm across all T2D duration quartiles. |
| In this post hoc analysis, fixed-ratio combination therapy with iGlarLixi benefitted patients regardless of T2D duration, including patients with longstanding T2D who can be challenging to treat because of progressive loss of β-cell function. |
Introduction
Type 2 diabetes (T2D) is characterized by hyperglycaemia, which is related to defects in both insulin action (resistance, in most patients [1]) and insulin secretion (β-cell dysfunction in all patients) [2]. By the time T2D is clinically manifest, β-cell function is diminished by 40–65% and continues to decline progressively with longer duration of disease [3]. Therapies relying on endogenous insulin and β-cell function may become less effective [3]. Thus, due to the progressive nature of the disease, many patients with longer duration of T2D will eventually require exogenous insulin therapy.
The short-acting glucagon-like peptide-1 receptor agonist (GLP-1 RA) lixisenatide does not depend on endogenous insulin and β-cell function only [4], as in addition to suppression of glucagon secretion it also has substantial effects on retardation of gastric emptying and, as a result, blunting of postprandial glucose excursions [5]. Therapy with both exogenous basal insulin and a GLP-1 RA such as lixisenatide may use complementary modes of action to enhance efficacy in longstanding diabetes. Moreover, this combination minimizes the potential for weight gain seen with insulin use, without increased risk for hypoglycaemia. iGlarLixi, a once-daily titratable fixed-ratio combination of basal insulin glargine 100 U/ml (iGlar) and lixisenatide, combines the ability of insulin to address basal requirements during fasting periods with the glucose homeostatic effects of GLP-1 agonism, including prolonged absorption leading to reduced postprandial glucose excursions [4, 6].
The phase 3 LixiLan-L trial (N = 736) evaluated the use of iGlarLixi in patients whose T2D was inadequately controlled by basal insulin with or without up to two oral antihyperglycaemic drugs (OADs). In this population, iGlarLixi showed greater reductions in glycated haemoglobin (HbA1c) from baseline compared with iGlar, while documented symptomatic hypoglycaemia (≤ 70 mg/dl) was comparable between groups [7]. Post hoc analyses of LixiLan-L trial data provide an opportunity to better understand areas of clinical relevance including whether the benefit of iGlarLixi compared with basal insulin is affected by patient characteristics. The current post hoc analysis of the LixiLan-L trial was conducted to assess the effects of iGlarLixi versus iGlar 100 U/ml stratified by reported duration of T2D, with the hypothesis that iGlarLixi, compared with iGlar, would demonstrate a benefit in efficacy and safety for patients regardless of duration of T2D.
Methods
Study Design and Participants
LixiLan-L (NCT02058160; Fig. S1) was an open-label, randomized, multinational, phase 3 clinical trial [7]. Briefly, eligible patients (> 1-year history of T2D treated with basal insulin for > 6 months before screening with or without up to 2 OADs) entered a 6-week run-in period. During this period any OAD other than metformin was discontinued (approximately 90% of patients were taking metformin in both groups), patients previously receiving another basal insulin were switched to iGlar, and the dose of iGlar was optimized for all patients. At the end of the run-in period, patients with HbA1c 7–10% (53–86 mmol/mol), fasting self-measured plasma glucose ≤ 140 mg/dl (≤ 7.8 mmol/l) and daily iGlar dose of 20–50 U were randomized 1:1 to receive iGlarLixi or iGlar, with or without metformin, for 30 weeks.
The trial was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation guidelines for good clinical practice and all applicable laws and regulations. The protocol was reviewed and approved by independent ethics committees and institutional review boards. All patients gave their informed consent prior to their inclusion in the study.
Post Hoc Analysis and Endpoints
This post hoc analysis was conducted to evaluate treatment effects in subgroups of patients stratified by quartiles of T2D duration: ≤ 7.305 years (quartile [Q]1), > 7.305 to ≤ 10.75 years (Q2), > 10.75 to ≤ 15.67 years (Q3) and > 15.67 years (Q4). In these quartiles, the effects of iGlarLixi versus iGlar were compared for the following: the primary endpoint of change in HbA1c at 30 weeks, change in insulin dose, change in weight and rate of hypoglycaemia. Hypoglycaemia was defined as symptomatic hypoglycaemia and included documented (accompanied by a measured plasma glucose concentration of ≤ 70 mg/dl [≤ 3.9 mmol/l]), probable (successfully treated with oral carbohydrate without testing plasma glucose levels) and severe (requiring assistance to administer carbohydrate, glucagon or other resuscitative actions). Proportions with the composite endpoint of achieving HbA1c < 7% (< 53 mmol/mol) with no weight gain at week 30 and no hypoglycaemia events during the 30-week treatment period were also compared. An additional analysis evaluated rates of clinically important hypoglycaemia events (documented with a plasma glucose concentration of < 54 mg/dl [< 3.0 mmol/l], which is the glucose value corresponding to the International Hypoglycaemia Study Group’s ‘Level 2’) [8], and this threshold was also used in further analysis of the composite endpoint. Further safety information included a summary of gastrointestinal adverse events.
Statistical Methods
Data were analysed in SAS® version 9.4 (SAS, Marlow, UK). Least squares mean differences between treatments by subgroups of patients stratified by T2D duration in change from baseline in HbA1c, insulin dose and body weight were each compared with an analysis of covariance model with treatment arms, randomization strata of HbA1c (< 8.0% [< 64 mmol/mol], ≥ 8.0% [≥ 64 mmol/mol]) at screening, randomization strata of metformin use at screening (yes, no) and country as fixed effects and baseline values of dependent variables as a covariate. Missing data were handled using last observation carried forward. A test of interaction for treatment effect across disease duration categories for the primary endpoint of change in HbA1c at 30 weeks was performed with randomization strata of HbA1c (< 8.0% [< 64 mmol/mol] and ≥ 8.0% [≥ 64 mmol/mol]), randomization strata of metformin use, country and baseline HbA1c also included in the model. The relationship between the duration of insulin use and change of insulin dose (baseline to week 30) was evaluated by Pearson correlation.
Differences between treatments by duration quartile in symptomatic hypoglycaemia events per patient-year were compared with Poisson regression adjusted for log-transformed patient-year. Differences between treatments by duration quartile in the proportion of patients with the composite endpoint were evaluated using a Cochran-Mantel-Haenszel test.
For a sensitivity analysis, patients were also grouped by baseline insulin dose (which may also relate inversely to β-cell dysfunction, among other factors [3]) to evaluate the combined relevance of disease duration and insulin dose to treatment effect on the primary endpoint of change in HbA1c at 30 weeks, using a similar model as described for the primary analysis above. Gastrointestinal adverse events were summarized descriptively.
Results
Patient Characteristics
Age increased across the quartiles of T2D duration in both treatment arms; the difference in mean age between patients in Q4 and those in Q1 was about 8 years (Table S1). However, within each quartile, there were no meaningful differences between the iGlarLixi and iGlar treatment arms in age or other baseline characteristics (Table S1).
Endpoints
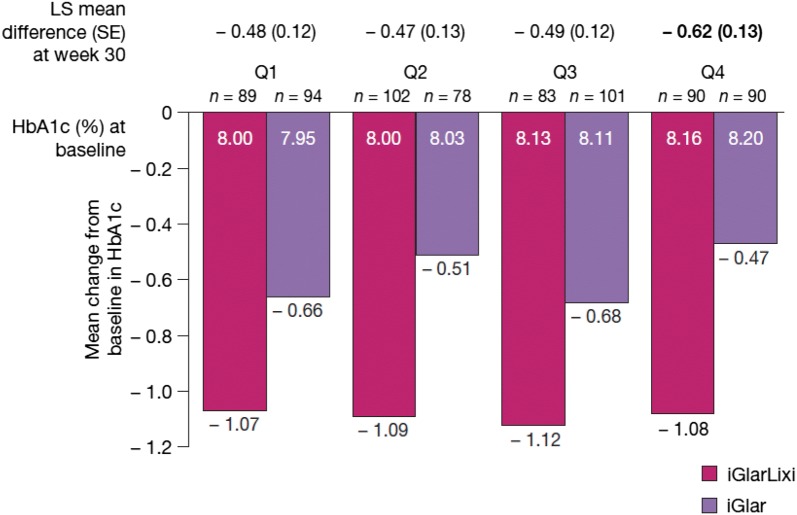
At the end of the study (week 30), HbA1c reduction was significantly (P < 0.01) greater with iGlarLixi compared with iGlar across all T2D duration quartiles and was most pronounced in patients with the longest duration of T2D (least squares mean ± standard error treatment difference in Q4: − 0.62 ± 0.13, P < 0.0001; Fig. 1), among whom was seen the least decrease for iGlar. Although there were numerical differences in treatment effects among quartiles, no significant interaction was seen between treatment and duration of T2D (i.e., there was no significant difference in treatment effect across disease duration quartiles [P = 0.1452]) for the primary endpoint of change in HbA1c at 30 weeks.
Fig. 1.
Change from baseline in HbA1c by T2D duration. P < 0.01 for iGlarLixi versus iGlar in all quartiles. Based on the analysis of covariance model with treatment arms, randomization strata of HbA1c (< 8.0% [< 64 mmol/mol], ≥ 8.0% [≥ 64 mmol/mol]) at screening, randomization strata of metformin use at screening (yes/no) and country as fixed effects, and baseline HbA1c as a covariate. N values indicate patients with data available at baseline. Week 30 and change from baseline to week 30 values are based on last observation carried forward. Bold text indicates the quartile with the greatest numerical difference between treatment arms. HbA1c glycated haemoglobin, iGlar insulin glargine 100 U/ml, iGlarLixi fixed-ratio combination of insulin glargine 100 U/ml and lixisenatide, LS least squares, Q quartile, SD standard deviation, SE standard error, T2D type 2 diabetes
Despite similar insulin doses at baseline across duration quartiles, the change in basal insulin dose fell with increasing quartiles of T2D duration: from 11.9 U for iGlarLixi and 14.4 U for iGlar in Q1 to 8.8 U and 8.9 U, respectively, in Q4 (Fig. S2), indicating that longer T2D duration was associated with less insulin uptitration. In addition, there was an inverse correlation between patient-reported duration of insulin use (a mean of 3.12 and 3.31 years in the iGlarLixi and iGlar group, respectively) and increase in insulin dose at the end of the study in both treatment arms (Pearson correlation coefficients of − 0.16 for iGlarLixi [P = 0.0027] and − 0.18 for iGlar [P = 0.0007]). Overall, mean weight increased with iGlar and decreased with iGlarLixi across all T2D duration quartiles, although the difference was less pronounced in those in Q4 (P = not significant; P < 0.01 in other quartiles) (Fig. S3).
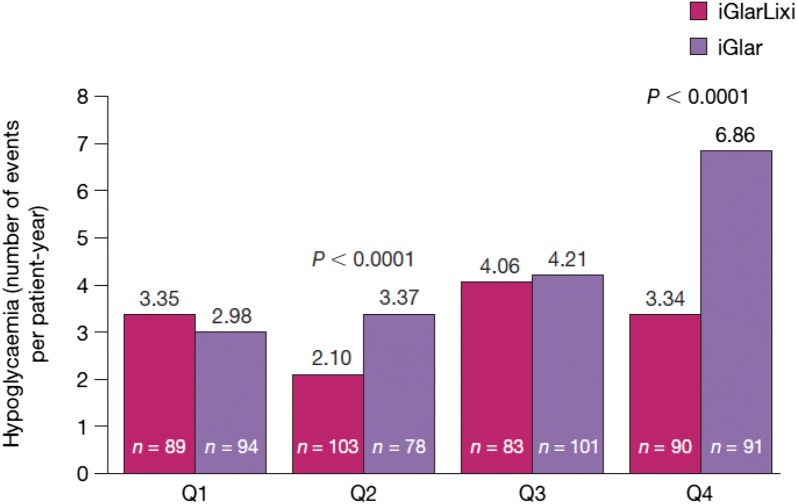
Hypoglycaemia rates were significantly lower in the iGlarLixi versus iGlar arm in Q2 and Q4, with a larger difference in Q4 (Fig. 2). However, no significant differences were observed between treatment arms in Q1 and Q3. In examination of the rates of clinically important hypoglycaemia, significant differences between arms were only observed in Q1 (0.87 events per patient-year with iGlarLixi vs. 0.26 with iGlar, P < 0.0001) (Table S2). As previously reported [7], severe symptomatic hypoglycaemic events were rare (five events in the overall iGlarLixi group vs. 1 in the iGlar group), with most being likely related to contributing circumstances (e.g., excessive exercise and/or diminished oral intake).
Fig. 2.
Hypoglycaemia events per patient-year by T2D duration. P = NS for iGlarLixi versus iGlar in Q1 and Q3. Hypoglycaemia was recorded on the electronic case report form and included severe, documented or probable. Documented symptomatic hypoglycaemia was accompanied by a measured plasma glucose concentration ≤ 70 mg/dl (≤ 3.9 mmol/l). P value is calculated by Poisson regression adjusted for log-transformed patient-year. iGlar insulin glargine 100 U/ml, iGlarLixi fixed-ratio combination of insulin glargine 100 U/ml and lixisenatide, NS not significant, Q quartile, T2D type 2 diabetes
Across all duration quartiles, iGlarLixi was associated with higher proportions of patients achieving the composite endpoint (HbA1c < 7% [< 53 mmol/mol] with no weight gain at week 30 and no hypoglycaemia [with threshold of ≤ 70 mg/dl (≤ 3.9 mmol/l)] up to week 30) than was iGlar. The differences between treatment arms were significant in Q2 (22.1% vs. 9%, respectively, P = 0.0167) and Q4 (18.9% vs. 7.7%, respectively, P = 0.0264). Similarly, when the threshold of < 54 mg/dl (< 3.0 mmol/l) for hypoglycaemia was used, the proportion of patients achieving composite endpoints was higher with iGlarLixi versus iGlar, and the between-treatment difference was significant (P ≤ 0.0170) in all four quartiles. Using this threshold, the proportions of patients achieving the composite endpoint for iGlarLixi and iGlar, respectively, were 29.2% versus 14.9% in Q1, 30.8% versus 12.8% in Q2, 28.9% versus 9.9% in Q3 and 31.1% versus 12.1% in Q4 (Fig. S4).
In sensitivity analyses, patients with both the longest reported disease duration (≥ 15.67 years [Q4]) and highest baseline insulin dose (≥ 42 U [Q4 for insulin dose quartiles]) showed the greatest difference between treatment arms in HbA1c reduction (Fig. S5).
Rates of gastrointestinal adverse events in the iGlarLixi and iGlar arms, respectively, were 10.1% versus 9.6% in Q1, 19.4% versus 7.7% in Q2, 22.9% versus 5.9% in Q3 and 15.6% versus 8.8% in Q4 (Table S2). Overall, four patients in the iGlarLixi arm discontinued due to gastrointestinal adverse events (all nausea), versus zero in the iGlar arm.
Discussion
In the LixiLan-L trial, iGlarLixi provided a superior reduction of HbA1c compared with iGlar. This post hoc analysis confirmed this was true regardless of T2D duration. Moreover, across all quartiles of T2D duration, significantly higher proportions of patients in the iGlarLixi arm achieved the composite endpoint of HbA1c < 7% (< 53 mmol/mol) with no weight gain at week 30 and no clinically important hypoglycaemia over 30 weeks.
Some differences across quartiles of T2D duration were seen in this analysis; while the baseline insulin dose was similar across quartiles, at the end of the study, those with a longer duration of diabetes required less uptitration of iGlar or iGlarLixi doses, with the lowest increase in dose among patients with the longest duration of disease. While the reason for this inverse association is not clear, we speculate that this may be related to the older age of participants with increasing quartiles of T2D duration and less aggressive titration of both iGlarLixi and iGlar in older participants. Another possible explanation for this observation could be that patients with longer T2D duration also had longer duration of insulin use and thus needed less of an increase in their insulin dose.
Interestingly, despite less increase in insulin dose in older patients with T2D duration of ≥ 15.67 years (Q4), hypoglycaemia event rates were higher for this long-duration quartile in the iGlar treatment arm (but not in the iGlarLixi arm; Fig. 2). Older age and longer duration of diabetes have been associated with an increased risk of hypoglycaemia and adverse events related to hypoglycaemia [9], possibly due to a reduced counter-regulatory hormone response to hypoglycaemia with increasing age and duration of diabetes [9–11]. Event rates of clinically important hypoglycaemia (< 54 mg/dl [< 3.0 mmol/l]), which were not examined in the primary analysis, were low overall; rates were higher in the iGlarLixi arm compared with iGlar in patients with duration of diabetes < 7.305 years (Q1) (Table S2) but similar between treatment arms in all other duration quartiles (Q2, Q3 and Q4).
T2D is a progressive disease; β-cell function deteriorates over time, and in disease duration > 10 years the cellular changes appear to be irreversible [12]. The benefits of other GLP-1 RAs in combination with insulin have been observed in patients with long duration of T2D. In a post hoc analysis of DUAL I (insulin-naïve patients) and DUAL II (patients on basal insulin), IDegLira (the combination of insulin degludec and liraglutide) showed superior reduction of HbA1c compared with basal insulin alone across baseline HbA1c categories and after adjustment for diabetes duration and previous insulin dose [13].
Therapies with extrapancreatic mechanisms of glucose lowering may play a role in longstanding diabetes in which pancreatic β-cell function is reduced. A main effect of short-acting GLP-1 RAs, for example, is retardation of gastric motility. As a consequence, short-acting GLP-1 RAs exert a greater postprandial effect than long-acting GLP-1 RAs [5] and may provide a mechanism of glucose lowering independent of β-cell function that might be relevant to patients with longstanding diabetes. A previous post hoc analysis found results consistent with the current study, in that the effects of lixisenatide were consistent across patients with varying levels of β-cell function [14].
Among the strengths of this analysis are a population in which a quarter of patients had a > 15-year history of T2D and the balanced baseline characteristics across subgroups. Additionally, results of the sensitivity analysis by both disease duration and insulin dose were consistent with those of the primary analysis.
These findings should be regarded as hypothesis generating, as they are limited by the post hoc nature of this research. This study was not prespecified nor designed or powered to test for differences in these subgroups, and the size of the sample in each subgroup was relatively small. It is important to note that the LixiLan-L trial used an open-label design, which carries the risk of bias. Finally, the statistical tests employed in this post hoc study did not include adjustment for multiple comparisons. Despite these limitations, this post hoc analysis of LixiLan-L trial data provided an opportunity to investigate the clinically important question of whether the benefit of iGlarLixi compared with basal insulin was affected in degree by duration of T2D at baseline.
Conclusion
In this post hoc analysis, further exploration of data from the LixiLan-L trial revealed that the improved change in HbA1c and weight associated with iGlarLixi versus iGlar in that trial occurred across subgroups of disease duration. The benefit with iGlarLixi versus iGlar in both comparative glycaemic efficacy and hypoglycaemia (plasma glucose concentration of ≤ 70 mg/dl [≤ 3.9 mmol/l]) rates was seen even among patients with the longest T2D duration (e.g., > 15.67 years). Fixed-ratio combination therapy with iGlarLixi benefitted patients regardless of T2D duration, including patients with longstanding T2D who can be challenging to treat because of progressive loss of β-cell function.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Sponsorship for this study and the Rapid Service Fee were funded by Sanofi. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Editorial Assistance
Editorial assistance in the preparation of this article was provided by Rob Coover of Caudex, New York. Support for this assistance was funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
LB, LB, VRA and DR conceived the analyses. YH was involved in data acquisition and analysed the data. AS contributed to study design, data acquisition and conceived the analyses. All authors contributed to the review and interpretation of results, revised the manuscript for intellectual content and approved the final version of the manuscript.
Prior Presentation
These results were previously presented as poster 1094-P at the American Diabetes Association (ADA; Orlando, FL, USA) 2018 and poster 787 at the European Association for the Study of Diabetes (EASD; Berlin, Germany) 2018.
Disclosures
Lawrence Blonde has received consultancy fees from AstraZeneca, Gilead Sciences, Janssen, Merck & Co., Novo Nordisk and Sanofi; he and/or his institution has received grant/research support from Janssen, Lexicon, Merck & Co., Novo Nordisk and Sanofi; and has received speaker fees from Janssen, Novo Nordisk and Sanofi. Lori Berard has received consultancy/speaker fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck & Co., Novo Nordisk and Sanofi. Aramesh Saremi was an employee of Sanofi at the time of study completion and is now an employee of Intercept Pharmaceuticals, San Diego, CA, USA. Yao Huang has received consultancy fees from Sanofi. Vanita R. Aroda reports a conflict with Merck Research Laboratories (employee: spouse); has received consultancy fees from Adocia, AstraZeneca, BD, Novo Nordisk, Sanofi and Zafgen; has received research support from AstraZeneca, Bristol-Myers Squibb, Calibra, Eisai, Fractyl, Janssen, Novo Nordisk, Sanofi and Theracos. Denis Raccah has received advisory board/speaker fees from AstraZeneca, Eli Lilly, Janssen, Novartis, Novo Nordisk and Sanofi.
Compliance with Ethics Guidelines
The trial was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation guidelines for good clinical practice and all applicable laws and regulations. The protocol was reviewed and approved by independent ethics committees and institutional review boards. All patients gave their informed consent prior to their inclusion in the study.
Data Availability
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies and process for requesting access can be found at: https://www.clinicalstudydatarequest.com/.
Open Access
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11920092.
References
- 1.Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care. 2012;35:717–722. doi: 10.2337/dc11-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia. 2009;52:1003–1012. doi: 10.1007/s00125-009-1321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saisho Y. β-cell dysfunction: its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015;6:109–124. doi: 10.4239/wjd.v6.i1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yabe D, Ambos A, Cariou B, et al. Efficacy of lixisenatide in patients with type 2 diabetes: a post hoc analysis of patients with diverse beta-cell function in the GetGoal-M and GetGoal-S trials. J Diabetes Complicat. 2016;30:1385–1392. doi: 10.1016/j.jdiacomp.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 6.Werner U. Effects of the GLP-1 receptor agonist lixisenatide on postprandial glucose and gastric emptying—preclinical evidence. J Diabetes Complicat. 2014;28:110–114. doi: 10.1016/j.jdiacomp.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39:1972–1980. doi: 10.2337/dc16-1495. [DOI] [PubMed] [Google Scholar]
- 8.International Hypoglycaemia Study Group Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155–157. doi: 10.2337/dc16-2215. [DOI] [PubMed] [Google Scholar]
- 9.Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med. 2008;25:245–254. doi: 10.1111/j.1464-5491.2007.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meneilly GS, Cheung E, Tuokko H. Counterregulatory hormone responses to hypoglycemia in the elderly patient with diabetes. Diabetes. 1994;43:403–410. doi: 10.2337/diab.43.3.403. [DOI] [PubMed] [Google Scholar]
- 11.Sesti G, Antonelli Incalzi R, Bonora E, et al. Management of diabetes in older adults. Nutr Metab Cardiovasc Dis. 2018;28:206–218. doi: 10.1016/j.numecd.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 12.White MG, Shaw JA, Taylor R. Type 2 diabetes: the pathologic basis of reversible beta-cell dysfunction. Diabetes Care. 2016;39:2080–2088. doi: 10.2337/dc16-0619. [DOI] [PubMed] [Google Scholar]
- 13.Rodbard HW, Buse JB, Woo V, et al. Benefits of combination of insulin degludec and liraglutide are independent of baseline glycated haemoglobin level and duration of type 2 diabetes. Diabetes Obes Metab. 2016;18:40–48. doi: 10.1111/dom.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonadonna RC, Blonde L, Antsiferov MB, et al. Lixisenatide as add-on treatment among patients with different B-cell function levels as assessed by HOMA-B index. Diabetes. 2014;63(Suppl 1):1018-P. doi: 10.1002/dmrr.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies and process for requesting access can be found at: https://www.clinicalstudydatarequest.com/.