See Clinical Research on Page 459
The past several years have been witness to a dramatic increase in the number of hepatitis C virus (HCV)–infected organ donors in the United States as a direct consequence of the opioid epidemic that has ravaged parts of the country.1 In an era of exceedingly long waiting times for a deceased donor kidney and a wait list approaching 100,000 patients, transplant centers have explored the possibility of using these organs as part of an effort to increase access to transplantation for more patients. Early on, Organ Procurement Organizations were either not retrieving these kidneys or had to discard them as concerns for transmission of disease dampened enthusiasm for their use.2 The availability of direct-acting antiviral (DAA) agents with proven safety and efficacy in HCV-infected patients with chronic kidney disease, end-stage kidney disease, and post kidney transplantation has completely altered the clinical landscape. This has created an opportunity to study whether a kidney from an HCV nucleic acid test (NAT)–positive donor could be safely transplanted into a patient with end-stage kidney disease either with or without HCV infection. Initially, kidneys from HCV-infected donors were transplanted exclusively into patients with chronic HCV infection, followed by early treatment with DAAs post-transplant; excellent sustained viral response (SVR) rates were reported using this strategy.3 Implementation of these protocols at a number of U.S. transplant centers translated into increased retrieval and decreased discard rates for these kidneys along with significantly shorter waiting times for patients who consented to accept a kidney from an HCV-infected donor.1,S1
The success of these early efforts and the remarkable efficacy of the DAAs set the stage to study the transplantation of HCV NAT-positive kidneys into HCV-negative recipients. The Transplanting Hepatitis C Kidneys into Negative Recipients (THINKER) trial recruited a small number of HCV-negative patients (n = 20) to test the efficacy and safety of early treatment with DAAs after transplantation of a kidney from an HCV NAT-positive donor.4 All patients were treated with grazoprevir/elbasvir once they demonstrated confirmed viremia post-transplant (mean of 3 days). The SVR12 was 100% and 1-year allograft outcomes were excellent. The EXPANDER-1 trial (Exploring Renal Transplants Using Hepatitis-C Infected Donors for HCV-Negative Recipients) used a slightly different strategy of initiating DAA therapy immediately before transplantation from an HCV NAT-positive donor (n = 10). In this trial, Durand and colleagues5 reported an SVR12 of 100% and excellent 1-year allograft outcomes.
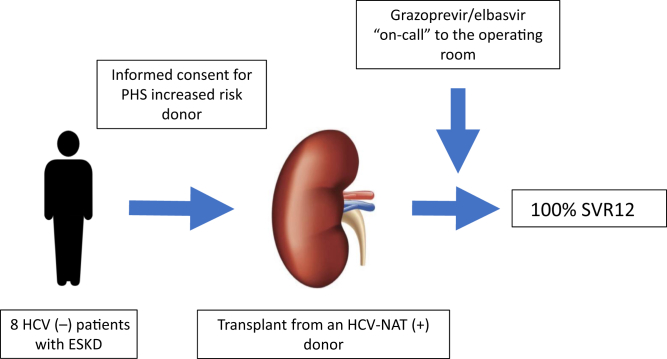
In the current issue of KI Reports, Sise and colleagues6 report the results from a trial of 8 HCV-negative patients who received a kidney from an HCV NAT-positive donor (genotype 1 or 4) (Figure 1). The mean age of the patients was 55.9 ± 9.4 years, 75% were male, 87.5% were white, and the median wait time to transplantation was 207.5 days (interquartile range, 86.75–426.75 days) after informed consent was obtained. All patients had an undetectable viral load within 2 weeks post-transplant and 100% achieved an SVR12. In addition, the authors reported excellent allograft function at 6 months post-transplant and improved quality-of-life metrics at the time of SVR12 when compared with baseline data. This important study adds to the existing literature and confirms the short-term safety and effectiveness of preemptive DAA therapy as a strategy for transplanting kidneys from HCV NAT-positive donors into HCV-negative patients.
Figure 1.
Kidney transplantation from a hepatitis C virus (HCV)–nucleic acid test (NAT)–positive donor into an HCV-negative recipient followed by preemptive direct-acting antiviral medications. ESKD, end-stage kidney disease; PHS, public health service; SVR12, sustained viral response at 12-weeks post-treatment.
THINKER, EXPANDER, and the current study were performed under strict research protocols with careful patient selection criteria. All 3 trials were externally funded with DAA agents provided by the study sponsor and thus readily available. How the results of these important studies will translate into a real-world experience is critical to the question of whether this strategy could be adapted on a large scale. To this point, Molnar and colleagues7 published their experience with 53 HCV-negative kidney transplant recipients who received a kidney from an HCV NAT-positive donor. All patients became viremic after transplantation and completed 12 weeks of DAA therapy. The mean time to initiation of DAA treatment post-transplant was 76 days, in sharp contrast to the preemptive or early post-transplant treatment used in THINKER, EXPANDER, and the study by Sise et al.6 Twenty-one percent of the insurance applications for the DAAs required an appeal for final approval. Importantly, the authors reported an increased incidence of cytomegalovirus and polyoma virus viremia when compared with historical controls, and an increase in the development of de novo donor-specific antibodies. They also observed an increased rate of transient transaminitis, with 1 patient developing fibrosing cholestatic hepatitis. Fortunately, this patient experienced normalization of liver function accompanying clearance of the virus by DAA therapy. In a similar study, Kapila et al.8 reported the results from 64 HCV-naïve patients transplanted with a kidney from an HCV NAT-positive donor. All eligible patients received DAA agents post-transplant. The median time to initiate DAA therapy was 72 days (interquartile range: 9–198 days). One patient was a nonresponder to initial DAA therapy due to N5SA resistance and required a change to sofosbuvir, velpatasvir, and voxilaprevir. There were 2 cases of fibrosing cholestatic hepatitis (both responded to DAA therapy) and 1 patient death that was not attributable to HCV infection.
For years, the transplant community has been pressed to increase access to kidney transplantation by finding novel ways to safely expand the number of organ donors. The introduction of DAAs to treat HCV infection has revolutionized the use of HCV NAT-positive donors and challenged transplant physicians to study whether these organs can be safely transplanted and not discarded in large numbers, as had been the case for several years. Whereas the transplantation of a kidney from an HCV NAT-positive donor into an HCV-infected recipient with post-transplant DAA therapy has been generally accepted as a standard of care not requiring further study, the transplantation of the same kidney into an HCV-negative patient continues to require careful study of both the short- and long-term consequences of such a strategy. Clinical trials with small numbers of patients have demonstrated that either preemptive or early post-transplant initiation of DAA treatment on confirmation of viremia in the recipient are effective strategies to obtain an SVR following transmission of HCV.4, 5, 6 Real-world studies in larger numbers of patients have extended these findings outside of the clinical trial setting and demonstrated consistent achievement of an SVR12 after DAAs were initiated.7,8 However, in contrast to THINKER, EXPANDER, and the current report from Sise et al.,7 the studies from Molnar et al.7 and Kapila et al.8 have raised important questions about the utility and safety of this strategy. As an example, given that third-party payers will almost universally be involved, the possibility of insurance denials and/or the added effort required from the clinical staff to have the medications approved must be factored into the decision process. Unquestionably, this will result in a delay in the initiation of DAA therapy post-transplantation, allowing for other unexpected consequences, such as liver injury with fibrosing cholestatic hepatitis, cytomegalovirus, and polyoma viremia and the development of de novo donor-specific antibodies to occur.7,8
The remarkably short waitlist times that have been reported for patients willing to accept a kidney from an HCV NAT-positive donor are understandably very appealing to patients with end-stage kidney disease facing the prospect of many years on dialysis. It must be recognized, however, that if the use of these kidneys becomes more the standard of care at many transplant centers, this perceived benefit may significantly diminish. In contrast, these kidneys are often retrieved from younger donors and may offer the patient a kidney of excellent quality, despite being identified as carrying a higher kidney donor profile index (KDPI) under the current allocation system. The KDPI was introduced into transplantation before the emergence of DAAs, in an era when HCV was essentially not treatable in kidney transplant recipients. With the strong evidence we now possess demonstrating the safety and efficacy of DAAs to eradicate HCV in kidney recipients, it has been suggested that the KDPI should be reassessed with HCV-positive status being removed from the equation. To this point, Cannon et al.9 performed a retrospective analysis of data from the United Network of Organ Sharing and demonstrated that patients who received a kidney from an HCV-positive donor had similar 1-year allograft survival when compared with matched pairs who received a kidney from an HCV-negative donor. Graham et al.S2 recalculated the KDPI of a cohort of patients who received an HCV-positive donor kidney by removing HCV status from the equation; this translated into an adjusted KDPI of 40.9% from 64.0%. As we move forward, this will undoubtedly be an area that requires further study and reassessment.
Conclusion
The challenge to improve access to transplantation and decrease the long-term morbidity and mortality associated with end-stage kidney disease requires that strategies that may have previously been considered to be unacceptable or too high risk must be reexamined in the context of advances in the diagnosis and treatment of prevalent diseases. Such is the case with HCV infection, whereby DAAs have revolutionized the treatment and made cure a realistic outcome for patients infected with the virus. Patients and transplant physicians must carefully weigh the potential benefits associated with accepting a kidney from an HCV NAT-positive donor (i.e., shorter wait times, higher quality kidneys) against the potential burden of additional risks that are still being defined. Further study is necessary so that larger numbers of patients have been followed for meaningful periods of time after transplantation. Until these data are available, we would suggest that transplanting a kidney from an HCV-infected donor into an uninfected recipient should be considered an experimental therapy that is studied under strict clinical trial protocols with a thorough informed consent process in place.
Disclosure
DR reports having been on Advisory Boards for Gilead, Merck, and AbbVie. The other authors declared no competing interests.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Goldberg D.S., Blumberg E., McCauley M. Improving organ utilization to help overcome the tragedies of the opioid epidemic. Am J Transplant. 2016;16:2836–2841. doi: 10.1111/ajt.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reese P.P., Abt P.L., Blumberg E.A., Goldberg D.S. Transplanting hepatitis C positive kidneys. N Engl J Med. 2015;373:303–305. doi: 10.1056/NEJMp1505074. [DOI] [PubMed] [Google Scholar]
- 3.Pagan J., Ladino M., Roth D. Treating hepatitis C virus in dialysis patients: how, when, and why? Semin Dial. 2019;32:152–158. doi: 10.1111/sdi.12764. [DOI] [PubMed] [Google Scholar]
- 4.Reese P.P., Abt P.L., Blumberg E.A. Twelve-month outcomes after transplant of hepatitis C-infected kidneys into uninfected recipients: a single-group trial. Ann Intern Med. 2018;169:273–281. doi: 10.7326/M18-0749. [DOI] [PubMed] [Google Scholar]
- 5.Durand C.M., Bowring M.G., Brown D.M. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients: an open-label nonrandomized trial. Ann Intern Med. 2018;168:533–540. doi: 10.7326/M17-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sise M.E., Strohbehn I.A., Chute D.F. Preemptive treatment with elbasvir and grazoprevir for hepatitis C–viremic donor to uninfected recipient kidney transplantation. Kidney Int Rep. 2020;5:459–467. doi: 10.1016/j.ekir.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molnar M.Z., Nair S., Cseprekal O. Transplantation of kidneys from hepatitis C-infected donors to hepatitis C-negative recipients: single center experience. Am J Transplant. 2019;11:3046–3057. doi: 10.1111/ajt.15530. [DOI] [PubMed] [Google Scholar]
- 8.Kapila N, Menon KVN, Al-Khalloufi K, et al. HCV NAT positive solid organ allografts transplanted into HCV negative recipients: a real-world experience [e-pub ahead of print]. Hepatology.https://doi.org/10.1002/hep.31011. Accessed February 1, 2020. [DOI] [PubMed]
- 9.Cannon R, Locke J, Orandi B, et al. Impact of donor hepatitis C virus on kidney transplant outcomes for hepatitis C positive recipients in the direct acting antiviral era:time to revise the kidney donor risk index? [e-pub ahead of print] Transplantation. https://doi.org/10.1097/TP.0000000000002949. Accessed February 1, 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.