Abstract
Introduction
Long wait times for kidney transplants have prompted investigation into strategies to decrease the discarding of potentially viable organs. Recent reports suggest that kidneys from hepatitis C virus (HCV)−infected donors may be transplanted into HCV-naive donors followed by direct-acting antiviral therapy.
Methods
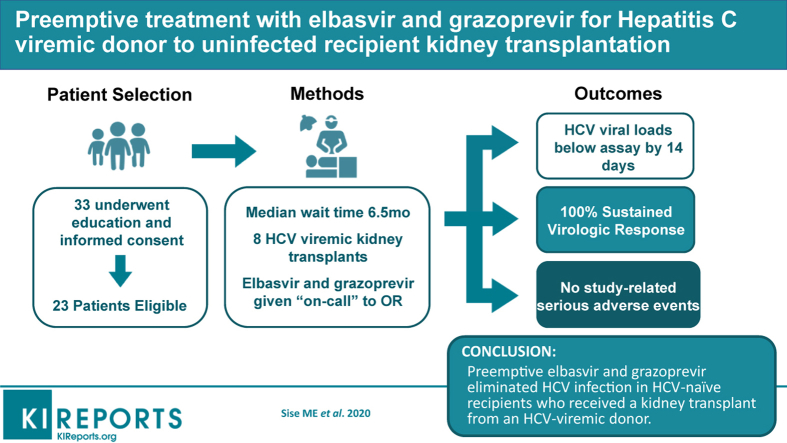
This was a pilot clinical trial to transplant kidneys from HCV-infected donors into HCV-naive recipients with preemptive use of elbasvir and grazoprevir for 12 weeks. The primary outcome was sustained virologic response 12 weeks after completion of therapy. Secondary outcomes were safety, quality of life, and early viral kinetics.
Results
A total of 33 patients were screened, and 8 underwent kidney transplantation from a HCV-viremic donors from August 2017 to March 2019. The median donor kidney donor profile index was 31% (range, 29%−65%), and patients who underwent transplantation waited a median of 6.5 months (range, 1−19 months). None had detectable HCV viremia beyond 2 weeks post-transplantation, and all achieved sustained virologic response 12 weeks after therapy (SVR12). There were no study-related severe adverse events. One patient experienced early graft loss due to venous thrombosis, whereas the remaining 7 patients had excellent allograft function at 6 months.
Conclusion
Preemptive elbasvir and grazoprevir eliminated HCV infection in HCV-naive patients who received a kidney transplant from an HCV-infected donor.
Keywords: direct-acting antivirals, hepatitis C virus, kidney transplantation, organ allocation
Graphical abstract
See Commentary on Page 386
According to the Organ Procurement and Transplantation Network (OPTN), as of June 13, 2019, nearly 95,000 people in the United States with end-stage kidney disease are waiting for a kidney transplant. Despite the rising incidence of end-stage kidney disease, the number of annual transplantations has not increased at the same rate as the number of patients waiting for transplants. Over the last 5 years, the number of deceased organ donors with hepatitis C virus (HCV) infection has increased substantially, largely because of the opioid epidemic.1 Because HCV-infected donors are typically younger and have fewer comorbidities than average donors, there has been tremendous interest in increasing use of kidneys from these donors.1
Hepatitis C−infected donor kidneys can be safely transplanted into HCV-infected recipients2,3; however, there has still been a high discard rate of organs from HCV-seropositive donors.3 The advent of direct-acting antiviral therapies (DAAs) for HCV infection has transformed HCV into a curable infection, producing >95% sustained virologic responses even in the post-transplantation setting.4, 5, 6, 7 This has opened the door for clinical trials investigating whether transplantation from actively viremic HCV-infected donors to HCV-naive recipients, managed with preemptive or post-transplantation treatment with DAAs, can lead to viable patient and allograft outcomes. Currently, the outcomes of 2 small trials have demonstrated 100% cure rates using a 12-week course of DAAs begun in the peri-transplantation period; nevertheless, the total number of patients reported in the literature is still small.8,9
The American Society of Transplantation Consensus Conference on the Use of HCV Donors in Solid Organ Transplantation has highlighted the urgent need for more prospective investigation into the risks and benefits of using organs from HCV-infected donors.2 In this study, we sought to evaluate the safety, efficacy, and viral kinetics when elbasvir and grazoprevir, a fixed-dose combination antiviral regimen, were administered preemptively (on-call to the operating room) and continued for 12 weeks post-transplantation from an HCV-viremic donor to an HCV-naive recipient.
Methods
This was a proof-of-concept, single-center study conducted at Massachusetts General Hospital and approved by the Institutional Review Board (ClinicalTrials.gov: NCT03093740). The goal of this pilot study was to determine the safety and efficacy of DAAs in HCV-naive patients undergoing kidney transplantation from HCV-viremic donors.
Eligible patients were 40 to 70 years old and were previously listed for isolated kidney transplants at Massachusetts General Hospital. Full inclusion and exclusion criteria are available in Supplementary Table S1A and B. This study specifically targeted patients who had long expected wait times for kidney transplantation; thus, eligibility based on maximum accrued waitlist time depended on blood type. We also selected patients with lower-than-average expected post-transplantation mortality, and excluded patients with a history of liver disease based on chart review, clinical evidence of liver disease at screening, or alanine aminotransferase above the upper limit of normal. FibroScan or other liver imaging was not required to exclude liver disease. The rationale for inclusion and exclusion criteria as well as our process for selecting and educating participants has been previously published.10 Patients who met inclusion criteria were reviewed by the pretransplantation coordinators to eliminate patients with a known live donor undergoing workup. Potentially eligible patients were invited for a one-on-one information session; all patients who attended this session ultimately decided to participate and provided signed informed consent.
Eligible donors had detectable circulating HCV RNA with genotype 1 or 4 infection (genotypes 2, 3, 5 and 6 were rejected), a kidney disease profile index score of 65% or below, had not previously been treated for HCV using DAAs, and did not have evidence of HIV or active hepatitis B virus infection.
Because only HCV genotype 1−infected and HCV genotype 4−infected organs were accepted, genotyping occurred prior to transplantation. Upon provisionally accepting and matching a deceased donor kidney to a recipient in our study, the local organ procurement organization (New England Organ Bank/New England Donor Services) provided donor peripheral blood that was sent via courier to the Molecular Pathology laboratory at the Hospital of the University of Pennsylvania, which performed rapid genotyping on plasma using the eSensor HCVg Direct Test (GenMark Diagnostics, Carlsbad CA).11 Upon confirmation of the donor’s HCV genotype as 1a, 1b, or 4, the deceased donor’s kidney was formally accepted, and transplantation proceeded.
Patients were given the first dose of elbasvir and grazoprevir (fixed-dose combination elbasvir 50 mg and grazoprevir 100 mg) on-call to the operating room, and then continued on a once-daily dose for 12 weeks. Because NS5A resistance-associated substitutions may occur in patients with HCV genotype 1a, any 1a donor sample underwent NS5A resistance-associated substitutions testing, results of which became available 10 to 14 days post-transplantation. Per protocol, if resistance-associated substitutions were present, the recipient’s course of elbasvir/grazoprevir was extended to 16 weeks, and weight-based ribavirin was added within 2 weeks post-transplantation.12
Seven participants received our standard induction immunosuppressive regimen of i.v. corticosteroids (methylprednisolone 250 mg) and rabbit anti-thymocyte globulin (1.5 mg/kg; range, 125−150 mg) both given intraoperatively, followed by oral tacrolimus, mycophenolate mofetil, and steroid taper (methylprednisolone on the first 2 postoperative days, and prednisone thereafter). One patient received 20 mg basiliximab for induction because of prior transplantation. Changes in immunosuppression were made at the treating transplant nephrologists’ discretion. Prophylaxis for cytomegalovirus infection and screening for BK virus took place per standard-of-care protocols (Supplementary Table S2). To minimize performance bias, patients received standard-of-care post-transplantation care. Study visits occurred on days 1, 3, 7, 14, 28, 42, 56, 70, 84, 91, 95, 112, 168, 252, and 365. Targeted physical examinations, vital signs, and laboratory testing were performed, and concomitant medications and adverse events were noted. Plasma HCV RNA levels were determined using the cobas 6800 System (Roche Diagnostics, Indianapolis, IN). The lower limit for both detection and quantification was 15 IU/ml, irrespective of genotype. Women of childbearing potential underwent pregnancy tests at screening, at post-transplantation weeks 4, 8, and 12, and at post-treatment weeks 4, 12, and 24. A quality of life (QOL) assessment (Research and Development (RAND) Corporation 36-Item Short Form Health Survey) was done at the screening visit, week 8 of treatment, and 12 weeks post-treatment.
Outcomes
The primary objective was to determine whether administration of 12 or 16 weeks of elbasvir and grazoprevir (with or without ribavirin) would eliminate HCV infection in a recipient of a kidney transplant from an HCV-viremic donor with genotype 1 or 4 infection. Cure was defined by sustained virologic response (negative HCV RNA) 12 weeks after treatment (SVR12).
We also evaluated the following secondary outcomes: (i) safety, (ii) QOL, and (iii) early viral kinetics. Safety was assessed by recording all serious and nonserious adverse events, transplant rejection, or patient mortality. Relatedness of adverse events was determined by the primary investigator and was considered to be study related if the event resulted from either active HCV viremia or from treatment with elbasvir or grazoprevir. Adverse events related to the transplantation procedure itself were not considered study related. For safety, we evaluated creatinine, estimated glomerular filtration rate, proteinuria, hemoglobin, and liver function tests results.13 Serious adverse events within the first 6 months post-transplantation were evaluated by patient report at each visit, and by chart review to minimize detection bias. QOL was assessed at screening and at post-treatment week 12 (post-transplantation week 24). Patients completed the SF-36 QOL questionnaire in person.14 We presented the average scores broken down by the Physical Component Summary and the Mental Component Summary. These were calculated in 3 steps: first, standardized z scores for each SF-36 scale were calculated using means and SDs from the general U.S. population; second, aggregate scores for physical and mental components were calculated by multiplying each scale z score by a corresponding physical or mental score coefficient; third, each aggregate score was transformed to a norm-based score by multiplying by 10 and adding the resulting product to 50.15 Early viral kinetics and time to undetectable viral load in patients receiving preemptive elbasvir and grazoprevir were determined by analysis of HCV RNA at each visit.
Statistical Analysis
Patient characteristics are presented with summary statistics for baseline demographics and clinical variables of all patients providing signed informed consent and all who underwent transplantation from an HCV-viremic donor. The mean, SD, and range were evaluated for on-treatment laboratory values and are presented to analyze safety.
Role of Funding Source
Merck provided elbasvir and grazoprevir and funding for this study through an investigator-initiated grant. The investigators designed the trial, wrote the protocol, and managed data collection, analysis, interpretation, manuscript writing, and decision to submit manuscript for publication.
Results
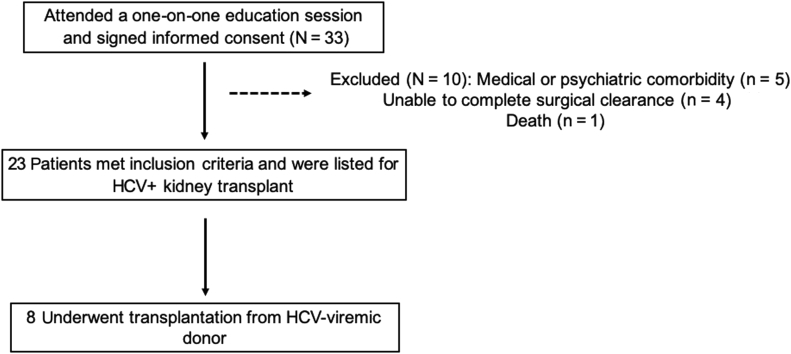
A total of 33 patients agreed to participate, provided signed informed consent, and underwent screening. After screening, 10 patients were excluded based on medical or psychiatric comorbidities. Of the 23 who were deemed to be eligible, 8 underwent kidney transplantation with an HCV-viremic donor kidney between August 2017 and March 2019 (Figure 1). All patients were followed up for at least 6 months post-transplantation to determine SVR12. Table 1 shows the baseline demographics of the patients who consented and those who underwent transplantation. The mean age of HCV-viremic kidney transplant recipients was 57 years (SD, 9.6); 75% were male; 7 were white non-Hispanic; and 1 was Hispanic. Five recipients (62%) had blood type A, and 3 recipients (38%) had blood type O. One had had a previous kidney transplantation. The median wait time to transplantation after signing consent was 6.5 months (range, 1−19 months). Length of hospital stay for the index transplant admission was 4 to 6 days (Table 1). The 1 recipient who had immediate graft failure (details below) was hospitalized for 4 days. Supplementary Table S3 shows the baseline characteristics of the 10 excluded patients.
Figure 1.
Flow of patients from enrollment to transplantation. HCV, hepatitis C virus.
Table 1.
Baseline characteristics of overall eligible subjects and transplant recipients
| Characteristic | Eligible subjects (n = 23) | HCV-viremic kidney transplant recipients (n = 8) |
|---|---|---|
| Age at time of consent, yr, mean (SD) | 56.2 (7.7) | 55.9 (9.4) |
| Female, n (%) | 10 (43.5) | 2 (25) |
| Race/ethnicity, n (%) | ||
| White, non-Hispanic | 19 (82.6) | 7 (87.5) |
| Hispanic | 3 (13.0) | 1 (12.5) |
| Black | 1 (4.4) | 0 |
| ESRD cause, n (%) | ||
| Diabetes/hypertension | 15 (65.2) | 3 (37.5) |
| IgA nephropathy | 3 (13.0) | 3 (37.5) |
| Polycystic kidney disease | 2 (8.7) | 1 (12.5) |
| Systemic lupus erythematosus | 1 (4.4) | 1 (12.5) |
| GPA | 1 (4.4) | 0 |
| Chronic reflux | 1 (4.4) | 0 |
| Blood type, n (%) | ||
| A | 12 (52.2) | 5 (62.5) |
| O | 8 (34.8) | 3 (37.5) |
| B | 4 (13.0) | 0 |
| Prior transplantation, n (%) | 1 (4.4) | 1 (12.5) |
| BMI, median (IQR) | 29.2 (26.6–31.8) | 29.9 (28.0–31.6) |
| History of diabetes, n (%) | 14 (60.9) | 4 (50) |
| Days on waitlist prior to consent, median (IQR) | 506 (340–657) | 482.5 (367.25–625.25) |
| Days from consent to transplantation, median (IQR) | – | 207.5 (86.75–426.75) |
| Length of hospital stay, da | 4 (25%) 5 (38%) 6 (38%) |
BMI, body mass index; ESRD, end-stage renal disease; GPA, granulomatosis with polyangiitis; HCV, hepatitis C virus; IQR, interquartile range.
Length of hospital stay refers to the index hospitalization for transplantation procedure.
Donor Characteristics
There were 6 donors for 8 recipients (2 donors provided 2 kidneys). All 6 donors were infected with genotype 1a HCV. The median donor kidney disease profile index score was 31% (range, 29%−65%). No donor had detectable NS5A resistance-associated variants; thus, all 8 recipients received 12 weeks of elbasvir/grazoprevir alone, without ribavirin.
All donors were of white, non-Hispanic ethnicity. Their median age was 27 years (range, 25−30 years). The cause of death for all donors was anoxia secondary to drug intoxication. None had diabetes, and 1 had hypertension. The median admission and terminal creatinine values were 1.06 mg/dl (range, 0.4−2.11) and 0.835 mg/dl (range, 0.5−1.29), respectively.
Viral Kinetics and Allograft Function
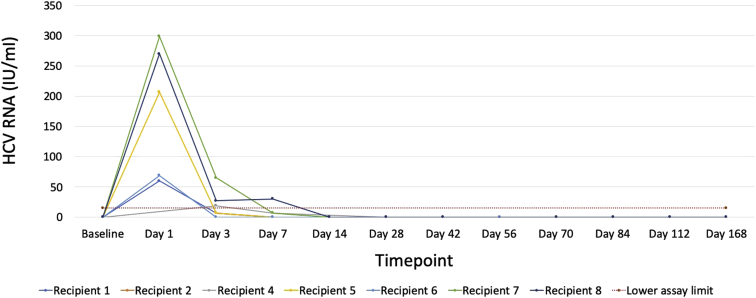
All but 1 recipient had detectable viremia at some point in the postoperative period. Viremia was highest on postoperative day 1; all but 1 patient had either undetectable or unquantifiable (i.e., below limits of quantification) HCV RNA by postoperative day 7 (Figure 2). The median peak viral load detected for the 7 recipients with detectable viremia was 69 IU/ml (range, <15 IU/ml to 299 IU/ml). Once the viral load became undetectable, all patients remained suppressed and achieved SVR12.
Figure 2.
Hepatitis C–virus (HCV) RNA levels among recipients of HCV-viremic kidneys. Detectable but unquantifiable HCV RNA is shown as 14 IU/ml, as the lower limit of quantification for our assay was 15 IU/ml. End of treatment occurred on day 84 post-treatment (PT). Twelve weeks post-treatment occurred on day 168.
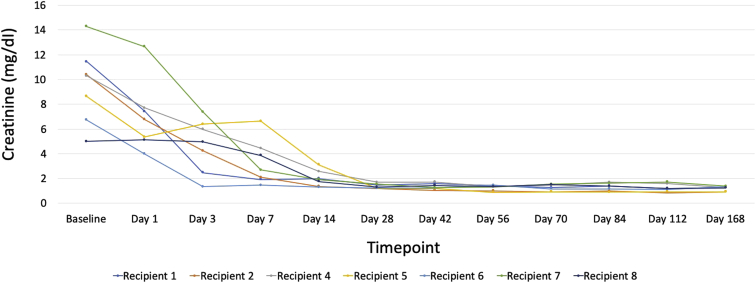
One recipient had immediate graft failure due to renal vein thrombosis (details below); this was the same patient who had undetectable viremia postoperatively. Among the other 7 recipients, 3 had delayed graft function requiring 1 dialysis session each. The 6-month graft function was excellent; the mean creatinine was 1.27 mg/dl (range, 0.91−1.38 mg/dl). There were no episodes of biopsy-proven or clinically suspected allograft rejection. Because of the lack of clinical suspicion of rejection, de novo donor-specific antibodies were not evaluated in the post-transplantation period, per our center protocol. Trends in creatinine values at all study visits are shown in Figure 3.
Figure 3.
Post-transplantation kidney function among recipients of hepatitis C–virus (HCV)−viremic kidneys. Serum creatinine values for recipients at each study visit. The clinical course of the recipient who had renal vein thrombosis is show in in Supplementary Figure S1 and is not represented here.
Supplementary Figure S1 shows the clinical course of the patient who experienced primary graft dysfunction and allograft explantation on postoperative day 1; pathology confirmed the diagnosis of renal vein thrombosis. The patient was anticoagulated, returned to dialysis, and underwent a workup for coagulopathy demonstrating a positive test result for lupus anticoagulant. His postoperative HCV RNA was never detectable after his transplant; he completed a 12-week course of elbasvir and grazoprevir as per protocol and achieved SVR12. He remained transplant listed with wait time reinstatement, and after an extensive second consent process and specific single-case protocol approval by the Partners Institutional Review Board, he underwent a second kidney transplantation from an HCV-viremic donor approximately 8 months after his first transplantation. He was treated with glecaprevir and pibrentasvir beginning on-call to the operating room, and again achieved SVR12. He experienced delayed graft function after the second transplantation, but regained function after 2 weeks and had a creatinine value of 0.7 mg/dl 6 months after his second transplantation.
Adverse Events and QOL Scores
Four patients experienced a severe adverse event extending hospitalization or requiring rehospitalization during the 6 months following transplantation (Table 2). None of the events were deemed to be related to study participation (either from HCV-viremia or antiviral therapy). Three patients had liver function test abnormalities that developed post-transplantation; 2 patients had a transient transaminase elevation within 2 weeks post-transplantation, and 1 developed elevated transaminases 11 weeks post-transplantation. In each case, the abnormalities were mild, with none >5 times the upper limit of normal. No patients developed clinical signs or symptoms of liver disease or required hospitalization or intervention. Trends in liver function test results at all study visits are shown in Supplementary Figure S2A−C.
Table 2.
Severe adverse events, relatedness, and total inpatient days in the first 6 months post-transplantationa
| SAEs/hospitalization | Total inpatient days | Relatedness to HCV viremia or elbasvir/grazoprevir |
|---|---|---|
| Lymphocele requiring drain placement Laparoscopic fenestration of perinephric lymphocele Repeat laparoscopic fenestration of perinephric lymphocele |
6 (3 separate hospitalizations) | Unrelated |
| Renal vein thrombosis | 4 | Unrelated |
| Diarrhea, abdominal pain, hematoma (surgical site), AKI, hypophosphatemia, hypomagnesemia | 1 | Unrelated |
| Readmission for delayed graft function | 1 | Unrelated |
AKI, acute kidney injury; HCV, hepatitis C virus; SAEs, serious adverse events.
Relatedness to HCV infection or elbasvir and grazoprevir treatment was determined by the primary investigator. Severe adverse events that were deemed to be related to the transplantation procedure itself were not considered study related.
One patient developed polyoma virus (BK virus) infection post-transplantation, which was detected as a part of routine screening. At 87 days post-transplantation, BK viremia was first detected and peaked at 46,510 copies/ml, prompting a reduction in mycophenolate mofetil dose with resolution of viremia. No patients developed cytomegalovirus virus infection or viremia. There were no additional nonsevere adverse events that were deemed by the primary investigator to be related to study treatment.
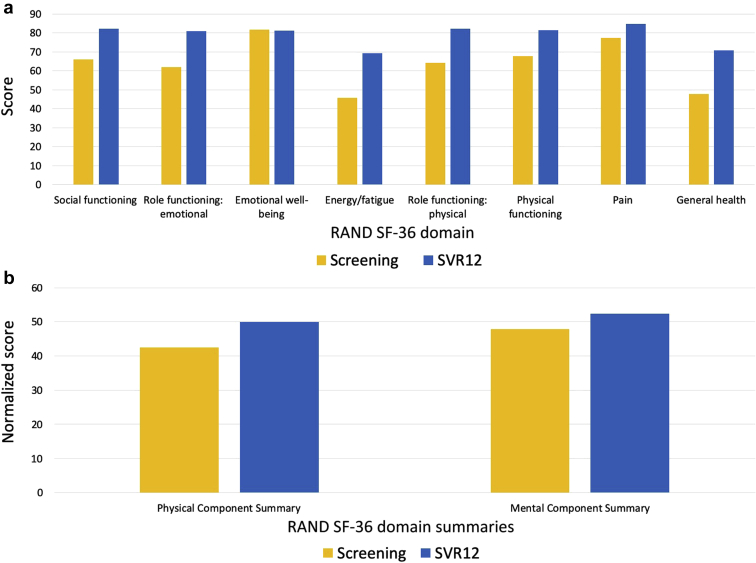
Quality of life scores improved for the physical and mental components when compared from baseline to SVR12. There were missing data at the post-transplantation week 8 visit for 50% of participants, and, in addition, not all patients have completed 1-year follow-up. Individual components and summary scores of the SF-36 QOL scores are shown in Figure 4a, b.
Figure 4.
(a) Research and Development (RAND) Corporation 36-Item Short Form Health Survey (SF-36) quality-of-life individual domain scores for recipients of hepatitis C virus (HCV)−viremic kidneys at baseline and 6 months post-transplantation (n = 7). Quality of life was assessed using the SF-36 at the screening and week 12 sustained virologic response (SVR12) visits. Recipient 3, who had renal vein thrombosis and underwent retransplantation, is not represented here. (b) SF-36 quality of life summary scores for recipients of HCV-viremic kidneys at baseline and 6 months post-transplantation (n = 7). Quality of life was assessed using the SF-36 at the screening and week 12 sustained virologic response (SVR12) visits. The SF-36 summary scores are calculated based on the 8 physical and mental domains. Recipient 3, who had renal vein thrombosis and underwent retransplantation, is not represented here.
Discussion
In this pilot trial of transplantation of HCV-infected donor kidneys into non−HCV-infected recipients, we demonstrated that 100% of non−HCV-infected recipients achieved an undetectable HCV viral load 12 weeks after completing treatment with a single 12-week course of elbasvir and grazoprevir. Beginning antiviral therapy preemptively (on-call to the operating room) led to low postoperative HCV viral loads, which became either undetectable or unquantifiable within 1 week post-transplantion. Four patients experienced SAEs, but none were deemed to be related to HCV infection or the study medication. One patient had immediate graft failure on the first postoperative day that was considered to be unrelated to HCV infection or to receipt of elbasvir/grazoprevir; however, he successfully underwent retransplantation 8 months after the first transplantation with another HCV-viremic donor kidney and was again cured with glecaprevir and pibrentasvir. All other recipients had excellent graft function at last follow-up, and there were no cases of acute rejection. Liver function test abnormalities were rare and low grade; no participant developed evidence of clinical liver disease. On average, self-reported QOL improved from the screening visit to the SVR12 visit (6 months post-transplantation) in the physical and mental domains.
Our trial results add to a growing body of literature supporting transplantation of HCV-viremic kidneys into non−HCV-infected recipients followed by immediate post-transplantation DAAs. In 2017, Goldberg et al. first reported on a trial in which 10 non−HCV-infected patients received kidneys from HCV-viremic donors with genotype 1 infection (THINKER-1 trial). Recipient HCV viral load was evaluated on postoperative day 3, and, if detected, treatment with elbasvir/grazoprevir was initiated immediately and continued for 12 weeks. All 10 patients required HCV treatment, and all achieved SVR12.16 One patient developed proteinuria and focal segmental glomerulosclerosis. In the THINKER-2 trial, 10 additional patients underwent transplantation in the same fashion. Again, all 10 patients were treated with elbasvir/grazoprevir post-transplantation and reached SVR12.8 All 20 recipients from both trials were reported to have excellent graft function 1 year post-transplantation.8,16 In 2018, Durand et al. showed that prophylactic treatment using elbasvir/grazoprevir (plus sofosbuvir, if necessary) prevented chronic HCV infection in HCV-naive patients undergoing transplantation with HCV-positive kidneys of genotype 1, 2, or 3 (EXPANDER trial).9 Ten patients received HCV-positive kidneys after initiating preemptive therapy; all 10 patients achieved SVR12. Two patients had sofosbuvir added to their treatment regimen because of non−genotype 1 HCV donor infection. Of note, 5 recipients never had detectable plasma HCV RNA. Data regarding the risks associated with delaying HCV antiviral treatment for more than 3 days post-transplantation are emerging. In 2019, Molnar et al. reported on 53 HCV-naive recipients of kidneys from HCV-infected donors who received treatment only after becoming viremic 4 to 8 weeks post-transplantation. All 53 patients became viremic and were treated with DAAs for at least 12 weeks. All achieved SVR12; however, 16 patients developed de novo donor-specific antibodies; one-third developed BK virus infection; more than one-half developed cytomegalovirus infection; and 1 patient developed fibrosing cholestatic hepatitis.17 Concerning reports of complications such as these should prompt the transplantation community to work with payers to ensure expeditious access to DAAs after HCV-viremic donor to non−HCV-infected recipient transplantation, especially in light of the overall cost-saving nature of these approaches.18 Given that there is almost universal detectable viremia in patients not receiving DAA therapy preemptively, donor viremia should be considered sufficient to approve this therapy in recipients of organs from such donors. Considering their safety with peri-transplantation administration, we advocate a preemptive course of DAAs to mitigate the morbidity of potentially serious acute HCV infection.
Our study had limitations. First, this was a single-center study with a limited number of recipients and a predominantly white population, limiting its generalizability. Because this was a pilot study, our inclusion and exclusion criteria aimed to decrease perioperative mortality and to ensure medication adherence; thus, this population may not be representative of the general transplantation population as a whole. In this series, there were no cases of rejection or cytomegalovirus infection, but 1 participant developed BK viremia requiring reduction in immunosuppression. However, our recipients were followed up for only 6 to 12 months post-transplantation. Multicenter studies with longer follow-up will be needed to more precisely determine the rates of post-transplantation complications in HCV-naive recipients of HCV-viremic kidney transplants. Although QOL scores improved in certain mental and physical domains, the absence of a control group and the small overall changes in summary scores are a limitation. Finally, because elbasvir/grazoprevir is approved only to treat genotype 1 or genotype 4 HCV infections, we relied on a multistep genotyping process prior to transplantation. Beyond the logistical hurdles involved to obtain this result in real time, it led to the discarding of potentially viable kidneys that were infected with genotype 2 or 3. New trials will assess pan-genotypic therapies for eradicating HCV after kidney transplantation, which would increase the donor pool (ClinicalTrials.gov: NCT03781726 and NCT03619837).
In summary, this study demonstrated that kidneys from HCV-viremic donors can be safely transplanted into non−HCV-infected recipients following initiation of preemptive DAA treatment. All 8 recipients were cured of HCV; viremia was transient and low level. There were no study-related serious adverse events; elevation of liver function test values was mild and not clearly related to viremia. A compelling case can be made for immediate DAA treatment in non−HCV-infected recipients of HCV-infected organs; these efforts may result in a large increase in the number and quality of kidneys available in the United States.
Disclosure
MES has received grant support from Gilead Sciences, Abbvie, Merck & Co. and has participated in scientific advisory board meetings for Abbvie, Gilead, and Merck & Co. RTC has received research grant support to his institution, Massachusetts General Hospital, from Abbvie, Gilead, Merck, Bristol-Myers Squibb, Janssen, Boehringer, and Roche. All the other authors declared no competing interests.
Acknowledgments
This study was supported by an investigator-initiated grant to Massachusetts General Hospital by Merck Sharp & Dohme Corp. to RTC. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp. In addition, MES was supported by NIH K23 DK117014. RTC was supported by NIH K24 DK078772 and the Massachusetts General Hospital Research Scholars Program. We acknowledge the contribution of the pretransplantation coordinators Linda Walsh, Laura Cornacchini, and Wendy Valerius; the post-transplantation coordinators Kim Sullivan, Colleen Dunbar, and Ana Chan; Peter Reese and David Goldberg from the University of Pennsylvania; and the New England Donor Services, particularly Jillian Wojtowicz and Christopher Curran. The authors would like to thank Xavier Vela Parada for creating the visual abstract.
Footnotes
Table S1A. Donor eligibility criteria.
Table S1B. Recipient eligibility criteria.
Table S2. Massachusetts General Hospital standard of care protocols for screening and prophylaxis of post-transplantation viral infections.
Table S3. Baseline characteristics of excluded subjects.
Figure S1. Clinical course of the recipient with immediate graft failure.
Figure S2A. Post-transplantation alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin in recipients of HCV-viremic kidneys.
Figure S2B. Post-transplantation AST in recipients of HCV-viremic kidney.
Figure S2C. Post-transplantation total bilirubin in recipients of HCV-viremic kidneys.
Supplementary Material
References
- 1.Chute D.F., Sise M.E. Effect of the opioid crisis on the donor pool for kidney transplantation: an analysis of national kidney deceased donor trends from 2010-2016. Am J Nephrol. 2018;47:84–93. doi: 10.1159/000486516. [DOI] [PubMed] [Google Scholar]
- 2.Levitsky J., Formica R.N., Bloom R.D. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant. 2017;17:2790–2802. doi: 10.1111/ajt.14381. [DOI] [PubMed] [Google Scholar]
- 3.Reese P.P., Abt P.L., Blumberg E.A. Transplanting hepatitis C-positive kidneys. N Engl J Med. 2015;373:303–305. doi: 10.1056/NEJMp1505074. [DOI] [PubMed] [Google Scholar]
- 4.Reau N., Kwo P.Y., Rhee S. Glecaprevir/pibrentasvir treatment in liver or kidney transplant patients with hepatitis C virus infection. Hepatology. 2018;68:1298–1307. doi: 10.1002/hep.30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo M., Aghemo A., Liu H. Treatment with ledipasvir-sofosbuvir for 12 or 24 weeks in kidney transplant recipients with chronic hepatitis C virus genotype 1 or 4 infection: a randomized trial. Ann Intern Med. 2017;166:109–117. doi: 10.7326/M16-1205. [DOI] [PubMed] [Google Scholar]
- 6.Chute D.F., Chung R.T., Sise M.E. Direct-acting antiviral therapy for hepatitis C virus infection in the kidney transplant recipient. Kidney Int. 2018;93:560–567. doi: 10.1016/j.kint.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Brown R.S., Jr., O'Leary J.G., Reddy K.R. Interferon-free therapy for genotype 1 hepatitis C in liver transplant recipients: real-world experience from the Hepatitis C Therapeutic Registry and Research Network. Liver Transpl. 2016;22:24–33. doi: 10.1002/lt.24366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reese P.P., Abt P.L., Blumberg E.A. Twelve-month outcomes after transplant of hepatitis C-infected kidneys into uninfected recipients: a single-group trial. Ann Intern Med. 2018;169:273–281. doi: 10.7326/M18-0749. [DOI] [PubMed] [Google Scholar]
- 9.Durand C.M., Bowring M.G., Brown D.M. Direct-acting antiviral prophylaxis in kidney transplantation from hepatitis C virus-infected donors to noninfected recipients: an open-label nonrandomized trial. Ann Intern Med. 2018;168:533–540. doi: 10.7326/M17-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sise M.E., Wojciechowski D., Chute D.F. Process of selecting and educating HCV-uninfected kidney waiting-list candidates for HCV-infected kidney transplantation. Artif Organs. 2019;43:913–920. doi: 10.1111/aor.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentile C., Van Deerlin V.M., Goldberg D.S. Hepatitis C virus genotyping of organ donor samples to aid in transplantation of HCV-positive organs. Clin Transplant. 2018;32 doi: 10.1111/ctr.13172. [DOI] [PubMed] [Google Scholar]
- 12.American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Treatment-naive genotype 1a without cirrhosis. 2017. https://www.hcvguidelines.org/treatment-naive/gt1a/no-cirrhosis Available at:
- 13.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware J.E., Jr., Sherbourne C.D. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 15.Ware J.E., Kosinski M., Keller S.D. Health Assessment Lab, New England Medical Center; Boston, MA: 1994. SF-36 Physical & Mental Health Summary Scales: A User’s Manual. [Google Scholar]
- 16.Goldberg D.S., Abt P.L., Blumberg E.A. Trial of transplantation of HCV-infected kidneys into uninfected recipients. N Engl J Med. 2017;376:2394–2395. doi: 10.1056/NEJMc1705221. [DOI] [PubMed] [Google Scholar]
- 17.Molnar M.Z., Nair S., Cseprekal O. Transplantation of kidneys from hepatitis C-infected donors to hepatitis C-negative recipients: single center experience. Am J Transplant. 2019;19:3046–3057. doi: 10.1111/ajt.15530. [DOI] [PubMed] [Google Scholar]
- 18.Eckman M.H., Woodle E.S., Thakar C.V. Transplanting hepatitis C virus-infected versus uninfected kidneys into hepatitis C virus-infected recipients: a cost-effectiveness analysis. Ann Intern Med. 2018;169:214–223. doi: 10.7326/M17-3088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.