Abstract
Objective:
To compare the effectiveness of mobile video-guided home exercise program and standard paper-based home exercise program.
Methods:
Eligible participants were randomly assigned to either experimental group with mobile video-guided home exercise program or control group with home exercise program in a standard pamphlet for three months. The primary outcome was exercise adherence. The secondary outcomes were self-efficacy for exercise by Self-Efficacy for Exercise (SEE) Scale; and functional outcomes including mobility level by Modified Functional Ambulatory Category (MFAC) and basic activities of daily living (ADL) by Modified Barthel Index (MBI). All outcomes were captured by phone interviews at 1 day, 1 month and 3 months after the participants were discharged from the hospitals.
Results:
A total of 56 participants were allocated to the experimental group and control group . There were a significant between-group differences in 3-months exercise adherence (experimental group: 75.6%; control group: 55.2%); significant between-group differences in 1-month SEE (experimental group: 58.4; control group: 43.3) and 3-month SEE (experimental group: 62.2; control group: 45.6). For functional outcomes, there were significant between-group differences in 3-month MFAC gain (experimental group: 1.7; control group: 1.0). There were no between-group differences in MBI gain.
Conclusion:
The use of mobile video-guided home exercise program was superior to standard paper-based home exercise program in exercise adherence, SEE and mobility gain but not basic ADL gain for patients recovering from stroke.
Keywords: Physiotherapy, stroke, rehabilitation, exercise, adherence, self-efficacy, functional outcome, video, home
Introduction
Stroke, also known as cerebrovascular accident, is an acute disturbance of focal or global cerebral function with signs and symptoms lasting more than 24 h or leading to death presumably of vascular origin.1 It is the third leading cause of death in Hong Kong after cancer and heart disease. More than 3000 people died in Hong Kong each year for this condition.2 The most widely recognized impairment caused by stroke is motor impairment, which restricts function in muscle movement or mobility.3,4 It was shown that patients with stroke who had as much practice as possible within 6 months after stroke onset could achieve the best functional outcome.5-7
In Hong Kong, under the service model of Hospital Authority, patients would be referred to ambulatory services, such as geriatric day hospital, or domestic physiotherapy services to continue their stroke rehabilitation after discharge from hospital. However, some patients could not attend ambulatory services due to various difficulties, such as transportation and absence of carers. The low frequency of domestic physiotherapy service also reduced the effectiveness of rehabilitation of patients. From our local data, around a quarter of home-care patients have not received any further rehabilitation training from ambulatory or domestic physiotherapy services. Among this population, there were approximately 56% of them rated to be Modified Functional Ambulatory Category (MFAC) 2–5, who may have had higher potential of improvement in stroke outcomes if appropriate rehabilitation training was given.8 Home-based exercise program would be a good choice for filling this post-discharge gap of rehabilitation continuation.
Traditionally, for continuation of exercise training program, physiotherapists would prescribe home exercises programs in paper-based format.9,10 However, evidence showed that home exercise prescription in paper-based format does not lead to better adherence to a home exercise program compared to having no written and pictorial instructions for patients with stroke less than four months.10
The recent increasing accessibility of smart technology11 offers an opportunity to advance the mode of delivery of home exercise program by mobile devices such as video-guided exercise on electronic tablets12 and video-guided exercise on mobile apps.13 However, the effects of mobile video-guided exercise programs were controversial. A study showed that home exercise programs filmed on an electronic tablet, with an automated reminder, were not superior to standard paper-based home exercise programs in terms of adherence, motor function, or satisfaction for patients recovering from stroke.12 In contrast, another study showed that people with musculoskeletal conditions who adhere better to their home exercise programs in video-based format are provided with an app with remote support compared to paper handouts; although the clinical importance of this added adherence is unclear.13
Since one of the major outcomes to evaluate the effectiveness of the mode of delivery of home exercise program is adherence,10,12,13 it is worthwhile to study the factor affecting adherence of mode of delivery of home exercise program. It was suggested that one of the barriers to adherence of home exercise is low self-efficacy.14,15 According to the theory of self-efficacy, self-efficacy is defined as a person’s confidence in their ability to perform a task.16 Thus, self-efficacy plays an important role in maintaining the exercises behavior after stroke17-19 and improving self-efficacy for exercise (SEE) could influence long-term exercise behavior as well as the early stages of exercise adoption.20-23 Another factor affecting adherence of home exercise was the delivery mode of training program.13 Studies showed that visual information brings more benefits to patients than verbal information alone.24-27 Adherence of home exercise program has positive correlation with physical function and physical performance of patients.15,28
It is worthwhile for physiotherapists to investigate the mode of delivery to enhance exercise adherence and self-efficacy of post-discharge home exercise for patients with stroke. The objective of the study is to compare the effectiveness of video-guided exercise program and standard paper-based home exercise program on adherence of exercise, self-efficacy and functional outcomes in patients with stroke within 3-month follow-up.
Methods
A randomized, controlled, assessor-blinded clinical trial was conducted between July 2018 and June 2019. Participants were recruited from the inpatient Stroke Rehabilitation Program in the Department of Medicine and Geriatrics of Tai Po Hospital and Shatin Hospital. The two hospitals provided multidisciplinary inpatient stroke rehabilitation to more than 1000 stroke patients yearly. All those stroke patients were diagnosed with acute stroke and were transferred from all the three acute hospitals in New Territories East Cluster. The hypothesis was that participants prescribed with video-guided home exercise will demonstrate higher adherence of exercise, better self-efficacy and better functional outcomes when compared with the participants in paper-based exercise prescription group.
Participants were eligible for inclusion if their principle diagnosis was stroke, participants or their carers have smart devices such as smart phones or tablets that are able to scan QR code and connect to the Internet as well. All the participants or carers could read Chinese. They were excluded if (i) could not follow gesture and instructions; (ii) MFAC upon discharge was below 2 or above 5, (iii) no smart device for video and (iv) refusal.
This was a single-blinded randomized study. The study procedure flowchart is presented in Appendix B. Before enrolment and randomization, potential participants were screened by their case therapists to ensure they met the inclusion criteria and none of the exclusion criteria. Eligible participants were then randomly assigned to either Intervention Group or Control Group in a 1:1 ratio. Investigators who were responsible for data collection were blinded to the group allocation. A randomization list was developed by personnel who were not involved in this study and who would not have any contact with the study participants. The details of the list were unknown to any of the investigators and study coordinators, and were contained in a set of sealed, sequentially numbered envelopes. Each enrolled participant was allocated to the next sequential number on the list. All research study personnel, including those who collect data and assess outcomes, were blinded to the group assignment. The envelopes and the randomization list were not revealed to any of the study personnel until completion of recruitment and data collection.
Research team adopted per-protocol analysis method and provided two brief sessions to all physiotherapists of the stroke units to inform the process of subject recruitment of potential participants. Case physiotherapists would inform research team for subject recruitment once potential participants have discharge plan, usually 1–2 weeks before discharged. Case physiotherapists provided brief information about the study to potential participants or their carers before their discharge from hospitals. Research team approached the participants and gave detailed information about the study and verifies their interest in participating once verbal consent was obtained. The Joint Chinese University of Hong Kong — New Territories East Cluster Clinical Research Ethics Committee (The Joint CUHK-NTEC CREC) approval was obtained prior to the commencement of the study. This trial design was registered prospectively with the ClinicalTrials.gov Protocol Registration and Results System (ClinicalTrials.gov ID: NCT03509363). Written informed consent was obtained from all participants.
Pre-discharge training sessions lasting for 10–15 min were provided to participants, and their carers if any, of both groups in order to make them familiar with the selected home exercises and the technique of using mobile phone to scan QR for intervention group. Participants allocated to the intervention group were prescribed a set of exercise video with QR code provided in home exercise pamphlets and they had to perform the prescribed exercises under the guidance of the videos. On the other hand, participants in control group were given instructions for their home exercise program in a traditional pamphlet includes photographs and instructions of exercise demonstration. The content of home exercise program in both groups was the same and was based on the recommendations from the National Stroke Foundation Clinical Guidelines, included nine arm control exercises, six leg control exercises, six truck control exercises and four mobility exercises (Table 1).
Table 1.
Type of home exercises in both experimental group and control group.
| Arm control exercise |
|
| Leg control exercise |
|
| Trunk control exercise |
|
| Mobility |
|
The doses for the participants were prescribed by their case physiotherapists according to the needs and abilities of participants. The total number of home exercise varied from 3 to 5 and the treatment frequencies of each exercise varied from daily to 3 times per day so the course length varied from 10 to 30 min daily. Participants’ needs and the abilities were defined as amount of assistance and the mobility by participants’ pre-discharge MFAC.
All participants were screened by their case physiotherapists according to exercise prescription guidelines of American College of Sports Medicine29 for any contraindication for exercise. Only patients who were medically fit for exercise were recruited. Exercise-related adverse events such as chest tightness or pain, dizziness, and/or trip, stumbles, or falls might happen during exercise but which was not as higher risk than the usual prescription of home exercises. Suitability of participating home exercise program will be assessed by physiotherapists based on environmental risk, fall risk, and competence of participants or carers in performing exercise with participants.
All participants’ data related to the research were collected in data collection sheets by case therapists of the patients. The data in the data collection sheets were transferred to a database in Excel format that could be accessed only by the research team members. Therefore, we could judge which participants were included in the analysis. Data on patients’ demographics, gender, age, site of lesion, side of stroke, type of stroke, education level, experience of using mobile device, present of complication such as neglect and dysphasia, cardiovascular risk factors and availability of carer, were retrieved from Central Management System of Hospital Authority by case therapists of the patients.
Outcome measures including self-reported exercise adherence, self-efficacy for exercise (SEE-C), MFAC, and Chinese version of the Modified Barthel Index (MBI) were assessed on phone follow-up basis by a blinded-assessor at 1 day, 1 month, and 3 months after the participants were discharged from hospitals. For between group comparisons, since participants had difference baseline functional status, baseline functional status affects MBI and MFAC but not SEE and Adherence, therefore MBI gain and MFAC gain were used to compare the improvement of functional status for fair comparisons. In addition, since we directly compared Adherence and SEE that 3 time points were tested; while we compared MBI gain and MFAC gain that only 2 time points were tested i.e., 1-month gain (1-month minus baseline) and 3-month gain (3-month minus baseline).
Self-reported exercise adherence
Since there has no well-developed measures that capture self-reported adherence to prescribed but unsupervised home-based rehabilitation exercises; and since we interviewed the participants by phone, some details could not be recalled. So we adopt the concept visual analogue scale to ask the adherence by percentage (0–100%) as a total effect of adherence of number of session, daily frequent, repetition, set and quality of movement of the whole review period. The exercise adherence was measured by asking participants to report their percentage of exercise completion between 3 period of time i.e., from discharge to 1 day post-discharge, from 1 day post-discharge and 1-month post-discharge and from 1-month post-discharge to 3-month post-discharge during phone follow-up. Log sheets were not used since it was reported that log sheet that needs to be filled out regarding the completion of each exercise would serve as a reminder and a motivational track record for the patient and assists patients in improving their adherence.31
Chinese version of self-efficacy for exercise (SEE-C)
An original English version of the SEE scale was designed to test people’s confidence to continue exercising in the face of barriers to exercise.32 This scale has a range of total scores from 0 to 90. A higher score indicates higher SEE. Estimates of the reliability and validity of the nine item SEE scale have been widely tested and shown to be valid for use in various settings, with internal consistency and validity with efficacy expectations significantly related to exercise activity, and factor loadings all greater than 0.50.33-35 The Cronbach’s alpha coefficient of Chinese version of the SEE scale was 0.7535 and Pearson’s correlation revealed a statistically significant correlation between perceived health and SEE-C score , .35
Modified functional ambulatory category (MFAC)
The MFAC was a 7-point Likert Scale (1–7) that was used to classify a patient’s walking capacity. Gait was divided into seven categories, ranging from no ability to walk and requires manual assistance to sit or is unable to sit for 1 minute without back or hand support (MFAC 1) to the ability to walk independently on level and non-level surfaces, stairs, and inclines (MFAC 7).36 The inter-rater reliability of the MFAC (intraclass coefficient [ICC]) was 0.982 (0.971–0.989), with a kappa coefficient of 0.923 and a consistency ratio of 94% for stroke patient37 and the ICC of the MFAC in patients with hip fractures is 0.96, with a construct validity of on the Elderly Mobility Scale (EMS).36 Participants will be asked to describe their current mobility status via phone follow-up.
Chinese version of the modified barthel index (MBI-C)
MBI was used to assess patients’ basic activities of daily living (ADL) in this study. MBI measures the participant’s performance on 10 functional items including self-care, continence, and locomotion. The values assigned to each item was based on the amount of physical assistance required to perform the task and added to give a total score ranging from 0 to 100 ( dependent, independent) with higher score indicating higher levels of physical function.38 There was no subtotal score because there was no subscale.38 The internal consistency reliability coefficient for MBI was 0.90.38 The Chinese version of MBI has been found to have good validity and reliability for assessing stroke patients.39 A study showed that the correlations between the performance-based ADL and the interview-based ADL were greater than 0.97 for the total score and greater than 0.85 for most of the individual items.40
Although the randomization design of the study reduced the biases which could compromise the outcomes, we analyzed the confounding variables such as age, gender, type of stroke, side of hemiplegia, present of complication such as neglect and dysphasia,41 follow-up physiotherapy such as ambulatory day hospital and domiciliary physiotherapy42 availability of carer,43 and experience of smart device44 to indicate any significant association in the study.
Pair -test and Chi-Square test were used to analysis the between-groups differences of baseline characteristics. Chi-Square test was used to analysis the between-groups of exercise adherence at all three time points i.e., baseline, 1-month, and 3-months. Independent -test was used to analysis the between-groups of SEE, MFAC gain, and MBI gain at all three time points i.e., baseline, 1-month, and 3-months. Pair -test was used to analysis the within-group differences between baseline to 1-month and baseline to 3-months of MFAC and MBI. The significance level was set at 0.05 for between-group analysis. All of these analyses were performed with SPSS version 18.0. Those participants with missing data due to loss of contact in phone interviews in three consecutive working days were omitted.
Results
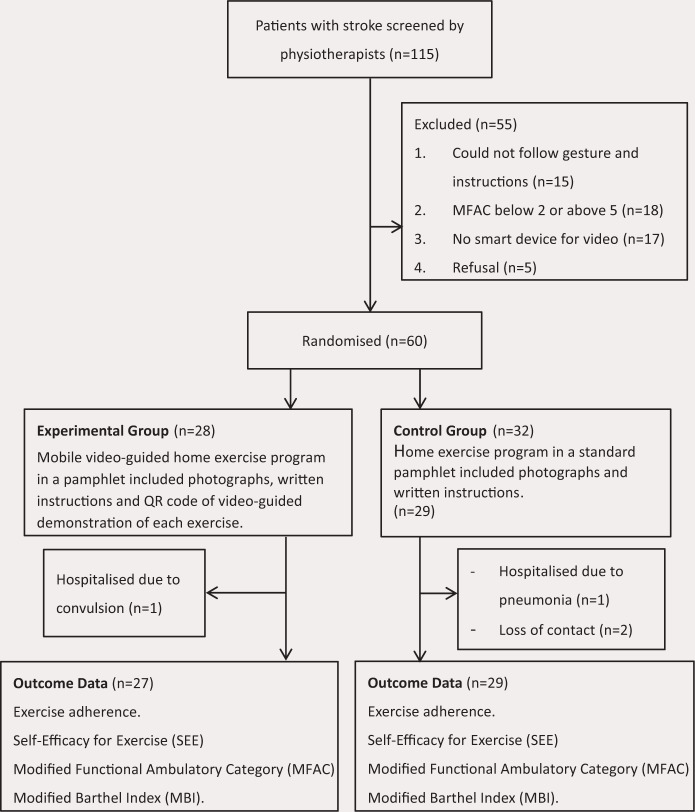
There was a total of 115 stroke patients who were screened by physiotherapists for this study from August 2018 to March 2019. Of these, 55 participants were excluded, in which, 15 could not follow gesture and instruction; 18 had mobility below MFAC 5 or above MFAC 5; 17 had no smart device for video watching and 5 refused. The remaining 60 participants were recruited; with 28 randomly allocated to the experience group and 32 randomly allocated to the control group (Fig. 1). There were no adverse events recorded as a result of participation in this trial. Of the 60 participants, there were four withdrawals. One hospitalized due to convulsion, one hospitalized due to pneumonia and two were lost of contact. A total of 56 participants completed the study without missing data.
Fig. 1.
Design and flow of participants through the trial.
The groups appeared well matched and no between-group difference in age, gender, time since stroke, type of stroke, side of hemiplegia, complication, site of lesion, follow-up physiotherapy, type of carer, experience in smart device, baseline SEE and baseline MFAC but slight difference in baseline MBI. Mean age of participants was 69.8 years (SD 14.9). The mean time since stroke was 39.5 (SD 15.3) (Table 2).
Table 2.
Baseline characteristics of participants.
| Characteristic | Total | Exp | Con | -tests | Chi-Square |
|---|---|---|---|---|---|
| Participants Age (years), mean (SD), (Range) | 69.8(14.9), 38–93 | 66.9(14.0), 40–93 | 72.5(15.5), 38–93 | 0.166 | |
| Gender, males (%) | 31(55.4) | 14(51.9) | 17(58.6) | 0.611 | |
| Time since stroke (days), mean (SD), (Range) | 39.5(15.3), 11–89 | 41.3(16.5), 23–89 | 37.9(14.2), 11–64 | 0.407 | |
| Type of stroke (%) | 0.838 | ||||
| Infract | 45(80.4) | 22(81.5) | 23(79.3) | ||
| Hemorrhage | 11(19.6) | 5(18.5) | 6(20.7) | ||
| Side of hemiplegia (%) | 0.571 | ||||
| Left | 25(44.6) | 11(40.7) | 14(48.3) | ||
| Right | 31(55.4) | 16(59.3) | 15(51.7) | ||
| Complication (%) | 0.568 | ||||
| Neglect | 5(8.9) | 3(11.1) | 2(6.9) | ||
| Dysphasia | 9(16.1) | 3(11.1) | 6(20.7) | ||
| Nil | 42(75.0) | 21(77.8) | 21(72.4) | ||
| Site of lesion (%) | 0.635 | ||||
| Cortex | 11(19.6) | 6(22.2) | 5(17.2) | ||
| Corona radiata | 7(12.5) | 4(14.8) | 3(10.3) | ||
| Internal capsule | 8(14.3) | 5(18.5) | 3(10.3) | ||
| Putamen | 1(1.8) | 1(3.7) | 0 | ||
| Thalamus | 6(10.7) | 2(7.4) | 4(13.8) | ||
| Other | 23(41.1) | 9(33.3) | 14(48.3) | ||
| Follow-up physiotherapy (%) | 0.151 | ||||
| Ambulatory day hospital | 38(67.9) | 21(77.8) | 17(58.6) | ||
| Domiciliary physiotherapy | 3(5.4) | 2(7.4) | 1(3.5) | ||
| Other | 2(3.6) | 0 | 2(6.9) | ||
| Nil | 13(23.2) | 4(14.8) | 9(31.0) | ||
| Type of carer (%) | 0.572 | ||||
| No | 15(26.8) | 10(37.0) | 5(17.2) | ||
| Spouse | 11(19.6) | 5(18.5) | 6(20.7) | ||
| Maid | 6(10.7) | 1(3.7) | 5(17.2) | ||
| Children | 21(37.5) | 10(37) | 11(37.9) | ||
| Other | 3(5.4) | 1(3.7) | 2(6.9) | ||
| Experience in smart device | 0.598 | ||||
| year | 8(14.3) | 3(11.1) | 5(17.2) | ||
| 1–2 years | 8(14.3) | 5(18.5) | 3(10.3) | ||
| years | 40(71.4) | 19(70.4) | 21(72.4) | ||
| MFAC | 4.0(1.4) | 4.3(1.0) | 3.8(1.7) | 0.173 | |
| MBI | 58.0(26.3) | 65.1(20.7) | 51.4(29.4) | 0.048* |
Notes: Exp: experimental group; Con: control group; difference between groups by independent -test or Chi-Square test. *.
There were no between-group difference in baseline adherence (), 1 month adherence () but significant difference in 3 months adherence () (Table 3).
Table 3.
Exercise adherence and self-efficacy for exercise of experience and control groups.
| Groups | Between-group | Groups | Between-group | Groups | Between-group | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1-Month | 3-Month | |||||||
| Exp | Con | -value | Exp | Con | -value | Exp | Con | -value | |
| Adherence | |||||||||
| Mean (SD) | 74.1 | 64.1 | 0.214 | 73.7 | 58.6 | 0.072 | 75.6 | 55.2 | 0.021* |
| (24.4) | (34.0) | (21.5) | (37.3) | (26.2) | (35.8) | ||||
| SEE | |||||||||
| Mean (SD) | 52.5 | 48.1 | 0.307 | 58.4 | 43.3 | 0.001* | 62.2 | 45.6 | * |
| (15.2) | (16.7) | (11.7) | (20.0) | (10.7) | (18.8) | ||||
Notes: Exp: experimental group; Con: control group; difference between groups of Adherence by Chi-Square test; difference between groups of SEE by independent -test.
.
There were significant between-group difference in 1 month change of SEE () and 3 month change in SEE (). There were no between-group difference in 1 month change of MFAC but significant between-group difference in 3 month change of MFAC (). There were no between-group difference in 1 month change of MBI () and 3 month change in MBI () (Table 4).
Table 4.
Functional outcomes of experience and control groups.
| Groups | Within-groups | Between-group | Within-groups | Between-group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1-month | 3-month | 1-month gain (1-month minus baseline) | 3-month gain (3-month minus baseline) | ||||||||
| Exp | Con | Exp | Con | Exp | Con | Exp | Con | -value | Exp | Con | -value | |
| MFAC | ||||||||||||
| Mean (SD) | 4.3 | 3.8 | 5.1 | 4.3 | 6.0 | 4.8 | 0.9 | 0.5 | 1.7 | 1.0 | ||
| (1.0) | (4.3) | (1.1) | (1.6) | (1.2) | (1.8) | (0.9) | (0.6) | (1.2) | (1.0) | |||
| -value | 0.000* | 0.000* | 0.124 | 0.000* | 0.000* | 0.036* | ||||||
| MBI | ||||||||||||
| Mean (SD) | 65.1 | 51.4 | 79.0 | 63.2 | 85.4 | 70.8 | 13.9 | 11.9 | 20.9 | 19.4 | ||
| (20.7) | (29.4) | (15.6) | (30.0) | (17.3) | (29.0) | (12.0) | (8.4) | (13.9) | (13.1) | |||
| -value | 0.000* | 0.000* | 0.474 | 0.000* | 0.000* | 0.808 | ||||||
Notes: Exp: experimental group; Con: control group; difference within groups by pair t-test; difference between groups by independent -test.
0.05.
Discussion
The main finding of the study was that mobile video-guided home exercise program was superior to standard paper-based home exercise programs in terms of exercise adherence, SEE and mobility gain but not basic ADL gain for patients recovering from stroke. This finding contrasted to the study of Emmerson et al. which showed that home exercise programs filmed on an electronic tablet was not superior to standard paper-based home exercise programs in terms of adherence, motor function, or satisfaction for patients recovering from stroke.12 There are several possible explanations for these finding, the adherence of home exercise may depend on the type of exercise and interest of patients. It was suggested that mobility decline is an essential concern in chronic stroke patients.42 Emmerson’s study12 only provided upper limb exercises that patients may have less interest to participate than the exercises in this study which consisted for arm, leg, trunk and mobility. We also found that there was a close relationship between exercise adherence and SEE. During the 3 months follow-up period, the SEE slightly increased (from 52.5 to 62.2 in video group) with the exercise adherence (from 74.1% to 75.6%) in experimental group; whereas the SEE was slightly decreased (from 48.1 to 45.6) with exercise adherence (from 64.1% to 55.2%) in control group (Table 3). The present results echoed with the theory of self-efficacy, the stronger the individual’s self-efficacy, the more likely it is that people will initiate and persist with a given activity16,45 that SEE was positive proportion to exercise adherence in both groups. The study also found that video-guided home exercise program could improve exercise adherence and SEE even without any regular encouragement, or automated reminder as in previous studies by Lambert et al.13 and Emmerson et al.12 The higher mobility gain of patients in experimental group than control group could be explained by adherence of home exercise program that has positive correlation with physical function and physical performance of patients.28,29 No difference in MBI gain in experimental group when compared to control group means the video-guided exercise program improves mobility or ambulation (as measured by MFAC) but its effects could not be translated to improvement of ADL (as measured by MBI). This could be explained by specificity principle46 that in training the home exercise program in this study was motor control and mobility orientated but not ADL orientated.
The limitation of the study included information bias introduced by outcome collection by telephone interview; selection bias to Chinese population since all the videos and pamphlets were in Chinese; and confounding bias by confounding variables such as, by chance, the experimental group had significant higher baseline MBI than that of control group. However, the effects of the confounding bias have been minimized by randomization and statistical analysis. The intervention fidelity monitoring is another limitation of the study; a pre-discharge training session was used to minimize the limitation. Further research studies with large sample size would allow comparing the most effective groups of the video-guided home exercise. In addition, as mentioned in the introduction session, it seems that adherence was affected by both self-efficacy and delivery mode of training program. The relationship between self-efficacy and delivery mode of training program such as video-guided element is still unclear and needs further study.
Conclusion
The use of mobile video-guided home exercise program was superior to standard paper-based home exercise program in exercise adherence, SEE and mobility gain but not basic ADL gain for patients recovering from stroke.
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this paper.
Funding/Support
The authors received no financial support for the research, authorship, and/or publication of this paper.
Author Contributions
-
(1)
Bryan Ping Ho Chung: Specifying the question, designing the study, analyzing the data, collecting and handling data, interpreting the data, writing — lead authorship.
-
(2)
Wendy Kam Ha Chiang: Specifying the question, designing the study, collecting and handling data, interpreting the data, writing — contributing significant text.
-
(3)
Herman Lau: Specifying the question, designing the study.
-
(4)
Titanic Fuk On Lau: Specifying the question, translating a protocol into practice.
-
(5)
Charles Wai Kin Lai: Translation of protocol into practice.
-
(6)
Claudia Sin Yi Sit: Identifying data needed, identifying relevant references.
-
(7)
Ka Yan Chan: Translation of protocol into practice.
-
(8)
Chau Yee Yeung: Translation of protocol into practice.
-
(9)
Tak Man Lo: Translation of protocol into practice.
-
(10)
Elsie Hui: Translation of protocol into practice, reading, editing, checking.
-
(11)
Jenny Shun Wah Lee: Translation of protocol into practice, reading, editing, checking.
References
- 1.World Health Organization. Recommendations on stroke prevention, diagnosis, and therapy; report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 1989;20:1407–31. [DOI] [PubMed] [Google Scholar]
- 2. Hospital Authority. Hospital Authority Statistical Report 2014; 2012–2013.
- 3. Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: A systematic review. Lancet Neurol 2009;8:741–54. [DOI] [PubMed] [Google Scholar]
- 4. Bohannon RW, Horton MG, Wikholm JB. Importance of four variables of walking to patients with stroke. Int J Rehabil Res 1991;14(3):246–50. [DOI] [PubMed] [Google Scholar]
- 5. Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke 2006;37(9):2348–53. [DOI] [PubMed] [Google Scholar]
- 6. Inouye M, Kishi K, Ikeda Y, Takada M, Katoh J, Iwahashi M, Hayakawa M, Ishihara K, Sawamura S, Kazumi T. Prediction of functional outcome after stroke rehabilitation. Am J Phys Med Rehabil 2000;79(6):513–8. [DOI] [PubMed] [Google Scholar]
- 7. Louie SW, Wong SK, Wong CM. Profiles of functional outcomes in stroke rehabilitation for Chinese population: A cluster analysis. NeuroRehabil 2009;25(2):129–35. [DOI] [PubMed] [Google Scholar]
- 8. Chung BPH. Stratification of stroke rehabilitation: 5-year profiles of functional outcomes. Hong Kong Physiother J 2018;38(2):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneiders AG, Zusman M, Singer KP. Exercise therapy compliance in acute low back pain patients. Man Ther 1998;3:147–52. [Google Scholar]
- 10. Kara S, Ntsiea MV. The effect of a written and pictorial home exercise prescription on adherence for people with stroke. Hong Kong J Occup Ther 2015;26:33e41. [Google Scholar]
- 11. Mander J. 80% of internet users own a smartphone, 2015. http://www.globalwebindex.net.
- 12. Emmerson KB, Harding KE, Taylor NF. Home exercise programmes supported by video and automated reminders compared with standard paper-based home exercise programmes in patients with stroke: A randomized controlled trial. Clin Rehabil 2017;31(8):1068–77. [DOI] [PubMed] [Google Scholar]
- 13. Lambert TE, Harvey LA, Avdalis C, Chen LW, Jeyalingam S, Pratt CA, Tatum HJ, Bowden JL, Lucas BR. An app with remote support achieves better adherence to home exercise programs than paper handouts in people with musculoskeletal conditions: A randomised trial. J Physiother 2017;63(3):161–67. [DOI] [PubMed] [Google Scholar]
- 14. Stenstrom CH, Arge B, Sundbom A. Home exercise and compliance in inflammatory rheumatic diseases: A prospective clinical trial. J Rheumatol 1997;24(3):470–76. [PubMed] [Google Scholar]
- 15. Chen CY, Neufeld PS, Feely CA, Skinner CS. Factors influencing compliance with home exercise programs among patients with upper-extremity impairment. The Am J Occup Ther 1999;53(2):171–80. [DOI] [PubMed] [Google Scholar]
- 16. Bandura AS, Adams NE. Analysis of self-efficacy theory of behavioral change. Cognitive Ther Res 1977;1:287–10. [Google Scholar]
- 17. Resnick B, Michael K, Shaughnessy M, Nahm ES, Kobunek S, Sorkin J, Orwig D, Goldberg A, Macko RF. Inflated perceptions of physical activity after stroke: Pairing self-report with physiologic measures. J Phys Act Health 2008;5(2):308–18. [DOI] [PubMed] [Google Scholar]
- 18. Morris J, Oliver T, Kroll T, MacGillivray S. The importance of psychological and social factors in influencing the uptake and maintenance of physical activity after stroke: A structured review of the empirical literature. Stroke Res Treatment 2012; Article ID 195249. [DOI] [PMC free article] [PubMed]
- 19. Michael KM, Allen JK, Macko RF. Fatigue after stroke: Relationship to mobility, fitness, ambulatory activity, social support, and falls efficacy. Rehabil Nurs 2006;31(5):210–17. [DOI] [PubMed] [Google Scholar]
- 20. Aittasalo M, Miilunpalo S, Stahl T, Kukkonen-Harjula K. From innovation to practice: Initiation, implementation and evaluation of a physician-based physical activity promotion programme in Finland. Health Promot Int 2007;22:19–27. [DOI] [PubMed] [Google Scholar]
- 21. Garcia AW, King AC. Predicting long-term adherence to aerobic exercise: A comparison of two models. J Sport Exercise Psychol 1991;13:394–410. [Google Scholar]
- 22. McAuley E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. J Behav Med 1992;15:65–88. [DOI] [PubMed] [Google Scholar]
- 23. Sallis JF, Criqui MH, Kashani IA, Rupp JW, Calfas KJ, Langer RD, Nader PR, Ross J Jr. A program for health behavior change in a preventive cardiology center. Am J Prev Med 1990;6:43–50. [PubMed] [Google Scholar]
- 24. Hill B, Perri-Moore S, Kuang J, Bray BE, Ngo LDA, Zeng-Treitler Q. Automated pictographic illustration of discharge instructions with Glyph: Impact on patient recall and satisfaction. J Am Med Inform Assoc 2016;23(6):1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schoo AMM, Morris ME, QMB. The effects of mode of exercise instructions on compliance with a home exercise program in older adults with osteoarthritis. Physiotherapy 2004;91(2):79–86. [Google Scholar]
- 26. Miller JS, Litva A, Gabbay M. Motivating patients with shoulder and back pain to self-care: Can a videotape of exercise support physiotherapy? Physiotherapy 2009;95(1):29–35. [DOI] [PubMed] [Google Scholar]
- 27. Sheetal K, Mokgobadibe VN. The effect of a written and pictorial home exercise prescription on adherence for people with stroke. Hong Kong Occupat Ther Assoc 2015;26:33–41. [Google Scholar]
- 28. Pisters MF, Veenhof C, Schellevis FG, Twisk JW, Dekker J, De Bakker DH. Exercise adherence improves long-term patient outcome in patients with osteoarthritis of the hip and/or knee. Arthritis Care Res 2010;62:1087. [DOI] [PubMed] [Google Scholar]
- 29. American College of Sports Medicine (ACSM). (2010). In ACSM’s Guidelines for Exercise Testing and Prescription, 8th edition. [DOI] [PubMed]
- 30. Bollen JC, Dean SG, Siegert RJ, Howe TE, Goodwin VA. A systematic review of measures of self-reported adherence to unsupervised home-based rehabilitation exercise programmes, and their psychometric properties. BMJ Open 2014;4(6):e005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor NF, Dodda KJ, McBurney H, Graham HK. Factors influencing adherence to a home-based strength-training programme for young people with cerebral palsy. Physiotherapy 2004;90(2):57–63. [Google Scholar]
- 32. Resnick B, Jenkins LS. Testing the reliability and validity of the self-efficacy for exercise scale. Nurs Res 2000;49(3):154–59. [DOI] [PubMed] [Google Scholar]
- 33. Resnick B. Reliability and validity testing of the self-efficacy for functional activities scale. J Nurs Measure 1999;7(1):5–20. [PubMed] [Google Scholar]
- 34. Resnick B. Geriatric rehabilitation: The influence of efficacy beliefs and motivation. Rehabil Nurs 2002;27(4):152–59. [DOI] [PubMed] [Google Scholar]
- 35. Lee LL, Perng SJ, Ho CC, Hsu HM, Lau SC, Arthur A. A preliminary reliability and validity study of the Chinese version of the self-efficacy for exercise scale for older adults. Int J Nurs Stud 2009;46(2):230–8. [DOI] [PubMed] [Google Scholar]
- 36. Chau MWR, Chan SP, Wong YW, Lau MYP. Reliability and validity of the modified functional ambulation classification in patients with hip fracture. Hong Kong Physiother J 2013;31(1):41–44. [Google Scholar]
- 37. Park CS, An SH. Reliability and validity of the modified functional ambulation category scale in patients with hemiparalysis. J Phys Ther Sci 2016; 28(8):2264–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 1989;42:703–9. [DOI] [PubMed] [Google Scholar]
- 39. Leung SO, Chan CC, Shah S. Development of a Chinese version of the Modified Barthel Index — validity and reliability. Clin Rehabil 2007;21(10):912–22. [DOI] [PubMed] [Google Scholar]
- 40. Shinar D1, Gross CR, Bronstein KS, Licata-Gehr EE, Eden DT, Cabrera AR, Fishman IG, Roth AA, Barwick JA, Kunitz SC. Reliability of the activities of daily living scale and its use in telephone interview. Arch Phys Med Rehabil 1987;68(10):723–8. [PubMed] [Google Scholar]
- 41. van de Port IG, Kwakkel G, van Wijk I, Lindeman E. Susceptibility to Deterioration of Mobility Long-Term After Stroke A Prospective Cohort Study. Stroke 2006;37:167–71. [DOI] [PubMed] [Google Scholar]
- 42. Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, Kwakkel G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE [Electronic Resource] 2014;9(2):e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kongsawasdi S, Klaphajone J, Wivatvongvana P, Watcharasaksilp K. Prognostic factors of functional outcome assessed by using the modified rankin scale in subacute ischemic stroke. J Clin Med Res 2019;11(5):375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mahmood A, Blaizy V, Verma A, Stephen Sequeira J, Saha D, Ramachandran S, Manikandan N, Unnikrishnan B, Solomon JM. Acceptability and attitude towards a mobile-based home exercise program among stroke survivors and caregivers: A cross-sectional study. Int J Telemed Appl 2019;2019:5903106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bandura A. Self-efficacy: The exercise of control. NY, US: W H Freeman/Times Books/Henry Holt & Co, 1997. [Google Scholar]
- 46. Williams LR, Daniell-Smith JH, Gunson LK. Specificity of training for motor skill under physical fatigue. Med Sci Sports 1976;8(3):162–7. [DOI] [PubMed] [Google Scholar]