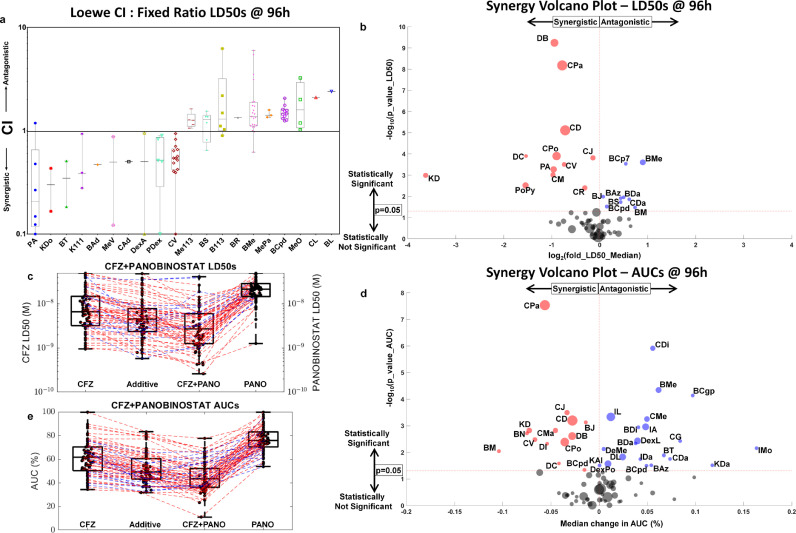
Fig. 3.
High-throughput combination screening based on ex vivo response measurements using CI, and a novel use of volcano plot to show statistical significance in synergy by LD50s and AUCs to demonstrate the relative merits and demerits of each method. a, CIs presented as whisker box plots: CIs are shown as box-and-whisker plots for 20 combinations (the 10 most synergistic and antagonistic by median CI; the rest can be found in Supplemental Information S5, which features 62 combinations) tested ex vivo, where the CI values are computed at LD50, 50% effect (cell kill), at 96 hours, estimated using EMMA and SAM models that capture tumour heterogeneity in a patient-specific manner. b, High-throughput combination screening by LD50: High-throughput combination screenings for 56 combinations were tested using at least 10 patients' specimens each via a volcano plot. Each disc is a two-drug combination with an x-coordinate that represents the log2 fold-change in LD50 at 96 hours for the median patient to signify the extent of combination effect, and the y-axis represents the -log10 p-value (for a two-tailed paired t-test) comparing the computed (from the two single-agent responses) additive responses (BLISS) to the combination responses to signify the statistical significance of the combination effect. Many combinations in A have sparse CI data, despite having ex vivo data from several patients (like BL and CL, which had 76 and 74 patients tested ex vivo), only one patient had a response, where both the single agents reached LD50. The volcano plot is a better approach to screen for synergistic combinations when using patient samples in diseases like MM as the combination response is compared to the additive response, which is computed from the response surfaces of the two single agents. This helps to consider combinations involving drugs that aren't equipotent in the high-throughput screen. c, Carfilzomib and panobinostat synergy by LD50 shown using a box-and-whisker plot: A box-and-whisker plot of LD50s for 60 MM patient samples treated ex vivo with carfilzomib (column 1), panobinostat (column 4), and their combination (column 3) is shown. The combination LD50s are compared to the additive LD50s (column 2) estimated from the additive response surface, which is the pointwise product of fraction population remaining at 96 hours for each of the two drugs. The red dashed lines indicate patients exhibiting synergy ex vivo for the combination, and the blue dashed lines indicate patients showing antagonism ex vivo. d, Carfilzomib and panobinostat synergy by AUC using a box-and-whisker plot: Similar to c, the additive response whisker box plot is compared to the combination response for the same 60 MM patients to estimate the P value for a two-tailed paired t test. e, High-throughput combination screening by AUC: Similar to b, a high-throughput combination screen is presented for 76 combinations, where the P value (of the two-tailed paired t test), estimated by comparing the additive and combination AUCs in d, is plotted along the y-coordinate and the x-coordinate shows the median change in AUC (%) between the additive and combination responses. The number of combinations and the criteria for studying them in a, b, and e is presented in Supplemental Information S9. Abbreviations: B113, bortezomib and 113; BAd, bortezomib and adavosertib; BAz, bortezomib and AZ-628; BCgp, bortezomib and CGP-60474; BCp7, bortezomib and CP-724714; BCpd, bortezomib and CPD22; BDa, bortezomib and dabrafenib; BJ, bortezomib and JNK-IN-8; BL, bortezomib and lenalidomide; BM, bortezomib and MARK-INHIBITOR; BMe, bortezomib and melphalan; BN, bortezomib and NU-7441; BR, bortezomib and R406; BS, bortezomib and silmitasertib; BT, bortezomib and TAI-1; CAd, carfilzomib and adavosertib; CDa, carfilzomib and dabrafenib; CD, carfilzomib and dexamethasone; CDi, carfilzomib and dinaciclib; CG, carfilzomib and GDC-0980; CJ, carfilzomib and JNK-IN-8; CL, carfilzomib and lenalidomide; CM, carfilzomib and MARK-INHIBITOR; CMe, carfilzomib and melphalan; CPa, carfilzomib and panobinostat; CPo, carfilzomib and pomalidomide; CR, carfilzomib and R406; CV, carfilzomib and volasertib; DB, daratumumab and bortezomib; DC, daratumumab and carfilzomib; DI, daratumumab and ixazomib; DL, daratumumab and lenalidomide; DeMe, defactinib and melphalan; DexA, dexamethasone and ABT-199; DexL, dexamethasone and lenalidomide; DexPo, dexamethasone and pomalidomide; IA, ixazomib and ABT-199; IMo, ixazomib and motesanib; K111, selinexor and 111; KAl, selinexor and alisertib; KDa, selinexor and dabrafenib; KDo, selinexor and doxorubicin; Me113, melphalan and 113; MePa, melphalan and panobinostat; MeV, melphalan and VS4718; MeO, melphalan and ONX; PA, panobinostat and ABT-199; PDex, panobinostat and dexamethasone; PoPy, pomalidomide and pyrvinium; LD50, the dose that achieves 50% cell kill; AUC, average area under the dose-response curve over all time points; CI, Loewe's Combination Index; CFZ, carfilzomib; PANO, panobinostat; h, hours; M, molar.