Abstract
Hepatic adenomas are benign liver tumors typically found in females of reproductive age. Though benign, hepatic adenomas are highly vascularized tumors, thus rupture and consequent hemorrhage present a feared complication. We report a case of a 31-year-old woman with hepatic adenoma who underwent preoperative portal vein embolization and subsequently suffered a rupture of her tumor. We postulate that the change in blood flow after portal vein embolization, a phenomenon known as the hepatic artery buffer response, may have contributed to the tumor rupture, though the possibility that the rupture was purely incidental remains. There is currently no prior report of such rupture occurring following portal vein embolization, and this case brings to light a potentially fatal complication of a generally safely regarded procedure in patients with hepatic adenoma.
Keywords: Hepatic adenoma, Portal vein embolization, Adenoma rupture, Bleed
Introduction
Hepatic adenomas (HA) are benign liver neoplasms classically found primarily in females of reproductive age who have a history of oral contraceptive use [8]. HA are typically asymptomatic and discovered incidentally, however, as highly vascularized tumors, rupture and subsequent hemorrhage is a common complication found in approximately 15%-27% of patients with HA [8]. Furthermore, malignant transformation into hepatocellular carcinoma has been found to occur in 4%-5% of these tumors [8]. The risks of both bleeding and malignant transformation increase with increasing tumor size, thus surgical resection is recommended for tumors greater than 5 cm [8], as well as for those with other factors associated with increased risk of malignant transformation including: male gender, tumors with cytologic atypia and/or beta-catenin mutation [3].
The baseline liver function and size of the future liver remnant (FLR), is a strong, independent predictor of liver function following surgical resection and an important consideration prior to surgery [5]. Preoperative portal vein embolization (PVE) is used to induce hypertrophy of the FLR in cases with marginal FLR size [5]. PVE is generally a safe procedure, with 0% procedure-related mortality reported by most series and few side effects [1].
Case report
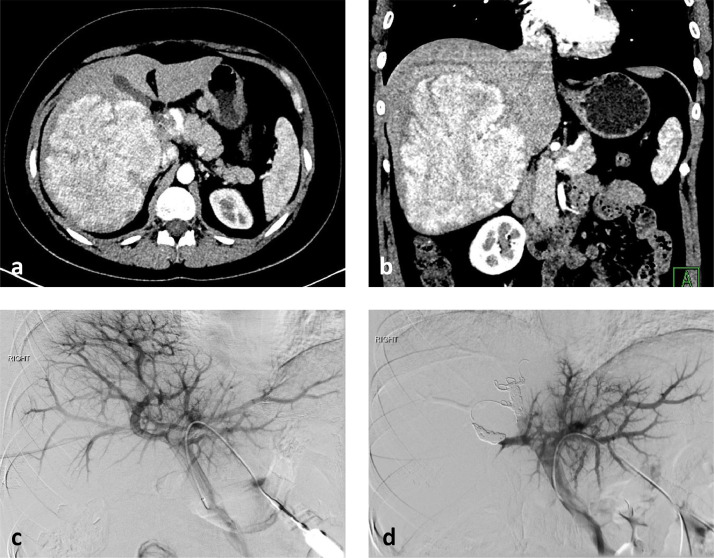
Here, we report a case of a 31-year-old woman on oral contraceptives for 14 years with a large HA who underwent preoperative PVE complicated with subsequent rupture of her tumor. For this type of study informed consent is not required. The patient was diagnosed with highly elevated plasma alkaline phosphatase level (1340 U/L) at routine medical check-up. Subsequent ultrasound evaluation demonstrated a 15 cm right hepatic lobe mass and percutaneous liver biopsy revealed nonbeta-catenin-mutated HA. CT scan demonstrated a 14.9 × 13.9 × 11.9 cm hypervascular mass replacing almost the entire right lobe with mass effect on the right portal vein and IVC (Fig. 1a,b). The multidisciplinary tumor board recommended right hepatectomy and preoperative PVE due to the small size of the FLR left lobe. She underwent embolization of the anterior and posterior divisions of the right portal vein from contralateral approach with portal vein access via a peripheral segment 3 portal vein branch. The PVE was performed using a total of 4 mL vials of 100 micron hydrogel microspheres (Embozene, Boston Scientific, Marlborough, MA) 4 mL of 100-300 micron tris-acryl gelatin microspheres (Embospheres, Merit Medical, South Jordan, UT) and 2 mL of 300-500 micron tris-acryl gelatin microspheres followed by coil embolization of the anterior and posterior branches of the right portal vein with 6, 8, and 10 mm Nester coils (Cook). Postembolization portal venogram showed no portal venous flow and no parenchymal opacification in the right lobe of the liver and unrestricted portal venous flow in the left main portal vein and its branches with brisk parenchymal opacification in the left lobe and segment 1 (Fig. 1c,d). She was discharged home 3 hours after the PVE in stable condition with minimal mid-abdominal pain at the left portal venous access site. She presented back to the hospital with intractable vomiting, dizziness and lethargy 4 hours after discharge. Her hemoglobin dropped from 12.0 g/dl to 8.7 g/dl. CT scan demonstrated a large intratumoral hematoma in the right liver adenoma with multiple foci of active extravasation with subcapsular and intraperitoneal hemorrhage (Fig. 2 a-c). The findings were consistent with adenoma rupture. She received a blood transfusion and underwent emergent visceral arteriogram which demonstrated multiple small foci of extravasation in the right lobe adenoma (Fig. 2d). Embolization of the posterior division of the right hepatic artery was performed with 2 mL of 500-700 micron tris-acryl gelatin microspheres and 2 mL of 900 micron tris-acryl gelatin microspheres. Postembolization angiogram demonstrated no evidence of extravasation. She was discharged home 5 days after the embolization; her extended hospitalization was due to severe abdominal pain and leukocytosis. Subsequent follow-up in 5 weeks revealed adequate hypertrophy of the FLR and she underwent successful right hepatectomy 2 months after the PVE (Fig. 3a-c). Follow-up 4 months after the liver resection revealed normal liver function and CT scan showed enlarged left liver lobe (Fig. 3d). She is completely asymptomatic 6 months after the hepatectomy.
Fig. 1.
Axial (a) and coronal (b) contrast-enhanced CT images demonstrate a large, hyperenhancing adenoma in the right lobe of the liver. Pre-embolization portal venogram (c) demonstrates decreased portal venous perfusion in the right lobe in the region of the hepatic adenoma. Postembolization portal venogram (d) shows redirection of the entire portal venous flow to the left hepatic lobe and absent portal venous flow in the embolized right lobe.
Fig. 2.
Axial (a, c) and coronal (b) contrast-enhanced CT images demonstrate large intratumoral hemorrhage with multiple foci of active extravasation (black arrows) and intraperitoneal hemorrhage (white arrows). Hepatic artery angiogram (d) confirmed foci of arterial extravasation (black arrows).
Fig. 3.
Photographs show the resected right liver lobe specimen (a) and the bisected right lobe of the liver with exposed adenoma (b). Low power (20×) view of hematoxylin and eosin staining of the resected right liver lobe specimen demonstrates embolization beads in a portal microvessel (c). Axial contrast-enhanced CT image 4 months after right lobe resection demonstrates enlarged left liver lobe (d).
Discussion
There is no existing literature regarding rupture of HA following PVE. A plausible cause of the rupture is the dramatic change in blood flow after PVE. It has been shown that changes in portal venous flow affect the flow in the hepatic artery, a phenomenon known as the hepatic artery buffer response [4]. The mediator of hepatic artery buffer response is adenosine, a potent vasodilator of the hepatic artery secreted into the space of Mall by hepatocytes that is typically constantly being washed away by portal venous flow. After PVE, due to the cessation of portal venous flow, adenosine accumulates and induces vasodilation of the hepatic artery, thus increasing hepatic arterial flow in the embolized lobe. This immediate increase in hepatic artery blood flow following PVE has been demonstrated both in experimental animal models and also clinically [2,4,6,7]. Hepatic tumors are almost exclusively supplied by the hepatic artery, and hypervascular tumors, like the large HA in this case, are known to syphon blood flow from surrounding normal liver parenchyma. It is therefore possible that the significantly increased arterial blood flow following PVE contributed to the rupture of the HA in our patient. However, PVE has been performed extensively to make HA resection possible without a reported complication of rupture. Therefore, besides the blood flow changes, other factors, like the large size of the tumor and possibly inherent abnormal tumoral vascularity could also have contributed to the tumor rupture in this patient. It is also possible that the rupture was completely incidental. Nonetheless, this case may suggest that for large, hypervascular tumors transarterial embolization prior to PVE may be warranted to reduce the risk of rupture.
Footnotes
Acknowledgments: This study was not supported by any funding.
Competing Interests: The authors declare that they have no conflict of interest.
References
- 1.Anaya D.A., Blazer D.G., Abdalla E.K. Strategies for resection using portal vein embolization: hepatocellular carcinoma and hilar cholangiocarcinoma. Semin Interv Radiol. 2008;25(2):110–122. doi: 10.1055/s-2008-1076684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kito Y., Nagino M., Nimura Y. Doppler sonography of hepatic arterial blood flow velocity after percutaneous transhepatic portal vein embolization. AJR Am J Roentgenol. 2001;176(4):909–912. doi: 10.2214/ajr.176.4.1760909. [DOI] [PubMed] [Google Scholar]
- 3.Laurent A., Dokmak S., Nault J.C., Pruvot F.R., Fabre J.M., Letoublon C. European experience of 573 liver resections for hepatocellular adenoma: a cross-sectional study by the AFC-HCA-2013 study group. HPB (Oxford) 2016;18(9):748–755. doi: 10.1016/j.hpb.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lautt W.W. Regulatory processes interacting to maintain hepatic blood flow constancy: Vascular compliance, hepatic arterial buffer response, hepatorenal reflex, liver regeneration, escape from vasoconstriction. Hepatol Res. 2007;37(11):891–903. doi: 10.1111/j.1872-034X.2007.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.May B.J., Madoff D.C. Portal vein embolization: rationale, technique, and current application. Semin Interv Radiol. 2012;29(2):81–89. doi: 10.1055/s-0032-1312568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagino M., Nimura Y., Kamiya J., Kanai M., Hayakawa N., Yamamoto H. Immediate increase in arterial blood flow in embolized hepatic segments after portal vein embolization: CT demonstration. AJR Am J Roentgenol. 1998;171(4):1037–1039. doi: 10.2214/ajr.171.4.9762992. [DOI] [PubMed] [Google Scholar]
- 7.Rocheleau B., Ethier C., Houle R., Huet P.M., Bilodeau M. Hepatic artery buffer response following left portal vein ligation: its role in liver tissue homeostasis. Am J Physiol. 1999;277(5):G1000–G1007. doi: 10.1152/ajpgi.1999.277.5.G1000. [DOI] [PubMed] [Google Scholar]
- 8.van Aalten S.M., de Man R.A., IJzermans J.N., Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99(7):911–916. doi: 10.1002/bjs.8762. [DOI] [PubMed] [Google Scholar]