Abstract
Background
Disruption of bile acid (BA) homeostasis plays a key role in intestinal inflammation. The gut-liver axis is the main site for the regulation of BA synthesis and BA pool size via the combined action of the nuclear Farnesoid X Receptor (FXR) and the enterokine Fibroblast Growth Factor 19 (FGF19). Increasing evidence have linked derangement of BA metabolism with dysbiosis and mucosal inflammation. Thus, here we aimed to investigate the potential action of an FGF19 analogue on intestinal microbiota and inflammation.
Methods
A novel engineered non-tumorigenic variant of the FGF19 protein, M52-WO 2016/0168219 was generated. WT and FXRnull mice were injected with AAV-FGF19-M52 or the control AAV-GFP and subjected to Sodium Dextran Sulphate-induced colitis.
Findings
FGF19-M52 reduced BA synthesis and pool size, modulated its composition and protected mice from intestinal inflammation. These events were coupled with preservation of the intestinal epithelial barrier integrity, inhibition of inflammatory immune response and modulation of microbiota composition. Interestingly, FGF19-M52-driven systemic and local anti-inflammatory activity was completely abolished in Farnesoid X Receptor (FXR)null mice, thus underscoring the need of FXR to guarantee enterocytes’ fitness and complement FGF19 anti-inflammatory activity. To provide a translational perspective, we also show that circulating FGF19 levels are reduced in patients with Crohn's disease.
Interpretation
Reactivation of the FXR-FGF19 axis in a murine model of intestinal inflammation could bona fide provide positive changes in BA metabolism with consequent reduction of intestinal inflammation and modulation of microbiota. These results point to the therapeutic potential of FGF19 in the treatment of intestinal inflammation with concomitant derangement of BA homeostasis.
Funding
A. Moschetta is funded by MIUR-PRIN 2017 <- 2017J3E2W2; Italian Association for Cancer Research (AIRC, IG 23239); Interreg V-A Greece-Italy 2014-2020-SILVER WELLBEING, MIS5003627; HDHL-INTIMIC EuJPI-FATMAL; MIUR PON “R&I” 2014-2020-ARS01_01220. No money has been paid by NGM Biopharmaceuticals or any other agency to write this article.
Keywords: Bile acids, Intestinal inflammation, Enterokine, Nuclear receptors, DSS-colitis
Research in context.
Evidence before this study
Bile acids (Bas) are detergent-like molecules synthesized from cholesterol in the liver, stored in the gallbladder, and released into the small intestine after food intake to facilitate the absorption of dietary lipids and liposoluble vitamins. The farnesoid X receptor (FXR) is a nuclear receptor highly expressed in the gut-liver axis that acts as the master regulator of BA homeostasis, mainly via the gut hormone Fibroblast growth factor FGF19 (FGF15/19). Disrupted BA metabolism has been associated with an expanding record of chronic diseases, including chronic intestinal inflammation. Strategies aimed at restoring BA homeostasis through activation of the intestinal FXR-FGF19 signalling system hold promise in intestinal disorders with concomitant BA derangements. Fifty percent of patients with chronic intestinal inflammation, and particularly Crohn's disease (CD) patients with inflammation of the terminal ileum, present with bile acid malabsorption and diarrhoea. This is due to decreased ASBT expression leading to impaired BA ileal re-uptake, lower FXR intestinal activation and consequent decreased production of FGF19 ultimately leading to loss of inhibition of hepatic Cyp7a1 and increased BA synthesis. This detrimental cascade worsens the vicious cycle in which higher BA levels undergo colonic spill over due to the decreased ileal ability to reabsorb them. Pharmacological FXR activation inhibits intestinal inflammation and preserves the intestinal barrier integrity in chemically-induced models of intestinal inflammation and modulates the immune response in patients with inflammatory bowel diseases, putatively via the action of FGF19.
Added value of this study
FGF19 is a putative major player in the inhibition of intestinal inflammation. In this study, we show for the first time the preclinical therapeutic exploitation of the enterokine Fibroblast Growth Factor 19 (FGF19) in experimental colitis. Our data powerfully show the multilevel ability of FGF19 in counteracting intestinal inflammation. First, FGF19 reduces intraluminal BA levels thus contributing to prevent the detergent cytotoxic events that further increase susceptibility to intestinal inflammation. This create the perfect environment for the subsequent anti-inflammatory boost generated by the beneficial shift in the intestinal microbiota. Concomitantly, FGF19 reduces local intestinal inflammation and immune response. Finally, it regulates enterocyte turnover and epithelial barrier integrity in mice competent for nuclear bile acid receptor FXR. In addition, in previously published papers, a negative relation between intestinal inflammation and FGF19 levels has only been shown in patient with CD (w/wo ileal resection) and concomitant BA diarrhea. This could theoretically influence data interpretation due to the lower intestinal ability to reabsorb BAs. Here, for the first-time data are presented from patients solely affected by CD without ileal resection and other concomitant conditions.
Implication of all the available evidence
Our data further strengthen the link between the regulation of BA homeostasis and inhibition of intestinal inflammation, highlighting the bona fide therapeutic potential of the FGF19 analogue M52 in the treatment of patient with intestinal inflammation and concomitant derangement of BA homeostasis. Our results are highly timely since similar FGF19 analogues are currently tested in phase 3 clinical trials for hepatic disease.
Alt-text: Unlabelled box
1. Introduction
The Fibroblast growth factor FGF19 (FGF15/19) is an atypical member of the FGFs family, acting as a signalling gut hormone regulating bile acid (BA) metabolism, protein and glycogen synthesis and gluconeogenesis (reviewed in [1]). It is expressed in the small intestine under the transcriptional control of the bile acids sensor Farnesoid X receptor (FXR) and its main physiological activities consist of the controlling BAs homeostasis during the transition from the fed to the fasted state [2], [3], [4], [5]. BAs are detergent-like molecules synthesized from cholesterol in the liver, stored in the gallbladder, and released into the small intestine after food intake to facilitate the absorption of dietary lipids and liposoluble vitamins. Following the enterohepatic circulation, 95% of BA are then reabsorbed in the terminal ileum, secreted into the portal vein, and subsequently re-uptaken in the liver.
BAs act as signalling molecules via FXR [6], [7], [8]. FXR is a nuclear receptor highly expressed in the gut-liver axis that acts as the master regulator of BA homeostasis. BA-activated FXR finely controls BA homeostasis, regulating tissue-specific gene networks orchestrating their synthesis, transport and metabolism (reviewed in [9]). De novo BA synthesis occurs in the liver via the classical biosynthetic pathways converting cholesterol into BAs. This pathway is controlled by a negative gut-liver feedback acting on the hepatic rate-limiting enzyme of BA synthesis Cholesterol 7 alpha-hydroxylase (CYP7A1). In the ileum, BA-dependent FXR activation induces the expression/production of the enterokine FGF19 (FGF15 in the mouse), a hormone secreted in the portal circulation, able to reach the liver and bind to the fibroblast growth factor receptor 4 (FGFR4)/β-Klotho complex. FGF19-FGFR4/β-Klotho binding initiates a c-jun N-terminal kinase-dependent pathway [4], which ultimately leads to CYP7A1 repression.
Disrupted BA metabolism has been associated with an expanding record of chronic diseases, including metabolic [10,11], liver [12,13] and intestinal diseases [14,15]. Recent studies in humans have shown important relationships between FGF19 levels, BA synthetic rates and intestinal disorders [16], [17], [18], including intestinal inflammation. BAs have potent secretory effects on the colonic mucosa and when an excess of BA reaches the small bowel, the ileal capacity for BA absorption may be overwhelmed thus spilling them over into the colonic lumen and accelerating colonic transit. As a result, colonic motility and secretion are stimulated and diarrhea occurs. Patients suffering from BA malabsorption and diarrhea often exhibit increased levels of C4, a marker of hepatic BA synthesis, and reduced levels of the FGF19 [16], [17], [18]. The integrity of the enterohepatic circulation of BAs depends on the apical sodium bile acid transporter (ASBT) [19] and therapy with glucorticoids per se is able to induce human ABST in patients with Crohn's disease (CD) [20]. Also, it has been shown that the use of BA sequestrant in combination with glucocorticoids is beneficial in patients with CD with concomitant downregulation of ASBT [21]. Moreover, treatment of Asbtnull mice with either a FXR agonist or Fgf15 downregulates hepatic Cyp7a1 mRNA levels, reduces the BA pool size, and reduces fecal BA excretion [22]. For these reasons, strategies aimed at restoring BA homeostasis through activation of the intestinal FXR-FGF19 signaling system hold promise in intestinal disorders with BA derangements. Indeed, pharmacological FXR activation inhibits intestinal inflammation and preserves the intestinal barrier integrity in chemically-induced models of intestinal inflammation and modulates the immune response in patients with inflammatory bowel disease (IBD) [15]. In addition, FXRnull mice display a disrupted intestinal barrier at baseline, with severe degeneration after disruption of the BA flow via bile duct ligation [15,23].
Notably, there is a strong interplay between the intestinal flora and BA metabolism. In fact, several studies have shown that the size and composition of circulating BAs have a crucial role not only in digestion, but also in shaping the gut microbial community [24]. BAs have intrinsic antimicrobial properties and via FXR activation they also regulate the expression of genes promoting innate defense [23,25]. Viceversa, the intestinal microbiota plays a key role in regulating BA metabolism, and transforms primary into secondary BAs via bacterial-induced BA deconjugation and epimerization. This, in turn, shapes the magnitude of BA-induced FXR activation in the gut [26], [27], [28], [29]. In experimental colitis, accumulation of BAs in the intestine leads to their disposal via the PPARα-UGT pathway [30]. This causes deregulation of the FXR transcriptional activity and compromises the Fxr-Fgf15 pathway activation, ultimately inducing continuous activation of Cyp7a1, increased de novo BA synthesis and colitis exacerbation [30]. Finally, it is worth to state that several studies have suggested the therapeutic potential of FXR pharmacological activators as putative strategy in IBD [15,31,32]. In this study, we aimed to investigate the potential intestinal anti-inflammatory activity of FGF19. Since safety concerns have been raised about potential hepatic mitogenic FGF19 activity [33], [34], [35], [36], [37], recent studies dissected the FGF19 domains separately implicated in the retention of BA synthesis regulatory activity and its proliferative one [38,39]. Therefore, we pre-clinically investigated the therapeutic potential of a novel engineered variant of the FGF19 protein, called FGF19-M52 (US patent US8951966B2), which is devoid of FGF19 hepatic pro-mitogenic capacity [12]. Here, we show for the first time that FGF19-M52 protects from experimental colitis in mice that are FXR competent. Since FGF19 analogues are being tested in clinic for liver disease, this discovery might bona fide open novel avenues in the therapeutic approach to IBD with concomitant BA derangements.
2. Material and methods
2.1. Animals and Sodium Dextran Sulphate induced colitis
Wild-type (WT) C57BL/6J mice were obtained from Charles River Laboratories [Calco (Lecco), Italy]. Pure strain C57BL/6J Fxrnull mice were originally kindly provided by Dr Frank Gonzalez (NIH, Bethesda, Maryland, USA). Mice were fed ad libitum and housed in a temperature and light-controlled room. Male 8-weeks-old WT and Fxrnull mice (n = 7–10 and n = 7, respectively) received a single intravenous dose of 1 × 1011 vector genome of adeno-associated virus (AAV) in a volume of 100µl containing genes encoding either the FGF19-M52 form or control green fluorescent protein (GFP, NGM Biopharmaceuticals, San Francisco, CA). All mice were housed under a standard 12-hour light/dark cycle and fed standard rodent chow diet and autoclaved tap water ad libitum. After 4 weeks, colitis was induced by administration of 3% (w/v) dextran sodium sulfate (DSS; molecular mass 36-50 kDa; MP Biochemicals Inc, Amsterdam, The Netherlands) in drinking water for 7 days. Daily changes in body weight and visible rectal bleeding were assessed. Visible rectal bleeding was scored on a scale from 0 to 5, indicating no (0) to very severe (5) rectal bleeding. The presence of occult blood in the stool was also monitored (Hemoccult, Beckman Coulter, Milano, Italy). Hemoccult was scored as follows: 1, normal; 2, trace positive; 3, strong positive; and 4, gross bleeding. The Ethical Committee of the University of Bari approved this experimental set-up, which was also certified by the Italian Ministry of Health in accordance with internationally accepted guidelines for animal care.
2.2. Bile salt measurements
Chenodeoxycholic Acid (CDCA), Cholic Acid (CA), Tauro-CDCA (T-CDCA), TCA and other endogenous BAs were purchased from Sigma-Aldrich (St. Louis, MO). All the studied BAs were identified and quantified by high-pressure liquid chromatography-electrospray-mass spectrometry/mass spectrometry (HPLC-ES-MS/MS) by optimized methods [40] suitable for use in pure standard solution, plasma and liver samples after appropriate clean-up preanalytical procedures. Liquid chromatography analysis was performed using an Alliance HPLC system model 2695 from Waters combined with a triple quadruple mass spectrometer QUATTRO-LC (Micromass; Waters) using an electrospray interface. BAs were separated by elution gradient mode with a mobile phase composed of a mixture ammonium acetate buffer 15 mM, pH 8.0 (Solvent A) and acetonitrile:methanol = 75:25 v/v (Solvent B). Chromatograms were acquired using the mass spectrometer in multiple reactions monitoring mode. Biliary BAs were measured with the Total Bile Acid Assay (Dyazime, Dresden, Deutschland), according to the manufacturer's instructions.
2.3. Histology and Immunohistochemistry
Colon specimens were snap-frozen or fixed in 10% formalin (24h), dehydrated, and embedded in paraffin. Distal colon sections (5 mm) were stained with hematoxylin and eosin (H&E). Histopathological scoring was performed using an established semiquantitative score ranging from 0 to 6 based on infiltration of inflammatory cells and epithelial damage (1 = few inflammatory cells, no epithelial degeneration; 2 = mild inflammation, few signs of epithelial degeneration; 3 = moderate inflammation, few epithelial ulcerations; 4 = moderate to severe inflammation, ulcerations in 25–50% of the tissue section; 5 = moderate to severe inflammation, large ulcerations in >50% of the tissue section; 6 = severe inflammation and ulcerations of >75% of the tissue section) [41]. Depletion of goblet cells was scored using a scoring index from 0 to 4 (0 = no depletion; 1 = 0–10% depletion; 2 = 10–25% depletion; 3 = 25–50% depletion; 4 = 50–100% depletion). Immunohistochemical (IHC) detection was performed on thin section (4um) of paraffin embeeded tissue. After Antigen retrieval, the sections were blocked with proteine blocking serum free (X0909, Dako). Antibody to F4/80 (CI:A3-1; 1:200) was incubated on slides at RT for 1h and detected by the Dako REAL EnVision Detection System Peroxidase/DAB according to manufacturer's instruction (K5007, Dako). Hematoxylin was used to counterstain. Stained slides were scanned on an AperioScanScope AT and image analysis and quantification was performed using AperioImageScope software (Leica Biosystems, Nussloch, Germany). Signal was normalized to area and image analyses were done using Aperio algorithms (Leica Biosystems). Analysis was performed by an investigator blinded to study design with results confirmed by an independent investigator.
2.4. In vivo intestinal permeability assay
In vivo intestinal permeability was assessed in mice on the day of sacrifice, as previously described [42]. Mice were gavaged with 0.6 mg/g body weight of fluorescein isothiocyanate (FITC)-conjugated dextran (Sigma, S Louis, Missouri, USA; molecular mass 3e5 kDa) for 4h. Blood was collected, and FITC concentrations were measured in plasma with the Microplate fluorometer VICTOR™ EnLite™ (PerkinElmer, Inc., Italy). Serum fluorescence intensity positively correlates with increased intestinal permeability.
2.5. mRNA extraction and quantitative real time qRT-PCR analysis
RNA was isolated from liver and colon of mice using RNeasy Micro kit (Qiagen, Milano, Italy). cDNA was generated from 4µg total RNA using High Capacity DNA Archive Kit (Applied Biosystem, Foster City, CA) and following the manufacturer's instructions. Primers to detect the mRNA expression level of each gene were designed using Primer Express software (Applied Biosystem) based on Gene Bank sequence data. mRNA expression levels were quantified by qRTPCR using Power Syber Green chemistry and normalized to cyclophillin mRNA levels. Validated primers for qRT-PCR are available upon request.
2.6. Metagenomic sequencing of cecal content samples
Microbiome analysis was performed by Vaiomer (www.vaiomer.com/). Bacterial populations contained in the samples were determined using next generation high throughput sequencing of variable regions (V3-V4) of the 16S rDNA bacterial gene and a metagenomic workflow, exclusive to bacteria and established by Vaiomer [43], was used to identify organisms from a sample by amplifying specific regions in the 16S ribosomal RNA gene. PCR amplification was performed using 16S universal primers targeting the V3-V4 region of the bacterial 16S ribosomal gene (Vaiomer universal 16S primers). The joint pair length was set to encompass 467 base pairs amplicon thanks to 2 × 300 paired-end MiSeq kit V3. For each sample, a sequencing library was generated by addition of sequencing adapters. The detection of the sequencing fragments was performed using MiSeq Illumina® technology. The targeted metagenomic sequences from microbiota were analyzed using the bioinformatic pipeline established by Vaiomer from the FROGS guidelines [44]. Briefly, after demultiplexing of the bar-coded Illumina paired reads, single read sequences are cleaned and paired for each sample independently into longer fragments. Operational taxonomic units (OTUs) were produced with via single-linkage clustering and taxonomic assignment was performed in order to determine community profiles. Reads obtained from the MiSeq sequencing system have been processed using Vaiomer bioinformatic pipeline. The steps include quality-filtering, clustering into OTUs with the Swarm algorithm and taxonomic affiliation. Data are available on the ENA database (accession number PRJEB36966).
2.7. RNA extraction and quantitative real time qRT-PCR analysis in IBD patients
Ileal patients’ biopsies from CD patients (n = 6 with inflamed mucosa localization; n=6 from adjacent macroscopically healthy mucosa) were obtained from Humanitas Institute. RNA was isolated using RNeasy Micro kit (Qiagen, Milano, Italy). cDNA was generated from 1µg total RNA using High Capacity DNA Archive Kit (Applied Biosystem, Foster City, CA) and following the manufacturer's instructions. Human primers to detect mRNA expression level of each gene were designed using Primer Express software (Applied Biosystem) based on Gene Bank sequence data. mRNA expression levels were quantified by qRTPCR using Power Syber Green chemistry and normalized to cyclophillin mRNA levels. Validated human primers for qRT-PCR are available upon request.
2.8. FGF19 quantification in IBD patients
Plasma samples from CD patients (n = 57 all with ileal disease localization, 19 with active disease and 38 in remission) and healthy controls (n=23) were obtained from Humanitas Institute and our own biobank, respectively. Written informed consent was obtained from the participants of the study. Ethical approval was obtained by the independent ethical committees of the Humanitas Institute ethical committee (Dept. of Gastroenterology and Digestive Endoscopy; n.345, 2006) and the University Hospital of Bari (Interdisciplinary Department of Medicine; n.311, MSC/PBMC/2015). ELISA for human FGF19 was performed using biotin-labelled antibody pairs and standards provided by Biovendor (Bologna, Italy), according to the manufacturers’ procedures.
2.9. Statistical Analysis
All results are expressed as means±standard error of the mean (SEM). Significant differences between two or more groups were determined by Mann-Whitney's U and Kruskal-Wallis tests, as appropriate. All statistical analyses were performed with GraphPad Prism software (v5.0; GraphPad Software Inc., San Diego, CA) and conducted as a two-sided alpha level of 0.05.
3. Results
3.1. FGF19 M52 analogue retains BA-synthesis regulatory activity
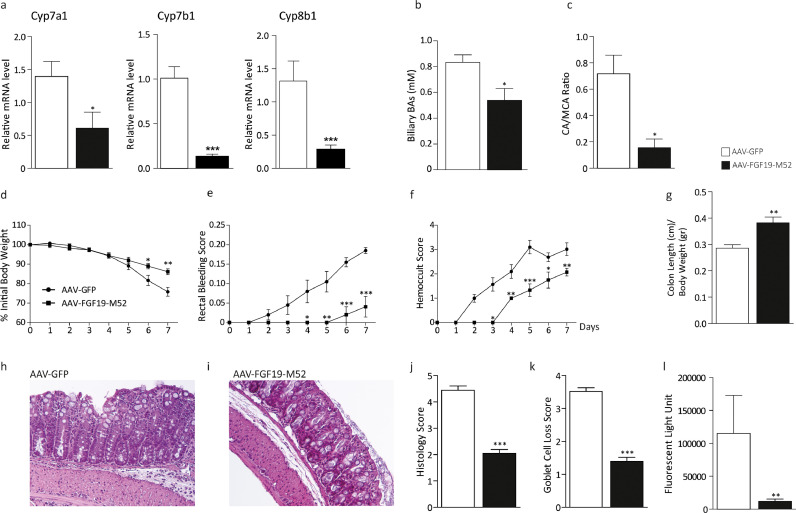
In order to study the therapeutic potential of FGF19 in intestinal inflammation, a novel engineered variant of the FGF19 protein, M52 (see WO 2016/0168219), was obtained from NGM Biopharmaceuticals, Inc. M52 differs from WT FGF19 by five amino acid substitutions (A30S, G31S, H33L, V35L, H36Q) and five–amino acid deletion at the N terminus. When compared to the full-length form in vivo, this variant retains its biological activity of repression of de novo BA synthesis while showing no hepatic tumorigenic activity [12]. We then tested M52 capability of controlling BA homeostasis in our colitis model. WT mice were injected with either the adeno-associated virus (AAV) containing either the FGF19-M52 analogue or GFP, and then subjected to DSS-induced colitis. Compared to the control group, AAV-FGF19-M52-injected mice displayed a significant lower hepatic mRNA expression of the key limiting enzymes of BA synthesis Cyp7a1, Cyp7b1 - a cytochrome that has a minor role in the conversion of cholesterol into BAs - and Cyp8b1 that regulates the relative amount of CDCA and CA (Fig. 1a). These changes were translated into a 50% reduction of the plasmatic BA pool size (AAV-GFP 2.37 ± 0.75 vs AAV-FGF19-M52 1.65 ± 0.93 μM), 35% reduction of the biliary bile acid pool size (Fig. 1b) and a shift in plasma BA composition to a more hydrophilic BA pool profile due to the enrichment in muricholic acid (MCA) (Fig. 1c).
Fig. 1.
The non-tumorigenic FGF19 analogue M52 retains BA synthesis regulatory activity in WT C57/Bl6 mice and confers protection against DSS-induced colitis. (a) qRT-PCR of hepatic Cyp7a1, Cyp7b1 and Cyp8b1. Expression was normalized to Cyclophillin. (b) Biliary BA concentration and (c) plasma BA composition (CA/MCA Ratio). (d) Percentage of initial body weight during DSS treatment, (e) visible rectal bleeding score, (f) hemoccult score (g) colon length in AAV-FGF19-M52- vs AAV-GFP-injected mice. Representative H&E-stained colonic sections for (h) GFP- and (i) AAV-FGF19-M52-injected mice (Magnification 200X). (j) Histology and (k) goblet cell loss scores. (l) In vivo intestinal permeability measurement after DSS-induced intestinal inflammation in WT mice. All values represent means±SEM. Statistical significance comparing AAV-FGF19-M52 versus control AAV-GFP assessed by Mann-Whitney's U test (*p < 0.05, **p < 0.01, ***p < 0.001).
3.2. FGF19 M52 analogue protects from DSS-induced colitis
We then investigated whether the FGF19 analogue M52 confers protection against DSS-induced colitis. In WT C57Bl/6J mice, AAV-FGF19-M52 injection significantly reduced the typical symptoms of intestinal inflammation, including body weight loss, visible rectal bleeding and occurrence of occult blood in mouse stools (Fig. 1d–f). Moreover, FGF19-M52 prevented colonic shortening compared to control AAV-GFP injected mice (Fig. 1g).
Histological analysis showed that DSS-induced colitis was associated with a severe disruption of the epithelial layer and acute inflammatory infiltrates in WT mice injected with AAV-GFP (Fig. 1h). In sharp contrast, AAV-FGF19-M52-injected WT mice showed significantly less morphological alteration and decreased inflammatory infiltrates (Fig. 1i). Patients with chronic intestinal inflammation presenting with clinically and endoscopically significant colitis display various degrees of mucin-secreting goblet cells loss [45], and the epithelial barrier is often already compromised at early stages of the disease, leading to bacterial translocation and inflammation [46,47]. Therefore, we assessed this disease index in our experimental model. Goblet cell loss due to DSS-induced inflammation was significantly less in AAV-FGF19-M52-injected WT mice compared to the control AAV-GFP group. A histopathological score method was applied and quantification of histological disease index and Goblet cell loss is shown (Fig. 1j, k). In a preliminary experiment performed to test appropriate DSS concentration, WT mice were treated with 5% DSS for 7 days. Results show a strong significant FGF19-M52-dependent protection from mortality compared to AAV-GFP-injected mice (10% mortality in FGF19-M52- vs 60% in GFP-injected mice, Supplementary Figure 1).
3.3. FGF19 analogue M52 decreases DSS-induced intestinal permeability in vivo
Protection from goblet cell loss due to FGF19-M52 treatment suggested a preservation of the intestinal epithelial barrier integrity. We assessed this in vivo with a FITC-dextran based intestinal permeability assay. FGF19-M52 protected from DSS-dependent increased epithelial permeability. Plasma levels of FITC-conjugated dextran were almost completely abolished in AAV-FGF19-M52-injected mice compared to the control group (Fig. 1l). These data point to a putative direct protective effect of FGF19-M52 in the enterocyte turnover and integrity.
3.4. FGF19 analogue M52 strengthens the integrity of the epithelial barrier and decreases local inflammatory response
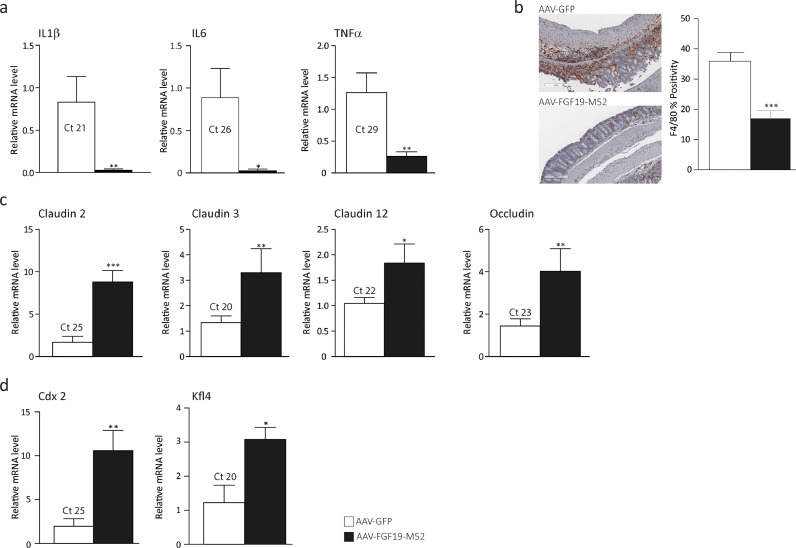
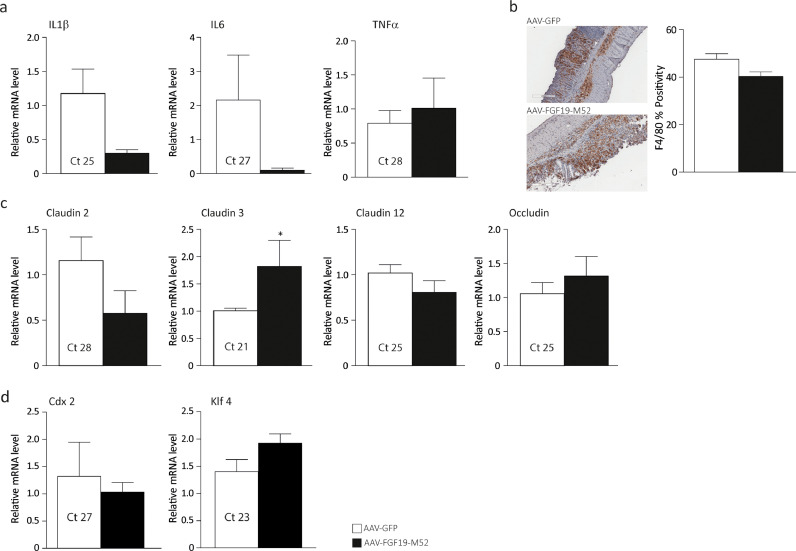
The intestinal epithelium is a physical barrier consisting of enterocytes tightly connected via intercellular junctions. It is constantly renewed every 3-5 days by stem cells residing at the bottom of the intestinal crypts. The majority of these cells migrate towards the top of intestinal villi while their molecular signature undergoes a switch from proliferative to differentiative pattern. The “enterocyte” phenotype is coupled with a progressive increased expression of differentiation markers such as the caudal homeobox domain 2 (CDX2) [48] and the Krüppel-like factor 4 (KLF4) [49], a gene involved in appropriate localization of the different intestinal cell lineage and epithelium homeostasis in the fully differentiated enterocytes. The intestinal epithelium separates the intestinal lumen from the lymphoid tissue associated with the gastrointestinal system, preventing external antigens and micro-organisms from entering the body while maintaining tolerance to harmless organisms [50]. Additionally, enterocytes contribute to trigger the inflammatory response by secreting pro-inflammatory cytokines and chemokines. Together with loss of epithelial barrier integrity, human intestinal chronic inflammation arises concomitantly with dysregulation of the mucosal immune response caused by a shift of balance from secretion of anti-inflammatory mediators towards pro-inflammatory molecules. For this reason, we investigated the ability of the non-tumorigenic FGF19-M52 analogue of modulating inflammatory and intestinal barrier gene expression during colitis. AAV-FGF19-M52-injected mice displayed a striking decrease of the colonic mucosa pro-inflammatory cytokine interleukin (IL)-1β, IL-6 and Tumor Necrosis Factor α (Fig. 2a). Intestinal immunohistochemical analysis of the inflammatory marker F4/80 shows that AAV-FGF19-M52 remarkably decreases macrophage recruitment at the site of inflammation compared to AAV-GFP-injected mice (Fig. 2b). This is associated with a strong induction of several members of tight junction proteins that have been shown to be downregulated in patients affected by IBD [51], namely Claudin 2, Claudin 3, Claudin 12 and Occludin (Fig. 2c). At the same time, the snapshot of gene expression pattern shows a dramatic increase of the differentiation markers Cdx2 and Klf4 in AAV-FGF19-M52-injected mice compared to the control group (Fig. 2d). Taken together, these data indicate that FGF19-M52 on the one hand decreases intestinal BA cytotoxicity and on the other hand, it inhibits the mucosal inflammatory immune response and preserves the epithelial barrier integrity by boosting the enterocytes ability to renew the intestinal epithelial barrier.
Fig. 2.
FGF19-M52 inhibits colonic expression of genes involved in local pro-inflammatory immune response and promotes genes involved in the maintenance of the epithelial barrier integrity. (a) qRT-PCR of proinflammatory genes IL-1β, IL-6 and TNFα. (b) Intestinal immunohistochemical staining of the inflammatory marker F4/80 (magnification 10X). Quantification of the signal assessed with Aperio Image Scope Software. qRT-PCR of (c) tight junctions’ genes Claudins 2, 3, 12 and Occludin and (d) intestinal differentiation markers Cdx2 and Klf4. Ct values are indicated. All values represent means±SEM. Statistical significance comparing AAV-FGF19-M52 versus control AAV-GFP assessed by Mann-Whitney's U test (*p<0.05, **p<0.01, ***p<0.001).
3.5. AAV-FGF19-M52 provides a beneficial shift in the intestinal flora of WT mice
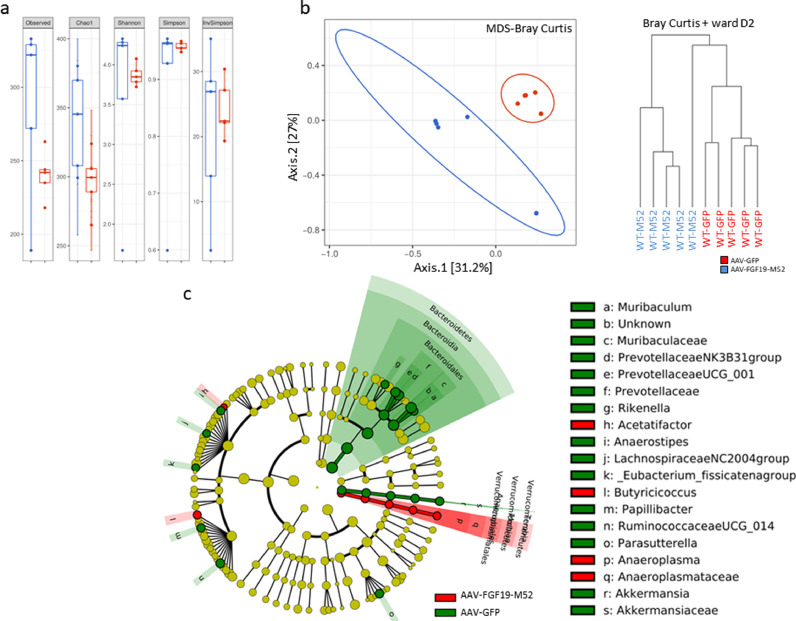
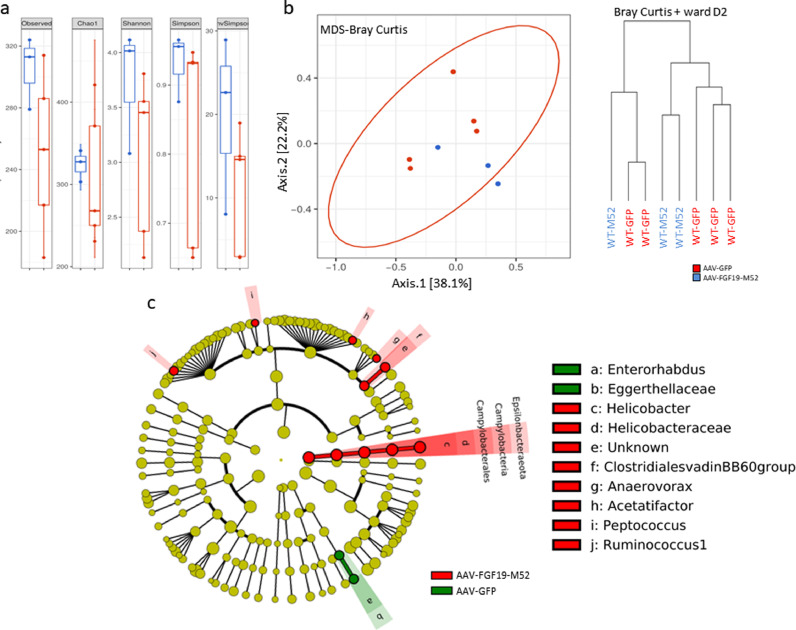
In order to understand whether FGF19-dependent reduction of BA synthesis and modulation of its composition together with protection from experimental colitis is paralleled by a change in the intestinal microbial composition we have sequenced the 16S rDNA extracted from cecal content of mice of our experimental groups. Alpha diversity analysis at OTU level shows a significant difference between WT mice treated with AAV-FGF19-M52 and AAV-GFP (Fig. 3a). Bray Curtis beta diversity analysis and hierarchical clustering show a major separation between WT mice injected with AAV-GFP and AAV-FGF19-M52 (Fig. 3b). Then, we focused on clade abundance to identify which microbial clades where modified by AAV-FGF19-M52 in WT mice and LEfSe analysis was performed on the sequence data [52]. AAV-FGF19-M52-injected mice subjected to experimental colitis show a beneficial bacterial shift compared to the control group. The highest mean of differential features at genus level are represented by tenericutes, mollicutes, acetatifactor, butyricicoccus, anaeroplasmateles, anaeroplasma and anaeroplasmataceae (Fig. 3c).
Fig. 3.
AAV-FGF19-M52 increases alpha diversity and generate a beneficial shift in the gut microflora of WT mice. (a) Graphs represent the alpha diversity at the OTU level in WT and Fxrnull mice. Observed and Chao1 indexes calculate the alpha diversity in term of richness (number of taxa that are present in the samples). Shannon, Simpson and inversed Simpson indexes calculate the alpha diversity regarding the evenness of taxa in the samples. (b) Graphs represent the distance between samples using the OTU distribution of each sample. The distance is here represented on 2 axes summarizing the entire distribution of all the OTU present in the samples (MDS representation) and a hierarchical clustering tree (dendogram). (c) Visual comparison of the relative abundance barplots indicates differences between the groups as also seen in the pairwise group LEfSe analyses (log(LDA Score)>2.0). The cladograms represented here indicate the bacterial taxa that are significantly different between the 2 groups being compared. This analysis helps to identify a first selection of differential bacterial taxa in the considered groups. (Overlapping taxa on the green section: Verrucomicrobia, Verrucomicrobiae, Verrucomicrobiales. Overlapping taxa on the red section:Tenericutes, Mollicutes, Anaeroplasmatales)
3.6. FXR is necessary for FGF19 analogue M52-dependent protection against colitis
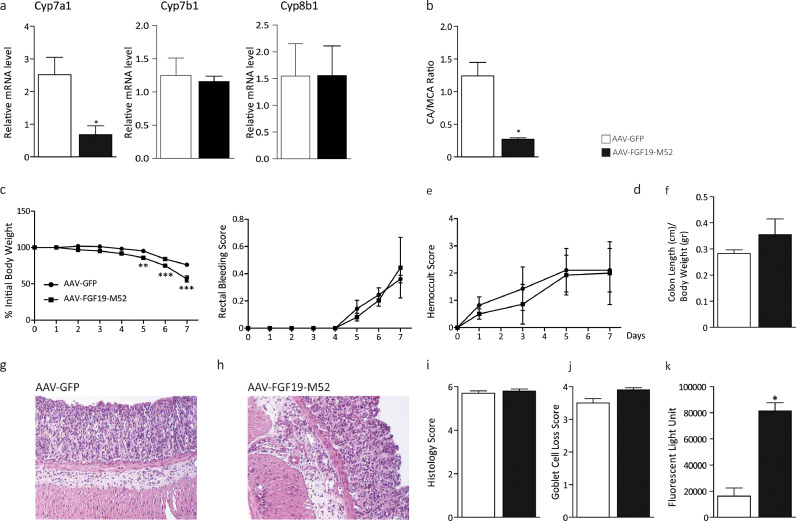
FGF19 is an intestinal target of FXR and loss of FXR has been show to increase susceptibility to inflammation and cancer [15,53]. We then tested if AAV-FGF19-M52 is able to reduce BA concentration in Fxrnull mice via repression of hepatic Cyp7a1 and if this event is coupled with protection against inflammation even if in the absence of FXR. We then treated FXRnull mice with AAV-GFP or AAV-FGF19-M52 and subjected these mice to DSS colitis. Hepatic Cyp7a1 mRNA expression (Fig. 4a) was significantly decreased in AAV-FGF19-M52 treated mice and this was associated with 50% decrease of plasmatic BA pool size that is chronically high in FXRnull mice (AAV-GFP 5.09 ± 0.45 vs AAV-FGF19-M52 2.19 ± 0.54 μM). No changes in hepatic expression of Cyp7b1 and Cyp8b1 were observed (Fig. 4a). Regardless, we observed a shift in plasma BA composition to a more hydrophilic BA pool profile due to the enrichment in MCA in AAV-FGF19-M52 mice compared to control mice (Fig. 4b). Difference in regulation of Cyp7a1 and Cyp8b1 via FGF19 is possibly ascribable to DSS ability to change BA metabolism and expression of related cytochromes [30]. Interestingly, no evident signs of FGF19-M52-dependent protection against DSS-induced symptoms were detected in FXRnull mice (Fig. 4c–f). These results were paralleled by no changes of the microscopic intestinal structure features, as indicated by HE staining and analysis of the colonic morphology and assessment of inflammatory infiltrate and goblet cell loss (Fig. 4g–j). In line with these data, FGF19-M52 did not provide an improvement of the intestinal barrier integrity (Fig. 4k) nor any significant difference in macrophage recruitment at the inflammation site and pro-inflammatory and tight-junctions gene expression levels, except Claudin 3 (Fig. 5a–c). Moreover, no changes in Cdx2 and Klf4 gene expression levels were observed (Fig. 5d). Taken together, these data indicate first that FGF19 is sufficient to modulate BA homeostasis even in the absence of FXR. However, the presence of FXR is necessary for the FGF19 anti-inflammatory response in the gut and for enterocyte differentiation and preservation of the intestinal barrier integrity.
Fig. 4.
The non-tumorigenic FGF19 analogue M52 retains BA synthesis regulatory activity in Fxrnull mice but does not protect Fxrnull mice against DSS-induced colitis. (a) qRT-PCR of hepatic Cyp7a1, Cyp7b1 and Cyp8b1. Expression was normalized to Cyclophillin. (b) Plasma BA composition (CA/MCA Ratio). (c) Percentage of initial body weight during DSS treatment, (d) visible rectal bleeding score, (e) hemoccult score and (f) colon length in AAV-FGF19-M52- vs AAV-GFP-injected Fxrnull mice. Representative H&E-stained colonic sections for (g) AAV-GFP- and (h) AAV-FGF19-M52-injected FXRnull mice (Magnification 200X). (i) Histology and (j) goblet cell loss scores. (k) In vivo intestinal permeability measurement after DSS-induced intestinal inflammation in FXRnull mice. All values represent means±SEM. Statistical significance comparing AAV-FGF19-M52 versus control AAV-GFP assessed by Mann-Whitney's U test (*p < 0.05, **p < 0.01, ***p < 0.001).
Fig. 5.
FGF19-M52 does not affect colonic expression of genes involved in local pro-inflammatory immune response and epithelial barrier homeostasis In FXRnull mice. (a) qRT-PCR of proinflammatory genes IL-1β, IL-6 and TNFα. (b) Intestinal immunohistochemical staining of the inflammatory marker F4/80 (magnification 10X). Quantification of the signal assessed with Aperio Image Scope Software. qRT-PCR of (c) tight junctions’ genes Claudins 2, 3, 12 and Occludin and (d) intestinal differentiation markers Cdx2 and Klf4. Ct values are indicated. All values represent means±SEM. Statistical significance comparing AAV-FGF19-M52 versus control AAV-GFP assessed by Mann-Whitney's U test (*p < 0.05).
3.7. AAV-FGF19-M52 does not provide a beneficial shift in the intestinal flora of FXRnull mice
In order to explore FGF19-M52-dependent changes in intestinal microbial composition of Fxrnull mice we have sequenced the 16S rDNA extracted from cecal content of mice of our experimental groups. Alpha and beta diversity analysis did not show a significant difference between Fxrnull mice injected with either AAV-GFP or AAV-FGF19-M52 (Fig. 6a, b). Moreover, clade abundance query indicates that the positive modulation of intestinal microbiota obtained in WT mice via FGF19-M52 treatment is lost in FXRnull mice, just as lost is the protection against inflammation and epithelial integrity (Fig. 6c).
Fig. 6.
AAV-FGF19-M52 does not generate a beneficial shift in the gut microflora of Fxrnull mice. (a) Graphs represent the alpha diversity at the OTU level in Fxrnull mice. Observed and Chao1 indexes calculate the alpha diversity in term of richness (number of taxa that are present in the samples). Shannon, Simpson and inversed Simpson indexes calculate the alpha diversity regarding the evenness of taxa in the samples. (b) Graphs represent the distance between samples using the OTU distribution of each sample. The distance is here represented on 2 axes summarizing the entire distribution of all the OTU present in the samples (MDS representation) and a hierarchical clustering tree (dendogram). (c) Visual comparison of the relative abundance barplots indicates differences between the groups as also seen in the pairwise group LEfSe analyses (log(LDA Score)>2.0). The cladograms represented here indicate the bacterial taxa that are significantly different between the 2 groups being compared. This analysis helps to identify a first selection of differential bacterial taxa in the considered groups.
3.8. Systemic FGF19 levels are lower in patients affected by Crohn's Disease
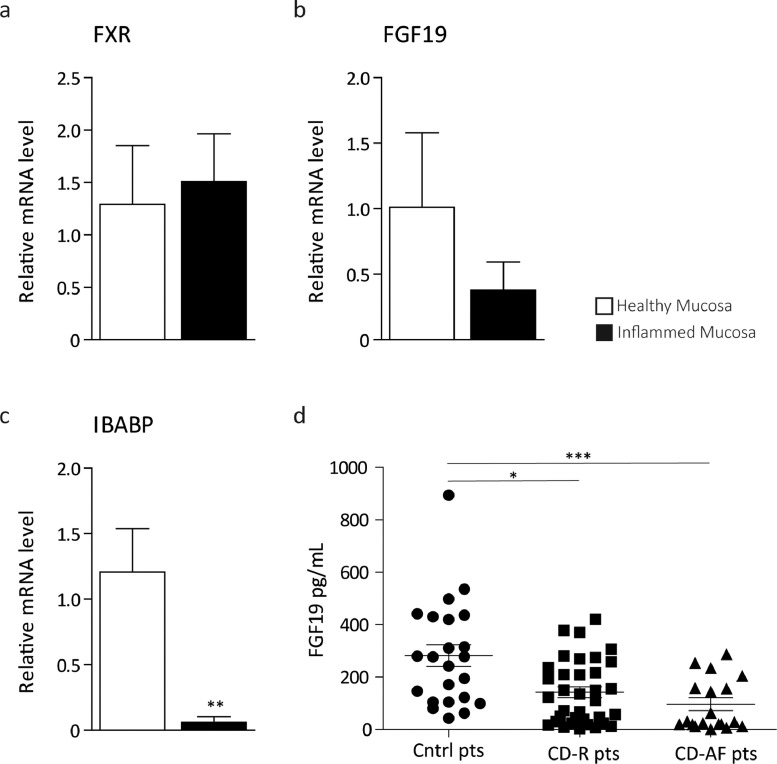
Since chronic active inflammation in patients with CD is known to affect the normal structure of the intestinal mucosa and decrease FXR intestinal transcriptional activity [54], we wanted to asses intestinal and systemic expression of FGF19. Using two different biopsies from the same CD patient (tot. n = 6 pts), one located in the actively inflamed area and the other one from the adjacent macroscopically not inflamed one, we assessed levels of genes that are normally expressed in the differentiated compartment of the intestinal mucosa. Relative mRNA levels of the differentiation markers Villin, Sucrose Isomaltase (SI) and CDX2 are significantly lower in the region affected by active inflammation compared to the uninflamed one (Supplementary Fig. 2a–c). We then assessed whether the expression of the FXR targets FGF19 and intestinal bile acid binding protein (IBABP) were affected. mRNA levels of IBABP resulted significantly decreased and FGF19 showed a strong decreasing trend (Fig. 7a–c) in the actively inflamed mucosa compared to the adjacent macroscopically healthy one. We then measured FGF19 circulating levels in the serum of healthy controls and CD patients. Circulating FGF19 levels are strikingly lower in patients with CD in remission and even lower in CD patients with active inflammation compared to control subjects (Fig. 7d). Overall, these data point to the existence of a downregulation of intestinal FXR activity with decreased circulating levels of FGF19 in patients with CD.
Fig. 7.
The FXR-FGF19 axis activity is impaired in Crohn's disease patients. qRT-PCR of (a) FXR and its targets (b) FGF19 and (c) IBABP in n = 6 patients with Crohn's disease collected from ileal actively inflamed mucosa and adjacent macroscopically not inflammed tissue. Expression was normalized to Cyclophillin. Statistical significance comparing inflamed mucosa versus healthy adjacent one assessed by Mann–Whitney's U test (**p < 0.01). (d) Scatter plot of ELISA assay performed on plasma samples collected from controls subjects (n = 23, circles ●) and fasting Crohn's patients either in remission (n = 38, squares ■) or with active inflammation (n = 19, triangles ▲). Horizontal lines indicate mean values. Statistical significance comparing the groups assessed by Kruskal-Wallis test followed by Dunn post hoc test comparing all pair of columns (*p < 0.05, **p < 0.01, ***p < 0.001).
4. Discussion
BA metabolic derangements are one of the wide causal phenotype heterogeneity of patients presenting with chronic intestinal inflammation. In fact, fifty percent of IBD patients, and particularly CD patients with inflammation of the terminal ileum, present with bile acid malabsorption and diarrhea [55]. This is putatively due to decreased ASBT expression [56] leading to increased intraluminal detergent BA via impaired BA ileal re-uptake, lower FXR intestinal activation [18,54] and consequent decreased production of the FXR major target FGF19 [16,57]. This, ultimately cause a vicious cycle priming loss of inhibition of hepatic Cyp7a1 and increased BA synthesis. In experimental colitis, accumulation of BAs in the intestine leads to their disposal via the PPARα-UGT pathway [30]. This causes deregulation of the FXR transcriptional activity and a compromised Fxr-Fgf15 pathway activation, ultimately leading to continuous activation of Cyp7a1, increased de novo BA synthesis and colitis exacerbation [30]. In this study we describe a novel non-tumorigenic FGF19 variant, FGF19-M52, as a potential therapeutic agent for intestinal inflammation. For the first time, here we show that FGF19-M52 successfully protects WT mice from DSS-induced symptoms of inflammation, reduces mucosal pro-inflammatory gene expression and immune response, preserves the intestinal barrier integrity and induces a beneficial microbial shift contributing to the resolution of the inflammatory phenotype. In particular, FGF19-M52 reduced biliary and plasmatic BA pool size and induced a beneficial shift in plasma BA composition to a more hydrophilic pool profile by repressing hepatic BA synthesis, thereby protecting from colitis development. In contrast with previously published data [58], Asbt expression level do not change upon FGF19 treatment (data not shown). This is likely due to the fact that in our model inflammation is induced upon DSS administration, resulting in colonic inflammation, a condition in which ileal Asbt expression does not appear to be affected [59]. Moreover, FGF19-M52 also showed a local anti-inflammatory and pro-turnover activity. In fact, FGF19-M52 inhibited the mucosal inflammatory immune response and boosted enterocytes’ ability to renew the intestinal epithelial barrier preserving its integrity during an inflammatory stimulus.
Since FGF19 is an intestinal target of FXR and loss of FXR has been show to increase susceptibility to inflammation and cancer [15,53], we asked ourselves whether FGF19-M52 is able to reduce BA concentration in FXRnull mice via repression of hepatic Cyp7a1 and if this event is coupled with protection against inflammation even in the absence of FXR. Our results in FXRnull mice indicate that FXR is necessary in the FGF19-mediated protection from DSS-induced intestinal inflammation and that its expression/activity has probably a local and basal physiological function in the maintenance of the intestinal barrier integrity. In fact, Fxrnull mice display a disrupted intestinal barrier at baseline, with severe degeneration after obstruction of the BA flow via bile duct ligation [15,23]. Here, we show that FXR is unconditionally necessary in protecting from intestinal inflammation and most likely it complements FGF19 anti-inflammatory activity in the preservation of intestinal homeostasis. Mechanistically, this could be explained considering the beneficial action of FGF19 in rendering circulating BA pool more favourable for the coordination of intestinal self-renewal of intestinal cells [60].
In both human and mice, the exact nature of the host-microbiome interaction contributing to chronic intestinal inflammation development has not been completely defined. Metagenomic techniques are greatly aiding the identification of bacterial species, functions and metabolites involved in the development of chronic intestinal inflammation. In an attempt to discover whether FGF19 anti-inflammatory effect was exploited also via a shift in the gut flora, we embarked in microbiome analysis of cecal samples of WT and FXRnull mice. Our results show that FGF19-M52 generates a beneficial shift in the intestinal bacterial composition of WT mice, with an increased abundance of butyricicoccus, tenericutes and acetatifactor, belonging to firmicutes and butyrate producers, known to be decreased in patient with inflammatory bowel disease. Although we are far from a complete understanding of bacterial dysbiosis in chronic intestinal inflammation and other gut disorders and the relative importance of bacterial species is still under debate, the species we have unveiled in our study have previously been associated with protection against IBD [61], [62], [63], [64] and therefore may be beneficially involved in the maintenance of gut fitness. Interestingly, it has previously been shown that an advantageous intestinal milieu contributes to the inhibition of BAs synthesis by promoting the intestinal activity of the Fxr-Fgf15 duo [29]. On the contrary, it is striking to note that the positive effects of FGF19-M52 on the intestinal microbiota are lost in FXRnull mice just as lost is the protective effects against colitis and the promotion of cell renewal.
Taken together, these data indicate that the FXR-FGF19 duo protects from intestinal inflammation by modulating the BA pool size and composition, counteracting the local inflammatory response and tuning the gut bacterial composition, with FXR playing a critical role in promoting intestinal cell renewal in the context of an inflammatory injury. First, FGF19 reduces the biliary and intraluminal BA levels thus reducing the detergent cytotoxic events that further increase susceptibility to intestinal inflammation in IBD patients. This creates the perfect environment for the subsequent anti-inflammatory boost generated by the beneficial shift in the intestinal microbiota. Concomitantly, FGF19 reduces local intestinal inflammation and immune responses and preserves the intestinal barrier integrity. Finally, FGF19 regulates enterocyte turnover and preserves the epithelial barrier integrity only in mice competent for FXR. Several clinical trials for hepatic and metabolic diseases using FGF19 analogues have been recently successfully concluded [2,[65], [66], [67], [68]] while others are ongoing, indicating that its therapeutic potential has just started to be uncovered and might soon be extended to other pathological condition. Safety profile for another FGF19 analogue, namely M70/NGM282, have indicated mild to moderate adverse event in the trialled patients, such as diarrhea, increased intestinal transit and abdominal discomfort. Therefore, despite its efficacy in treating different metabolic and inflammatory conditions, patients might benefit in the future also from studies with the novel analogue M52.
In conclusion, our data strengthen the link between the BA homeostasis, intestinal inflammation and microbiota composition, highlighting the bona fide therapeutic potential of the FGF19 analogues in the treatment of IBD patients where concomitant derangement of BA homeostasis is diagnosed.
Declaration of Competing Interest
Brian Ko and Jian Luo are employers of NGM Pharmaceuticals that retains the Patent for FGF19-M52 (WO 2013/006486). Brian Ko and Jian Luo had no role in study design, data collection, data analysis, interpretation, writing of the report. Gionatha Fiorino reports personal fees from AbbVie, MSD, Takeda, Janssen, Amgen, Sandoz, Pfizer, Mylan and Ferring, outside the submitted work. Silvio Danese reports personal fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ely Lilly, Enthera, Ferring Pharmaceuticals inc, Gilead, Hospira, Janssen, Johnson and Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB Inc and Vifor outside the submitted work. Antonio Moschetta reports grants from NGM Biopharmaceuticals, during the conduct of the study; grants from Intercept Pharmaceuticals, outside the submitted work. All other authors have declared no conflict of interest.
Acknowledgments
Acknowledgments
We thank A. Contursi for helping during the animal studies.
Funding
A. Moschetta is funded by MIUR-PRIN 2017 <- 2017J3E2W2; Italian Association for Cancer Research (AIRC, IG 23239); Interreg V-A Greece-Italy 2014-2020-SILVER WELLBEING, MIS5003627; HDHL-INTIMIC EuJPI-FATMAL; MIUR PON “R&I” 2014-2020-ARS01_01220. No money has been paid by NGM Biopharmaceuticals or any other agency to write this article. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author contributions
RMG contributed to study design, performed experiments, analyzed the data, performed statistical analysis and wrote the paper; OGI, MC and NS contributed to perform the experiments; CP performed histology; SV, GF and SD provided human samples and translational expertise; EP performed bile acid measurements; AR provided bile acid profiling expertise, JL and BK provided the adenoviruses; CS provided medical expertise and double checked the clinical relevance of the human data; AM designed the study, supervised the project and wrote the paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102719.
Appendix. Supplementary materials
References
- 1.Gadaleta R.M., Moschetta A. Metabolic Messengers: fibroblast growth factor 15/19. Nature Metabolism. 2019 doi: 10.1038/s42255-019-0074-3. [DOI] [PubMed] [Google Scholar]
- 2.Choi M., Moschetta A., Bookout A.L., Peng L., Umetani M., Holmstrom S.R. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12(11):1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 3.Holt J.A., Luo G., Billin A.N., Bisi J., McNeill Y.Y., Kozarsky K.F. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17(13):1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C.L., McDonald J.G. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Song K.H., Li T., Owsley E., Strom S., Chiang J.Y. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49(1):297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 7.Parks D.J., Blanchard S.G., Bledsoe R.K., Chandra G., Consler T.G., Kliewer S.A. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284(5418):1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 8.Wang H., Chen J., Hollister K., Sowers L.C., Forman B.M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 9.Gadaleta R.M., Cariello M., Sabba C., Moschetta A. Tissue-specific actions of FXR in metabolism and cancer. Biochim Biophys Acta. 2015;1851(1):30–39. doi: 10.1016/j.bbalip.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 11.Patti M.E., Houten S.M., Bianco A.C., Bernier R., Larsen P.R., Holst J.J. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17(9):1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gadaleta R.M., Scialpi N., Peres C., Cariello M., Ko B., Luo J. Suppression of Hepatic Bile Acid Synthesis by a non-tumorigenic FGF19 analogue Protects Mice from Fibrosis and Hepatocarcinogenesis. Sci Rep. 2018;8(1):17210. doi: 10.1038/s41598-018-35496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridlon J.M., Alves J.M., Hylemon P.B., Bajaj J.S. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4(5):382–387. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duboc H., Rajca S., Rainteau D., Benarous D., Maubert M.A., Quervain E. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62(4):531–539. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 15.Gadaleta R.M., van Erpecum K.J., Oldenburg B., Willemsen E.C., Renooij W., Murzilli S. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60(4):463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 16.Lenicek M., Duricova D., Komarek V., Gabrysova B., Lukas M., Smerhovsky Z. Bile acid malabsorption in inflammatory bowel disease: assessment by serum markers. Inflamm Bowel Dis. 2011;17(6):1322–1327. doi: 10.1002/ibd.21502. [DOI] [PubMed] [Google Scholar]
- 17.Nolan J.D., Johnston I.M., Walters J.R. Altered enterohepatic circulation of bile acids in Crohn's disease and their clinical significance: a new perspective. Expert Rev Gastroenterol Hepatol. 2013;7(1):49–56. doi: 10.1586/egh.12.66. [DOI] [PubMed] [Google Scholar]
- 18.Walters J.R. Bile acid diarrhoea and FGF19: new views on diagnosis, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2014;11(7):426–434. doi: 10.1038/nrgastro.2014.32. [DOI] [PubMed] [Google Scholar]
- 19.Meihoff W.E., Kern F., Jr. Bile salt malabsorption in regional ileitis, ileal resection and mannitol-induced diarrhea. J Clin Invest. 1968;47(2):261–267. doi: 10.1172/JCI105722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung D., Fantin A.C., Scheurer U., Fried M., Kullak-Ublick G.A. Human ileal bile acid transporter gene ASBT (SLC10A2) is transactivated by the glucocorticoid receptor. Gut. 2004;53(1):78–84. doi: 10.1136/gut.53.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon R.S., Carey M.C. Do steroids ameliorate bile acid malabsorption in Crohn's disease? Gut. 2004;53(1):10–11. doi: 10.1136/gut.53.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung D., Inagaki T., Gerard R.D., Dawson P.A., Kliewer S.A., Mangelsdorf D.J. FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. Journal of Lipid Research. 2007 doi: 10.1194/jlr.M700351-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Inagaki T., Moschetta A., Lee Y.K., Peng L., Zhao G., Downes M. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103(10):3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridlon J.M., Kang D.J., Hylemon P.B., Bajaj J.S. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30(3):332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofmann A.F., Eckmann L. How bile acids confer gut mucosal protection against bacteria. Proc Natl Acad Sci U S A. 2006;103(12):4333–4334. doi: 10.1073/pnas.0600780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islam K.B., Fukiya S., Hagio M., Fujii N., Ishizuka S., Ooka T. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141(5):1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 27.Jones M.L., Martoni C.J., Ganopolsky J.G., Labbe A., Prakash S. The human microbiome and bile acid metabolism: dysbiosis, dysmetabolism, disease and intervention. Expert Opin Biol Ther. 2014;14(4):467–482. doi: 10.1517/14712598.2014.880420. [DOI] [PubMed] [Google Scholar]
- 28.Sagar N.M., Cree I.A., Covington J.A., Arasaradnam R.P. The interplay of the gut microbiome, bile acids, and volatile organic compounds. Gastroenterol Res Pract. 2015;2015 doi: 10.1155/2015/398585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayin S.I., Wahlstrom A., Felin J., Jantti S., Marschall H.U., Bamberg K. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhou X., Cao L., Jiang C., Xie Y., Cheng X., Krausz K.W. PPARalpha-UGT axis activation represses intestinal FXR-FGF15 feedback signalling and exacerbates experimental colitis. Nat Commun. 2014;5:4573. doi: 10.1038/ncomms5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Schaik F.D., Gadaleta R.M., Schaap F.G., van Mil S.W., Siersema P.D., Oldenburg B. Pharmacological activation of the bile acid nuclear farnesoid X receptor is feasible in patients with quiescent Crohn's colitis. PLoS One. 2012;7(11):e49706. doi: 10.1371/journal.pone.0049706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vavassori P., Mencarelli A., Renga B., Distrutti E., Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183(10):6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- 33.French D.M., Lin B.C., Wang M., Adams C., Shek T., Hotzel K. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS One. 2012;7(5):e36713. doi: 10.1371/journal.pone.0036713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latasa M.U., Salis F., Urtasun R., Garcia-Irigoyen O., Elizalde M., Uriarte I. Regulation of amphiregulin gene expression by beta-catenin signaling in human hepatocellular carcinoma cells: a novel crosstalk between FGF19 and the EGFR system. PLoS One. 2012;7(12):e52711. doi: 10.1371/journal.pone.0052711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholes K., Guillet S., Tomlinson E., Hillan K., Wright B., Frantz G.D. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol. 2002;160(6):2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawey E.T., Chanrion M., Cai C., Wu G., Zhang J., Zender L. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell. 2011;19(3):347–358. doi: 10.1016/j.ccr.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaap F.G., van der Gaag N.A., Gouma D.J., Jansen P.L. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology. 2009;49(4):1228–1235. doi: 10.1002/hep.22771. [DOI] [PubMed] [Google Scholar]
- 38.Wu A.L., Coulter S., Liddle C., Wong A., Eastham-Anderson J., French D.M. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS One. 2011;6(3):e17868. doi: 10.1371/journal.pone.0017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X., Ge H., Lemon B., Vonderfecht S., Baribault H., Weiszmann J. Separating mitogenic and metabolic activities of fibroblast growth factor 19 (FGF19) Proc Natl Acad Sci U S A. 2010;107(32):14158–14163. doi: 10.1073/pnas.1009427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roda A., Gioacchini A.M., Cerre C., Baraldini M. High-performance liquid chromatographic-electrospray mass spectrometric analysis of bile acids in biological fluids. J Chromatogr B Biomed Appl. 1995;665(2):281–294. doi: 10.1016/0378-4347(94)00544-f. [DOI] [PubMed] [Google Scholar]
- 41.Melgar S., Karlsson L., Rehnstrom E., Karlsson A., Utkovic H., Jansson L. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol. 2008;8(6):836–844. doi: 10.1016/j.intimp.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 42.Cario E., Gerken G., Podolsky D.K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132(4):1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 43.Lluch J., Servant F., Paisse S., Valle C., Valiere S., Kuchly C. The Characterization of novel tissue microbiota using an optimized 16S metagenomic sequencing pipeline. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escudie F., Auer L., Bernard M., Mariadassou M., Cauquil L., Vidal K. FROGS: find, rapidly, OTUs with galaxy solution. Bioinformatics. 2018;34(8):1287–1294. doi: 10.1093/bioinformatics/btx791. [DOI] [PubMed] [Google Scholar]
- 45.Sommers S.C., Korelitz B.I. Mucosal-cell counts in ulcerative and granulomatous colitis. Am J Clin Pathol. 1975;63(3):359–365. doi: 10.1093/ajcp/63.3.359. [DOI] [PubMed] [Google Scholar]
- 46.Hollander D. Permeability in Crohn's disease: altered barrier functions in healthy relatives? Gastroenterology. 1993;104(6):1848–1851. doi: 10.1016/0016-5085(93)90668-3. [DOI] [PubMed] [Google Scholar]
- 47.Peeters M., Geypens B., Claus D., Nevens H., Ghoos Y., Verbeke G. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 1997;113(3):802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 48.Stringer E.J., Duluc I., Saandi T., Davidson I., Bialecka M., Sato T. Cdx2 determines the fate of postnatal intestinal endoderm. Development. 2012;139(3):465–474. doi: 10.1242/dev.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghaleb A.M., McConnell B.B., Kaestner K.H., Yang V.W. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev Biol. 2011;349(2):310–320. doi: 10.1016/j.ydbio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hooper L.V., Bry L., Falk P.G., Gordon J.I. Host-microbial symbiosis in the mammalian intestine: exploring an internal ecosystem. Bioessays. 1998;20(4):336–343. doi: 10.1002/(SICI)1521-1878(199804)20:4<336::AID-BIES10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 51.Landy J., Ronde E., English N., Clark S.K., Hart A.L., Knight S.C. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22(11):3117–3126. doi: 10.3748/wjg.v22.i11.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Modica S., Murzilli S., Salvatore L., Schmidt D.R., Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68(23):9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- 54.Nijmeijer R.M., Gadaleta R.M., van Mil S.W., van Bodegraven A.A., Crusius J.B., Dijkstra G. Farnesoid X receptor (FXR) activation and FXR genetic variation in inflammatory bowel disease. PLoS One. 2011;6(8):e23745. doi: 10.1371/journal.pone.0023745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cosnes J., de P.V., Carbonnel F., Beaugerie L., Ngo Y., Gendre J.P. Classification of the sequelae of bowel resection for Crohn's disease. Br J Surg. 1994;81(11):1627–1631. doi: 10.1002/bjs.1800811122. [DOI] [PubMed] [Google Scholar]
- 56.Neimark E., Chen F., Li X., Magid M.S., Alasio T.M., Frankenberg T. c-Fos is a critical mediator of inflammatory-mediated repression of the apical sodium-dependent bile acid transporter. Gastroenterology. 2006;131(2):554–567. doi: 10.1053/j.gastro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Nolan J.D., Johnston I.M., Pattni S.S., Dew T., Orchard T.R., Walters J.R. Diarrhea in Crohn's disease: investigating the role of the ileal hormone fibroblast growth factor 19. J Crohns Colitis. 2015;9(2):125–131. doi: 10.1093/ecco-jcc/jju022. [DOI] [PubMed] [Google Scholar]
- 58.Ghosh A., Chen F., Banerjee S., Xu M., Shneider B.L. c-Fos mediates repression of the apical sodium-dependent bile acid transporter by fibroblast growth factor-19 in mice. Am J Physiol Gastrointest Liver Physiol. 2014;306(2):G163–G171. doi: 10.1152/ajpgi.00276.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rau M., Stieger B., Monte M.J., Schmitt J., Jahn D., Frey-Wagner I. Alterations in enterohepatic Fgf15 signaling and changes in bile acid composition depend on localization of murine intestinal inflammation. Inflamm Bowel Dis. 2016;22(10):2382–2389. doi: 10.1097/MIB.0000000000000879. [DOI] [PubMed] [Google Scholar]
- 60.Fu T., Coulter S., Yoshihara E., Oh T.G., Fang S., Cayabyab F. FXR regulates intestinal cancer stem cell proliferation. Cell. 2019;176(5):1098–1112. doi: 10.1016/j.cell.2019.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J., Chang R., Zhang X., Wang Z., Wen J., Zhou T. Non-isoflavones diet incurred metabolic modifications induced by constipation in rats via targeting gut microbiota. Front Microbiol. 2018;9:3002. doi: 10.3389/fmicb.2018.03002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bajer L., Kverka M., Kostovcik M., Macinga P., Dvorak J., Stehlikova Z. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol. 2017;23(25):4548–4558. doi: 10.3748/wjg.v23.i25.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Machiels K., Joossens M., Sabino J., De P.V., Arijs I., Eeckhaut V. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 64.Yeom Y., Kim B.S., Kim S.J., Kim Y. Sasa quelpaertensis leaf extract regulates microbial dysbiosis by modulating the composition and diversity of the microbiota in dextran sulfate sodium-induced colitis mice. BMC Complement Altern Med. 2016;16(1):481. doi: 10.1186/s12906-016-1456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harrison S.A., Rinella M.E., Abdelmalek M.F., Trotter J.F., Paredes A.H., Arnold H.L. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391(10126):1174–1185. doi: 10.1016/S0140-6736(18)30474-4. [DOI] [PubMed] [Google Scholar]
- 66.Hirschfield G.M., Chazouilleres O., Drenth J.P., Thorburn D., Harrison S.A., Landis C.S. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol. 2019;70(3):483–493. doi: 10.1016/j.jhep.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 67.Mayo M.J., Wigg A.J., Leggett B.A., Arnold H., Thompson A.J., Weltman M. NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun. 2018;2(9):1037–1050. doi: 10.1002/hep4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oduyebo I., Camilleri M., Nelson A.D., Khemani D., Nord S.L., Busciglio I. Effects of NGM282, an FGF19 variant, on colonic transit and bowel function in functional constipation: a randomized phase 2 trial. Am J Gastroenterol. 2018;113(5):725–734. doi: 10.1038/s41395-018-0042-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.