Abstract
Background
Bipolar disorder (BD) is a mental disorder characterized by mood fluctuations between an acute episodic state of either mania or depression and a clinically remitted state. Dysfunction of large-scale intrinsic brain networks has been demonstrated in this disorder, but it remains unknown whether those network alterations are related to different states.
Methods
In the present study, we performed a meta-analysis of whole-brain seed-based resting-state functional connectivity (rsFC) studies in BD patients to compare the intrinsic function of brain networks between episodic and remitted states. Thirty-nine seed-based voxel-wise rsFC datasets from thirty publications (1047 BD patients vs 1081 controls) were included in the meta-analysis. Seeds were categorized into networks by their locations within a priori functional networks. Seed-based d mapping analysis of between-state effects identified brain systems in which different states were associated with increased connectivity or decreased connectivity within and between each seed network.
Findings
We found that BD patients presented decreased connectivity within the affective network (AN) in acute episodes but not in the remitted state of the illness. Similar decreased connectivity within the default-mode network (DMN) was also found in the acute state, but it was replaced by increased connectivity in the remitted state. In addition, different patterns of between-network dysconnectivity were observed between the acute and remitted states.
Interpretation
This study is the first to identify different patterns of intrinsic function in large-scale brain networks between the acute and remitted states of BD through meta-analysis. The findings suggest that a shift in network function between the acute and remitted states may be related to distinct emotional and cognitive dysfunctions in BD, which may have important implications for identifying clinically relevant biomarkers to guide alternative treatment strategies for BD patients during active episodes or remission.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81171488, 81671669 and 81820108018) and by a Sichuan Provincial Youth Grant (2017JQ0001).
Keywords: Bipolar disorder (BD), Resting-state functional connectivity (rsFC), Meta-analysis, Functional network, Mood states
Research in context.
Background of previous research
Bipolar disorder (BD) is characterized by acute episodes of mania and depression separated by phases of relative remission. Dysfunctions in large-scale functional networks have been implicated in both emotional and cognitive dysregulation, which may contribute to the clinical symptoms of BD.
Clarifying the neural mechanisms that underlie the similarities and differences between acute episodes and remission would advance the neurobiological understanding of BD and potentially provide objective markers for treatment planning. Thus, the databases PubMed, Web of Science and Embase were searched for articles published before Jan 1st, 2020, for a comprehensive and systematic meta-analysis to address this issue.
Added value of this study
The current study demonstrated that BD patients had an acute-state-related functional shift toward hypoconnectivity within the affective network (AN) and trait-related abnormalities within the default-mode network (DMN), which might provide clinically useful information for targeted therapeutic interventions in BD.
Implications of the available evidence
Hypoconnectivity within the AN may be characteristic of acute episodes, reflecting deficits in mood regulation, while altered connectivity within the DMN across mood states implies trait-related cognitive impairments. Dysfunctions in large-scale functional networks in the acute state compared to the remitted state might reflect core emotional and cognitive dysfunctions in BD patients.
Alt-text: Unlabelled box
1. Introduction
Bipolar disorder (BD), a debilitating psychiatric disorder whose lifetime prevalence varies from 0.6 to 5% in different countries [1,2], is characterized by mood fluctuations between an acute episodic state of either mania or depression and a remitted or euthymic state. The treatment for BD is to stabilize the acute mood episode, with the aim of bringing the patient from mania or depression to a symptomatic recovery with normalized (stable) mood [3]. Even with ongoing psychiatric care, approximately 73% of BD patients receiving pharmacotherapy experience another acute episode of affective illness within 5 years [4]. Therefore, clarifying the distinct neural mechanisms of the acute and remitted states is particularly important in preventing recurrent episodes and keeping patients’ symptom free, as well as selecting treatments for individual patients [5,6].
Previous studies have proposed that abnormal communications in large-scale functional networks may underlie the pathophysiology of BD [7,8]. Among these studies, functional magnetic resonance imaging (fMRI) studies have revealed aberrant resting-state functional connectivity (rsFC) in the default-mode network (DMN), the frontoparietal network (FPN), the salience network (SN) and the affective network (AN) or limbic network in patients with BD compared to healthy controls (HCs) [7, 8], but these abnormalities varied depending on whether mood was controlled or not [9,10]. For instance, abnormal functional connectivity in the DMN has often been reported in BD patients during acute episodic states [11] but not during remission. This difference may reflect a normalization of DMN functional connectivity in the remitted state [12]. In addition, hypoconnectivity between the AN and the anterior DMN was found in BD patients during acute episodes [13], but hyperconnectivity between the AN and the anterior DMN [14,15] was found in clinically remitted BD patients compared to HCs. Moreover, one study directly compared FC patterns of the ANs, which consists of emotion-processing areas such as the amygdala, subgenual anterior cingulate cortex (sgACC) and ventrolateral prefrontal cortex (VLPFC), between patients in acute mood states and in the remitted state, and they found that the connectivity between the sgACC and the amygdala is critically affected during acute mood episodes, while sgACC–VLPFC coupling plays a key role in mood normalization [16]. A recent systematic review of all rsFC studies in individuals with remitted BD suggested that stability of the DMN, FPN and SN might reflect a state of remission [12], but the authors admitted that the study was limited by the heterogeneity of the analytical methods.
Taken together, these results suggest that clinically remitted BD patients do not have entirely normalized intrinsic cerebral function but may have some characteristic changes related to the next mood relapse. Network differences between different mood states may have important implications for personalized treatment and preventive strategies in BD management [17]. However, the exact neural mechanism underlying different mood states in BD patients remains unclear, and few studies have directly compared the patterns of network functions related to these different states [18]. Thus, synthesizing results from published studies will be an important way to help clarify whether those network alterations are static traits of BD or represent different states of mood control.
In the present study, we aimed to perform a meta-analysis of whole-brain seed-based resting-state functional connectivity (rsFC) studies in BD patients to synthesize the findings of each large-scale brain network and compare the intrinsic function of brain networks between active and remitted states. We applied the strategy proposed by Kaiser and his colleagues, which categorized seeds and corresponding effect regions into a priori functional network parcellations based on their coordinate locations [19]. A meta-analysis was then performed for each seed network to identify consistent patterns of functional connectivity alterations across studies. This approach has been applied to study many psychiatric disorders, including major depressive disorder [19], schizophrenia [20], obsessive–compulsive disorder [21] and early psychosis [22], but it has not yet been applied in BD. We hypothesized that BD patients in acute episodes and those in remission would display distinct patterns of abnormal functional connectivity in their large-scale brain networks.
2. Materials and methods
2.1. Search strategy
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, an online search was conducted in the databased PubMed, Web of Science and Embase for literature published before Jan 1st, 2020. The keywords used in the search were (“bipolar disorder” OR “bipolar affective disorder”) AND (“functional magnetic resonance imaging” OR “fMRI” OR “resting state” OR “functional connectivity”). We then manually searched the references of the included studies and pertinent review articles.
2.2. Study selection criteria
The inclusion criteria were as follows:
The study was an original work of peer-viewed fMRI research;
The patients included in the study were diagnosed with BD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases (ICD) criteria;
The study directly compared BD patients in different mood states with HCs;
Whole-brain seed-based rsFC analysis was performed;
The peak coordinates were reported in standard stereotaxic spaces (e.g., Montreal Neurological Institute (MNI) or Talairach).
Studies were excluded if 1) they did not differentiate patients according to whether they were in an acute mood state or a remission state; 2) they did not compare seed-based rsFC between BD and HCs at the whole-brain level; or 3) the coordinates of between-group effects could not be retrieved even by contacting the author. If two studies had overlapping samples and selected the same seed, the one with the larger sample was included in the analysis. Importantly, studies on the same samples using different seeds were considered separate datasets. Meanwhile, studies in which distinct BD groups were compared with a single HC group were coded as distinct datasets.
2.3. Data extraction
First, we classified the mood states of BD patients in each study. Patients in periods of clinical remission (euthymia) were considered the remitted group (BD in remission, BDR). Conversely, patients having acute mood episodes (mania, depression, or mixed) were coded as the acute group (BD in acute state, BDA).
Second, location information (e.g., the coordinates, a prior template, or a standard atlas) of each seed and peak coordinates of each significant between-group effect for each seed were extracted. We categorized each seed into one of seven seed networks according to its location within the a priori network parcellation [23], [24], [25], which included the DMN, AN, FPN, dorsal attention network (DAN), ventral attention network (VAN), somatomotor network (SMN) and visual network (VIS).
Third, the effects of rsFC were also categorized into two groups according to the direction of the effect: hyperconnectivity (BD patients > HC) and hypoconnectivity (BD patients < HC). Studies with null findings (i.e. those that reported no between-group differences in rsFC or whose effects did not survive statistical correction) were also included. Two investigators independently conducted the data extraction and double-checked the information, and disagreements were resolved by discussion.
2.4. Meta-analysis
The meta-analysis was performed using the anisotropic effect size version of seed-based d mapping (AES-SDM) software package v5.141 (http://www.sdmproject.com/software), which is a statistical technique for meta-analysis of neuroimaging studies based on peak coordinates of identified effects [26]. It first recreated the effect-size maps of differences between groups for each study based on the peak coordinates of the effects and statistics (T-value, Z-value or p-value) by assigning an effect size to each voxel relying on its distance to close peak coordinates [27]. Both positive and negative coordinates were reconstructed in the same map, which is important for preventing a particular voxel from erroneously appearing to have effects in opposite directions at the same time [28]. Then, individual maps from each study were combined using meta-analytic calculations, and meta-analytic maps were constructed for each seed network of the BDR and BDA groups separately. Subsequently, quantitative comparisons between the BDR and BDA groups were performed for each seed network to directly calculate the rsFC differences between the two groups.
Statistical significance was determined using standard permutation tests with an uncorrected p = 0.005 as the main threshold. This value has been suggested to ensure an optimal balance between sensitivity and specificity and to be an approximate equivalent to a corrected p value of 0.05 in AES-SDM based on empirical validation [29]. To further reduce the false positive errors, we used an additional Z-based threshold of |Z| >1 and an extent threshold of 100 voxels [30].
2.5. Sensitivity analysis and meta-regression analysis
A jackknife sensitivity analysis was conducted to evaluate the robustness and reliability of the results. This analysis repeats the main statistical analysis multiple times, excluding a different study each time. If a previous significant finding remains significant in all combinations of studies, or in at least 80% of the studies, it can be regarded as replicable.
To investigate the potential effects of relevant demographic and clinical variables, we performed meta-regression analyses with sex ratio, mean age and percentage of medicated patients as the regressors. The results were thresholded at a lower uncorrected value of p < 0.0005 as well as an extent threshold of 100 voxels to minimize the detection of false correlations [28].
2.6. Heterogeneity analysis and publication bias
Interstudy heterogeneity was assessed by converting the QH statistics (a specific Q statistic in SDM to assess inter-study heterogeneity) to Z scores. Clusters that showed significant heterogeneity and overlapped with the main results were considered heterogeneous between studies.
Funnel plots and Egger's test in AES-SDM were used to test the possibility of any publication bias [31]. Funnel plots are generated to visualize any possible publication bias, while Egger's test is a quantitative method of assessing asymmetry in the funnel plots and can therefore be used as an indicator of publication bias. Results showing p < 0.05 on Egger's test were considered to have significant publication bias.
3. Results
3.1. Included studies and sample characteristics
The search yielded 39 datasets from 30 publications with a total of 1047 BD patients, which included 554 patients in the acute mood state (405 depression, 142 mania, 7 mixed), 493 patients in the clinical remission state, and 1081 HCs. Detailed sample characteristics are shown in Table 1 in the supplement. After the seeds from each study were categorized into a priori functional networks, 22 datasets with 32 DMN seeds, 19 datasets with 25 AN seeds, 9 datasets with 9 FPN seeds, 10 datasets with 8 VAN seeds, 4 datasets with 4 SMN seeds and 3 datasets using the thalamus (a region that does not belong to any of the 7 networks) as the seed were included (see Table S1 in the online Supplement). Ultimately, studies with DMN and AN seeds were included in the quantitative meta-analysis, while studies with seeds in other networks were not subjected to meta-analysis because the datasets were insufficient to provide acceptable statistical power [32]. To make up for this shortcoming, we added the detailed findings of each study to specify the FC alterations in each state (see Table S2 in the online Supplement). A flowchart of the search strategy and study selection is shown in Fig. 1.
Table 1.
Summary of the demographic characteristics of studies included in the meta-analysis.
| Study | BD (female) | State | HCs (female) | Mean age ± SD |
Subtype | Illness duration | Medicated (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| BD | HCs | |||||||||
| Dickstein et al. 2010 [80] | 15(5) | BDR | 15 (8) | 13.7 ± 3.3 | 14.0 ± 3.1 | BD-I | – | 100% | ||
| Chai et al. 2011 [101] | 14 (5) | BDA-mania | 15 (6) | 32.7 ± 3.0 | 37.3 ± 2.4 | BD-I | – | 100% | ||
| Torrisi et al. 2013 [102] | 20 (10) | BDR | 20 (10) | 42.1 ± 11.4 | 39.8 ± 12.6 | BD-I | 22.7 ± 11 Y | 85% | ||
| Najt et al. 2013 [103] | 13 (7) | BDR | 15 (6) | 43.08 ± 11.37 | 36.13 ± 12.13 | BD-I | – | 100% | ||
| Reinke et al. 2013 [104] | 21 (9) | BDR | 20 (8) | 35.67 ± 10.68 | 36.9 ± 11.06 | NA | – | 100% | ||
| Favre et al. 2014 [105] | 20 (10) | BDR | 20 (10) | 42.0 ± 10.7 | 43.7 ± 11.1 | BD-I = 13, BD-II=5, NOS=2 | 15.6 ± 9.3 Y | 100% | ||
| Knochel et al. 2014 [106] | 21 (9) | BDR | 21 (9) | 35.67 ± 10.68 | 36.95 ± 11.10 | BD-I | 7.62 ± 5.82 Y | 100% | ||
| Anticevic et al. 2014 [107] | 40 (32) | BDR | 56 (32) | 30.2 ± 11.5 | 31.25 ± 10.3 | NA | 11.48 ± 9.1 Y | 82% | ||
| Stoddard et al. 2015 [108] | 14 (3) | BDR | 20 (11) | 14.6 ± 2.5 | 14.3 ± 2.3 | BD-I = 11, BD-II=3 | – | 71% | ||
| Li ML et al. 2015a [82] | 10 (3) | BDA-depression | 28 (12) | 30.90 ± 8.94 | 31.05 ± 7.53 | BD-I = 7, BD-II=3 | 79.80 ± 78.59 M | 100% | ||
| Li ML et al. 2015b [82] | 18 (8) | BDA-mania | 28 (12) | 31.67 ± 6.98 | BD-I = 16, BD-II=2 | 62.89 ± 74.24 M | 100% | |||
| Magioncalda et al. 2015 [46] | 40 (27) | BDA-depression=11, BDA-mania=11, BDA-mixed=7, BDR=11 | 40 (26) | 44.6 ± 11.8 | 43.9 ± 12.8 | BD-I | 20 ± 11.4 M | 97.5% | ||
| Oertel-Knöchel et al. 2015 [109] | 21 (9) | BDR | 20 (8) | 35.67 ± 10.68 | 36.90 ± 11.06 | BD-I = 21 | 7.62 ± 5.82 Y | 100% | ||
| Singh et al. 2015 [61] | 20 (11) | BDA-mania | 23 (14) | 17.21 ± 1.89 | 16.86 ± 1.43 | BD-I = 20 | – | 100% | ||
| Lui et al. 2015 [110] | 57 (39) | BDR | 59 (33) | 34 ± 13 | 38 ± 17 | NA | 16.89 ± 12.71 Y | 100% | ||
| Li ct et al. 2015 [79] | 20 (6) | BDR | 20 (7) | 41.6 ± 11.3 | 41.8 ± 10.6 | BD-I = 20 | 16.1 ± 10.3 Y | 100% | ||
| Martino et al. 2016a [81] | 21 (18) | BDA-mania | 42 (27) | 45.6 ± 11.8 | 44.3 ± 12.7 | BD-I | 20.9 ± 14.6 Y | 99% | ||
| Martino et al. 2016b [81] | 20 (13) | BDA-depression | 42 (27) | 44.9 ± 10.9 | 44.3 ± 12.7 | BD-I | 19.5 ± 10.8 Y | 100% | ||
| Martino et al. 2016c [81] | 20 (12) | BDR | 42 (27) | 43.1 ± 11 | 44.3 ± 12.7 | BD-I | 18.2 ± 9 Y | 99% | ||
| Lv et al. 2016a [111] | 23 (13) | BDA-depression | 28 (15) | 26.17 ± 4.42 | 24.82 ± 6.62 | NA | 53.81 ± 45.37 M | 86.95% | ||
| Lv et al. 2016b [111] | 19 (9) | BDR | 28 (15) | 27.79 ± 6.71 | 24.82 ± 6.62 | NA | 65.33 ± 55.59 M | 100% | ||
| Altinay et al. 2016a [83] | 30 (17) | BDA-depression | 30 (18) | 34 ± 11 | 31 ± 10 | BD-I = 12, BD-II=18 | 35 ± 30 W | 0% | ||
| Altinay et al. 2016b [83] | 30 (19) | BDA-mania | 30 (18) | 33 ± 11 | 31 ± 10 | BD-I = 16, BD-II=14 | 25 ± 32 W | 0% | ||
| Brady et al. 2016a [13] | 28 (8) | BDA-mania | 23 (7) | 27.5 ± 10.7 | 29.7 ± 10.9 | BD-I | – | 89% | ||
| Brady et al. 2016b [13] | 24 (8) | BDR | 23 (7) | 30.9 ± 11.9 | 29.7 ± 10.9 | BD-I | – | 95.8% | ||
| Ambrosi et al. 2017 [14] | 36 (20) | BDA-depression | 40 (16) | 31.0 ± 11.3 | 35.5 ± 14.4 | BD-1, BD-II | – | 100% | ||
| Li J et al. 2017 [112] | 46 (18) | BDR | 66 (30) | 31.5 ± 9.7 | 31.6 ± 9.4 | NA | 55.58 ± 60.5 M | 78.26% | ||
| Minuzzi et al. 2017 [76] | 32 (32) | BDR | 36 (36) | 29.0 ± 8.07 | 32.8 ± 8.32 | BD-I = 18, BD-II=14 | 18.63 ± 6.8 Y | 100% | ||
| Chen LX et al. 2018 [15] | 43 (26) | BDA-depression | 47 (25) | 27.9 ± 9.1 | 29.7 | BD-II=43 | 34.2 ± 54.8 M | 0% | ||
| Gong et al. 2018 [47] | 96 (44) | BDA-depression | 100 (55) | 27.33 ± 9.2 | 29.32 ± 9.01 | BD-II=96 | 47.84 ± 61.05 M | 44.79% | ||
| Whittaker et al. 2018 [113] | 35 (22) | BDR | 23 (14) | 44.71 ± 5.51 | 44.00 ± 4.48 | BD-I = 16, BD-II=19 | – | 88.6% | ||
| Li GZ et al. 2018 [114] | 19 (9) | BDR | 25 (10) | 38.79 ± 12.03 | 33.40 ± 8.21 | BD-I = 6, BD-II=13 | 3.95 ± 3.30 Y | 100% | ||
| Wang et al. 2018 [115] | 25 (16) | BDR | 25 (17) | 28.55 ± 9.76 | 28.65 ± 9.66 | BD-II=25 | 40.20 ± 44.86 M | 100% | ||
| Yin et al. 2018 [77] | 21 (11) | BDA-depression | 70 (39) | 29.29 ± 8.35 | 29.36 ± 8.082 | NA | NA | 42.86% | ||
| Chen GM et al. 2019 [85] | 90 (42) | BDA-depression | 100 (55) | 26.74 ± 8.73 | 28.32 ± 0.01 | BD-II=90 | 48 ± 61.54 M | 0% | ||
| He et al. 2019 [84] | 25 (12) | BDA-depression | 34 (18) | 34.28 ± 8.65 | 33.53 ± 11.08 | BD-1 = 14, BD-II=11 | – | 84% | ||
| Total | 1047 | 1081 | ||||||||
Abbreviations: bipolar disorder (BD), healthy controls (HCs), standard deviation (SD), not available (NA), BD in remission (BDR), BD in acute state (BDA), not otherwise specified (NOS).
Fig. 1.
Flowchart of the research strategy and literature selection. Abbreviations: bipolar disorder (BD); healthy controls (HC); affective network (AN); default mode network (DMN); regional homogeneity (ReHo); amplitude of low-frequency fluctuation (ALFF); fractional ALFF (fALFF); independent component analysis (ICA).
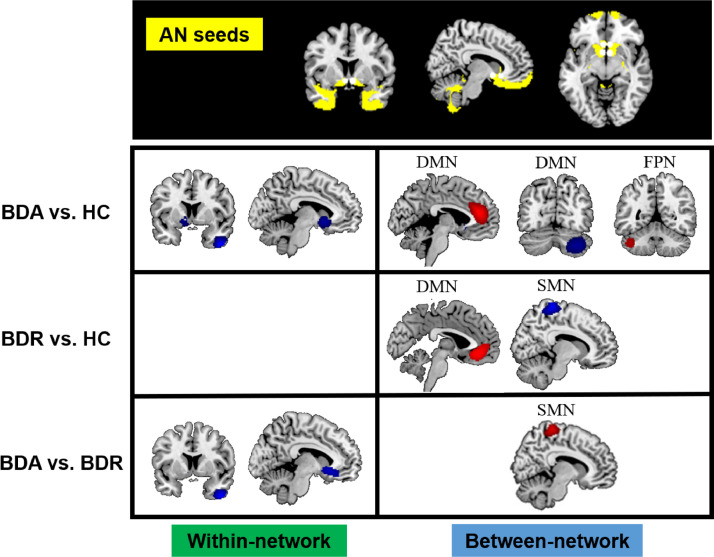
3.2. Abnormal connectivity within the AN
Hypoconnectivity was found between the AN seeds and the right inferior temporal gyrus (ITG) and the left subgenual anterior cingulate cortex (sgACC) in BDA patients relative to HCs (see Table 2 and Fig. 2). However, there was no altered within-AN connectivity in BDR patients compared with HCs. Meanwhile, direct comparison between the active and remitted groups revealed hypoconnectivity within the AN (located in the right ITG and the left sgACC) in the BDA patients.
Table 2.
Results of meta-analysis of altered AN resting-state functional connectivity in BD patients compared with HCs.
| Seed network | Seed region | Effect network | Effect region | MNI coordinates | SDM-Z | P value | Voxels |
|---|---|---|---|---|---|---|---|
| AN | amygdala, sgACC, OFC, ventral striatum | ||||||
| BDA vs. HC (datasets=10) | |||||||
| BDA > HC | DMN | Left dACC extending to DMPFC | −4, 40, 18 | 1.574 | <0.0001 | 1508 | |
| FPN | Left cerebellum | −44, −52, −38 | 1.072 | 0.0016 | 439 | ||
| DMN and FPN | Right cerebellum | 42, −66, −34 | −1.239 | 0.0010 | 1883 | ||
| AN | Right ITG | 44, 6, −44 | −2.082 | <0.0001 | 517 | ||
| AN | Left NAcc extending to OFC, sgACC | −12, 14, −4 | −1.512 | 0.0005 | 443 | ||
| BDR vs. HC (datasets=9) | |||||||
| BDR > HC | DMN | Left rACC extending to VMPFC | −2, 36, −8 | 1.149 | 0.0005 | 899 | |
| BDR < HC | SMN | Right SMA | 12, −36, 72 | −2.474 | <0.0001 | 1067 | |
| BDA vs. BDR | |||||||
| BDA > BDR | SMN | Right SMA | 12, −36, 72 | 1.895 | <0.0001 | 887 | |
| BDA < BDR | AN | Left NAcc extending to OFC, sgACC | −12, 16, −6 | −1.250 | 0.0002 | 506 | |
| AN | Right ITG | 44, 6, −44 | −1.454 | <0.0001 | 364 | ||
Abbreviations: bipolar disorder (BD), BD in remission (BDR), BD in acute state (BDA), healthy controls (HC), default-mode network (DMN), affective network (AN), frontoparietal network (FPN), somatomotor network (SMN), dorsal attention network (DAN), superior frontal gyrus (SFG), orbitofrontal cortex (OFC), medial prefrontal cortex (MPFC), dorsolateral prefrontal cortex (DLPFC), dorsomedial prefrontal cortex (DMPFC), rostral anterior cingulate cortex (rACC), ventromedial prefrontal cortex (VMPFC), ventrolateral prefrontal cortex (VLPFC), anterior cingulate cortex (ACC), subgenual anterior cingulate cortex (sgACC), posterior cingulate cortex (PCC), nucleus accumbens (NAcc), hippocampus (HIPP), parahippocampus (Para-HIPP), superior temporal gyrus (STG), middle temporal gyrus (MTG), supplementary motor area (SMA).
Fig. 2.
Results of meta-analysis of altered resting-state functional connectivity for the affective network (AN) in patients with bipolar disorder (BD) compared with the healthy control (HC) group. The top line shows seeds (indicated by white dots) located in the a priori AN mask (yellow). The second-to-last line separately illustrates patients with BD in the acute state (BDA) relative to the HC group, patients with BD in remission (BDR) relative to the HC group and a comparison between BDA (vs. HC) and BDR (vs. HC). Red refers to hyperconnectivity (BD>HC), and blue refers to hypoconnectivity (BD<HC).
3.3. Abnormal connectivity between the AN and regions of the DMN, FPN, SMN
Hyperconnectivity was found between the AN seeds and regions of the ventromedial prefrontal cortex (VMPFC) and the dorsal medial prefrontal cortex (DMPFC) in the DMN in BDA and BDR patients relative to HCs (see Table 2 and Fig. 2). Both hyperconnectivity and hypoconnectivity were found between the AN seeds and the left and right regions of the cerebellum, spreading across the DMN and the FPN, respectively, in the BDA patients. In addition, hypoconnectivity was found between the AN seeds and areas of the supplementary motor area (SMA) in the SMN in the BDR group; however, in a direct comparison between the acute and remitted groups, hyperconnectivity was found between the AN seeds and regions of the SMA in the BDA group.
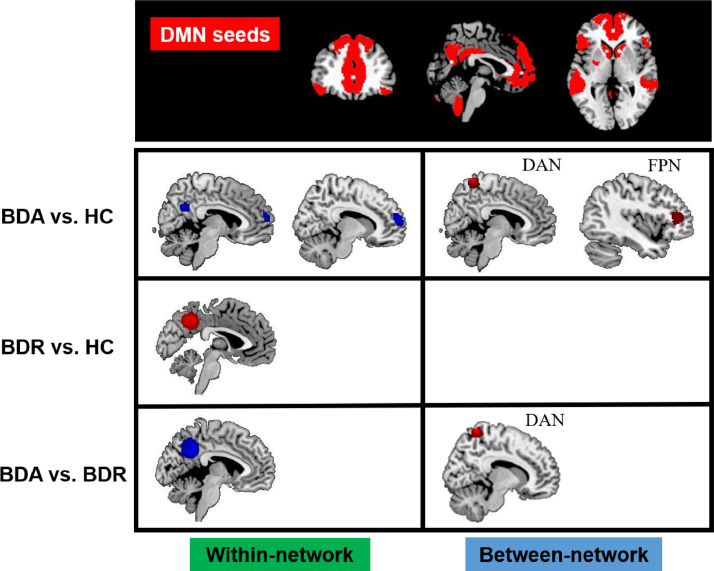
3.4. Abnormal connectivity within the DMN
BD patients in acute mood episodes showed hypoconnectivity between the DMN seeds and regions of the posterior cingulate cortex (PCC) and medial prefrontal cortex (MPFC) compared with HCs (see Table 3 and Fig. 3). In contrast, compared with HCs, BD patients in clinical remission showed hyperconnectivity between the DMN seeds and regions of the PCC relative to HCs. When comparing acute and remitted groups directly, we found that BDA patients exhibited within-DMN hypoconnectivity relative to BDR patients, also peaking in the PCC.
Table 3.
Results of meta-analysis of altered DMN resting-state functional connectivity in BD patients compared with HCs.
| Seed network | Seed region | Effect network | Effect region | MNI coordinate | SDM-Z | P value | Voxels |
|---|---|---|---|---|---|---|---|
| DMN | ACC, PCC, MPFC, MTG, VLPFC, HIPP, VSS, caudate, cerebellum, precuneus | ||||||
| BDA vs. HC (datasets=14) | |||||||
| BDA > HC | FPN | Left DLPFC | −34, 38, 12 | 1.659 | 0.0002 | 408 | |
| DAN | Right dorsal-anterior precuneus | 8, −52, 72 | 2.001 | <0.0001 | 435 | ||
| BDA < HC | DMN | Right PCC/precuneus | 4, −54, 28 | −1.705 | 0.0002 | 188 | |
| DMN | Right DMPFC/SFG | 16, 62, 16 | −2.259 | 0.0003 | 545 | ||
| BDR vs. HC (datasets=8) | |||||||
| BDR>HC | DMN | Left PCC/precuneus | −10, −54, 38 | 2.585 | 0.0002 | 885 | |
| BDA vs. BDR | |||||||
| BDA > BDR | DAN | Right dorsal-anterior precuneus | 8, −52, 72 | 1.042 | <0.0001 | 385 | |
| BDA < BDR | DMN | Left PCC/precuneus | −10, −54, 38 | −2.473 | <0.0001 | 1428 | |
Abbreviations: bipolar disorder (BD), BD in remission (BDR), BD in acute state (BDA), healthy controls (HC), default-mode network (DMN), affective network (AN), frontoparietal network (FPN), somatomotor network (SMN), dorsal attention network (DAN), superior frontal gyrus (SFG), orbitofrontal cortex (OFC), medial prefrontal cortex (MPFC), dorsolateral prefrontal cortex (DLPFC), dorsomedial prefrontal cortex (DMPFC), rostral anterior cingulate cortex (rACC), ventromedial prefrontal cortex (VMPFC), ventrolateral prefrontal cortex (VLPFC), anterior cingulate cortex (ACC), subgenual anterior cingulate cortex (sgACC), posterior cingulate cortex (PCC), nucleus accumbens (NAcc), hippocampus (HIPP), parahippocampus (Para-HIPP), superior temporal gyrus (STG), middle temporal gyrus (MTG), supplementary motor area (SMA).
Fig. 3.
Results of meta-analysis of altered resting-state functional connectivity for the default mode network (DMN) in patients with bipolar disorder (BD) compared with the healthy control (HC) group. The top line shows seeds (indicated by white dots) located within the a priori DMN mask (red). The second-to-last line separately illustrates patients with BD in the acute state (BDA) relative to the HC group, patients with BD in remission (BDR) relative to the HC group and a comparison between BDA (vs. HC) and BDR (vs. HC). Red refers to hyperconnectivity (BD>HC), and blue refers to hypoconnectivity (BD<HC).
3.5. Abnormal connectivity between the DMN and regions of the DAN and FPN
BD patients in acute episodes showed hyperconnectivity between the DMN seeds and regions of the right dorsal-anterior precuneus in the DAN as well as regions of the left dorsolateral prefrontal cortex (DLPFC) in the FPN (see Table 3 and Fig. 3). However, no altered between-network connectivity was observed in the BDR patients relative to HCs. When the acute and remitted groups were compared directly, hyperconnectivity was found between the DMN seeds and the dorsal-anterior precuneus in acute BD patients relative to remitted patients.
3.6. Sensitivity analysis and meta-regression analysis
The jackknife sensitivity analysis revealed that the result in the right SMA remained in all combinations of datasets. All the other results remained in all but one or two combinations of datasets. Details on the results of the sensitivity analysis are listed in Tables S4–7 (online Supplement).
Linear regression analyses showed that the mean age, the percentage of female patients and the percentage of medicated patients were not associated with BD-related rsFC changes in either the remitted group or the acute group.
3.7. Heterogeneity analysis and publication bias
There was no significant between-group heterogeneity observed in the results for AN and DMN data.
None of the clusters reported above showed significant publication bias based on Egger's test (p > 0.05) except the right DMPFC/SFG (Egger's test p = 0.042). Funnel plots are presented in Fig. S1-12.
4. Discussion
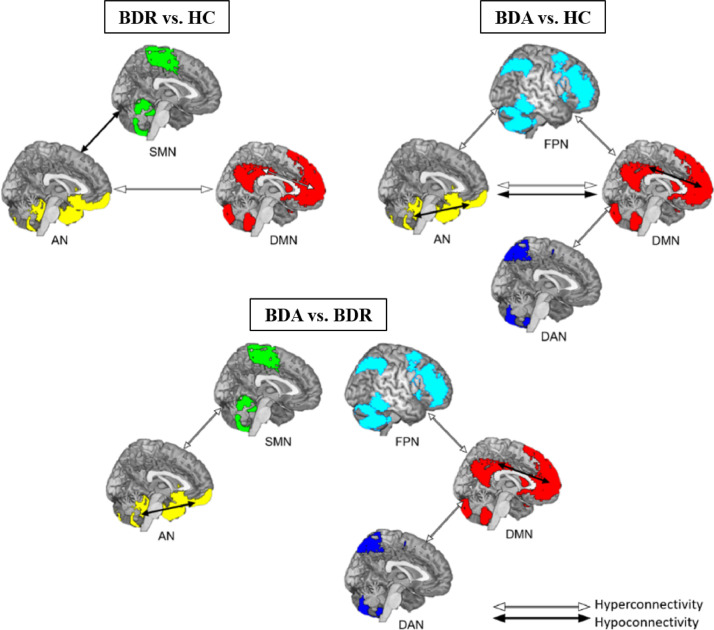
This study provided the first meta-analytic evidence of abnormal rsFC within and between several functional brain networks in BD patients in the acute episodic state of illness and during clinical remission. We found that hypoconnectivity within the AN existed in the acute episodic state but not in the remitted state. Similar hypoconnectivity within the DMN was observed in acute episodes, but it was replaced by hyperconnectivity in the remitted state. Additionally, we observed different between-network dysconnectivity patterns between these two groups; the patterns are summarized in Fig. 4. These observations indicate a functional shift of the AN and DMN between the acute and remitted states; this shift appears to be involved in dysfunctions of emotion processing and cognitive regulation and may provide clinically useful information for targeted therapeutic interventions in BD.
Fig. 4.
Dissociated abnormalities in large-scale brain networks between acute and remitted patients with bipolar disorder (BD). Acute-state-related hypoconnectivity within the AN and DMN and AN–DMN hyperconnectivity might reflect dysregulated emotional processing and cognition in BD patients during the active phase. However, there is also remitted-state-related hyperconnectivity within the DMN and between the AN and DMN, which may underlie abnormal cognitive regulation during remission. Both findings indicate that abnormal emotional processing is a state-related impairment that is evident in acutely ill patients but normalized with remission. Cognitive dysregulation is a trait-related impairment in BD patients that is common in both acute and remission states. BDA, BD in acute state; BDR, BD in remission; HC, healthy control.
4.1. Abnormalities within the AN
Reduced rsFC was observed in the AN, encompassing seed regions such as the amygdala, sgACC and striatum and effect regions including the right ITG, the left nucleus accumbens (NAcc), the sgACC and the orbitofrontal cortex (OFC), which are involved in emotion processing. These limbic regions have strong anatomical and functional connections and form circuits that regulate mood and emotion as well as the response of the amygdala to environmental stress [33]. This finding supports the theoretical model that BD is closely related to emotional dysregulation. Interestingly, these abnormalities were apparent only in the acutely ill state. Many studies have reported that amygdala responses to emotional stimuli are increased in BD during the acute state [34,35]. However, these studies have not found such activity increases in individuals with BD during remission. In addition, some studies have suggested that prefrontal cortex (PFC) activity during remission may compensate for or reduce amygdala overactivation to maintain attentional and memory performance [36,37]. Moreover, previous network-based studies also revealed abnormal connectivity within the affective-related network in acutely ill bipolar patients [38,39] but found no differences in the rsFC of the AN between remitted BD patients and controls [40,41]. Thus, our finding of hypoconnectivity within the AN during acute illness compared to both BDR patients and HCs supports the hypothesis of disruption in the neural emotion regulatory model in symptomatic episodes in BD [32,36,42,43]. Meanwhile, normalized FC within the AN in BDR patients compared to HCs and increased functional connection of AN modules in BDR patients compared to acutely ill patients may contribute to the improved function of the network and the improved emotion processing observed during remission [44].
4.2. Abnormalities within the DMN
Connectivity between elements of the DMN is increased in BDR patients (vs. HCs) and, in the case of midline cortical structures, is decreased during acute episodes of BD (vs. both HCs and BDR patients). Imbalanced connectivity in this circuitry might represent a mood-state-dependent abnormality across acute/remitted states [8,17,45]. Previous studies found that the hypoconnectivity between the anterior and posterior DMN in manic BD patients versus HCs might be related to an attention pattern that is excessively focused on external stimuli at the expense of internal reflection [46]. In contrast, the hypoconnectivity within the posterior DMN in depressive BD patients versus HCs might be related to rumination and working memory impairment [47]. Hyperconnectivity between the anterior DMN and posterior DMN in BDR compared to HCs might indicate heightened planning in relation to the visual environment; it may also predispose these patients to relapse or new episodes and may help to explain why psychotherapies, such as mindfulness, are effective in BDR patients [48]. However, previous studies using independent component analysis found no differences in the rsFC of the DMN between BD patients during clinical remission and HC [40, 49] or even hypoconnectivity in remitted BD patients [39,50]. This inconsistency might arise from the heterogeneity of samples, such as different BD types or histories of psychosis. Abnormalities within the DMN were common but inverted between acute and remitted states, which implies that impairment of the DMN is a trait in this disorder and affects individuals with BD differentially during different mood states.
4.3. Abnormalities between the AN and the DMN
The present meta-analysis also revealed hyperconnectivity in BD patients between the AN and regions located in the MPFC and ACC. Previous studies have indicated that emotional and cognitive processing are principally modulated by ventral and dorsal medial prefrontal regions [51], respectively, and the reciprocal interaction between these regions may be modulated by the ACC [52], [53], [54]. During remission, the rostral ACC (rACC) may be overactivated [55], whereas the dorsal ACC (dACC) is underactivated [56], indicating a possible dissociation within the ACC related to the control of an affective and cognitive activity or persistent/residual illness. We observed hyperconnectivity in the BDR between the rACC/VMPFC and the AN as well as hyperconnectivity in acutely ill patients between the dACC/DMPFC and the AN compared to HCs. The former hyperconnectivity pattern might be associated with increased top-down processes in emotion regulation at rest during remission [44, 45, 57, 58]. However, the latter hyperconnectivity pattern might relate to attention toward self-referential or introspectively oriented mental activity [51,[59], [60], [61], [62], [63]] or increased reward processing [58,61,64,65] during acute states of BD.
4.4. Abnormalities between the AN and the cerebellum
Our study also provides evidence for imbalanced connectivity between AN and regions in the cerebellum supporting cognitive control or emotion. Lobules VI, VII, VIII of the posterior lobe, as well as the crus, have been linked to cognition; the posterior vermis has been associated with emotion; and lobules I–V have been related to sensorimotor function [66]. The cerebellum has anatomical connections to the limbic system, which may underlie its role in affective processes [67], [68], [69]. The role of the cerebellum, particularly the vermis, in emotional behaviors is increasingly recognized, and individuals with cerebellar lesions have been noted to have mood symptoms [70]. Thus, altered input from the cerebellum to the AN in acute BD (involving the DMN and FPN) may contribute to cognitive and affective dysregulation during acute episode BD.
4.5. Abnormalities between the DMN and networks involved in cognitive regulation
Although mixed, the present meta-analysis also demonstrated hyperconnectivity between brain systems involved in self-referential and frontal parietal systems involved cognition and execution, also known as task-positive networks (TPNs). These regions, such as the DLPFC, are preferentially active during tasks that demand external attention [71]. TPN activity typically has an inverse relation to DMN activity, reflecting the switching between directed activity/planning and reflective activity at rest [72]. Previous studies have proposed that the ventral precuneus is part of the DMN, while the dorsal-anterior precuneus is part of the DAN [73], [74], [75]. Moreover, Zhang et al. reported that the dorsal-anterior precuneus is relevant to the attentional monitoring of spatial behavior [75]. Thus, hyperconnectivity between the DMN and the left DLPFC in acute BD and hyperconnectivity between the DMN and dorsal-anterior precuneus might be related to compensatory cognitive activity aimed at restraining affect and behavior, which may be impairments specific to acute illness.
4.6. Abnormalities in the FPN, VAN and SMN
Meta-analyses of the FPN, VAN, DAN, SMN and VIS were not performed due to insufficient data in the original studies, so we narratively reviewed the findings of these studies. No study chose seeds in the DAN and VIS, suggesting a bias in seed selection across the existing rsFC studies. Hypoconnectivity between the VAN seeds (insula, putamen) and regions of the SMN (somatosensory cortex, superior temporal gyrus) was found both in the BDR and BDA groups [76,77]. Altered functional connectivity between FPN seeds (DLPFC, SFG) and regions of the AN [78,79] and SMN [80] was observed in the BDR patients, but whether these abnormalities existed in the BDA patients needs to be further clarified.
4.7. Narrative review of abnormalities in manic and depressed states
In our included studies, four studies analyzed BD mania (BDM) or BD depression (BDD) separately [46,[81], [82], [83]]. Altinay M et al. [83] found that two caudate regions showed uniquely increased FC in BDM and two putamen regions showed uniquely increased FC in BDD; these changes are likely to be state-related changes. However, several common FC abnormalities, which might be trait-related, were detected in both the BDM and BDD patients compared to the HCs. In one study, Martino M and Magioncalda P [81] et al. found decreased FC between the anterior and posterior parts of the cingulum in BDM patients compared to both BDD patients and HCs. In another study [46], different FC abnormalities were shown between BDM and BDD patients, and these FC findings during mania and depression correlated with the severity of the manic and depressive symptoms, respectively. In Li's study [82], both BDM and BDD patients showed similar amygdala FC reductions, but subtle opposing FC abnormalities were found between BDM and BDD patients. We visually inspected the findings from other studies referring to BDM or BDD only, and similar [83,84] and different [83,85] network dysfunctions were reported (with the exception of opposing results) between the two states. There are also studies investigated functional abnormalities in different phases of BDA using other measures such as global signal topography, which showed distinct and in some cases opposing network abnormalities in BDM and BDD [86,87]. However, there are also a number of studies uncovered many similar findings between BDM and BDD [88], [89], [90]. Limited by insufficient datasets for BDM and BDD, we were not able to conduct separate analyses for the two episodes in a meta-analytic way. Our findings regarding BDA are the merged results of both mania and depression, so different alterations between mania and depression may be obscured or missed. Future studies specifically detecting FC abnormalities in both BDM and BDD are needed to discover biomarkers of active episodes of illness.
4.8. Medication effects
A major consideration is that the majority of studies included in the current meta-analysis (20 out of 23) recruited medicated individuals with BD, such that subgroup analysis of medication-naïve patients could not be conducted given the small number of studies in this category (n = 3); therefore, interpretation specifically with respect to bipolar pathophysiology is challenging. However, we conducted a meta-regression analysis between the percentage of medicated patients and rsFC changes in this study, and no correlation was shown. Medication seemed to normalize abnormal fMRI changes rather than cause spurious results in BD patients [91].
We found hypoconnectivity between the SMN and the AN in remitted patients compared to both acutely ill patients and HCs, which might be an effect of medication that causes a reduction in FC between the amygdala and posterior and premotor areas [44]. In contrast to the amygdala, which is involved in motivation-related aspects of emotion recognition [92], [93], [94], the right SMA has been linked to emotion recognition processes that entail reliance on internal representation of body states [62,63,95,96]. Notably, Kanske et al. [97] observed increased activity in the SMA during an emotion regulation task that was negatively coupled with amygdala activity in healthy adults. Aron et al. [98] proposed that the SMA, DLPFC, and striatum comprise a network mediating motor and cognitive inhibition, which is an area of dysfunction in BD [99]. On the other hand, atrophy of the postcentral gyrus [76] in BDR patients (versus HCs) might also result in a reduced FC. Both factors lead to impairment of motor and cognitive inhibition [99] and a disruption in internal representation of body states in BDR patients [62,63,95,96]. Future studies will be needed to confirm whether medication mediates these rsFC abnormalities in BD patients.
4.9. Limitations and future directions
It is important to consider the limitations of this study when interpreting the findings. First, an important question unanswered by the current meta-analysis is how rsFC abnormalities differ between during manic and depressive episodes. The insufficient primary data of the included studies limited our analysis of this issue. In addition, DMN hyperconnectivity could be also related to residual depressive symptoms (more so than to residual manic symptoms) during euthymia [100]. Given this information, future longitudinal studies are needed to understand the evolution of network functioning and symptomology across states of depression, mania, and euthymia in BD. Moreover, the correlations between abnormalities and depression/mania symptoms should be analyzed to understand which abnormalities are mood-state non-specific and which are emotional valance related. Second, the present meta-analysis focused entirely on whole-brain seed-based rsFC studies, which do not cover findings from alternative analytic methods, such as edge-based statistics and independent component analysis. Since relatively few prior studies have implemented these methods with BD samples, it was not possible to implement these alternative methods for meta-analytic purposes. Third, the effects of head motion, physiological influences, and arousal in the scanner may substantially affect the results. Unfortunately, it was not possible to test the moderating effects of such variables (Table S3 in the online Supplement), which merit future investigation. Fourth, almost all the patients in our sample were undergoing pharmacotherapy. Thus, it is unclear to what extent these rsFC abnormalities were related to acute or chronic treatment effects. In addition, the numbers of studies for medicated and unmediated patients and the subtypes of BD (BD-I and BD-II) were not sufficient for separate analysis.
5. Conclusion
In summary, our findings highlight the different patterns of intrinsic function in large-scale brain networks between acute and remitted patients with BD, which were associated with distinct emotional and cognitive characteristics between the two states. The function of the affective network is likely to depend on the patient's emotional state, while the DMN plays a regulatory role throughout the disease course. Our findings provide clinically useful information for the development of alternative treatment strategies for BD patients during active episodes or remission.
Declaration competing of interest
The authors report no potential conflicts of interest.
Acknowledgments
Acknowledgments
The authors would like to thank their tutors and colleagues for providing valuable help.
Funding sources
This study was supported by grants from the National Natural Science Foundation of China (81171488, 81671669 and 81820108018) and by a Sichuan Provincial Youth Grant (2017JQ0001). The funding sources had no involvement in the study design, collection, analysis, or interpretation of data.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102742.
Appendix. Supplementary materials
References
- 1.Merikangas K., Akiskal H.J., Greenberg P., Hirschfeld R., Petukhova M., Kessler RJAoGP. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. 2007;64(5):543–52. [DOI] [PMC free article] [PubMed]
- 2.Huang Y., Wang Y., Wang H., Liu Z., Yu X., Yan J. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi: 10.1016/S2215-0366(18)30511-X. [DOI] [PubMed] [Google Scholar]
- 3.Geddes J.R., Miklowitz D.J. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672–1682. doi: 10.1016/S0140-6736(13)60857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gitlin M.J., Swendsen J., Heller T.L., Hammen C. Relapse and impairment in bipolar disorder. Am J Psychiatry. 1995;152(11):1635–1640. doi: 10.1176/ajp.152.11.1635. [DOI] [PubMed] [Google Scholar]
- 5.Viktorin A., Ryden E., Thase M.E., Chang Z., Lundholm C., D'Onofrio B.M. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341–348. doi: 10.1176/appi.ajp.2016.16040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abé C., Liberg B., Song J. Longitudinal cortical thickness changes in bipolar disorder and the relationship to genetic risk, mania, and lithium use. Biol Psychiatry. 2020;87(3):271–281. doi: 10.1016/j.biopsych.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Sha Z., Wager T.D., Mechelli A., He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry. 2019;85(5):379–388. doi: 10.1016/j.biopsych.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Perry A., Roberts G., Mitchell P.B., Breakspear M. Connectomics of bipolar disorder: a critical review, and evidence for dynamic instabilities within interoceptive networks. Mol Psychiatry. 2019;24(9):1296–1318. doi: 10.1038/s41380-018-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chase H.W., Phillips M.L. Elucidating neural network functional connectivity abnormalities in bipolar disorder: toward a harmonized methodological approach. Biol Psychiatry Cogn Neurosci Neuroimag. 2016;1(3):288–298. doi: 10.1016/j.bpsc.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pomarol-Clotet E., Alonso-Lana S., Moro N., Sarro S., Bonnin M.C., Goikolea J.M. Brain functional changes across the different phases of bipolar disorder. Br J Psychiatry. 2015;206(2):136–144. doi: 10.1192/bjp.bp.114.152033. [DOI] [PubMed] [Google Scholar]
- 11.Vargas C., López-Jaramillo C., Vieta E. A systematic literature review of resting state network–functional MRI in bipolar disorder. J Affect Disord. 2013;150(3):727–735. doi: 10.1016/j.jad.2013.05.083. [DOI] [PubMed] [Google Scholar]
- 12.Syan S.K., Smith M., Frey B.N. Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review. J Psychiatry Neurosci. 2018;43(5):298–316. doi: 10.1503/jpn.170175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady R.O., Masters G.A., Mathew I.T. State dependent cortico-amygdala circuit dysfunction in bipolar disorder. J Affect Disord. 2016;201:79–87. doi: 10.1016/j.jad.2016.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosi E., Arciniegas D.B., Madan A. Insula and amygdala resting-state functional connectivity differentiate bipolar from unipolar depression. Acta Psychiatr Scand. 2017;136(1):129–139. doi: 10.1111/acps.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L., Wang Y., Niu C. Common and distinct abnormal frontal-limbic system structural and functional patterns in patients with major depression and bipolar disorder. Neuroimage Clin. 2018;20:42–50. doi: 10.1016/j.nicl.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rey G., Piguet C., Benders A., Favre S., Eickhoff S.B., Aubry J.M. Resting-state functional connectivity of emotion regulation networks in euthymic and non-euthymic bipolar disorder patients. Eur Psychiatry. 2016;34:56–63. doi: 10.1016/j.eurpsy.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Phillips M.L., Swartz H.A. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829–843. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaffer J.J., Jr, Johnson C.P., Fiedorowicz J.G., Christensen G.E., Wemmie J.A., Magnotta V.A. Impaired sensory processing measured by functional MRI in bipolar disorder manic and depressed mood states. Brain Imaging Behav. 2018;12(3):837–847. doi: 10.1007/s11682-017-9741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-Scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong D., Wang Y., Chang X., Luo C., Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44(1):168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gursel D.A., Avram M., Sorg C., Brandl F., Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev. 2018;87:151–160. doi: 10.1016/j.neubiorev.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 22.O'Neill A., Mechelli A., Bhattacharyya S. Dysconnectivity of large-scale functional networks in early psychosis: a meta-analysis. Schizophr Bull. 2019;45(3):579–590. doi: 10.1093/schbul/sby094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeo B.T., Krienen F.M., Sepulcre J. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi E.Y., Yeo B.T., Buckner R.L. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108(8):2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radua J., Mataix-Cols D. Meta-analytic methods for neuroimaging data explained. Biol Mood Anxiety Disord. 2012;2:6. doi: 10.1186/2045-5380-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radua J., Rubia K., Canales-Rodriguez E.J., Pomarol-Clotet E., Fusar-Poli P., Mataix-Cols D. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radua J., Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195(5):393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 29.Radua J., Mataix-Cols D., Phillips M.L. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiat. 2012;27(8):605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Tang S., Lu L., Zhang L. Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: a comparative meta-analysis. EBioMedicine. 2018;36:436–445. doi: 10.1016/j.ebiom.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egger M., Smith G.D., Phillips A.N. Meta-analysis: principles and procedures. BMJ. 315(7121):1533–7. [DOI] [PMC free article] [PubMed]
- 32.Carlisi C.O., Norman L.J., Lukito S.S., Radua J., Mataix-Cols D., Rubia K. Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol Psychiatry. 2017;82(2):83–102. doi: 10.1016/j.biopsych.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Marchand W.R. Self-referential thinking, suicide, and function of the cortical midline structures and striatum in mood disorders: possible implications for treatment studies of mindfulness-based interventions for bipolar depression. Depress Res Treat. 2012;2012 doi: 10.1155/2012/246725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida J.R., Versace A., Hassel S., Kupfer D.J., Phillips M.L. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010;67(5):414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foland-Ross L.C., Bookheimer S.Y., Lieberman M.D. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage. 2012;59(1):738–744. doi: 10.1016/j.neuroimage.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strakowski S.M., Adler C.M., Holland S.K., Mills N., DelBello M.P. A preliminary fMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29(9):1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 37.Pavuluri M.N., O'Connor M.M., Harral E., Sweeney J.A. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62(2):158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Meda S.A., Gill A., Stevens M.C., Lorenzoni R.P., Glahn D.C., Calhoun V.D. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khadka S., Meda S.A., Stevens M.C., Glahn D.C., Calhoun V.D., Sweeney J.A. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74:458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lois G., Linke J., Wessa M. Altered functional connectivity between emotional and cognitive resting state networks in euthymic bipolar i disorder patients. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0107829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Syan S.K., Minuzzi L., Smith M. Resting state functional connectivity in women with bipolar disorder during clinical remission. Bipolar Disord. 2017;19:97–106. doi: 10.1111/bdi.12469. [DOI] [PubMed] [Google Scholar]
- 42.Cummings J.L. Frontal-subcortical circuits and human behavior. J Psychosom Res. 1998;44(6):627–628. doi: 10.1016/s0022-3999(98)00034-8. [DOI] [PubMed] [Google Scholar]
- 43.Teng S., Lu C.F., Wang P.S., Li C.T., Tu P.C., Hung C.I. Altered resting-state functional connectivity of striatal-thalamic circuit in bipolar disorder. PLoS ONE. 2014;9:e96422. doi: 10.1371/journal.pone.0096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blumberg H.P., Donegan N.H., Sanislow C.A. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005;183(3):308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 45.Blond B.N., Fredericks C.A., Blumberg H.P. Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar Disord. 2012;14(4):340–355. doi: 10.1111/j.1399-5618.2012.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magioncalda P., Martino M., Conio B. Functional connectivity and neuronal variability of resting state activity in bipolar disorder–reduction and decoupling in anterior cortical midline structures. Hum Brain Mapp. 2015;36(2):666–682. doi: 10.1002/hbm.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong J., Chen G., Jia Y. Disrupted functional connectivity within the default mode network and salience network in unmedicated bipolar ii disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:11–18. doi: 10.1016/j.pnpbp.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Das P., Calhoun V., Malhi G.S. Bipolar and borderline patients display differential patterns of functional connectivity among resting state networks. Neuroimage. 2014;98:73–81. doi: 10.1016/j.neuroimage.2014.04.062. [DOI] [PubMed] [Google Scholar]
- 49.Du Y., Pearlson G.D., Liu J. A group ICA based framework for evaluating resting fMRI markers when disease categories are unclear: application to schizophrenia, bipolar, and schizoaffective disorders. Neuroimage. 2015;122:272–280. doi: 10.1016/j.neuroimage.2015.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brady R.O., Jr, Tandon N., Masters G.A. Differential brain network activity across mood states in bipolar disorder. J Affect Disord. 2017;207:367–376. doi: 10.1016/j.jad.2016.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 53.Mayberg H.S., Liotti M., Brannan S.K. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 54.Yamasaki H., LaBar K.S., McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99(17):11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pavuluri M.N., Sweeney J.A. Integrating functional brain neuroimaging and developmental cognitive neuroscience in child psychiatry research. J Am Acad Child Adolesc Psychiatry. 2008;47(11):1273–1288. doi: 10.1097/CHI.0b013e318185d2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gruber S.A., Rogowska J., Yurgelun-Todd D.A. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. J Affect Disord. 2004;82(2):191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Krüger S., Seminowicz D., Goldapple K., Kennedy S.H., Mayberg H.S. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54(11):1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- 58.Fornito A., Harrison B.J., Goodby E. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70(11):1143–1151. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- 59.Frangou S., Haldane M., Roddy D., Kumari V. Evidence for deficit in tasks of ventral, but not dorsal, prefrontal executive function as an endophenotypic marker for bipolar disorder. Biol Psychiatry. 2005;58(10):838–839. doi: 10.1016/j.biopsych.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 60.Sheline Y.I., Price J.L., Yan Z.Z., Mintun M.A. Resting-state functional mri in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh M.K., Kelley R.G., Howe M.E., Reiss A.L., Gotlib I.H., Chang K.D. Reward processing in healthy offspring of parents with bipolar disorder. JAMA Psychiatry. 2014;71(10):1148–1156. doi: 10.1001/jamapsychiatry.2014.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adolphs R., Damasio H., Tranel D., Cooper G., Damasio A.R. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci. 2000;20(7):2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heberlein A.S., Adolphs R., Tranel D., Damasio H. Cortical regions for judgments of emotions and personality traits from point-light walkers. J Cognitive Neurosci. 2004;16(7):1143–1158. doi: 10.1162/0898929041920423. [DOI] [PubMed] [Google Scholar]
- 64.Phillips M.L., Ladouceur C.D., Drevets W.C. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13(9):829. doi: 10.1038/mp.2008.65. 833-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nusslock R., Almeida J.R., Forbes E.E. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14(3):249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 67.Anand B.K., Malhotra C.L., Singh B., Dua S. Cerebellar projections to limbic system. J Neurophysiol. 1959;22(4):451–457. doi: 10.1152/jn.1959.22.4.451. [DOI] [PubMed] [Google Scholar]
- 68.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7(7):511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 69.Snider R.S., Maiti A. Cerebellar contributions to the Papez circuit. J Neurosci Res. 1976;2(2):133–146. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- 70.Lauterbach E.C. Bipolar disorders, dystonia, and compulsion after dysfunction of the cerebellum, dentatorubrothalamic tract, and substantia nigra. Biol Psychiatry. 1996;40(8):726–730. doi: 10.1016/0006-3223(96)82516-9. [DOI] [PubMed] [Google Scholar]
- 71.Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 72.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic bold signal fluctuations. Neuropsychologia. 2006;44(14):2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 73.Margulies D.S., Vincent J.L., Kelly C. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci U S A. 2009;106(47):20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 75.Zhang S., Li C.S. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012;59(4):3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minuzzi L., Syan S.K., Smith M., Hall A., Hall G.B., Frey B.N. Structural and functional changes in the somatosensory cortex in euthymic females with bipolar disorder. Aust N Z J Psychiatry. 2018;52(11):1075–1083. doi: 10.1177/0004867417746001. [DOI] [PubMed] [Google Scholar]
- 77.Yin Z., Chang M., Wei S. Decreased functional connectivity in insular subregions in depressive episodes of bipolar disorder and major depressive disorder. Front Neurosci. 2018;12:842. doi: 10.3389/fnins.2018.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oertel-Knochel V., Reinke B., Matura S., Prvulovic D., Linden D.E., van de Ven V. Functional connectivity pattern during rest within the episodic memory network in association with episodic memory performance in bipolar disorder. Psychiatry Res. 2015;231:141–150. doi: 10.1016/j.pscychresns.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 79.Li C.T., Tu P.C., Hsieh J.C. Functional dysconnection in the prefrontal-amygdala circuitry in unaffected siblings of patients with bipolar I disorder. Bipolar Disord. 2015;17(6):626–635. doi: 10.1111/bdi.12321. [DOI] [PubMed] [Google Scholar]
- 80.Dickstein D.P., Gorrostieta C., Ombao H. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol Psychiatry. 2010;68:839–846. doi: 10.1016/j.biopsych.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martino M., Magioncalda P., Saiote C. Abnormal functional-structural cingulum connectivity in mania: combined functional magnetic resonance imaging-diffusion tensor imaging investigation in different phases of bipolar disorder. Acta Psychiatr Scand. 2016;134:339–349. doi: 10.1111/acps.12596. [DOI] [PubMed] [Google Scholar]
- 82.Li M., Huang C., Deng W. Contrasting and convergent patterns of amygdala connectivity in mania and depression: a resting-state study. J Affect Disord. 2015;173:53–58. doi: 10.1016/j.jad.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 83.Altinay M.I., Hulvershorn L.A., Karne H., Beall E.B., Anand A. Differential resting-state functional connectivity of striatal subregions in bipolar depression and hypomania. Brain Connect. 2016;6:255–265. doi: 10.1089/brain.2015.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He Z., Sheng W., Lu F. Altered resting-state cerebral blood flow and functional connectivity of striatum in bipolar disorder and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2019;90:177–185. doi: 10.1016/j.pnpbp.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 85.Chen G., Zhao L., Yes Jia. Abnormal cerebellum-DMN regions connectivity in unmedicated bipolar II disorder. J Affect Disord. 2019;243:441–447. doi: 10.1016/j.jad.2018.09.076. [DOI] [PubMed] [Google Scholar]
- 86.Martino M., Magioncalda P., Huang Z. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc Natl Acad Sci U S A. 2016;113(17):4824–4829. doi: 10.1073/pnas.1517558113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J., Magioncalda P., Huang Z. Altered global signal topography and its different regional localization in motor cortex and hippocampus in mania and depression. Schizophr Bull. 2018;45(4):902–910. doi: 10.1093/schbul/sby138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anand A., Li Y., Wang Y., Lowe M.J., Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171:189–198. doi: 10.1016/j.pscychresns.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen C.H., Lennox B., Jacob R., Calder A., Lupson V., Bisbrown- Chippendale R., Suckling J., Bullmore E. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 90.Hulvershorn L.A., Karne H., Gunn A.D., Hartwick S.L., Wang Y., Hummer T.A., Anand A. Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. Biol Psychiatry. 2012;71:603–610. doi: 10.1016/j.biopsych.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hafeman D.M., Chang K.D., Garrett A.S., Sanders E.M., Phillips M.L. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14(4):375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- 92.Berretta S., Pantazopoulos H., Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;62(8):884–893. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 93.Baxter M.G., Murray The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 94.Everitt B.J., Cardinal R.N., Parkinson J.A., Robbins T.W. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- 95.Heberlein A.S., Saxe R.R. Dissociation between emotion and personality judgments: convergent evidence from functional neuroimaging. Neuroimage. 2005;28(4):770–777. doi: 10.1016/j.neuroimage.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 96.Straube T., Miltner W.H. Attention to aversive emotion and specific activation of the right insula and right somatosensory cortex. Neuroimage. 2011;54(3):2534–2538. doi: 10.1016/j.neuroimage.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 97.Kanske P., Kotz S.A. Positive emotion speeds up conflict processing: ERP responses in an auditory Simon task. Biol Psychol. 2011;87(1):122–127. doi: 10.1016/j.biopsycho.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 98.Aron A.R., Durston S., Eagle D.M., Logan G.D., Stinear C.M., Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27(44):11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168(2):129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fagiolini A., Kupfer D.J., Masalehdan A., Scott J.A., Houck P.R., Frank E. Functional impairment in the remission phase of bipolar disorder. Bipolar Disord. 2005;7(3):281–285. doi: 10.1111/j.1399-5618.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 101.Chai X.J., Whitfield-Gabrieli S., Shinn A.K. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Torrisi S., Moody T.D., Vizueta No. Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar Disord. 2013;15:156–166. doi: 10.1111/bdi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Najt P. Durham University; 2013. Functional cerebral asymmetries of emotional processes in the healthy and bipolar brain. [Google Scholar]
- 104.Reinke B., Ven Vv, Matura S., Linden D.E., Oertel-Knöchel V. Altered intrinsic functional connectivity in language-related brain regions in association with verbal memory performance in euthymic bipolar patients. Brain Sci. 2013;3(3):1357–1373. doi: 10.3390/brainsci3031357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Favre P., Baciu M., Pichat C., Bougerol T., Polosan M. fMRI evidence for abnormal resting-state functional connectivity in euthymic bipolar patients. J Affect Disord. 2014;165:182–189. doi: 10.1016/j.jad.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 106.Knochel C., Stablein M., Storchak H. Multimodal assessments of the hippocampal formation in schizophrenia and bipolar disorder: evidences from neurobehavioral measures and functional and structural MRI. Neuroimage Clin. 2014;6:134–144. doi: 10.1016/j.nicl.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anticevic A., Brumbaugh M.S., Winkler A.M. Global prefrontal and fronto-amygdala dysconnectivity in bipolar i disorder with psychosis history. Biol Psychiatry. 2013;73(6):565–573. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stoddard J., Hsu D., Reynolds R.C. Aberrant amygdala intrinsic functional connectivity distinguishes youths with bipolar disorder from those with severe mood dysregulation. Psychiatry Res. 2015;231:120–125. doi: 10.1016/j.pscychresns.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oertel-Knochel V., Reinke B., Matura S., Prvulovic D., Linden D.E., van de Ven V. Functional connectivity pattern during rest within the episodic memory network in association with episodic memory performance in bipolar disorder. Psychiatry Res. 2015;231:141–150. doi: 10.1016/j.pscychresns.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 110.Lui S., Yao L., Yes Xiao. Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol Med. 2015;45:97–108. doi: 10.1017/S003329171400110X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lv D., Lin W., Xue Z. Decreased functional connectivity in the language regions in bipolar patients during depressive episodes but not remission. J Affect Disord. 2016;197:116–124. doi: 10.1016/j.jad.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 112.Li J., Tang Y., Womer F. Two patterns of anterior insular cortex functional connectivity in bipolar disorder and schizophrenia. World J Biol Psychiatry. 2018;19(sup3):S115–S123. doi: 10.1080/15622975.2016.1274051. [DOI] [PubMed] [Google Scholar]
- 113.Whittaker J.R., Foley S.F., Ackling E., Murphy K., Caseras X. The functional connectivity between the nucleus accumbens and the ventromedial prefrontal cortex as an endophenotype for bipolar disorder. Biol Psychiatry. 2018;84:803–809. doi: 10.1016/j.biopsych.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li G., Liu P., Andari E., Zhang A., Zhang K. The role of amygdala in patients with euthymic bipolar disorder during resting state. Front Psychiatry. 2018;9:445. doi: 10.3389/fpsyt.2018.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y., Zhong S., Chen G. Altered cerebellar functional connectivity in remitted bipolar disorder: a resting-state functional magnetic resonance imaging study. Aust N Z J Psychiatry. 2018;52(10):962–971. doi: 10.1177/0004867417745996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.