Abstract
Background
Untreated HIV infection leads to alterations in HIV-specific CD4+ T cells including increased expression of co-inhibitory receptors (IRs) and skewing toward a T follicular helper cell (Tfh) signature. However, which changes are maintained after suppression of viral replication with antiretroviral therapy (ART) is poorly known.
Methods
We analyzed blood CD4+ T cells specific to HIV and comparative viral antigens in ART-treated people using a cytokine-independent activation-induced marker assay alone or in combination with functional readouts.
Findings
In intra-individual comparisons, HIV-specific CD4+ T cells were characterized by a larger fraction of circulating Tfh (cTfh) cells than CMV- and HBV-specific cells and preferentially expressed multiple IRs and showed elevated production of the Tfh cytokines CXCL13 and IL-21. In addition, HIV-specific cTfh exhibited a predominant Th1-like phenotype and function when compared to cTfh of other specificities, contrasting with a reduction in Th1-functions in HIV-specific non-cTfh. Using longitudinal samples, we demonstrate that this distinct HIV-specific cTfh profile was induced during chronic untreated HIV infection, persisted on ART and correlated with the translation-competent HIV reservoir but not with the total HIV DNA reservoir.
Interpretation
Expansion and altered features of HIV-specific cTfh cells are maintained during ART and may be driven by persistent HIV antigen expression.
Funding
This work was supported by the National Institutes of Health (NIH), the Canadian Institutes of Health Research (CIHR) and the FRQS AIDS and Infectious Diseases Network.
Keywords: HIV, T Follicular helper T cells, HIV-specific CD4+ T cells, Antiretroviral therapy (ART)
Research in context.
Evidence before this study
Combination antiretroviral therapy (ART) is highly effective in controlling HIV but requires life-long medication due to the existence of a latent viral reservoir, and to the fact that, with rare exceptions, ART alone does not restore immune responses capable of suppressing HIV. T follicular helper cells (Tfh) are of high interest both as HIV reservoirs and for their critical role in enabling the development of potent broadly neutralizing antibodies (bNAbs). The identification of a circulating Tfh (cTfh) population in peripheral blood with strong similarities with lymphoid tissue Tfh has attracted much attention over the past few years. However, investigations of cTfh at the antigen (Ag)-specific level have been hampered by the lack of sensitive assays to detect them. We recently overcame this hurdle by using new experimental, cytokine-independent approaches to evaluate HIV-specific CD4+ T cell responses. We found an elevated Tfh signature in HIV-specific CD4+ T cells of chronically infected, untreated individuals that correlated with blood HIV viremia. Although this Tfh signature decreased after ART initiation at the transcriptional level, detailed phenotypic and functional analyses of the HIV-specific CD4+ T cell responses during ART are lacking.
Added value of this study
Here, we evaluated HIV-specific CD4+ T cells, and in particular cTfh, in a cohort of ART-treated individuals. Comparative phenotypic and functional analyses with Ag-specific responses (CMV, HBV) in the same study participant revealed that HIV-specific cTfh cells are abundant in ART-treated humans and represent a much larger fraction of the virus-specific CD4+ T cell response compared to their CMV- and HBV-specific counterparts. HIV-specific cTfh also differ from CMV-specific and HBV-specific cTfh by multiple phenotypic and functional features that are established during chronic viremic infection, which persist on ART and appear less responsive to viral suppression than in non-cTfh HIV-specific CD4+ T cells. This distinctive HIV-specific cTfh profile correlates with the translation-competent HIV reservoir, but not the total HIV DNA reservoir, suggesting that persistent HIV antigen expression maintains these altered features during ART.
Implications of all the available evidence
Increasing evidence suggest that cTfh are ontogenically related to lymphoid tissue Tfh. As animal models suggest that overabundant, qualitatively impaired Ag-specific Tfh may be detrimental and lead to low affinity Ab responses, it will be important to determine if priming of new, effective Tfh responses may be hampered in therapeutic vaccine trials by competition with such large pre-existing HIV-specific Tfh populations during ART.
Alt-text: Unlabelled box
1. Introduction
Virus-specific CD4+ T cell help is critical for pathogen control in chronic infections [1,2]. High-level antigen (Ag) exposure and inflammatory signals induce virus-specific CD8+ T cell exhaustion, a state characterized by decreased proliferative potential and loss of effector function [3]. While dysfunctional pathogen-specific CD4+ T cells share some characteristics with their CD8+ counterparts during chronic infections, such as increased co-inhibitory receptor (IR) expression, they also present distinct characteristics of altered differentiation and function [4]. These features include skewing toward T follicular helper cell (Tfh) function, a phenomenon seen in chronic LCMV clone 13 in mice [5], and HCV in humans [6]. Tfh cells are a subset of CD4+ T cells that express CXCR5 and provide help for B cell maturation and development of high affinity antibody (Ab) responses in the germinal center (GC) of secondary lymphoid organs. CXCR5+ cells can also be found in the peripheral blood as circulating memory cells (cTfh) [7], however, their origin is not fully understood. Although cTfh cells could originate from non-GC Tfh cells, recent studies highlight the clonal and phenotypic overlap between activated blood cTfh cells and GC Tfh cells during steady state or after vaccination [8,9], demonstrating that GC Tfh cells fuel at least in part the blood cTfh pool.
Tfh cell-dependant humoral responses are required for eventual viral control of chronic murine LCMV infection [10]. In chronic HIV infection, expansion of bulk GC Tfh cells occurs in lymph nodes [11], [12], [13]; while technical hurdles have thus far limited studies of the specificity of these cells, part of this expanded GC Tfh population appears to be HIV-specific [14]. This quantitative increase in GC Tfh in HIV infection correlates with markers of disease progression and qualitative defects in their capacity to provide help to B cells [15]. Studies of peripheral blood responses confirmed an Ag-driven component to these alterations: the transcriptome of blood HIV-specific CD4+ T cells exhibits a Tfh-like signature that directly correlates with viral load [4]. In contrast to LCMV, however, in HIV infection, this Tfh skewing does not result in virus clearance, perhaps due to the remarkable capacity of HIV to generate escape mutations and elude autologous Ab neutralization.
While current antiretroviral therapy (ART) regimens are highly effective at suppressing viral replication, resulting in improved immunity against opportunistic infections and remarkable reduction in morbidity and mortality, they do not lead to the restoration of an effective HIV-specific immune response capable of suppressing virus. Consequently, ART interruption generally leads to a rapid viral rebound from a reservoir of latently infected cells [16].
Frequencies of total GC Tfh decrease in lymphoid tissue with ART initiation but can stay elevated compared to uninfected controls [11], [12], [13]. Bulk cTfh cells from ART-treated donors show a reduced capacity to induce HIV antibody production and B cell differentiation in vitro when compared to uninfected controls [17,18]. We observed that cTfh constitute a readily detectable fraction of HIV-specific CD4+ T cells in individuals prior to ART initiation, this irrespective of viral load [4]. ART decreased the marked Tfh gene signature present in viremic persons at the transcriptional level [4], however, the impact of ART on the differentiation and function of HIV-specific CD4+ T cell has not yet been investigated. Whether immunological features of HIV-specific cTfh would be unique to this virus or shared with other Ag specificities in the same individual, remains to be determined.
Here, we used activation-induced marker (AIM) assays [4,19,20], and functional tests to define the frequency, phenotype and function of blood CD4+ T cells, in particular cTfh, specific to HIV, CMV and HBV in ART-treated individuals. We longitudinally investigated participants pre- and post-ART initiation to delineate features of HIV-specific CD4+ T cells that were modulated by therapeutic control of viral load from those that persisted despite suppressive ART. Finally, we identified features of these HIV-specific cTfh cells that correlated with the size of the translation-competent HIV reservoir.
2. Material and methods
2.1. Human sample collection and processing
Subject characteristics are summarized in Tables S1-3. HIV-infected, ART-treated participants were on ART for over 12 months with controlled viral load (<50 vRNA copies/ml) for at least 6 months. Donors on ART were not excluded when a single small viral blip (VL >50 but <200 vRNA copies/ml) occurred with below detection viral load on precedent and subsequent tests. Untreated participants were either treatment naive or untreated for at least 3 months. HIV-uninfected individuals were used as negative controls for HIV antibody and reservoir measurements. PBMCs were isolated from leukapheresis samples by the Ficoll-Hypaque density gradient centrifugation and cryo-preserved in liquid nitrogen until use.
2.2. CD69/CD40L AIM assay
Peripheral blood mononuclear cells (PBMCs) were thawed, washed and put in culture at a concentration of 10 million cells/ml in RPMI 1640 medium (Gibco by Life Technologies, Cat# 11,875-093) supplemented with 0.5% penicillin/streptomycin (Gibco by Life Technologies, Cat# 15140122) and 10% human serum (Sigma). After a rest of 3 h at 37 °C, a CD40 blocking antibody (Miltenyi Biotec, Cat# 130-094-133, RRID:AB_10839704) was added to the culture to prevent the interaction of CD40L with CD40 and its subsequent downregulation. In addition, antibodies for chemokine receptors CXCR5, CXCR3 and CCR6 were added into the culture medium. After 15 min incubation at 37 °C, cells were stimulated with 0.5 µg/ml staphylococcal enterotoxin B (SEB) or 0.5 µg/ml of overlapping peptide pools for CMV pp65 (Cat# PM-PP65), HBV HBsAg (Cat# PM-HBV-IEP), HIV Gag (Cat# PM-HIV-GAG), HIV Env (Cat# PM-HIV-ENV) or HIV Nef (Cat# PM-HIV-NEF) (all JPT) for 9 h at 37 °C. An unstimulated condition served as a negative control. Cells were stained for viability dye (Aquavidid, Thermofisher, Cat# L34957), surface markers (30 min., 4 °C) and fixed using 2% paraformaldehyde (PFA) before acquisition at the flow cytometer (LSRII, BD) (see Table S4 for antibodies). Analysis was performed using FlowJo version 10 for Mac (Treestar, RRID:SCR_008520) and Spice version 5.3 (RRID:SCR_016603) [21]. For phenotypic analysis of Ag-specific CD4+ T cells, only responses that were >2-fold over unstimulated condition were included to limit the impact of background staining. In contrast, for analysis of Ag-specific CD4+ T cells subsets as percentage of total CD4+ T cells, background-subtracted net values were used, which did not require excluding responses.
2.3. Standard intracellular cytokine staining
PBMCs were thawed, washed and put in culture at a concentration of 4 million cells/ml in RPMI 1640 medium (Gibco by Life Technologies, Cat# 11875-093) supplemented with penicillin/streptomycin (Gibco by Life Technologies, Cat# 15140122) and 10% fetal bovine serum (FBS) (Seradigm, Cat#1500-500). After a rest of 2 h, cells were stimulated with 0.5 µg/ml of overlapping peptide pools for CMVpp65, HBV HBsAg or HIV Gag for 6 h in presence of Brefeldin A (BD Biosciences, Cat# 555029) and monensin (BD Biosciences, Cat# 554724). For some experiments, anti-CD107A-BV785 (Biolegend, Cat#328644, RRID: AB_2565968) was added into culture. Cells were stained for viability marker, surface markers and intracellular cytokines using the IC Fixation/Permeabilization kit (eBioscience, Cat# 88-8824-00) before fixation with 2% PFA and acquisition on the flow cytometer (see Table S4 for antibodies). For the detection of CD107A, granzyme B and perforin within Ag-specific CD4+ T cells, we identified AIM+ cells by intracellular staining for CD69 and CD40L.
2.4. Delayed ICS assay
For the detection of some cytokines (IFNγ, IL-2, TNFα, IL-21, CXCL13) within AIM+ Ag-specific CD4+ T cells, cultured PBMCs were incubated with a CD40-blocking antibody, stimulated with peptide pools for 9 h as described above and further incubated for 12 h at 37 °C in the presence of Brefeldin A (BD Biosciences, Cat# 555029). Cells were surface stained, fixed and permeabilized using the IC Fixation/Permeabilization kit (Thermo Fisher, Cat# 88-8824-00) and incubated with antibodies against cytokines for 30 min at 4 °C (see Table S4 for antibodies).
2.5. Transcription factor staining
For the detection of transcription factors within AIM+ Ag-specific CD4+ T cells, cultured PBMCs were incubated with CD40-blocking antibodies and stimulated with peptide pools for 9 h as described above. Cells were stained for viability dye (Aquavidid, Thermofisher, Cat# L34957), surface markers (30 min., 4 °C), fixed (30 min, room temperature (RT)) and permeabilized using the Transcription Factor Staining Buffer Set (Invitrogen/ThermoFisher, Cat# 00–5523–00) before staining with antibodies against transcription factors for 1 h at RT (see Table S4 for antibodies).
2.6. HLA class II typing
Genomic DNA was extracted from PBMC using a QIAamp DNA blood kit (Qiagen, Cat# 51106). All subjects were typed for MHC class II alleles by sequence-based typing using kits from Atria Genetics (South San Francisco, CA). Assign software was used to interpret sequence information for allele typing (Conexio Genetics, Perth, Australia).
2.7. MHCII tetramer staining
CD4+ T cells were isolated from thawed PBMCs using a negative isolation kit (StemCell, Cat# 19052), rested for 2 h at 37 °C, washed and stained for 60 min at room temperature with PE-labeled MHC-II tetramers loaded with DV16 peptide (DRFYKTLRAEQASQEV) for the DRB1*01:01 allele or YV18 peptide (YVDRFYKTLRAEQASQEV) for the DRB1*11:01 allele (NIH Tetramer Core Facility at Emory University, Atlanta, GA). These sequences encompass an immunodominant, HLA Class II promiscuous epitope in Gag [22]. Control tetramers loaded with an irrelevant peptide (CLIP: PVSKMRMATPLLMQA) or HIV-uninfected donors with the same HLA-DRB1 genotype served as negative controls. Tetramer+ CD4+ T cells were column enriched using anti-PE beads (Miltenyi, Cat# 130-048-801). Cells were stained for viability marker (Aquavivid, Thermofisher, Cat# L34957), CXCR5 (45 min, 37 °C), surface markers (30 min, 4 °C) and fixed with 2% PFA before acquisition at the flow cytometer (LSRIIB, BD).
2.8. Quantification of total and integrated HIV DNA
Total and integrated HIV DNA were measured in CD4+ T cells isolated from PBMCs by magnetic bead-based negative selection (Stem Cell Technologies, Cat# 19052) by real time nested polymerase chain reaction (PCR) as described previously [23].
2.9. Detection of translation-competent reservoir by RNA flow-FISH
CD4+ T cells harbouring latent translation-competent reservoir were identified using the HIVRNA/Gag assay as previously described [24,25]. Briefly, CD4+ T cells were isolated by magnetic bead negative selection (StemCell, Cat# 19052) from PBMCs from ART-treated individuals, rested for 3 h and stimulated with PMA (50 ng/ml, Sigma-Aldrich, Cat# P1585) and Ionomycin (0.5 µg/ml, Sigma-Aldrich, Cat# I9657) for 12 h. Unstimulated cells and cells from HIV-uninfected individuals served as controls. Cells were stained with surface markers, anti-Gag KC57 (Beckman Coulter) by intracellular staining and labeled for HIV gag RNA with Alexa Fluor 750-coupled probes (ThermoFisher) using the PrimeFlow RNA Assay (ThermoFisher, Cat# 88-18005-210) (see Table S4 for antibodies). Translation-competent CD4+ T cells were identified as cells expressing both HIV Gag protein and gag RNA after PMA/Ionomycin stimulation.
2.10. Detection of p24-specific antibodies by ELISA
96 well plates (Thermo Scientific Nunc, FluoroNunc/LumiNunc, MaxiSorp Surface) were coated with 0.1 μg/ml of recombinant p24 (NIH AIDS Research and Reference Reagent Program, Cat# 12028) or bovine serum albumin (BSA) (Bioshop, Cat# ALB001.1) in PBS overnight at 4 °C. Plates were blocked for 90 min at RT with blocking buffer (TBS, Tween 0.1%, BSA 2%) and then washed 4 times with washing buffer (TBS, Tween 0.1%). Dilutions of human sera (1:3000) or rabbit anti-HIV p24 antiserum (NIH AIDS Reagent Program, Cat# 4250) in washing buffer containing 0.1% of BSA were incubated for 2 h at RT. Plates were washed 4 times with washing buffer before incubation for 90 min at RT with HRP-conjugated secondary Abs goat anti-human IgG HRP (Thermo Fisher Scientific Cat# 31410, RRID:AB_228269) or anti IgG rabbit HRP (Thermo Fisher Scientific Cat# 65-6120, RRID:AB_2533967). Plates were then washed 4 times with washing buffer before revealing with standard ECL (Perkin Elmer) with a TriStar luminometer (LB 941, Berthold Technologies).
2.11. Detection of gp120-specific antibodies
Gp120-specific antibodies were detected in plasma samples using a flow cytometry-based assay as described previously [26]. Briefly, CEM.NKr cells were coated with recombinant HIV-1YU2 gp120 (100 ng/ml) for 30 min at 37 °C and incubated with human plasma from HIV-infected ART-treated donors or uninfected controls (1:10,000 dilution) for 30 min at 37 °C. Cells were washed with PBS and stained with 1 μg/ml goat anti-human Alexa Fluor 647 (Thermo Fisher Scientific, Cat# A-21445 RRID:AB_2535862) secondary antibody for 15 min in PBS at room temperature. Cells were washed and fixed using 2% PFA before acquisition at the flow cytometer. The geometric mean of the Alexa Fluor 647 signal was used to express plasma gp120-antibody levels.
2.12. Statistics
Statistical analyses were done using GraphPad Prism version 8 using non-parametric tests. Two-group comparisons were performed using the Mann-Whitney and pairwise comparisons were performed using the Wilcoxon matched pair test. For comparisons between three or more groups, Kruskal–Wallis (for unpaired samples or when values were missing in paired samples) or Friedman one-way ANOVA (for paired samples) with Dunn's post-test was used. Permutation test (10,000 permutations) was applied for pie-chart comparison using the SPICE software. For correlations, Spearman's R correlation coefficient was applied. Statistical tests were two-sided and p < 0.05 was considered significant.
2.13. Ethic statement
Leukaphereses were obtained from study participants at the McGill University Health Centre, Montreal, Canada, and at the Centre Hospitalier de l'Université de Montréal (CHUM) in Montreal, Canada. The study was approved by the respective IRBs, written informed consent obtained from all participants prior to enrolment.
2.14. Data availability
Raw experimental data associated with the figures presented in the manuscript are available from the corresponding author upon reasonable request.
3. Results
3.1. AIM assay identifies HIV-specific CD4+ responses with cTfh expansion in ART-treated individuals
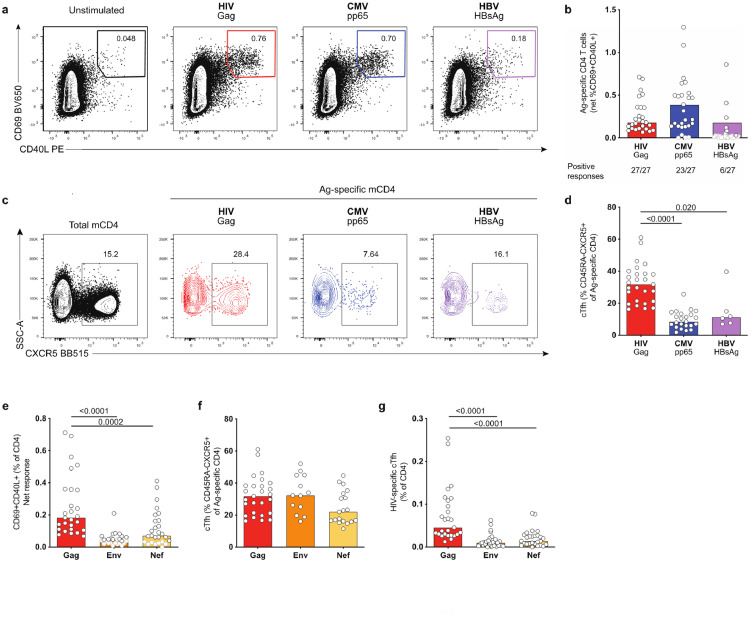
To study Ag-specific CD4+ T cells with diverse differentiation and functionality in HIV-infected ART-treated people, we used an approach based on the concurrent detection of activation-induced markers (AIM) on the cell surface after cognate Ag stimulation, as previously described [4,19,20]. PBMCs from a cohort of 27 HIV-infected individuals on ART (Participant characteristics: Table S1 (ART1-27)) were stimulated for 9 h with overlapping peptide pools spanning the sequence of the immunodominant HIV structural protein Gag (Fig. S1a). HIV Gag-specific T cells were identified by concurrent surface expression of AIM CD69 and CD40L (AIM+ cells) (Fig. 1a, S1b). In addition, we examined within the same individual CD4+ T cells specific for other Ags (CMV and HBV) to delineate characteristics that differentiate HIV-specific CD4+ T cells. AIM+ HIV Gag-specific responses were readily detectable in all ART-treated subjects examined, with low background in the absence of exogenous Ag (Fig. 1b, S1c) (responses were considered as positive when more than 2-fold over unstimulated condition). CMV-specific CD4+ T cell responses were detectable in all individuals with positive CMV serology and absent for the 4 CMV-seronegative participants, demonstrating the high specificity of the assay (Fig. 1b, Table S1). Due to the low number of donors with a detectable HBV-specific CD4+ T cell response (6 individuals of the 27 examined; 2 resolved infection, 4 vaccinated) (Fig. 1b, Table S1), we pooled responses elicited by infection or vaccination for further analysis, given the similarities of their profile.
Fig. 1.
HIV-specific cTfh are expanded compared to CMV- and HBV-specific cTfh in ART-treated donors. PBMCs were stimulated for 9 h with peptide pools for HIV Gag, CMV pp65, HBV HBsAg or left unstimulated. Ag-specific CD4+ T cells were identified by the concurrent upregulation of CD40L and CD69 (AIM+ cells). (a) Example plots showing gating for CD69+CD40L+ for one representative donor (pre-gated on CD4+). (b) Net frequency of AIM+ Ag-specific CD4+T cells. Responses greater than 2-fold over background are shown as black bordered circles, responses below this threshold are shown as gray-bordered symbols. Median values shown were calculated using responses greater than 2-fold over background only. Below each bar, numbers of individuals with positive responses for each antigen are shown. (c) Example plot showing gating of cTfh as memory CD4+ T cells expressing CXCR5. Left hand panel shows gating on total memory CD4+(black). Ag-specific memory CD4+ T cells are shown as colored plots. (d) Quantification of results in (c). (e) Net frequency of HIV-specific CD4+ T cell responses identified using AIM assay. (f) Frequency of cTfh (CXCR5+) within HIV-specific CD4+ T cells (g) Frequency of HIV-specific cTfh of total CD4+ T cells. n = 27 for (b), (e) and (g); for (d): HIV Gag: n = 27, CMV pp65: n = 23, HBV HBsAg n = 6; for (f): HIV Gag: n = 27, Env: n = 13, Nef: n = 19. Bars represent median values. Only significant p-values are shown and were calculated by Kruskal–Wallis test with Dunn's post test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The AIM assay identified significantly higher frequencies of virus-specific CD4+ T cells compared to IFNγ intra-cellular staining (ICS) (Fig. S1d-e), demonstrating that ICS underestimates the frequency of Ag-specific CD4+ T cell responses. This is particularly striking for HIV and HBV, but also apparent for known Th1-skewed responses such as CMV.
Ag-specific cTfh show limited cytokine production and are more likely to be missed by standard ICS, but are detectable by AIM assays [19,27]. Here, we broadly identified cTfh cells as memory (CD45RA−) CD4+ T cells expressing CXCR5 (Fig. S2a, 1c) to avoid the potential confounding factor of PD-1 upregulation in chronic infection [28,29]. Phenotypic analysis was only performed for donors with detectable AIM responses. Compared to CMV- and HBV-specific CD4+ T cells, HIV-specific CD4+ responses were characterized by a significantly higher proportion of CXCR5+ memory cells (Fig. 1d) with comparable CXCR5 MFI for all specificities (Fig. S2b). This skewing was not due to in vitro stimulation, as a similar frequency was observed by staining with MHC Class II tetramers loaded with an immunodominant HIV Gag epitope (Fig. S2c-e). To test whether the enrichment of cTfh cells was a general feature of HIV-specific CD4+ T cell responses, we analyzed responses against the HIV envelope glycoprotein Env and the accessory protein Nef, which showed a lower magnitude compared to HIV Gag as described previously (Fig. 1e) [22]. Despite these lower frequencies, responses were detectable in 13/27 participants for Env and 18/27 participants for Nef. HIV Env- and Nef-specific CD4+ T cell responses were characterized by a similar frequency of CXCR5+ memory cells when compared to Gag-specific CD4+ responses (Fig. 1f). Accordingly, due to the higher magnitude of HIV Gag-specific CD4+ responses, HIV Gag-specific cTfh were more prevalent in the total CD4+ populations compared to HIV Nef or Env (Fig. 1g). In conclusion, our results demonstrate the expansion of cTfh cells within HIV-specific CD4+ T cell responses compared to other specificities in ART-treated individuals.
3.2. HIV-specific cTfh and non-cTfh cells express multiple co-inhibitory receptors despite viral suppression
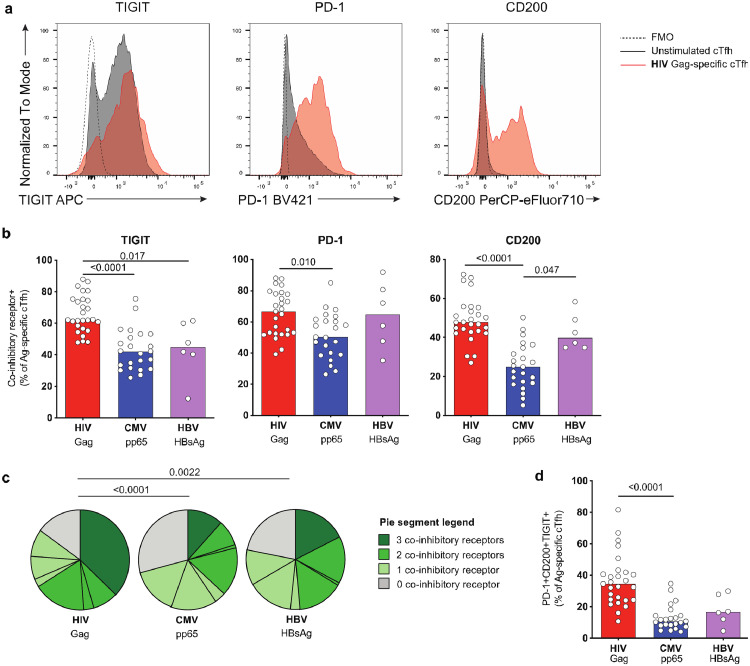
IRs are key modulators of T cell signaling for both the regulation of physiologic responses to Ag stimulation and in the context of diseases characterized by persistent Ag exposure. Consistent with a requirement for tight functional control, Tfh cells frequently express IRs such as PD-1, TIGIT and CD200 [30,31]. On the other hand, upregulation of multiple IRs on CD8+ and CD4+ T cells also occurs in cancer or chronic infections including HIV and contribute to exhaustion (reviewed in [32]). Given this dual role of IRs, we next analyzed IR expression on Ag-specific CD4+ responses in the context of the massive reduction in HIV Ag load due to viral suppression during ART.
HIV Gag-specific cTfh from ART-treated individuals were characterized by a significantly higher frequency of cells expressing the IRs TIGIT and CD200 compared to non-cTfh cells, while the frequency of PD-1+ cells was similar between both subsets (Fig. S3a). When we compared cTfh IR expression between Ags, we observed a high frequency of TIGIT+ for HIV-specific cTfh cells compared to CMV- and HBV-specific cTfh (Fig. 2a–b). In addition, high levels of CD200 and PD-1 characterized HIV- and HBV-specific cTfh cells (Fig. 2b). The frequency of cTfh cells specific for HIV co-expressing TIGIT, PD-1 and CD200 was significantly higher compared to CMV or HBV (Fig. 2c-d). The differences in IR expression observed for Ag-specific non-cTfh reflected those seen on cTfh, albeit at lower levels (Fig. S3b-c).
Fig. 2.
HIV-specific cTfh express multiple IRs despite viral suppression. cTfh specific for HIV, CMV and HBV were analyzed by flow cytometry for expression of the co-inhibitory receptors TIGIT, PD-1 and CD200. (a) Example plots showing expression of TIGIT, PD-1 or CD200 on unstimulated (black) or HIV-specific cTfh (red) from one representative donor. FMO controls are shown as dotted lines. (b) Frequency of TIGIT-, PD-1- or CD200-expressing Ag-specific cTfh. (c) Analysis of co-expression of TIGIT, PD-1 and CD200 on Ag-specific cTfh. (d) Comparison of frequency of TIGIT+CD200+PD-1+ Ag-specific cTfh. n = 27 (HIV), n = 23 (CMV) or n = 6 (HBV) for (b-d). Significant p-values are shown in figure and were calculated by Kruskal–Wallis test with Dunn's post test (b,d) or permutation test (c). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
IR expression can be transiently induced by activation [33], and may thus be impacted by the 9 h stimulation required for the AIM assay. Therefore, we used MHC Class II tetramers to phenotype HIV-specific cTfh cells in 3 ART-treated donors (Fig. S2b). We detected similar frequencies of TIGIT+ and PD-1+ cells on tetramer+ as on AIM+ Gag-specific cTfh cells, which were increased when compared to the total cTfh population (Fig. S3d). In contrast, more HIV Gag-specific cTfh cells expressed CD200 when the AIM assay was used. These findings show that a high frequency of HIV-specific cTfh cells in ART-treated donors pre-express multiple IRs, such as PD-1 and TIGIT, before stimulation with the cognate Ag. On the other hand, while a fraction of HIV-specific cTfh cells pre-expresses CD200, this molecule is further rapidly upregulated during stimulation, as shown previously [34]. Therefore, a higher capacity to upregulate CD200 after stimulation might contribute to its increased expression on HIV- vs HBV- and CMV-specific CD4+ T cells.
In summary, our results demonstrate that despite viral suppression on ART, HIV-specific cTfh and non-cTfh CD4+ T cells are characterized by a high frequency of cells expressing multiple IRs.
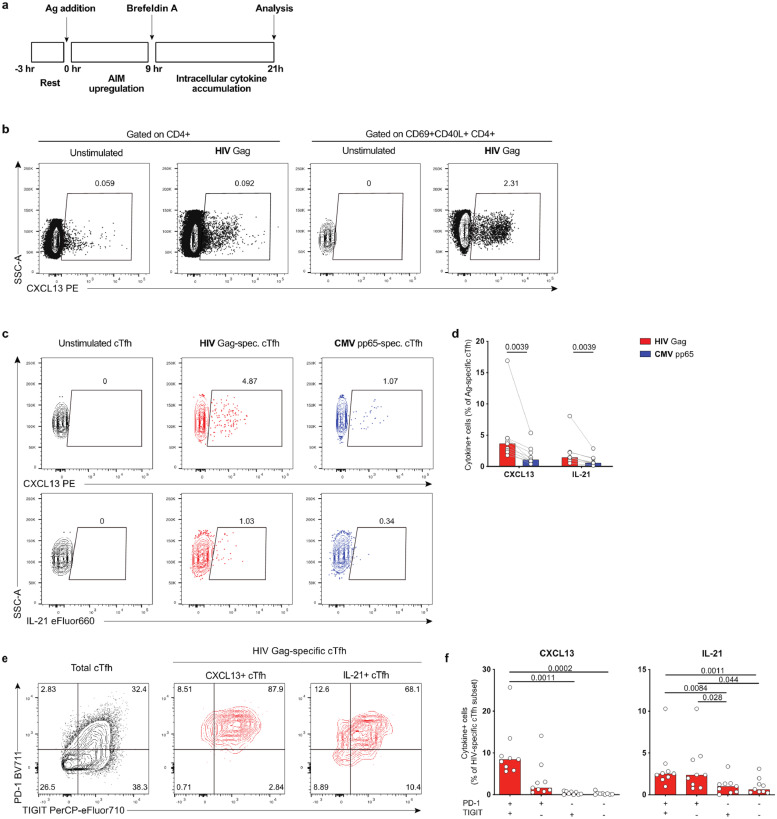
3.3. HIV-specific cTfh cells produce higher levels of Tfh cytokines than CMV-specific cTfh
To elucidate whether high expression of IRs on HIV-specific cTfh influences Tfh function, we assessed the production of the canonical Tfh-related cytokines CXCL13 and IL-21. IL-21 is an important cytokine for B cell help [7], and decreased IL-21 expression has been reported in HIV-specific CD4+ T cells of HIV+ individuals on and off ART [35,36]. Detection of CXCL13 and IL-21 at the protein level is challenging due to their limited expression upon Ag stimulation and unspecific background staining. To overcome this hurdle, we used a modified ICS assay (“delayed ICS”), which included an extended (9 h) stimulation prior to addition of Brefeldin A, thus allowing for upregulation of AIM markers on the cell surface before the phase of intracellular protein trapping (Fig. 3a). We analyzed cytokine expression by a sequential gating strategy: pre-gating on CD69+CD40L+ cTfh cells increased specificity for CXCL13 and IL-21 and enabled robust detection of rare cytokine-expressing cells (Fig. 3b). We detected a significantly higher frequency of CXCL13- and IL-21-producing cells in HIV-specific compared to CMV-specific cTfh cells (Fig. 3c-d). Consistent with the known role of IRs for Tfh function [37], Tfh-cytokine production was highest in cTfh cells expressing multiple IRs (Fig. 3e-f). Therefore, HIV-specific cTfh cells expressing multiple IRs demonstrate robust expression of Tfh-related functions.
Fig. 3.
HIV-specific cTfh produce higher levels of Tfh cytokines than CMV-specific cTfh. Ag-specific cTfh responses were analyzed by flow cytometry for expression of CXCL13 and IL-21 using a delayed ICS assay. (a) Schematic representation of the delayed ICS assay. (b) Example plot showing CXCL13 expression in unstimulated or HIV-stimulated CD4+ cells after gating on either total CD4+ or CD69+CD40L+ Ag-specific CD4+. Plots shown are from the same sample. (c) Example plot showing CXCL13 or IL-21 expression in CD69+CD40L+ cTfh. (d) Comparison of cytokine+ HIV-specific vs CMV-specific cTfh. (e) Example plot showing phenotype of total cTfh cells (black) or HIV-specific CXCL13+ or IL-21+ cTfh (shown in red) based on PD-1 and TIGIT expression. (f) Frequency of cytokine+ cells in cTfh subsets based on TIGIT/PD-1 expression. n = 9. Significant p-values shown were calculated by Wilcoxon test (d) or Friedman test with Dunn's post test (f). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
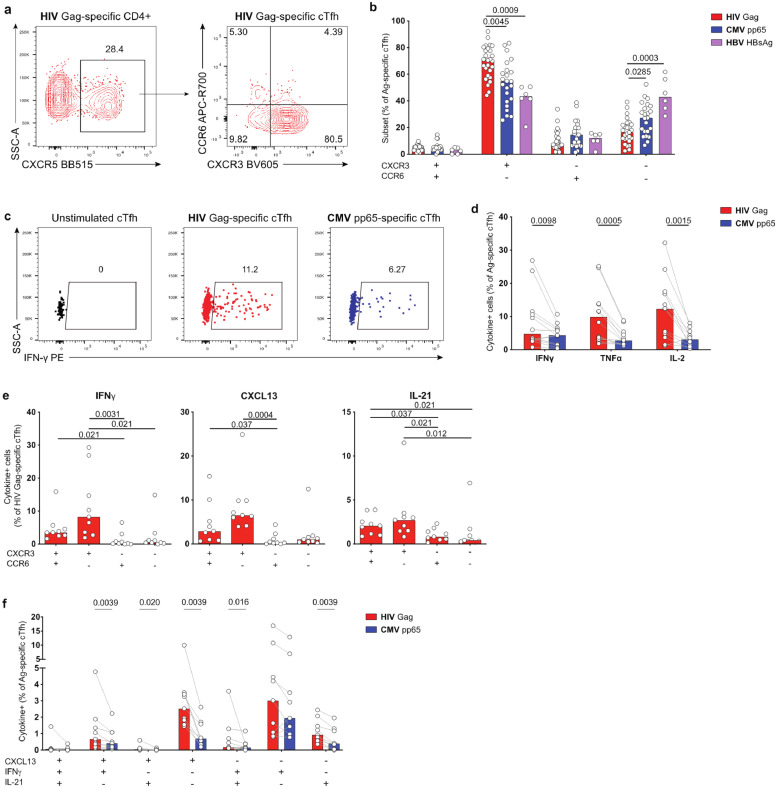
3.4. HIV-specific cTfh cells show preferential Th1-like phenotype and function
Co-expression patterns of the chemokine receptors CXCR3 and CCR6 have been associated with T helper cell differentiation and function [38,39], and can also provide an indication of cTfh polarization and of their capacity to provide help to B cells [40]. We thus next examined CXCR3 and CCR6 expression on AIM+ Ag-specific cTfh cells in ART-treated individuals (Fig. 4a). HIV- and CMV-specific cTfh predominantly had a Th1-like (CXCR3+CCR6−) phenotype, while HBV-specific cTfh responses showed a mixed cTfh profile with Th2-like (CXCR3−CCR6−) and Th1-like polarizations (Fig. 4a, S4a). HIV-specific cTfh cells expressed in addition higher levels of Eomes and/or T-bet, whereas RORγt or GATA3 was rarely detected (Fig. S4bc), confirming their preferential Th1-like polarization. Importantly, the frequency of Th1-polarized cTfh was significantly higher for HIV than for both CMV and HBV (Fig. 4b).
Fig. 4.
HIV-specific cTfh are predominantly Th1-polarized. (a) Example plot showing CXCR3/CCR6 expression on HIV-specific cTfh. (b) Comparison of Ag-specific cTfh subsets based on CXCR3/CCR6 coexpression. (c) Example plots showing expression of IFNγ in HIV- and CMV-specific cTfh. (d) Frequency of Th1-cytokine+ HIV- vs CMV-specific cTfh. (e) Frequency of HIV Gag-specific cTfh subsets expressing IFNγ, CXCL13 or IL-21. (f) Co-expression analysis of Ag-specific cTfh cells based on CXCL13, IFNγ and IL-21 expression. n = 27 (HIV), n = 23 (CMV) or n = 6 (HBV) for (b); n = 12 for (d), n = 9 for (e,f). P-values shown in figure were calculated by Kruskal–Wallis test with Dunn's post test (b), Wilcoxon test (d, f) or Friedman test with Dunn's post test (e).
In contrast, the proportion of CXCR3+CCR6− Th1-like cells within the HIV-specific non-cTfh subset was comparable to CMV and HBV (Fig. S4d). In addition, HIV-specific non-cTfh cells included a high frequency of CCR6+ Th1Th17-like (CCR6+CXCR3+) and Th17-like (CCR6+CXCR3−) (Fig. S4d). Accordingly, we identified subsets of HIV-specific non-cTfh cells characterized by Eomes/T-bet and RORγt expression (Fig. S4e). Eomes and/or T-bet expression was significantly lower in HIV-specific cTfh than non-cTfh (Fig. S4f). These data suggest that the distinctive Th1-like phenotype skewing of HIV-specific cTfh compared to other specificities might correspond to a mixed differentiation pattern that does not reach the full acquisition of all Th1-related features seen in non-cTfh cells.
We next investigated whether the phenotypic polarization of HIV-specific cTfh correlated with function. We analyzed expression of classical Th1-cytokines in HIV- or CMV-specific cTfh cells (Fig. 4c). We detected a significantly higher frequency of IFNγ, TNFα and IL-2-expressing cells in HIV- vs CMV-specific cTfh (Fig. 4c-d). This profile of elevated Th1-cytokine expression was not seen for non-cTfh cells; indeed, IFNγ secretion was significantly reduced in HIV-specific non-cTfh compared to CMV-specific, while IL-2 production was increased (Fig. S4g).
Consistent with previous findings at the bulk cTfh level [30], CXCR3+ HIV-specific cTfh populations (CXCR3+CCR6- Th1-like, and to a lesser extent CXCR3+CCR6+ Th1/Th17-like) produced IFNγ following stimulation (Fig. 4e). However, a large fraction of Ag-specific CXCR3+ cTfh cells also produced the Tfh-cytokines CXCL13 and IL-21. Thus, co-expression analyses revealed increased CXCL13 and IL-21 single-positive in addition to Tfh-cytokines/IFNγ co-expressing HIV-specific cTfh cells, compared to their CMV-specific counterparts (Fig. 4f).
We next compared the frequency of cytokine+ cells within Th1-like Ag-specific cTfh and found comparable levels for IFNγ but a significantly higher CXCL13 and IL-21 expression in HIV vs CMV (Fig. S4h). These findings suggest that the elevated production of Th1-cytokines in HIV-specific cTfh cells may be related to their predominant Th1-like polarization compared to CMV, but that polarization alone is not responsible for the elevated production of Tfh cytokines.
In contrast and as shown previously [41], cTfh cells, independent of their specificity, rarely expressed cytolytic markers such as CD107A, granzyme B or perforin (Fig. S5ab). These functions were, however, detectable in Ag-specific non-cTfh cells, especially for CMV (Fig. S5ab).
Collectively, these observations demonstrate a preferential Th1-associated phenotype and function of HIV-specific cTfh cells in ART-treated individuals, contrasting with lack of cytotoxic molecule expression.
3.5. Suppression of HIV viremia by ART does not reverse the phenotype of HIV-specific cTfh cells
Having identified several distinct features of HIV-specific CD4+ T cell responses in ART-treated individuals, we next investigated whether these characteristics were present prior to ART initiation, and if they were modulated by therapeutic control of Ag load. For this, we examined samples from 7 HIV-infected donors obtained during the chronic phase of untreated infection before ART initiation and after 1 to 3.2 years of therapy (Participant characteristics: Table S2). We first determined the magnitude of HIV-specific CD4+ T cell responses and detected little change after starting therapy using the AIM assay, contrasting with a clear contraction of the HIV-specific CD4+ T cell response when the IFNγ+ ICS was used (Fig. 5a).
Fig. 5.
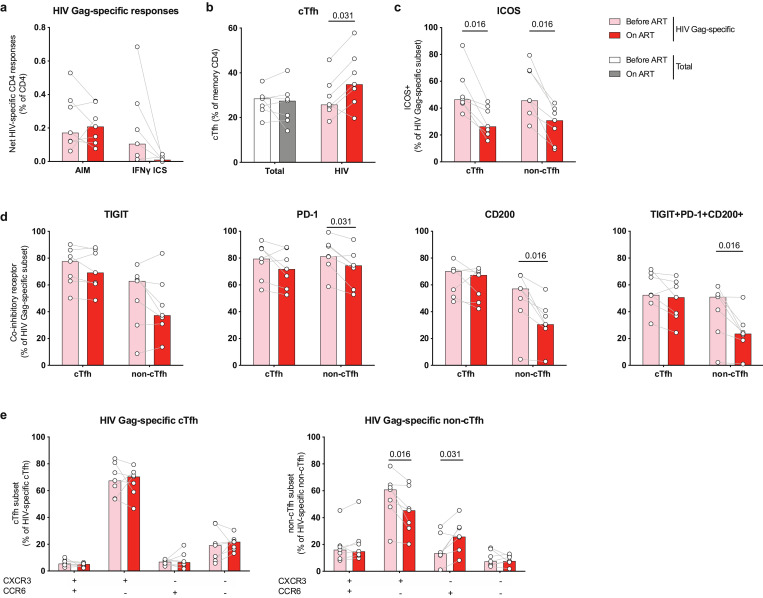
HIV-specific cTfh responses increase with initiation of ART and maintain expression of co-inhibitory receptors and a Th1-like phenotype. HIV-specific CD4+ responses were analyzed longitudinally using matched PBMC samples obtained during chronic HIV infection (Before ART) or after 1.0–3.2 years of ART (On ART). (a) Net frequency of Ag-specific CD4+ responses identified by CD69/CD40L AIM assay or standard IFNγ ICS. (b) Frequency of total (left) or Ag-specific cells with cTfh phenotype (right), defined as CXCR5+ memory CD4 T cells. (c) Frequency of Ag-specific CD4+ subsets expressing ICOS. (d) Single or co-expression analysis of IRs TIGIT, PD-1 or CD200 on HIV-specific cTfh or non-cTfh before vs after ART initiation. (e) Phenotypic analysis of HIV-specific cTfh (left) or non-cTfh (right) based on the expression of CXCR3 and CCR6 before and after ART initiation. n = 7 longitudinal pairs of untreated/treated HIV-infected donors. Significant p-values are shown in figure and were calculated by Wilcoxon test.
In a transcriptional analysis of HIV-specific CD4+ T cells, we recently demonstrated that ART decreased the Tfh gene signature present in viremic individuals off therapy [4]. As this analysis was performed at the RNA level on total AIM+ HIV-specific CD4+ cells, we next investigated whether HIV-specific cTfh and non-cTfh were affected differentially at the phenotypic level by ART.
Although the frequency of cTfh cells within the total memory CD4+ T cell pool did not change following ART initiation, we observed an increase in the frequency of cTfh cells within the HIV-specific CD4+ T cell population (Fig. 5b). We detected a significant decrease in ICOS+ HIV-specific cTfh and non-cTfh cells (Fig. 5c), consistent with a general reduction of activation of HIV-specific CD4+ T cells. In addition, we detected a significant decrease in the frequencies of PD-1+, CD200+ and PD-1+TIGIT+CD200+ HIV-specific non-cTfh cells (Fig. 5d). In contrast, viral suppression on ART had a limited impact on expression and co-expression of IRs on HIV-specific cTfh cells (Fig. 5d). Similarly to IR expression, we did not detect changes in the CXCR3/CCR6 phenotype of HIV-specific cTfh cells following ART initiation (Fig. 5e), contrasting with a significant decrease in Th1-polarization of HIV-specific non-cTfh cells.
In summary, our results demonstrate that the phenotype of cTfh, characterized by a preferential Th1-profile and high expression of multiple IRs, is established during chronic HIV infection and maintained despite suppressed viremia, this at least for the first three years after ART initiation.
3.6. Persistent levels of plasma HIV-specific antibodies in ART-treated individuals correlate with HIV-specific cTfh
Untreated HIV infection is associated with GC Tfh expansion and hypergammaglobulinemia [11]. Although HIV-specific Ab plasma levels decrease after ART initiation, they are detectable in the plasma of long-term treated individuals [42]. It has been suggested that ongoing Ag stimulation in lymphoid tissue may contribute to elevated HIV-specific plasma concentrations [43].
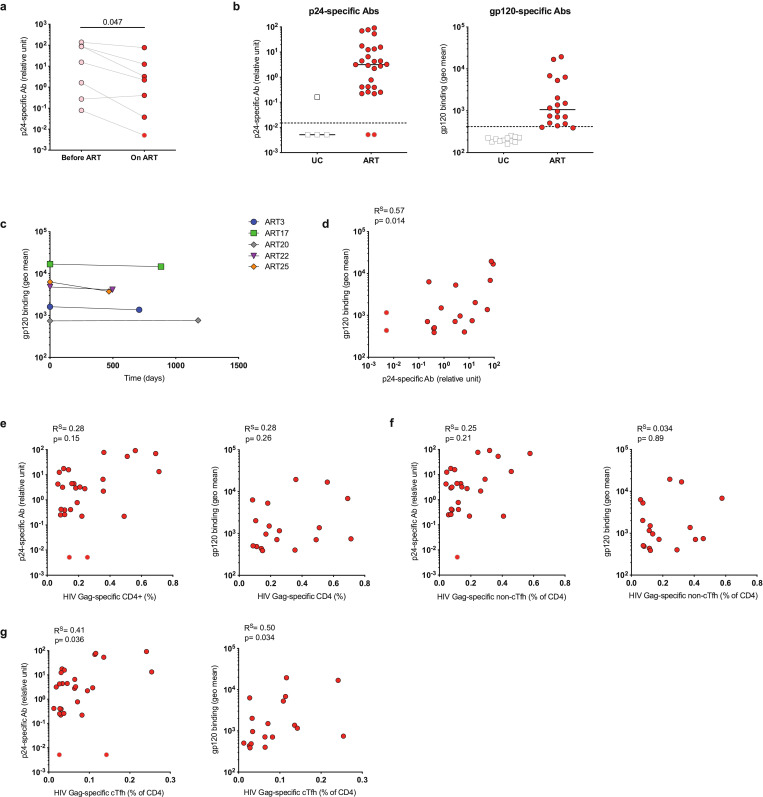
We therefore next assessed plasma levels of p24- and gp120-specific antibodies in our cohort of ART-treated individuals and how they relate to persistent Tfh responses. Despite their decrease after viral suppression on ART (Fig. 6a), HIV-specific Ab levels were detectable in nearly all ART-treated participants (Fig. 6b) and remained stable over time during ART (Fig. 6c). In addition, levels of p24- and gp120-specific Abs in plasma of ART-treated individuals were directly correlated (Fig. 6d). There was no association between total HIV-specific CD4+ T cell or HIV-specific non-cTfh responses and HIV-specific Abs (Fig. 6e-f). However, we detected a significant correlation between HIV-specific cTfh and persistent plasma HIV-specific Ab levels in ART-treated donors (Fig. 6g). Thus, our results suggest ongoing stimulation of HIV-specific B and T cells, which is associated with augmented Ab responses in ART-treated people in vivo.
Fig. 6.
HIV-specific cTfh responses correlate with p24- and gp120-specific antibody levels in ART-treated donors. Plasma samples from HIV+ donors were analyzed for HIV-specific Abs by ELISA (p24-Abs) or flow cytometry (gp120-Abs). (a) Longitudinal analysis of p24-specific Ab levels using matched plasma samples obtained during chronic untreated infection or during ART. (b) Level of p24- or gp120-specific Abs in ART-treated vs uninfected control (UC) donors. Dotted line represents limit of detection. (c) Longitudinal analysis of gp120-specific Abs using matched plasma samples from 2 different time points of long-term ART-treated subjects. (d) Correlation between p24- and gp120-specific Ab plasma levels. (e-g) Correlation between total HIV-specific CD4+ T cells (e), HIV-specific non-cTfh (f) or HIV-specific cTfh (g) and levels of p24- or gp120-specific Abs, respectively. n = 7 for (a); p24-Abs: n = 4 UC and n = 27 ART, gp120-Abs: n = 12 UC and n = 18 ART for (b); n = 5 for (c); n = 18 for (d); p24-Abs: n = 27, gp120-Abs: n = 18 for (e-g). Gray-bordered symbols represent values below limit of detection. P values were calculated by Wilcoxon test (a) or Spearman correlation (d-g).
3.7. The translation-competent HIV reservoir correlates with Th1-like phenotype and function of HIV-specific cTfh
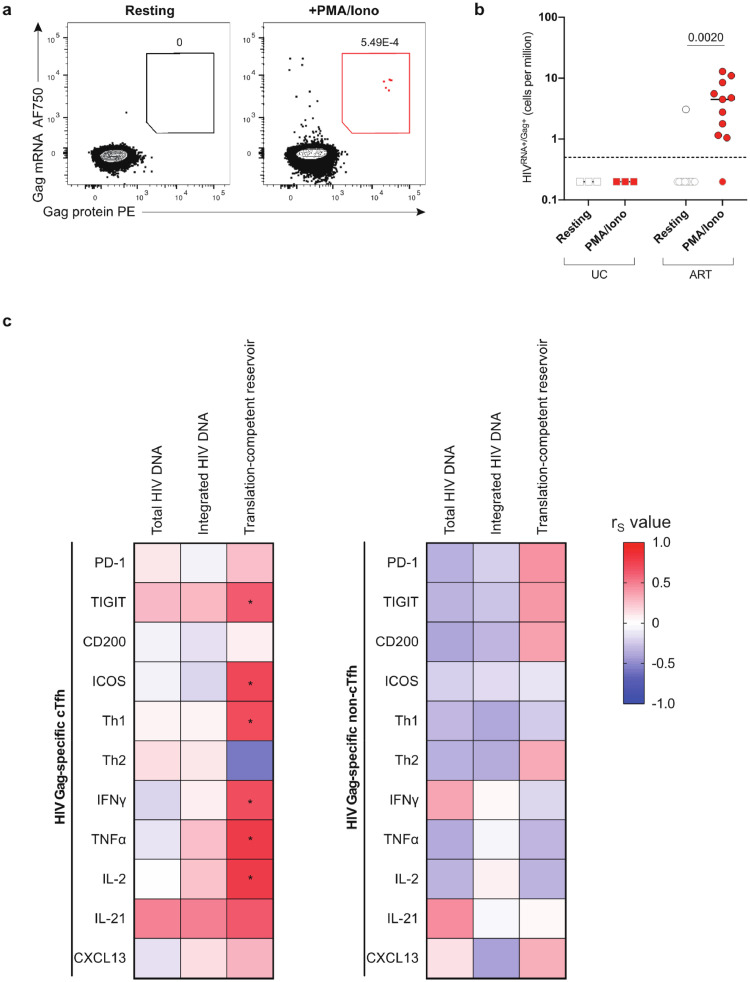
As the phenotypic profiles of HIV-specific cTfh cells during untreated infection and during ART were similar, we next explored possible links between these maintained features detected in our analyses and markers of HIV persistence during ART. We assessed the size of the HIV reservoir in CD4+ T cells using PCR for total viral DNA (vDNA), PCR for integrated vDNA and by measuring the inducible translation-competent reservoir by an RNA flow cytometric fluorescent in situ hybridization (RNA flow-FISH) assay. With this method, the translation-competent reservoir is defined as CD4+ T cells capable of co-expressing HIV gag RNA and Gag protein after in vitro stimulation (Fig. 7a) [24,25]. Translation-competent HIVRNA+/Gag+ CD4+ cells were undetectable in most ART-treated individuals in the absence of stimulation and in HIV-uninfected controls (Fig. 7b, Table S3), but could be identified in ART-treated donors after stimulation with PMA/Ionomycin with varying frequencies (Fig. 7b). DNA PCR-based methods detect defective viral genomes or proviruses unable to express viral proteins, and as expected the two PCR assays gave much larger estimates than the RNA flow-FISH method (Fig S6a). As low Ag persistence might contribute to maintain the pool of virus-specific T cells on ART, we examined the relationship between reservoir size and magnitude of HIV-specific CD4+ T cell responses. We observed no significant association between the frequency of HIV-specific CD4+ T cells or cTfh responses and vDNA reservoir measurements but a non-significant correlation between the magnitude of the HIV-specific CD4+ T cell response and the translation-competent reservoir (Fig. S6b).
Fig. 7.
Th1-like phenotype and function of HIV-specific cTfh correlates with size of the translation-competent reservoir. (a) Example plot showing CD4+ T cells from one representative ART-treated donor expressing HIV gag mRNA and HIV Gag protein after resting or stimulation with PMA/Ionomycin. (b) Frequency of HIVRNA+/Gag+ CD4+ T cells in uninfected control (UC) or ART-treated individuals in resting CD4+ or after PMA/Ionomycin stimulation. Gray-bordered symbols are below limit of detection (dotted line). Lines represent median values. (c) Heatmap showing associations between phenotype and function of HIV-specific cTfh (left graph) or HIV-specific non-cTfh (right graph) with total HIV DNA, integrated HIV DNA and translation-competent reservoir. Color represents R value for each association calculated and *p < 0.05 indicates significant correlations (Spearman correlation). n = 3 UC (uninfected control donors), n = 11 ART for (b); n = 23 ART for correlations with total or integrated HIV DNA, n = 11 for correlations with translation-competent reservoir (c). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We next explored potential relationships between the size of the total or integrated vDNA reservoir and phenotypic or functional features of HIV-specific cTfh or non-cTfh responses and found no significant association (Fig. 7c). In contrast, the size of the translation-competent HIV reservoir during ART was significantly positively associated with TIGIT, ICOS expression and the Th1-like phenotype and -function of HIV-specific cTfh (Fig. 7c, S6c-d). Of note, no association was detected between the size of the translation-competent reservoir and the phenotype or function of HIV-specific non-cTfh in ART-treated individuals (Fig. 7c). Therefore, our results indicate that the expression of IRs and Th1 functions of HIV Gag-specific cTfh cells correlate with a marker of the functional viral reservoir in individuals on ART. This suggests that the low but continuous levels of HIV Ag production may contribute to the maintenance of this population after prolonged therapy.
4. Discussion
Tfh cells are of high interest in HIV infection: at the total subset level, they serve as important sites of viral replication pre-ART [12] and reservoirs post-ART [13]. Studies of the total cTfh compartment show positive associations between memory Tfh cells and generation of HIV-specific broadly neutralizing antibodies (bNAbs) [30,44,45], but investigations of HIV-specific cTfh have been hampered by the lack of sensitive tools to assess them. It remained unclear whether frequencies of HIV-specific Tfh cells, and/or altered functions, set them apart from Tfh specific for other viruses. Here, we used cytokine-independent AIM assays, alone or in combination with functional readouts, to characterize HIV-specific cTfh. We found that compared to their CMV- and HBV-specific counterparts in the same donors, HIV-specific cTfh, defined as CXCR5+ memory cells, were more abundant and that this difference was exacerbated after ART initiation. On suppressive therapy, HIV-specific cTfh maintained a distinct phenotype with high expression of IRs, high production of cytokines, and a Th1-like skewing. Positive correlations between the magnitude, phenotype and function of HIV-specific cTfh responses with HIV-specific antibody levels and with the size of the translation-competent reservoir suggest that persistent exposure to viral products contribute to ongoing stimulation of HIV-specific Tfh despite undetectable HIV viremia on ART.
Our experimental approach gives a different picture of the impact of ART on HIV-specific CD4+ T cell responses compared to traditional assays such as IFNγ ICS. HIV-specific CD4+ T cell responses were detectable in all ART-treated individuals examined by the AIM assay, in contrast to IFNγ ICS. Matched samples obtained before and after ART initiation demonstrated a clear contraction of IFNγ+ HIV-specific CD4+ T cell responses, whereas AIM+ responses were maintained. Therefore, the impact of ART on the magnitude of HIV-specific CD4+ T cells may have been overestimated in previous reports using IFNγ as a readout [46]. Rather than massive attrition, smaller changes in frequency associated with more dramatic changes in polarization, differentiation and function of HIV-specific CD4+ T cells occur after viral suppression.
Many studies suggest that Tfh cells and GC play a critical role in the ability of the immune system to generate bNAbs, which are uncommon, leading to the hypothesis that rarity and/or qualitative defects of HIV-specific Tfh could be limiting factors in the bNAb generation in infected humans [47]. Here, we demonstrate that, surprisingly, the median fraction of cTfh within the HIV-specific CD4+ T cell population in ART-treated people is large, 3.7-fold greater than in CMV-specific and 2.8-fold greater than in HBV-specific CD4+ T cells. The frequencies of HIV-specific cTfh observed by AIM assay were similar to the frequencies measured by tetramer staining in our experiments and to those reported by another group [18]. These results also confirm the capacity of the CD69/CD40L AIM assay to sensitively detect cTfh to chronic pathogens, besides its capacity to detect acute cTfh responses, as has been recently shown for the flu vaccine [20]. As these comparisons were made on an intra-individual basis, they control for the frequent persistence of an inflammatory environment despite suppressive ART and potential for bystander changes in T cell responses, therefore demonstrating actual virus-specific differences in cTfh responses. These expanded HIV-specific cTfh also present distinct phenotypic and functional features: they express higher levels of the IRs PD-1, TIGIT and CD200 compared to their CMV- and HBV-specific counterparts yet produce more cytokines. This augmented production includes not only the Tfh-cytokines CXCL13 and IL-21, but also the Th1 cytokines IFN-γ, TNFα, and IL-2, consistent with the Th1-like phenotype of high CXCR3 expression. The production of Tfh-associated cytokines CXCL13 and IL-21 was highest in Ag-specific cTfh cells expressing multiple IRs. Previous studied demonstrated the importance of PD-1 for optimal Tfh cell positioning and function including IL-21 production in vivo [37].
In this manuscript, we compare our findings related to HIV-specific CD4+ T cells to responses specific to CMV, which also causes a chronic infection albeit with some differences. The length of CMV infection in our cohort is likely greater when compared to HIV. However, CMV viral loads are usually undetectable in ART-treated non-immunocompromised individuals [48]. Life-long CMV Ag exposure can induce a particularly large T cell response, which is characterized by high Th1-related cytokine expression including cytolytic markers and expansion of senescent cells [49]. However, we detected these functions only in the CMV-specific non-cTfh population. No cTfh expansion nor preferential IR upregulation in CMV-specific CD4+ T cell responses was detected in our cohort, similar to HBV-specific responses induced by vaccination or resolved infections. In contrast, other studies observed a Tfh expansion and Th1-phenotype in other chronic infections that share some features with HIV, such as LCMV clone 13 in mice [5], SIV in non-human primates [50], HCV [6], and malaria [51], in humans. The underlying mechanisms are poorly understood, but several studies suggested that increased duration of Ag exposure, high levels of Ag and inflammatory cytokines, common for these infections, favor this polarization (reviewed in [52]).
Two non-mutually exclusive mechanisms may be responsible for maintaining the characteristics of virus-specific CD4+ T cell responses during viral suppression on ART. First, persistent low-level HIV Ag expression could continuously stimulate Tfh responses on ART and maintain HIV-specific cTfh phenotype and function. Importantly, such a mechanism does not imply residual replication of the full virus, as abortive expression of some viral genes or trapped Ag, for example on follicular dendritic cells, may have similar consequences [53]. HIV Ags can be detected in lymphoid tissue of ART-treated donors [54,55]. In support of this hypothesis, we detected a significant correlation between the size of the translation-competent reservoir and expression of TIGIT, ICOS and Th1-like phenotype and function of HIV-specific cTfh but not non-cTfh cells. In contrast, HIV DNA measurements, which include a large fraction of defective genomes unable to produce viral Ags [56], were not associated. One possible explanation to our observations is that occasional reactivation of persistently infected cells leads to stochastic production of viral proteins within the GCs of lymphoid tissues and stimulation of HIV-specific Tfh cells. These cells eventually exit the GC when the burst of HIV products resolves, and subsequently become cTfh cells. A second possibility of persistent characteristics of HIV-specific CD4+ T cells is permanent epigenetic programming. Previous studies demonstrated that during chronic viral exposure, virus-specific CD8+ T cells in mice and humans are epigenetically altered so that PD-1 expression was persistently elevated even after Ag withdrawal [57], [58], [59], [60]. Thus, maintenance of certain phenotypic profiles in HIV-specific cTfh cells during ART could be related to an epigenetic reprogramming due to high levels of chronic Ag exposure during untreated infection.
We recently showed that HIV-specific CD4+ T cells in untreated HIV infection are characterized by an atypical Tfh signature in CXCR5− cells [4]. Longitudinal samples analyzed before and after ART initiation demonstrated a decrease of a Tfh transcriptional signature and a reduction of phenotypic markers including PD-1, TIGIT, CD200 and CXCR3 in HIV-specific CD4+T cells at the RNA level [4]. Here, we demonstrate that the proportion of CXCR5+ cTfh cells within HIV-specific CD4+ responses not only remains expanded, but increases on therapy. While the phenotype of HIV-specific cTfh cells does not change with ART, non-cTfh cells experience a decline in cells co-expressing multiple IRs and Th1-like phenotype. Taken together, these data suggest that some CXCR5− HIV-specific CD4+ T cells with Tfh-like properties identified in viremic infection [61], might gain (or recover) the ability to express CXCR5; and that the previously observed reduction of Tfh-related gene expression and phenotypic markers in HIV-specific CD4+ T cells on ART [4], is not due to a reduction of cTfh cells, but related to partial normalization of cTfh markers in HIV-specific non-cTfh responses.
This work raises questions that will need to be addressed in further studies. Our report focuses on the characterization of cTfh responses and the blood HIV reservoir. Studies on secondary lymphoid tissues could provide critical information regarding Ag persistence and its impact on HIV-specific Tfh alterations. Of note, HIV Gag-specific Tfh cells expressing Th1-like cytokines have been detected in lymphoid tissues of HIV-infected individuals [11,12,41], suggesting that some of the features we identified may be shared between the peripheral blood and secondary lymphoid tissue compartments. As these are very challenging to conduct in humans, investigations in non-human primates could prove powerful alternatives. Further detailed analyses on pre- vs post-ART samples will be essential to better understand subset-related changes in HIV-specific CD4+ T cells and their ontogeny. Taking into account our observations into the advanced immunomonitoring of interventional studies will allow addressing a key question: what are the immunological consequences of the distinct characteristics of HIV-specific cTfh on ART? Our results show a direct correlation of these responses with anti-p24 and anti-gp120 Ab titers, whereas there is no such association between Ab levels and magnitude of non-cTfh HIV-specific CD4+ T cell responses. This suggests that ongoing HIV Ag stimulation in lymphoid tissues leads to persistent induction and/or maintenance of HIV-specific B cell, Ab and cTfh expansion. As therapeutic vaccination to complement ART is an important strategy considered to achieve HIV cure, future studies should address how such pre-existing, expanded HIV-specific Tfh would impact development of new Thelper and/or B cell responses. Animal models suggest that overabundant Ag-specific Tfh, especially if qualitatively impaired, may be detrimental, leading to low affinity responses [62,63]. Such studies should address whether priming of new, effective Tfh responses may be hampered by competition from the boosting of large pre-existing cell populations. Finally, the presence of marked quantitative and qualitative differences in the cTfh specific for different viruses has likely relevance beyond HIV vaccine development and therapy and may be important for other pathogens for which the human system frequently fails to develop high-quality immune responses.
Declaration of Competing Interest
The authors have declared that no conflict of interest exists.
Acknowledgments
Acknowledgments
We thank Josée Girouard, the clinical staff at the McGill University Health Centre in Montreal and all study participants for their invaluable role in this project; Dr. Dominique Gauchat, Philippe St-Onge and the CRCHUM Flow Cytometry Platform as well as Dr. Olfa Debbeche and the CRCHUM BSL3 platform for technical assistance. The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: Anti-HIV-1 SF2 p24 Polyclonal and HIV-1 IIIB p24 Recombinant Protein from ImmunoDX, LLC. The following reagents were obtained through the NIH Tetramer Core Facility: HLA Class II tetramers DRB1*01:01 DRFYKTLRAEQASQEV-PE and DRB1*11:01-YVDRFYKTLRAEQASQEV-PE. Fig. S1A was prepared using images from Servier Medical Art by Servier, which is licensed under a Creative Commons Attribution 3.0 Unported License.
Funding Sources
This study was supported by the National Institutes of Health (no. HL092565 to D.E.K., no. AI100663 and no. AI144462 CHAVI-ID to D.E.K. (Consortium PI: Dennis Burton); the Canadian Institutes of Health Research grants (no. 137694 and no. 320721 to D.E.K.; foundation grant no. 352417 to A.F.; no. 36440 to N.C.); the Canada Foundation for Innovation Program Leader grant (no. 31756 to D.E.K.); and the FRQS AIDS and Infectious Diseases Network. D.E.K. is a FRQS Merit Research Scholar. N.C. is supported by a FRQS Research Scholar Career Award. J.N. is supported by scholarships from the FRQS, the Bavarian Research Alliance (BayFor) and the Department of Microbiology, Infectious Diseases and Immunology of the University of Montreal. A.E.B. is supported by a CIHR post-doctoral fellowship. J.R. is the recipient of a Mathilde Krim Fellowship in Basic Biomedical Research from AmfAR. I.T. is supported by a CIHR scholarship. J.P.R. is the holder of the Louis Lowenstain Chair in Hematology & Oncology, McGill University. A.F. is supported by a Canada Research Chair Award. The funding sources had no role in: study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102727.
Appendix. Supplementary materials
References
- 1.Matloubian M., Concepcion R.J., Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laidlaw B.J., Craft J.E., Kaech S.M. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat Rev Immunol. 2016;16:102–111. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLane L.M., Abdel-Hakeem M.S., Wherry E.J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 4.Morou A., Brunet-Ratnasingham E., Dubé M. Altered differentiation is central to HIV-specific CD4(+) T cell dysfunction in progressive disease. Nat Immunol. 2019;20:1059–1070. doi: 10.1038/s41590-019-0418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahey L.M., Wilson E.B., Elsaesser H., Fistonich C.D., McGavern D.B., Brooks D.G. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raziorrouh B., Sacher K., Tawar R.G. Virus-Specific CD4+ T cells have functional and phenotypic characteristics of follicular T-Helper cells in patients with acute and chronic HCV infections. Gastroenterology. 2016;150:696–706. doi: 10.1053/j.gastro.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vella L.A., Buggert M., Manne S. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J Clin Invest. 2019;129:3185–3200. doi: 10.1172/JCI125628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heit A., Schmitz F., Gerdts S. Vaccination establishes clonal relatives of germinal center T cells in the blood of humans. J Exp Med. 2017;214:2139–2152. doi: 10.1084/jem.20161794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greczmiel U., Krautler N.J., Pedrioli A. Sustained T follicular helper cell response is essential for control of chronic viral infection. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aam8686. [DOI] [PubMed] [Google Scholar]
- 11.Lindqvist M., van Lunzen J., Soghoian D.Z. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perreau M., Savoye A.-.L., De Crignis E. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banga R., Procopio F.A., Noto A. PD-1+ and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat. Med. 2016;22:754–761. doi: 10.1038/nm.4113. [DOI] [PubMed] [Google Scholar]
- 14.Wendel B.S., del Alcazar D., He C. The receptor repertoire and functional profile of follicular T cells in HIV-infected lymph nodes. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aan8884. eaan8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cubas R.A., Mudd J.C., Savoye A.-.L. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat. Med. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagard C., Oxenius A., Gunthard H. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med. 2003;163:1220–1226. doi: 10.1001/archinte.163.10.1220. [DOI] [PubMed] [Google Scholar]
- 17.Cubas R., van Grevenynghe J., Wills S. Reversible reprogramming of circulating memory t follicular helper cell function during chronic HIV infection. J Immunol. 2015;195:5625–5636. doi: 10.4049/jimmunol.1501524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claireaux M., Galperin M., Benati D. A high frequency of HIV-specific circulating follicular helper T cells is associated with preserved memory B cell responses in HIV controllers. MBio. 2018;9:e00317–e00318. doi: 10.1128/mBio.00317-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiss S., Baxter A.E., Cirelli K.M. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0186998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallikkuth S., de Armas L.R., Rinaldi S. Dysfunctional peripheral t follicular helper cells dominate in people with impaired influenza vaccine responses: results from the Florah study. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roederer M., Nozzi J., Nason M. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann D.E., Bailey P.M., Sidney J. Comprehensive analysis of human immunodeficiency virus type 1-Specific CD4 responses reveals marked immunodominance of gag and nef and the presence of broadly recognized peptides. J. Virol. 2004;78:4463–4477. doi: 10.1128/JVI.78.9.4463-4477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandergeeten C., Fromentin R., Merlini E. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J. Virol. 2014;88:12385–12396. doi: 10.1128/JVI.00609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baxter A.E., Niessl J., Fromentin R. Single-Cell characterization of viral translation- competent reservoirs in HIV-infected individuals. Cell Host Microbe. 2016;20:368–380. doi: 10.1016/j.chom.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baxter A.E., Niessl J., Fromentin R. Multiparametric characterization of rare HIV-infected cells using an RNA-flow FISH technique. Nat Protoc. 2017;12:2029–2049. doi: 10.1038/nprot.2017.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richard J., Veillette M., Batraville L.-.A. Flow cytometry-based assay to study HIV-1 gp120 specific antibody-dependent cellular cytotoxicity responses. J. Virol. Methods. 2014;208:107–114. doi: 10.1016/j.jviromet.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Dan J.M., Lindestam Arlehamn C.S., Weiskopf D. A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J Immunol. 2016 doi: 10.4049/jimmunol.1600318. published online June 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hokey D.A., Johnson F.B., Smith J. Activation drives PD-1 expression during vaccine-specific proliferation and following lentiviral infection in macaques. Eur J Immunol. 2008;38:1435–1445. doi: 10.1002/eji.200737857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porichis F., Kwon D.S., Zupkosky J. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 2011;118:965–974. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locci M., Havenar-Daughton C., Landais E. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing hiv antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi Y.S., Yang J.A., Yusuf I. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. J Immunol. 2013;190:4014–4026. doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuertes Marraco S.A., Neubert N.J., Verdeil G., Speiser D.E. Inhibitory receptors beyond T cell exhaustion. Front Immunol. 2015;6:1–14. doi: 10.3389/fimmu.2015.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Attanasio J., Wherry E.J. Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity. 2016;44:1052–1068. doi: 10.1016/j.immuni.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herati R.S., Muselman A., Vella L. Successive annual influenza vaccination induces a recurrent oligoclonotypic memory response in circulating T follicular helper cells. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aag2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chevalier N., Jarrossay D., Ho E. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 36.Iannello A., Boulassel M.-.R., Samarani S. Dynamics and consequences of IL-21 production in HIV-Infected individuals: a longitudinal and cross-sectional study. J Immunol. 2010;184:114. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- 37.Shi J., Hou S., Fang Q., Liu X., Liu X., Qi H. PD-1 controls follicular T helper cell positioning and function. Immunity. 2018;49:264–274. doi: 10.1016/j.immuni.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonecchi R., Bianchi G., Bordignon P.P. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acosta-Rodriguez E.V., Rivino L., Geginat J. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt N., Bentebibel S.-.E., Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35:436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buggert M., Nguyen S., McLane L.M. Limited immune surveillance in lymphoid tissue by cytolytic CD4+ T cells during health and HIV disease. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Gach J.S., Achenbach C.J., Chromikova V. HIV-1 specific antibody titers and neutralization among chronically infected patients on long-term suppressive antiretroviral therapy (ART): a cross-sectional study. PLoS ONE. 2014;9:e85371. doi: 10.1371/journal.pone.0085371. –10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binley J.M., Trkola A., Ketas T. The effect of highly active antiretroviral therapy on binding and neutralizing antibody responses to human immunodeficiency virus type 1 infection. J Infect Dis. 2000;182:945–949. doi: 10.1086/315774. [DOI] [PubMed] [Google Scholar]
- 44.Moody M.A., Pedroza-Pacheco I., Vandergrift N.A. Immune perturbations in HIV-1-infected individuals who make broadly neutralizing antibodies. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aag0851. aag0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen K., Altfeld M., Alter G., Stamatatos L. Early preservation of CXCR5+ PD-1+ helper T cells and B cell activation predict the breadth of neutralizing antibody responses in chronic HIV-1 infection. J. Virol. 2014;88:13310–13321. doi: 10.1128/JVI.02186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitcher C.J., Quittner C., Peterson D.M. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 47.Burton D.R., Ahmed R., Barouch D.H. A blueprint for HIV vaccine discovery. Cell Host Microbe. 2012;12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goossens V.J., Wolffs P.F., van Loo I.H., Bruggeman C.A., Verbon A. CMV DNA levels and CMV gB subtypes in ART-naive HAART-treated patients: a 2-year follow-up study in The Netherlands. AIDS. 2009;23:1425–1429. doi: 10.1097/QAD.0b013e32832c165c. [DOI] [PubMed] [Google Scholar]
- 49.Casazza J.P., Brenchley J.M., Hill B.J. Autocrine production of β-chemokines protects CMV-specific CD4+ T cells from HIV infection. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Velu V., Mylvaganam G.H., Gangadhara S. Induction of Th1-biased T follicular helper (Tfh) cells in lymphoid tissues during chronic simian immunodeficiency virus infection defines functionally distinct germinal center Tfh cells. J Immunol. 2016;197:1832–1842. doi: 10.4049/jimmunol.1600143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obeng-Adjei N., Portugal S., Tran T.M. Circulating Th1 cell-type Tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. CellReports. 2015;13:425–439. doi: 10.1016/j.celrep.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vella L.A., Herati R.S., Wherry E.J. CD4(+) T cell differentiation in chronic viral infections: the Tfh perspective. Trends Mol Med. 2017;23:1072–1087. doi: 10.1016/j.molmed.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baxter A.E., O'Doherty U., Kaufmann D.E. Beyond the replication-competent HIV reservoir: transcription and translation-competent reservoirs. Retrovirology. 2018;15:18. doi: 10.1186/s12977-018-0392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Estes J.D., Kityo C., Ssali F. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med. 2017;23:1271–1276. doi: 10.1038/nm.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stellbrink H.-.J., van Lunzen J., Westby M. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial) AIDS. 2002;16:1479–1487. doi: 10.1097/00002030-200207260-00004. [DOI] [PubMed] [Google Scholar]
- 56.Bruner K.M., Murray A.J., Pollack R.A. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016;22:1043–1049. doi: 10.1038/nm.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pauken K.E., Sammons M.A., Odorizzi P.M. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sen D.R., Kaminski J., Barnitz R.A. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Youngblood B., Noto A., Porichis F. Cutting edge: prolonged exposure to hiv reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol. 2013;191:540. doi: 10.4049/jimmunol.1203161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youngblood B., Oestreich K.J., Ha S.-.J. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35:400–412. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.del Alcazar D., Wang Y., He C. Mapping the lineage relationship between CXCR5(+) and CXCR5(-) CD4(+) T cells in HIV-Infected human lymph nodes. CellReports. 2019;28:3047. doi: 10.1016/j.celrep.2019.08.037. –7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Recher M., Lang K.S., Hunziker L. Deliberate removal of T cell help improves virus-neutralizing antibody production. Nat Immunol. 2004;5:934–942. doi: 10.1038/ni1102. [DOI] [PubMed] [Google Scholar]
- 63.Preite S., Baumjohann D., Foglierini M. Somatic mutations and affinity maturation are impaired by excessive numbers of T follicular helper cells and restored by Treg cells or memory T cells. Eur J Immunol. 2015;45:3010–3021. doi: 10.1002/eji.201545920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw experimental data associated with the figures presented in the manuscript are available from the corresponding author upon reasonable request.