Abstract
Purpose
Carbon ion beams have several physical and biological advantages compared with conventional radiation for cancer therapy. The objective of this study is to evaluate the safety and effectiveness of 2-fraction carbon ion radiation therapy (CIRT) in patients with hepatocellular carcinoma (HCC).
Methods and Materials
Between December 2008 and March 2013, 57 patients with localized HCC were treated with CIRT at a total dose of 45 Gy (relative biological effectiveness) in 2 fractions and retrospectively analyzed after long-term observation. The main endpoints of this study were treatment-related toxicity and local tumor control. Toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Changes in the Child-Pugh score from before to after CIRT were also examined to evaluate hepatic toxicity. Local control was defined as no progression of the irradiated lesion according to the modified Response Evaluation Criteria in Solid Tumors.
Results
The median age of the patients was 75 years (range, 49-89 years). Of these patients, 41 had a newly diagnosed lesion, and 16 had residual or recurrent lesions after previous treatments. The median follow-up duration was 54 months (range, 7-103 months). All surviving patients were followed for more than 51 months. Two patients experienced grade 3 acute skin reactions, but no other grade 3 or higher toxicities were observed in any organ. No patient exhibited an increase in the Child-Pugh score of 2 or more points after CIRT. The local tumor control rates at 1, 3, and 5 years were 98%, 91%, and 91% after CIRT, respectively. All lesions that failed to respond to previous treatments were successfully controlled by CIRT. The 1-, 3-, and 5-year overall survival rates were 97%, 67%, and 45%, respectively.
Conclusions
Two-fraction CIRT was a well-tolerated and effective treatment for patients with HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, and the incidence of HCC varies widely according to geographic location.1 Eastern Asia, including Japan, has one of the highest incidences of HCC. The majority of patients with HCC have chronic hepatitis B or C viral infection, and many already have liver dysfunction at the time of HCC diagnosis. In addition, they require repeated anticancer treatments due to multifocal carcinogenesis of HCC. Therefore, minimizing treatment invasiveness and improving therapeutic effectiveness are important for the treatment of HCC.
Historically, the role of radiation therapy in the treatment of liver tumors was limited in terms of radiation-induced hepatic insufficiency caused by whole-liver irradiation.2,3 However, recent progress in the development of irradiation devices and technology has enabled highly localized irradiation, thereby reducing the degree of toxicity,4, 5, 6 and has spurred advances in radiation therapy research for liver cancers.7
Carbon ion beams have several physical and biological advantages compared with conventional radiation used for cancer therapy.8,9 We have been using carbon ion beams for the treatment of HCC at the National Institute of Radiological Sciences (NIRS) in Japan since 1995.10 Four clinical studies were carried out to determine the optimal dose of carbon ion radiation therapy (CIRT) based on a dose-escalating protocol with 5 different fractionation schedules, using 15, 12, 8, 4, and 2 fractions.10, 11, 12 Most of the patients enrolled in these studies were not amenable to, or experienced recurrence after, previous treatments or had no prospect of an adequate treatment effect with any existing therapy. There were no severe adverse events with any of the fractionation treatments, and the duration of treatment was safely reduced from 5 weeks to 2 days. However, after long-term observation in the last phase 1/2 clinical trial of 2-fraction CIRT using a dose of 38.8 Gy (relative biological effectiveness [RBE]) or less, the local control rate was not as good as those of previous clinical trials. We speculated that the inferior local control rate was attributed to an inadequate dose. Thus, we gradually escalated the dose up to 45 Gy (RBE) and found in a preliminary analysis that the antitumor effect tended to improve.13
The objective of this retrospective study was to confirm the safety and effectiveness of high-dose (45 Gy [RBE]) 2-fraction CIRT in patients with HCC after long-term observation.
Methods and Materials
Patients
Patients who underwent 2-fraction CIRT at a dose of 45 Gy (RBE) and met the following conditions were analyzed in this study: (1) pathologically proven HCC or a clinical diagnosis of HCC by triphasic (arterial, portal, and delayed) dynamic contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI); (2) a solitary tumor or multiple tumors that were all treated within a single target volume as a whole; (3) no other active HCC; (4) Child-Pugh grade A or B liver function; (5) no tumor invasion into the main trunk of the portal vein or inferior vena cava; (6) tumors located more than 1 cm away from the digestive tract; (7) Eastern Cooperative Oncology Group performance status of 0 to 2; (8) no uncontrolled ascites; and (9) no other active cancers.
This study was conducted in accordance with the ethical standards set forth by the Declaration of Helsinki and was approved by the NIRS Ethics Committee on Human Clinical Research. Written informed consent for the treatment and follow-up study was obtained from all patients and their relatives before treatment.
Carbon ion radiation therapy
Before treatment planning, 1 or 2 metal markers, as landmarks for the target location, were implanted percutaneously into the hepatic parenchyma near the tumor under ultrasound imaging guidance. A customized cradle (Moldcare; Alcare, Tokyo, Japan) and a low-temperature thermoplastic sheet (Shellfitter; Kuraray, Osaka, Japan) were used for immobilization of patients to position the patient accurately. After immobilization, planning CT images were acquired using a respiratory gating system.14 Three-dimensional treatment planning using expiratory phase, 2.5 mm thick CT images was performed using the HIPLAN software program (NIRS, Chiba, Japan)15 or XiO-N (ELEKTA, Stockholm, Sweden and Mitsubishi Electric, Tokyo, Japan).
The gross target volume was identified as disease by contrast-enhanced CT or contrast-enhanced MRI. The clinical target volume was defined as the gross target volume plus a 5-mm margin to compensate for subclinical microscopic disease. The planning target volume (PTV) was defined as the clinical target volume plus a 5-mm margin to compensate for interfraction and intrafraction variations. For targets just below the diaphragm, we devised the treatment volume by projecting structures involving the liver on caudal-slice CT images so that the distal edge of the spread-out Bragg peak would reliably cover the distal end of the PTV. To assess the accuracy of the patient’s position and target localization, just before each treatment session, we obtained orthogonal 2-directional x-ray images and matched the skeletal structure on digital reconstructed 2-dimensional radiographic images using treatment planning CT data and verified the location of fiducial markers and organs such as the diaphragm.
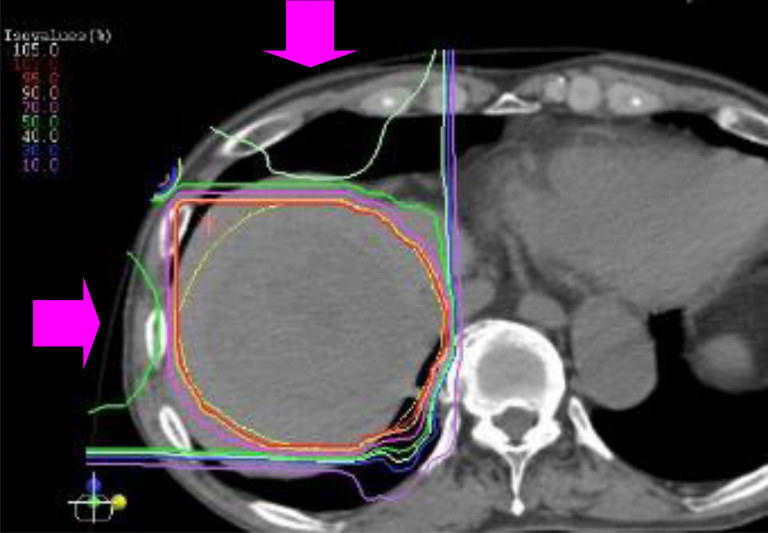
The accelerated energy of the carbon ion beams was 290 or 350 MeV vertically and 290 or 400 MeV horizontally, depending on the tumor location. Because beam ports are fixed horizontally and vertically, the patients were treated in a more favorable position (supine and/or prone) to minimize the volume of the liver irradiated and to meet the dose constraints for organs at risk (OARs), such as the gastrointestinal tract and skin. Furthermore, the treatment couch was tilted as needed. Carbon ion beams were delivered during the expiratory phase using the respiratory gating system. Radiation dose was expressed in gray (RBE), derived by multiplication of physical dose by the RBE of carbon ions beams.5,16 The prescribed dose was 45 Gy (RBE) in 2 fractions over 2 consecutive days. The dose constraints for OARs were as follows: a maximum dose to the gastrointestinal tract of 15 Gy (RBE) and a maximum dose to the skin of 30 Gy (RBE). The beams were delivered in combination via 2 or 3 ports. A typical dose distribution is shown in Figure 1.
Figure 1.
Dose distribution of carbon ion beams in a patient with hepatocellular carcinoma 8 cm in diameter in the right superior segment of the liver. Beams were delivered in combination with anterior and lateral ports (dose ratio 1:1) in the prone position. The yellow line represents the planning target volume, and the red, orange, pink, and green curves represent the 95%, 90%, 70%, and 50% isodose lines, respectively.
Follow-up and evaluation
After CIRT, patients were followed at least once every 3 months for the first 2 years and once every 3 to 6 months thereafter. Follow-up studies included history taking, physical examination, a complete blood count, blood chemistry analysis, and contrast-enhanced CT or MRI examinations. The main objectives of this study were to evaluate treatment-related toxicity and local tumor control. Intrahepatic recurrence and overall survival were also evaluated.
Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Newly developed toxicities or toxicities that had increased in grade compared with baseline were considered adverse events. Acute toxicities were defined as adverse events occurring within 90 days and late toxicities as those occurring 91 days or later from the start of CIRT. The change in the Child-Pugh score from before to after CIRT was also determined to assess hepatic toxicity.
Local control was defined as freedom from local failure. Local failure was defined as progression of the irradiated lesion according to the modified Response Evaluation Criteria in Solid Tumors.17 If progression of coexisting cirrhosis but no active HCC was observed at the time of death, the cause of death was defined as liver failure.
Statistical analysis
Local control and overall survival were calculated from the first day of CIRT using the Kaplan-Meier method. Statistical significance was defined as P < .05. Statistical analyses were performed using Stat View, version 5 (SAS Institute, Inc, Cary, NC).
Results
Patient characteristics and follow-up
Between December 2008 and March 2013, 57 consecutive patients with HCC who met the eligibility criteria were included in this study. The pretreatment characteristics of all patients are presented in Table 1. The patients consisted of 33 males and 24 females with a median age of 75 years (range, 49-89 years); 17 of the 57 patients (30%) were aged 80 years or older. Twenty-two patients (39%) had a history of treatment for HCC, including surgical resection (n = 8), transcatheter arterial chemoembolization (TACE, n = 14), and radiofrequency ablation (RFA, n = 5). The degree of liver impairment was classified as Child-Pugh grade A in 51 patients and grade B in 6 patients. The median maximum tumor diameter was 33 mm (range, 13-95 mm), with 31 patients (54%) having a diameter greater than 30 mm. There were 41 patients (72%) with a newly diagnosed lesion and 16 (28%) with a residual or recurrent lesion after previous treatments, such as TACE and RFA. Forty-three patients (75%) were judged inappropriate for surgical resection and local ablation therapy for medical reasons, including insufficient liver function, comorbidities, and tumor location.
Table 1.
Patient and disease characteristics
| Characteristics | No. of patients (%) (n = 57) |
|---|---|
| Age (y) | |
| Median | 75 |
| Range | 49-89 |
| Sex | |
| Male | 33 (58) |
| Female | 24 (42) |
| Performance status | |
| 0 | 39 (68) |
| 1 | 17 (30) |
| 2 | 1 (2) |
| Viral infection | |
| HCV | 35 (61) |
| HBV | 5 (9) |
| Neither | 17 (30) |
| Child-Pugh classification | |
| A | 51 (89) |
| B | 6 (11) |
| No. of tumors | |
| Single | 56 (98) |
| Multiple | 1 (2) |
| Maximum tumor diameter (mm) | |
| Median | 33 |
| Range | 13-95 |
| Previous treatment for HCC | |
| No | 35 (61) |
| Yes | 22 (39) |
| Previous treatment for the target lesion | |
| No | 41 (72) |
| Yes | 16 (28) |
Abbreviations: HBV = hepatitis B virus; HCC = hepatocellular carcinoma; HCV = hepatitis C virus.
All patients completed the prescribed treatment. The median follow-up duration was 54 months (range, 7-103 months) for all patients and 73 months (range, 51-103 months) for the survivors. No patients were lost to follow-up at the time of analysis (end of December 2018).
Toxicity
The acute and late toxicities observed in this study are shown in Table 2. Two patients who developed multiple recurrence in the liver and underwent TACE at 3 months after CIRT were excluded from the evaluation of late hepatic toxicities. No patient experienced grade 3 or higher treatment-related hepatic toxicities in either the acute or late phase. Two patients (4%) experienced grade 3 acute dermatitis with desquamation. These reactions were most prominent around 1 month after CIRT and improved to grade 1 skin pigmentation and grade 1 skin atrophy in the late phase. No other grade 3 or higher toxicity was seen in any organ in either the acute or late phase. One patient developed symptomatic rib fracture, which improved without any medication. No patient showed an increase of 2 or more points in the Child-Pugh score in either the acute or late phase after CIRT (Table 3).
Table 2.
Acute and late toxicities after carbon ion radiation therapy
| Acute (n = 57) |
Late (n = 57) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade |
Grade |
|||||||||
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | |
| Skin | 2 | 49 | 4 | 2 | 0 | 9 | 47 | 1 | 0 | 0 |
| Liver∗ | 36 | 16 | 5 | 0 | 0 | 24 | 28 | 3 | 0 | 0 |
| Gastrointestinal | 57 | 0 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 0 |
| Lung | 45 | 12 | 0 | 0 | 0 | 33 | 24 | 0 | 0 | 0 |
| Bone | 57 | 0 | 0 | 0 | 0 | 56 | 0 | 1 | 0 | 0 |
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Two patients who developed multiple recurrence in the liver and underwent TACE at 3 months after CIRT were excluded from evaluation for late hepatic toxicities.
Table 3.
Child-Pugh score before and after carbon ion radiation therapy
| Score before CIRT | Score after CIRT |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acute phase (n = 57) |
Late phase (n = 55)∗ |
|||||||||
| 5 | 6 | 7 | 8 | ≧9 | 5 | 6 | 7 | 8 | ≧9 | |
| 5 (n = 43) | 38 | 5 | 0 | 0 | 0 | 38 | 3 | 0 | 0 | 0 |
| 6 (n = 8) | 2 | 5 | 1 | 0 | 0 | 2 | 6 | 0 | 0 | 0 |
| 7 (n = 5) | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 4 | 1 | 0 |
| 8 (n = 1) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
Abbreviation: CIRT = carbon ion radiation therapy.
Two patients who developed multiple recurrence in the liver and underwent transcatheter arterial chemoembolization at 3 months after carbon ion radiation therapy were excluded from late phase evaluation.
Local control and recurrence
Local failure was observed in 4 of 57 patients (7%) at 8, 18, 21, and 31 months after CIRT. The tumor diameters before CIRT in these 4 patients were 48, 23, 62, and 22 mm, respectively. Local failure was observed in 2 of 26 patients (8%) with tumors 3 cm or smaller in diameter and in 2 of 31 patients (7%) with tumors larger than 3 cm. These patients underwent salvage treatments for local recurrence. Of the 2 patients with a locally recurrent lesion larger than 3 cm in diameter, 1 received surgery and the other TACE. Of the 2 patients with a locally recurrent lesion 3 cm or smaller in diameter, 1 received RFA and the other repeat CIRT at a dose of 52.8 Gy (RBE) in 4 fractions, which we recommended in previous clinical trials. All 4 of these recurrent lesions were successfully controlled by salvage treatments. The patient who was re-treated with CIRT was alive without any treatment-related adverse events at the time of analysis 60 months after the second CIRT administration.
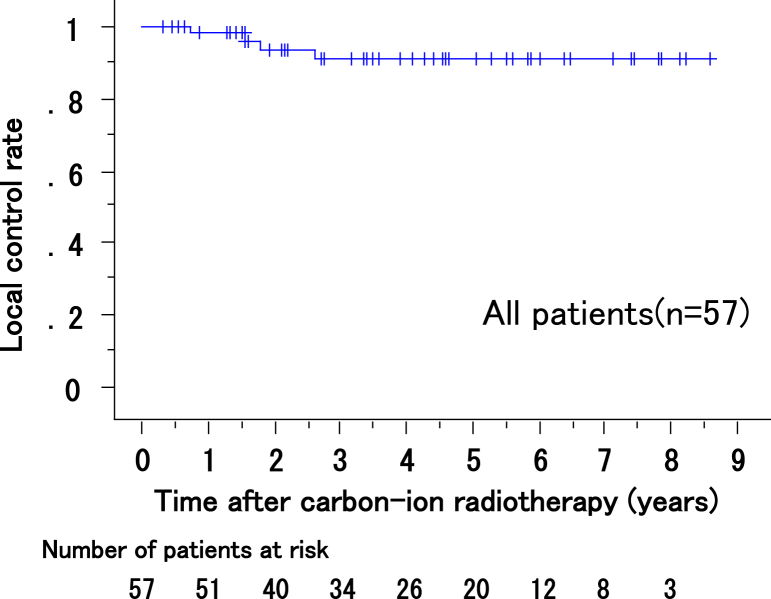
The local control rates at 1, 3, and 5 years for all patients were 98% (95% confidence interval [CI], 95%-100%), 91% (95% CI, 87%-95%), and 91% (95% CI, 87%-95%), respectively (Fig 2). All 16 lesions that failed to respond to previous treatment were successfully controlled by CIRT with an acceptable toxicity, the same as that of untreated lesions.
Figure 2.
Local control rates of all patients.
Intrahepatic recurrence outside of the irradiation field was seen in 38 of the 57 patients (67%). The intrahepatic recurrence-free rates at 1, 3, and 5 years were 77% (95% CI, 72%-83%), 43% (95% CI, 37%-50%), and 28% (95% CI, 21%-34%), respectively. The majority of the 38 patients who experienced intrahepatic recurrence underwent salvage treatments involving surgery, RFA, TACE, or CIRT. Four patients who showed localized out-of-field intrahepatic recurrence and were ineligible for other treatments were treated again with CIRT at a dose of 45 Gy (RBE) in 2 fractions. The cumulative dose in these 4 patients met the dose constraints for OARs described earlier, such as the digestive tract. These recurrent lesions were successfully controlled by the second round of CIRT without any severe toxicities. Distant metastasis was observed in 9 of the 57 patients (16%).
Survival
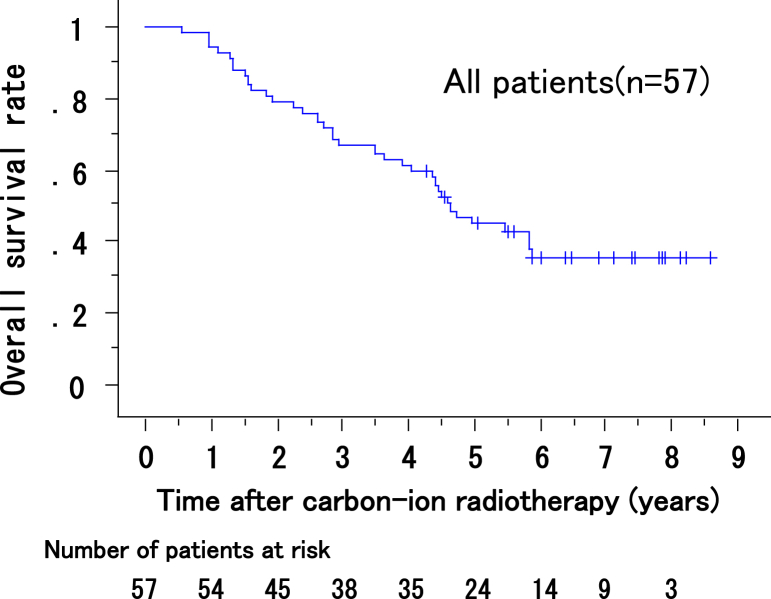
At the last follow-up, 23 of 57 patients were alive. Of the 34 patients who died, 21 died of HCC, and 6 died of hepatic failure. The remaining 7 patients died of nonhepatic causes, including other malignancies, ischemic cardiac disease, and pneumonia. Four of the 6 patients who died of hepatic failure experienced intrahepatic recurrence outside of the irradiation field after CIRT and received additional treatment; they died between 16 and 70 months after CIRT. The remaining 2 patients had no HCC progression and died of progression of coexisting liver cirrhosis at 43 and 53 months after CIRT, respectively. The overall survival rates of all 57 patents at 1, 3, and 5 years after CIRT were 97% (95% CI, 95%-100%), 67% (95% CI, 61%-74%), and 45%, (95% CI, 38%-51%) respectively (Fig 3). The median overall survival was 56 months.
Figure 3.
Overall survival rates of all patients.
Discussion
During the past 2 decades, as a result of advanced technologies, use of radiation therapy in patients with liver cancer has been increasing rapidly.7 To our knowledge, 2-fraction irradiation is the shortest radiation therapy schedule for HCC. In the present study, no severe treatment-associated complications occurred in the liver or any other organ with 2-fraction CIRT at a total dose of 45 Gy (RBE). Among all 57 patients, no grade 3 or higher hepatic toxicities were observed in either the acute or late phase. In the assessment of hepatic toxicity, no patient showed an increase in the Child-Pugh score of 2 or more points after CIRT. We reported previously that CIRT using various dose-fraction schedules caused only minor liver damage in patients with HCC.10, 11, 12,18,19 The present study demonstrated that CIRT can be administered safely using a much shorter schedule and a relatively high dose if the lesion is not near the digestive tract. These results may be attributed to the unique characteristics of carbon ion beams, which can deliver a highly concentrated radiation dose to a small portion of the liver, with limited exposure to noncancerous tissues, more easily than can be done with conventional radiation therapy.
Local failure was observed in 4 of the 57 patients (7%) after long-term observation in this study. All local failures developed within 3 years after CIRT, and the 3 to 5-year local control rate was 91%. This result is comparable with those of previous clinical trials using 15, 12, 8, and 4 fractions.10,11 Studies of patients with HCC treated with conformal radiation therapy and stereotactic body radiation therapy (SBRT) have demonstrated 2- or 3-year local control rates of 38% to 100%20, 21, 22 and 82% to 100%,23, 24, 25, 26, 27 respectively. In many of the studies of conformal radiation therapy for HCC, radiation therapy was delivered in more than 20 fractions and combined with TACE. We have used CIRT alone over shorter schedules as a definitive treatment for HCC.10, 11, 12, 13
SBRT is delivered in approximately 3 to 6 fractions for HCC treatment.23, 24, 25, 26, 27, 28 SBRT mainly targets relatively small tumors. Takeda et al reported a 3-year local control rate of 96.3% in a phase 2 study of SBRT with or without TACE in patients with solitary HCC 4 cm or less in diameter.27 In a retrospective study, Wahl et al reported that the rate of freedom from local progression for tumors 2 cm or larger was better with SBRT than with RFA.28 The majority of the tumors were less than 3 cm in their study. Our present study included relatively large tumors, in that 54% of all tumors were larger than 3 cm in diameter, and the local control rate was not affected by tumor size.
Carbon ion beams and proton beams can deliver a high, concentrated dose to the target volume conformably while sparing the normal liver tissue, even for large tumors, compared with SBRT.29 Studies of proton beam radiation therapy including large tumors also showed 2- or 3-year local control rates of 86% to 91% in patients with HCC.30, 31, 32 Sugahara et al demonstrated a 2-year local control rate of 87% in patients with large HCC treated with proton beams at a median total dose of 72.6 Gy (RBE) in 22 fractions.31 Fukumitsu et al reported a 5-year local control rate of 87.8% in a prospective study of hypofractionated proton beam therapy using 66 Gy (RBE) in 10 fractions.32 To our knowledge, their treatment schedule is the shortest course of proton beam treatment that has been used for HCC.
Whereas the fractionation schedules were substantially different, the local control rates were similar between CIRT and proton beam radiation therapy. Our institute attempted to decrease the fraction number11, 12, 13 because hypofractionation is theoretically advantageous with high linear energy transfer radiation such as carbon ion beams. As a result, this short 2-fraction CIRT course exerted an antitumor effect similar to that of conventionally fractionated proton therapy.
In this study, 30% of the patients were 80 years or older, with a median age of 75 years. Short-course radiation therapy may ease the physical and social burdens on patients, especially elderly patients. In addition, the local control rates after CIRT were the same for patients with residual or recurrent lesions after previous treatments and those with untreated lesions. CIRT seemed to be useful as a salvage treatment for local failure after previous treatments.
In contrast to the low incidence of local failure in the radiation field, the incidence of intrahepatic recurrence outside of the irradiation field was very high (67%). A high incidence of intrahepatic recurrence after curative treatment of HCC has also been reported by others.33 This is a major problem for patients with HCC with chronic viral infections, especially hepatitis C. Recently, a meta-analysis showed that adjuvant interferon therapy after curative treatment prevents recurrence of HCC in patients with chronic viral infections.34 In addition, direct-acting antivirals, newly developed antiviral agents that can eradicate hepatitis C virus, may reduce HCC development after curative treatment. It is also important to follow the treated patient properly to monitor the development of intrahepatic recurrence.
Charged particle therapies such as CIRT and proton therapy can often be applied safely to intrahepatic recurrent lesions after initial local treatment with radiation therapy. This may be one of the advantages of charged particle therapies over photon beam radiation therapy, possibly contributing to prolonged survival. In the present study, 5 patients who developed intrahepatic recurrence after CIRT were treated with CIRT again. All of their recurrent lesions were well controlled by the second administration of CIRT without any severe adverse events. Recently, Oshiro et al reported the feasibility and efficacy of repeated proton beam therapy for HCC in a retrospective analysis.35
In the present study, the overall survival rates after CIRT of patients with HCC were 97%, 67%, and 45% at 1, 3, and 5 years, respectively. Considering that the proportion of elderly patients who were not suitable for surgery or local ablation therapy was high in this study, these results suggest that 2-fraction CIRT is a minimally invasive therapeutic option even for elderly patients with HCC. Of the 34 patients who died, 27 died of liver-related causes (ie, HCC or hepatic failure). Antiviral therapy, which prevents progression of cirrhosis and development of HCC, may reduce liver-related deaths after curative HCC treatment in patients with chronic viral infections.
A limitation of our study was that it was single-institution study involving a small number of patients. However, all patients who met the inclusion criteria were treated with CIRT using a consistent radiation dose, and a sufficient follow-up period was included in the analysis. Additional studies involving larger cohorts are needed to confirm the results, and evaluation of the therapeutic indications based on intergroup analysis would be useful.
Based on the results of this study, we are now conducting another study on 2-fraction CIRT at an even higher dose than 45 Gy (RBE) using new technology involving a respiratory-gated scanning method, which can deliver a high, concentrated dose to the target conformably.36 It is expected that toxicities in the surrounding tissues will be reduced with this technology. Furthermore, in the future, we plan to introduce treatment using a rotating gantry, which will make it easier to avoid organs at risk such as the gastrointestinal tract, and thus expansion of adaptation is expected.
Conclusions
High-dose 2-fraction CIRT is well tolerated and effective in patients with HCC, potentially even residual and recurrent lesions after previous treatments. Additional improvement of therapeutic outcomes is expected with the introduction of new therapeutic technologies in combination with new antiviral agents.
Acknowledgments
The authors are grateful to the members of the Working Group for Liver Tumor, Shigeki Arii, Masaaki Ebara, Junji Furuse, Kiyoshi Ohara, Takuji Okusaka, Takehito Otsubo, Masayuki Otsuka, Fumihoko Kanai, Fukuo Kondo, Akiko Saito, Yoshiaki Shimizu, Kenichi Takayasu, Ikuo Udagawa, Hiroshi Yamamoto, Junji Yamamoto, Masakazu Yamamoto, Osamu Yokosuka, Hiroyuki Yoshidome, for their constructive discussion and precious advice.
Footnotes
Sources of support: This study was supported by the Research Project with Heavy Ions at the National Institute of Radiological Sciences, Japan.
Disclosures: None.
Contributor Information
Shigeo Yasuda, Email: shigeo_yasuda@chibah.johas.go.jp.
Working Group for Liver Tumor:
Shigeki Arii, Masaaki Ebara, Junji Furuse, Kiyoshi Ohara, Takuji Okusaka, Takehito Otsubo, Masayuki Otsuka, Fumihoko Kanai, Fukuo Kondo, Akiko Saito, Yoshiaki Shimizu, Kenichi Takayasu, Ikuo Udagawa, Hiroshi Yamamoto, Junji Yamamoto, Masakazu Yamamoto, Osamu Yokosuka, and Hiroyuki Yoshidome
References
- 1.Torre L.A., Bray F., Siegel R.L. Cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ingold J.A., Reed G.B., Kaplan H.S. Radiation hepatitis. Am J Roentgenol. 1965;93:200–208. [PubMed] [Google Scholar]
- 3.Reed G.B., Jr., Cox A.J., Jr. The human liver after radiation injury: A form of veno-occlusive disease. Am J Pathol. 1966;48:597–611. [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson J.M., Lawrence T.S., Dworzanin L.M. Treatment of primary hepatobiliary cancers with conformal radiation therapy and regional chemotherapy. J Clin Oncol. 1993;11:1286–1293. doi: 10.1200/JCO.1993.11.7.1286. [DOI] [PubMed] [Google Scholar]
- 5.Kanai T., Endo M., Minohara S. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201–210. doi: 10.1016/s0360-3016(98)00544-6. [DOI] [PubMed] [Google Scholar]
- 6.Xi M., Liu M.Z., Deng X.W. Defining internal target volume (ITV) for hepatocellular carcinoma using four-dimensional CT. Radiother Oncol. 2007;84:272–278. doi: 10.1016/j.radonc.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Klein J., Dawson L.A. Hepatocellular carcinoma radiation therapy: Review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87:22–32. doi: 10.1016/j.ijrobp.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 8.Kanai T., Furusawa Y., Fukutsu K. Irradiation of mixed beam and design of spread-out Bragg peak for heavy-ion radiotherapy. Radiat Res. 1997;147:78–85. [PubMed] [Google Scholar]
- 9.Ando K., Koike S., Uzawa A. Biological gain of carbon-ion radiotherapy for the early response of tumor growth delay and against early response of skin reaction in mice. J Radiat Res. 2005;46:51–57. doi: 10.1269/jrr.46.51. [DOI] [PubMed] [Google Scholar]
- 10.Kato H., Tsujii H., Miyamoto T. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59:1468–1476. doi: 10.1016/j.ijrobp.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Kasuya G., Kato H., Yasuda S. Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: Combined analyses of 2 prospective trials. Cancer. 2017;123:3955–3965. doi: 10.1002/cncr.30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato H, Yamada S, Yasuda S, et al. Two-fraction carbon ion radiotherapy for hepatocellular carcinoma: Preliminary results of a phase I/II clinical trial [Abstract]. J Clin Oncol. 2005;23(Suppl):338s.
- 13.Yasuda S. Hepatocellular carcinoma. In: Tsujii H., Kamada T., Shirai T., Noda T., Tsuji H., Karasawa K., editors. Carbon-ion radiotherapy. Springer; Tokyo: 2014. pp. 213–218. [Google Scholar]
- 14.Minohara S., Kanai T., Endo M. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1097–1103. doi: 10.1016/s0360-3016(00)00524-1. [DOI] [PubMed] [Google Scholar]
- 15.Endo M., Koyama-Ito H., Minohara S. HIPLAN: a heavy-ion treatment planning system at HIMAC. J Jpn Soc Ther Radiol Oncol. 1996;8:231–238. [Google Scholar]
- 16.Inaniwa T., Kanematsu N., Matsufuji N. Refoermulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the National Institute of Radiological Sciences, Japan. Phys Med Biol. 2015;60:3271–3286. doi: 10.1088/0031-9155/60/8/3271. [DOI] [PubMed] [Google Scholar]
- 17.Lencioni R., Llovet J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 18.Imada H., Kato H., Yasuda S. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity of the porta hepatis. Radiother Oncol. 2010;96:231–235. doi: 10.1016/j.radonc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Imada H., Kato H., Yasuda S. Compensatory enlargement of the liver after treatment of hepatocellular carcinoma with carbon ion radiotherapy – Relation to prognosis and liver function. Radiother Oncol. 2010;96:236–242. doi: 10.1016/j.radonc.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Cheng J.C.-H., Chuang V.P., Cheng S.H. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable HCC. Int J Radiat Oncol Biol Phys. 2000;47:435–442. doi: 10.1016/s0360-3016(00)00462-4. [DOI] [PubMed] [Google Scholar]
- 21.Park W., Lim do H., Paik S.W. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan S., Dawson L.A., Seong J. Radiotherapy for hepatocellular carcinoma: An overview. Ann Surg Oncol. 2008;15:1015–1024. doi: 10.1245/s10434-007-9729-5. [DOI] [PubMed] [Google Scholar]
- 23.Andolino D.L., Johnson C.S., Maluccio M. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:447–453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Kang J.K., Kim M.S., Cho C.K. Stereotactic body radiation for inoperable hepatocellular carcinoma as a local salvage treatment after in complete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 25.Honda Y., Kimura T., Aikata H. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:530–536. doi: 10.1111/jgh.12087. [DOI] [PubMed] [Google Scholar]
- 26.Sanuki N., Takeda A., Oku Y. Stereotactic body radiotherapy for small hepatocellular carcinoma: A retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53:399–404. doi: 10.3109/0284186X.2013.820342. [DOI] [PubMed] [Google Scholar]
- 27.Takeda A., Sanuki N., Tsurugai Y. Phase 2 study of stereotactic body radiotherapy and optimal transarterial chemoradiation for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016;122:2041–2049. doi: 10.1002/cncr.30008. [DOI] [PubMed] [Google Scholar]
- 28.Wahl D.R., Stenmark M.H., Tao Y. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe T., Saitoh J., Kobayashi D. Dosimetric comparison of carbon ion radiotherapy and stereotactic body radiotherapy with photon beams for the treatment of hepatocellular carcinoma. Radiat Oncol. 2015;10:187. doi: 10.1186/s13014-015-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawashima M., Furuse J., Nishio T. Phase II study of radiotherapy employing proton beam for HCC. J Clin Oncol. 2005;23:1839–1846. doi: 10.1200/JCO.2005.00.620. [DOI] [PubMed] [Google Scholar]
- 31.Sugahara S., Oshiro Y., Nakayama H. Proton beam therapy for large hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;76:460–466. doi: 10.1016/j.ijrobp.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 32.Fukumitsu N., Sugahara S., Nakayama H. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831–836. doi: 10.1016/j.ijrobp.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 33.Tateishi R., Shiina S., Yoshida H. Prediction of recurrence of hepatocellular carcinoma after curative ablation using three tumor markers. Hepatology. 2006;44:1518–1527. doi: 10.1002/hep.21408. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W., Song T.Q., Zhang T. Adjuvant interferon for early or late recurrence of hepatocellular carcinoma and mortality from hepatocellular carcinoma following curative treatment: A meta-analysis with comparison of different types of hepatitis. Mol Clin Oncol. 2014;2:1125–1134. doi: 10.3892/mco.2014.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oshiro Y., Mizumoto M., Okumura T. Analysis of repeated proton beam therapy for patients with hepatocellular carcinoma. Radiother Oncol. 2017;123:240–245. doi: 10.1016/j.radonc.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Ebner D.K., Tsuji H., Yasuda S. Respiration-gated fast-rescanning carbon-ion radiotherapy. Jpn J Clin Oncol. 2017;47:80–83. doi: 10.1093/jjco/hyw144. [DOI] [PubMed] [Google Scholar]