Abstract
Purpose
Current standard of care for patients with breast cancer with a positive node on sentinel lymph node biopsy (SLNB) after neoadjuvant chemotherapy is axillary dissection with irradiation of the regional nodes, but it is unknown whether axillary lymph node dissection (ALND) can be safely omitted if complete axillary radiation is delivered instead.
Methods and Materials
We identified 161 patients found to have a positive sentinel lymph node on SLNB after neoadjuvant chemotherapy for breast cancer between December 2006 and October 2017, who were treated with or without completion ALND. Local, regional, and distant recurrence and overall survival were analyzed using the Kaplan-Meier method. Patient, disease, and treatment factors potentially predictive of each outcome were entered into Cox regression analysis.
Results
Median follow-up was 28.8 months (range, 2.5-137.0). The 3-year regional control rate did not differ according to extent of axillary surgery (92.6% for SLNB alone vs 96.4% for SLNB with ALND, P = .616). Regional recurrence occurred as part of first recurrence in 9 patients (5.6%). Five patients failed in axillary levels 1 or 2, 6 failed in axillary level 3 or supraclavicular nodes, and 2 failed in internal mammary nodes, with some patients failing in multiple regional nodal areas. Extent of axillary dissection (SLNB only vs SLNB plus ALND) did not predict for disease control or survival. Patients who underwent ALND were significantly more likely to have lymphedema (25.0% vs 9.4%, P = .021).
Conclusions
Careful selection of patients with a positive sentinel node on SLNB after neoadjuvant chemotherapy for omission of completion ALND in favor of irradiation of the undissected axilla does not compromise local, regional, or distant control or overall survival and results in lower rates of lymphedema.
Introduction
Sentinel lymph node biopsy (SLNB) for breast cancer has a high negative predictive value and is associated with a decreased risk of lymphedema compared with axillary lymph node dissection (ALND).1, 2, 3 Although initially investigated in early stage (clinical T1-2 N0) breast cancer, SLNB has become increasingly used after neoadjuvant chemotherapy for locally advanced breast cancer.4 The American College of Surgeons Oncology Group (ACOSOG) Z1071 and SENTinel NeoAdjuvant (SENTINA) trials demonstrated the feasibility of SLNB after neoadjuvant chemotherapy, with a false-negative rate of 13% to 14%, which could be reduced with utilization of the dual tracer technique or with dissection of 3 or more sentinel nodes.5,6 However, uncertainty remains regarding management of the axilla for patients undergoing SLNB after neoadjuvant chemotherapy. The current standard of care for patients with a positive sentinel node in this setting is axillary dissection with irradiation of the regional nodes, but it is unknown whether axillary dissection can be omitted if complete axillary radiation is delivered instead. This question is the subject of the ongoing Alliance A011202 trial. This trial, which includes initially cT1-3 N1 patients with a persistent positive lymph node on SLNB after neoadjuvant chemotherapy, aims to compare ALND plus irradiation of the undissected axilla and regional nodes versus complete axillary and regional node irradiation without axillary dissection. While the results of Alliance A011202 are awaited, we aimed to determine the outcomes and pattern of recurrence for patients found to have node-positive disease on SLNB after neoadjuvant chemotherapy, with or without axillary dissection.
Methods and Materials
After institutional review board approval, we identified 840 patients with breast cancer who were treated with neoadjuvant chemotherapy between December 2006 and October 2017 from a prospectively collected breast tumor registry at our institution. Of these, 447 patients were clinically node-negative after completion of neoadjuvant chemotherapy and subsequently underwent SLNB. One hundred sixty-one patients were found to have at least 1 positive sentinel lymph node and were treated with or without completion axillary dissection, and these formed the cohort for analysis. Patients treated with neoadjuvant hormonal therapy only were excluded.
The decision to proceed with completion ALND was made based on clinical factors, pathology results, and joint patient–physician decision making. Neoadjuvant chemotherapy generally consisted of doxorubicin, cyclophosphamide, and taxane-based regimens for HER2-negative disease, whereas HER2-positive tumors were treated with docetaxol, carboplatin, and trastuzumab with or without pertuzumab. Patients underwent lumpectomy, total mastectomy, or modified radical mastectomy, and all underwent adjuvant radiation therapy to the breast or chest wall. Patients additionally received radiation to the undissected axilla when no ALND was done, and the regional nodes were included at the treating physician’s discretion based on clinical and pathologic factors. Patients were typically treated to a conventionally fractionated dose of 50 to 50.4 Gy to the whole breast or chest wall plus regional nodes, followed by a 10 to 14 Gy tumor bed boost. Only 6 (3.7%) lumpectomy patients received hypofractionated radiation, to a median dose of 42.56 Gy in 16 fractions, and no postmastectomy patient was treated with hypofractionation. Radiation treatment technique consisted of 3-dimensional tangential beams for 119 patients (75.3%) and intensity modulated radiation therapy for 39 (24.6%), and was unknown for 3. Regional nodes were contoured as per the Radiation Therapy Oncology Group contouring atlas. Adjuvant systemic therapy consisted of trastuzumab for HER2-positive patients and hormonal therapy for ER-positive and/or PR-positive patients. A subset of patients with high-risk triple-negative disease received adjuvant Xeloda.
Disease outcome endpoints included local recurrence, regional nodal recurrence, distant recurrence, disease-free survival, and disease-specific survival, all defined from date of completion of radiation therapy. Local recurrence was defined as ipsilateral breast tumor recurrence or new primary in the treated breast. Regional nodal recurrence was defined as recurrence in the ipsilateral axillary, supraclavicular, or internal mammary lymph nodes. All disease outcome and survival endpoints were analyzed using the Kaplan-Meier method. Patient, disease, and treatment factors potentially predictive of each outcome were entered into Cox regression analyses. Factors analyzed included extent of axillary dissection (SLNB only vs SLNB plus ALND), age, menopausal status, clinical T and N classification, ypT and ypN classification, clinical tumor size, pathologic tumor size, phenotype (luminal A [defined as hormone receptor positive with Ki67 <20%], luminal B [defined as hormone receptor positive with Ki67 ≥20%], triple negative, or HER2 positive), multifocal versus concentric regression pattern, number of positive sentinel nodes, extent of nodal burden (macrometastases vs micrometastases vs isolated tumor cells), extracapsular extension, angiolymphatic invasion, Nottingham grade, and use of regional nodal irradiation. A P value of <.05 was considered statistically significant. The incidence of lymphedema was collected based on retrospective review of clinical notes, and factors potentially predictive of lymphedema were analyzed using binary logistic regression. Upper extremity lymphedema was defined using circumferential tape measurements showing a difference of greater than 2 cm between the 2 arms. All statistical analyses were completed using SPSS Statistics version 24 (IBM Corp., Armonk, NY).
Results
Patient and treatment characteristics
Patient and treatment characteristics are shown in Table 1. Median follow-up was 28.8 months (range, 2.5-137.0) for the entire cohort, 25.1 months for the SLNB-alone group, and 32.4 months for the SLNB plus ALND group. Of the 161 patients with positive sentinel lymph node(s) included for analysis, 53 (32.9%) were managed without further axillary surgery, and 108 (67.1%) underwent completion ALND. Neoadjuvant chemotherapy regimens used consisted of doxorubicin, cyclophosphamide, and taxane in 99 (61.5%); docetaxol, carboplatin, and trastuzumab ± pertuzumab in 40 (24.8%); docetaxol and cyclophosphamide ± doxorubicin in 8 (5.0%); docetaxol, trastuzumab, and pertuzumab in 4 (2.5%); and other in 10 (6.2%).
Table 1.
Patient characteristics
| Characteristics | SLNB only (n = 53) | SLNB + ALND (n = 108) | P value |
|---|---|---|---|
| Age, median (IQR), y | 53 (44-62) | 52 (43-61) | .407 |
| Menopausal status | |||
| Premenopausal | 21 (39.6%) | 52 (48.6%) | .315 |
| Postmenopausal | 32 (60.4%) | 55 (51.4%) | |
| Clinical T classification | .203 | ||
| T0 | 0 (0.0%) | 1 (0.9%) | |
| T1 | 4 (7.5%) | 15 (13.9%) | |
| T2 | 36 (67.9%) | 54 (50.0%) | |
| T3 | 12 (22.6%) | 36 (33.3%) | |
| T4 | 1 (1.9%) | 2 (1.9%) | |
| Clinical N classification | .075 | ||
| N0 | 25 (47.2%) | 29 (26.9%) | |
| N1 | 26 (49.1%) | 72 (66.7%) | |
| N2 | 1 (1.9%) | 5 (4.6%) | |
| N3 | 1 (1.9%) | 2 (1.9%) | |
| Phenotype | .608 | ||
| Luminal A | 3 (5.7%) | 7 (6.9%) | |
| Luminal B | 21 (39.6%) | 46 (45.5%) | |
| HER2+ | 18 (34.0%) | 24 (23.8%) | |
| Triple negative | 11 (20.8%) | 24 (23.8%) | |
| Primary surgery | .056 | ||
| Mastectomy | 21 (39.6%) | 63 (58.3%) | |
| Lumpectomy | 32 (60.4%) | 44 (40.7%) | |
| Multifocal disease | |||
| No | 35 (66.0%) | 74 (69.2%) | .721 |
| Yes | 18 (34.0%) | 33 (30.8%) | |
| Primary tumor size, median (IQR), cm | |||
| Clinical | 3.0 (2.5-4.8) | 3.4 (2.5-5.6) | .290 |
| Pathologic | 2.3 (1.5-4.1) | 2.5 (1.7-4.5) | .124 |
| Nottingham grade | |||
| Grade 1 | 2 (4.8%) | 5 (6.0%) | .165 |
| Grade 2 | 26 (61.9%) | 37 (44.0%) | |
| Grade 3 | 14 (33.3%) | 42 (50.0%) | |
| Angiolymphatic invasion | .036 | ||
| Absent | 39 (75.0%) | 61 (57.5%) | |
| Present | 13 (25.0%) | 45 (42.5%) | |
| Nodal extracapsular extension | .084 | ||
| Absent | 31 (73.8%) | 52 (56.5%) | |
| Present | 11 (26.2%) | 40 (43.5%) | |
| Extent of residual nodal disease | <.001 | ||
| Isolated tumor cells | 8 (15.1%) | 1 (0.9%) | |
| Micrometastases | 20 (37.7%) | 8 (7.4%) | |
| Macrometastases | 25 (47.2%) | 99 (91.7%) | |
| RCB score, median (IQR) | 3.0 (2.2-3.4) | 3.4 (3.1-4.0) | .013 |
| RCB-I | 1 (5.8%)∗ | 1 (3.7%)∗ | |
| RCB-II | 10 (58.8%)∗ | 9 (33.3%)∗ | |
| RCB-III | 6 (35.3%)∗ | 17 (63.0%)∗ | |
| RCB unknown | 36 (67.9%) | 81 (75.0%) | |
| No. SLN dissected, median (IQR) | 3 (2-4.5) | 3 (2-4) | .493 |
| No. SLN involved, median (IQR) | 1 (1-1) | 1 (1-2) | .002 |
| Regional node irradiation | 40 (76.9%)∗ | 90 (86.5%)∗ | .171 |
Abbreviations: ALND = axillary lymph node dissection; IQR = interquartile range; RCB = residual cancer burden; SLNB = sentinel lymph node biopsy.
Percentages calculated using the denominator of patients for whom this information was known.
Patients receiving completion ALND had significantly higher rates of angiolymphatic invasion (42.5% vs 25.0%, P = .036), macrometastases versus micrometastases/isolated tumor cells (91.7% vs 47.2%, P <.001), and higher residual cancer burden (RCB) score (median 3.4 vs 3.0, P = .013), although RCB score was missing in 117 (72.7%) of cases because this parameter only began to be reported on pathology reports in more recent years. Patients receiving completion ALND also trended toward having higher clinical N classification (P = .075), more mastectomies (P = .056), and higher incidence of nodal extracapsular extension (P = .084). The number of involved sentinel nodes was higher in patients who underwent ALND (P = .002), but there was no difference in the number of sentinel nodes dissected, with both groups having a median of 3 sentinel nodes dissected (P = .493). The median total number of lymph nodes dissected in patients who underwent ALND was 15 (interquartile range, 12-20).
Data on extent of radiation therapy fields were available for 156 of 161 total patients, of whom 130 (83.3%) received irradiation to the regional nodes consisting of undissected axilla and supraclavicular nodes with or without internal mammary node coverage, whereas 26 (16.7%) did not. Use of regional nodal irradiation varied according to size of the sentinel node metastases, with 88.3% (n = 106), 64.3% (n = 18), and 75.0% (n = 6) of patients with macrometastases, micrometastases, and isolated tumor cells receiving regional nodal irradiation, respectively (P = .007). Among the 26 patients who did not receive regional nodal irradiation, 14 had macrometastases, 10 had micrometastases, and 2 had isolated tumor cells. As can be seen in Table 1, more patients in the SLNB plus ALND group received regional nodal irradiation (n = 40, 86.5%) compared with the SLNB-alone group (n = 90, 76.9%), although this difference was not statistically significant (P = .171). Of the 12 patients in the SLNB-alone group who did not receive regional nodal irradiation, 7 patients had radiation fields modified to cover the entire axillary levels 1 and 2, 2 patients did not have fields modified to provide coverage of axillary levels 1 and 2, and for 3 patients it could not be determined from chart review whether levels 1 and 2 were covered. Nodal extracapsular extension, angiolymphatic invasion, and Nottingham grade did not predict for use of nodal irradiation.
Of the 84 included mastectomy patients, all received chest wall irradiation, 79 received regional nodal irradiation, and 2 did not receive regional nodal irradiation; for 3 of 84, the extent of nodal coverage could not be determined based on chart review. In February 2015, we updated our institutional clinical pathway to mandate regional nodal irradiation for all pathologically node-positive patients after chemotherapy, resulting in an increase in rate of regional nodal irradiation usage from 73.8% (n = 59) to 93.4% (n = 71) before and after the clinical pathway change, respectively (P = .001).
Regional control
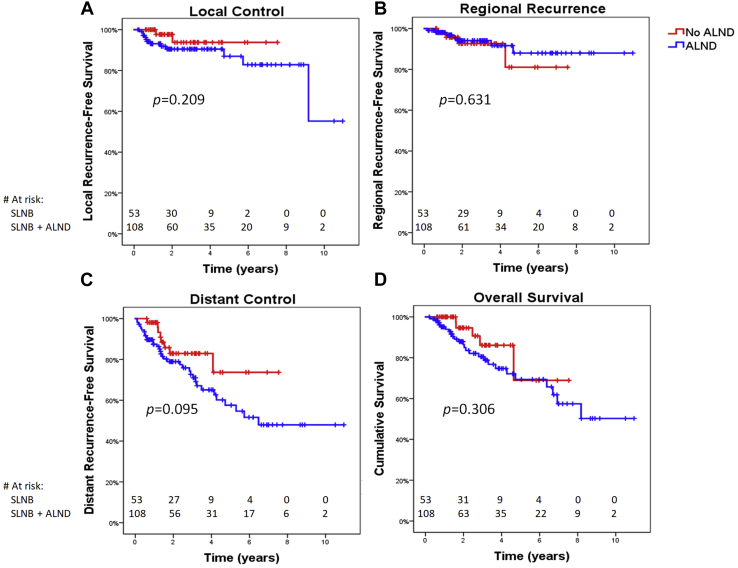
The overall 3-year regional control rate was 95.1%. The regional failure rate did not differ according to extent of axillary surgery (3-year regional control rate of 92.6% for SLNB alone vs 96.4% for SLNB with ALND; P = .616; Fig 1B). Regional recurrence occurred as part of first recurrence in 9 patients (5.6%). Of these, 2 had isolated regional failure, 2 had concurrent distant recurrence, 1 had concurrent local recurrence, and 4 had both concurrent local and distant failure. Four recurrences were biopsy proven, and the remaining 5 were identified on either positron emission tomography/computed tomography or computed tomography imaging. Five patients had failure in the axillary levels 1 or 2, 6 failed in axillary level 3 or supraclavicular nodes, and 2 failed in internal mammary nodes, with some patients failing in multiple regional nodal areas. Of the 5 patients who failed in axillary levels 1 or 2, 3 had been managed with SLNB alone, and 2 had received ALND. Thus, the crude axillary level 1 or 2 failure rate was 5.7% in the SLNB-alone group and 1.9% in the SLNB with ALND group (P = .332). For patients who had nodal macrometastases, micrometastases, and isolated tumor cells, the crude regional failure rates were 5.6% (n = 7), 7.1% (n = 2), and 0.0% (n = 0), respectively (P = .718). In the subset of 124 patients with macrometastases, of whom 25 underwent SLNB alone and 99 underwent SLNB plus ALND, the 3-year regional control rate was 84.7% for SLNB alone versus 96.0% for SLNB plus ALND (P = .112). Median time to regional recurrence for the entire cohort was 18.7 months (range, 2.2-58.4). No factors were predictive of regional recurrence on univariate Cox regression analysis, so no multivariate analysis was done.
Figure 1.
Kaplan-Meier analysis of (A) local control, (B) regional control, (C) distant control, and (D) overall survival according to whether patients underwent sentinel lymph node biopsy (SLNB) alone versus SLNB plus axillary lymph node dissection (ALND).
The crude regional failure rate was 11.5% (3 of 26) among patients who did not receive regional nodal irradiation and 4.6% (6 of 130) among patients who received regional nodal irradiation (P = .491). The analysis on regional control was repeated on the subset of patients who underwent regional nodal irradiation (n=130), since this represents current standard of care treatment. For the 130 patients who had regional nodal irradiation, the actuarial 3-year regional control rate was 93.7%, and their regional failure rate did not differ according to extent of axillary surgery (3-year regional control rate of 90.2% for SLNB alone vs 95.5% for SLNB with ALND, P = .362). Among the 6 patients who received nodal irradiation and later failed regionally, 3 failed in axillary levels 1 or 2, 4 failed in axillary level 3 or supraclavicular nodes, and 2 failed in internal mammary nodes, with some patients failing in multiple areas. All 6 patients had macrometastases. Among patients treated with regional nodal irradiation who had nodal macrometastases, micrometastases, and isolated tumor cells, the crude regional failure rates were 5.7% (n = 6), 0.0% (n = 0), and 0.0% (n = 0), respectively (P = .491).
Local control, distant control, and survival
The actuarial 3-year local and distant control rates were 91.7% and 76.8%, respectively. The disease-free survival, disease-specific survival, and overall survival rates were 73.5%, 85.1%, and 82.5%, respectively. On univariate Cox regression analysis, neither extent of axillary dissection (SLNB only vs SLNB plus ALND), extent of nodal burden (macrometastases vs micrometastases vs isolated tumor cells), nor receipt of nodal irradiation predicted for local recurrence, regional recurrence, distant recurrence, disease-free survival, disease-specific survival, or overall survival (Fig 1A-D). No predictive factors were identified for any endpoint on univariate Cox regression, so no multivariate analysis was attempted. The 3-year disease-free survival for patients with macrometastases, micrometastases, and isolated tumor cells was 70.8%, 86.1%, and 71.4%, respectively (P = .338). Compared with patients without regional recurrence, those who developed regional recurrence had significantly worse 3-year disease-specific survival (45.7% vs 88.3%, P = .002) and 3-year overall survival (45.7% vs 84.7%, P = .007).
Lymphedema
The incidence of upper extremity lymphedema was 19.9% (n = 32), and the incidence of breast lymphedema without upper extremity lymphedema was 5.0% (n = 8). Median time to development of upper extremity lymphedema from date of surgery was 9.3 months (range, 0.4-75.4). Having an axillary dissection significantly predicted for upper extremity lymphedema (25.0% vs 9.4%; hazard ratio, 3.20; 95% confidence interval, 1.16-8.86; P = .021), but irradiation of the regional nodes was not predictive (hazard ratio, 1.44; 95% confidence interval, 0.46-4.54; P = .532). Among the 108 patients who underwent ALND, the total number of lymph nodes dissected did not predict for lymphedema (P = .600).
Discussion
Current standard of care for node-positive disease identified on SLNB after neoadjuvant chemotherapy for breast cancer consists of completion axillary dissection. Our study demonstrates that when carefully selected, patients with a positive lymph node on SLNB after neoadjuvant chemotherapy have excellent regional control and disease-free survival even when completion ALND is omitted. We found that regional failure rate did not differ significantly according to extent of axillary surgery, with 3-year regional control rates of 90.2% and 95.5% for patients who were managed with SLNB alone and SLNB plus ALND, respectively, and subsequently received regional nodal irradiation.
Axillary dissection comes at the cost of significant morbidity, with acute complication rates ranging from 20% to 30% and risk of chronic lymphedema in 1 out of 4 patients.7, 8, 9 For these reasons, use of axillary dissection has declined in favor of SLNB for patients with early stage breast cancer treated in the upfront setting. The ACOSOG Z0011 trial demonstrated that among patients with 1 to 2 positive sentinel lymph nodes treated with breast conservation therapy, sentinel node dissection alone is noninferior to full axillary dissection, and the AMAROS trial (NCT00014612) showed that ALND and axillary radiation therapy after positive SLNB offered excellent and comparable axillary control.10,11
However, these studies supporting omission of ALND are based on patients treated in the upfront setting, not after neoadjuvant chemotherapy. Outcome data on the risk of regional recurrence for node-positive disease found on SLNB after neoadjuvant chemotherapy is currently limited. This has to do with the fact that use of SLNB for clinically node-positive disease after neoadjuvant chemotherapy did not become more widespread until after publication of the ACOSOG Z1071 and SENTINA trials, which showed the feasibility of this approach.5,6 However, these trials’ results were not ideal, demonstrating a false-negative rate of 13% to 14%, which did not meet the predefined acceptable threshold of <10%. Our study offers some reassurance that using SLNB to assess nodal disease after neoadjuvant chemotherapy is an acceptable approach, considering our overall 3-year regional control rate was high at 95.1%.
Because concern remains that in the postneoadjuvant chemotherapy setting, any microscopic residual nodal disease left behind after surgery may not be sterilized by radiation therapy, the standard of care is still completion ALND in case of a positive node on SLNB after neoadjuvant chemotherapy. Nevertheless, in practice the reality is that some patients decline axillary dissection and are instead offered radiation to the undissected axilla along with the regional nodes, even though there is limited data to suggest this approach is safe in patients who have received neoadjuvant chemotherapy. The ongoing Alliance A011202 trial, enrolling clinical T1-3 N1 patients with a persistent positive lymph node on SLNB after neoadjuvant chemotherapy, will answer this question by comparing ALND plus irradiation of the undissected axilla and regional nodes versus complete axillary and regional node irradiation without axillary dissection.
The differential risk of locoregional recurrence according to degree of nodal burden after neoadjuvant chemotherapy has not previously been well delineated. Although based on limited follow-up, our study is the first to define the risk of regional recurrence based on extent of pathologic nodal burden (macrometastases vs micrometastases vs isolated tumor cells). We found that among patients with nodal macrometastases, micrometastases, and isolated tumor cells receiving standard-of-care regional nodal irradiation, the crude regional failure rates were 5.7%, 0.0, and 0.0%, respectively (P = .491). In the subset of patients with macrometastases, the 3-year regional control rate was 84.7% for SLNB alone versus 96.0% for SLNB plus ALND (P = .112), although this finding is limited by small sample size and low event number. The joint NSABP B-18 and B-27 analysis offered some insight into the risk of locoregional recurrence after neoadjuvant chemotherapy in a cohort of patients who did not receive regional nodal irradiation, but it did not differentiate the risk among isolated tumor cells, micrometastases, or macrometastases.12 In the NSABP joint analysis, the cumulative risk of locoregional recurrence at 10 years for clinically node-positive, pathologically node-positive disease ranged from 14.7% to 22.4%, depending on patient risk factors, whereas for clinically node-negative, pathologically node-positive patients, the risk ranged from 7.2% to 14.6%. The predominant pattern of locoregional recurrence consisted of local failure, especially for clinically node-negative disease, where regional recurrence constituted <20% of locoregional failures in most patient groups.
We found that physicians seem to tailor surgical and radiation treatments according to the burden of nodal metastases, with significantly more patients with macrometastases receiving ALND and regional nodal irradiation. In our study 16.7% of patients did not receive standard-of-care regional nodal irradiation, about half of whom had either isolated tumor cells or micrometastases. Although this could have affected our results, we did a subset analysis of patients who underwent regional nodal irradiation and found that the regional control rates for SLNB alone versus SLNB plus ALND (90.2% vs 95.5%) for this subset seemed to be similar to the rates for the entire cohort.
A post hoc analysis of radiation treatment details from the ACOSOG Z1071 trial, which had no specific guidelines regarding radiation therapy indications or field design, showed wide variability in practice patterns with an even lower utilization rate of regional nodal irradiation compared with our study.13 In that trial, supraclavicular radiation was omitted in 47.3% of patients with residual node-positive disease. The ACOSOG Z1071 post hoc analysis highlights the need for standardization in radiation field design. Clinical pathways may be a helpful tool in standardizing treatment practice patterns in this regard. We have previously shown that at our institution, utilization of a clinical pathway mandating regional nodal irradiation for women with macrometastases on SLNB and adverse risk factors significantly increased the percentage of patients receiving regional nodal irradiation in the upfront setting, from 24.6% to 56.9%.14 We updated our clinical pathway in February 2015 to mandate regional nodal irradiation for pathologically node-positive patients after neoadjuvant chemotherapy as well, resulting in an increase in the rate of regional nodal irradiation usage from 73.8% to 93.4% (P = .001).
When identified in the upfront setting, isolated tumor cells are considered node-negative disease, as reflected in the current American Joint Committee on Cancer eighth edition staging. However, isolated tumor cells after neoadjuvant chemotherapy do not carry the same prognosis as in the upfront setting because their presence represents residual disease. Our 3-year disease-free survival for patients with macrometastases, micrometastases, and isolated tumor cells was 70.8%, 86.1%, and 71.4%, respectively, with no statistically significant difference among groups. In the recently published Sentinel Node Biopsy Following Neoadjuvant Chemotherapy (SN FNAC) phase 2 trial evaluating SLNB after neoadjuvant chemotherapy for initially node-positive patients, sentinel node metastases of any size were considered positive, including immunohistochemistry-only–detected micrometastases or isolated tumor cells.15 This trial had a design similar to ACOSOG Z1071, but it was unique in that it mandated immunohistochemistry in case of negative hematoxylin and eosin stain. The authors found no correlation between the size of sentinel node metastases and the rate of positive nonsentinel nodes, with positive nonsentinel nodes identified in 57%, 38%, and 56% of cases for isolated tumor cells, micrometastases, and macrometastases, respectively. Therefore, significant caution is warranted when considering omission of regional nodal irradiation after neoadjuvant chemotherapy for any node-positive patient. At present, the philosophy at our institution is to treat all patients with residual disease after neoadjuvant chemotherapy, including patients with isolated tumor cells and micrometastases.
Finally, it is important to note that there were imbalances in characteristics between the patients in our study who were treated with SLNB alone and those who went on to have completion ALND. Patients receiving completion ALND in general had characteristics consistent with more advanced and aggressive disease. They had significantly higher rates of angiolymphatic invasion, macrometastases versus micrometastases or isolated tumor cells, and higher RCB score, and trended toward having higher clinical N classification, more mastectomies, and higher incidence of nodal extracapsular extension. Thus, it is clear that surgeons’ decisions on whether to proceed with ALND took into careful consideration the clinical and pathologic risk factors of each individual patient, resulting in 32.9% of the patients in this study being spared the morbidity of full axillary dissection. As expected, the patients who were spared ALND had a significantly lower incidence of upper extremity lymphedema (9.4% vs 25.0%).
This study is limited by its retrospective nature, relatively small sample size, and limited follow-up. Nevertheless, our study offers important evidence that even after neoadjuvant chemotherapy, a subset of patients with low-risk clinical and pathologic risk factors can be safely managed without full ALND after a positive sentinel lymph node, thus sparing the morbidity of ALND and significantly lowering the risk of chronic lymphedema in around one-third of patients. Our study suggests that axillary radiation without ALND may be sufficient for patients with isolated tumor cells or micrometastases, but larger, prospective studies with longer follow-up are needed to confirm the viability of this approach, for both micrometastases and macrometastases.
Conclusions
Careful selection of patients with a positive sentinel node on SLNB after neoadjuvant chemotherapy for omission of completion ALND in favor of irradiation of the undissected axilla does not compromise local, regional, or distant control or overall survival and results in lower rates of lymphedema.
Footnotes
Sources of support: None
Disclosures: Dr Beriwal is assistant medical director for radiation oncology of Via Oncology (Pittsburgh, PA). Via Oncology is affiliated with Elsevier.
References
- 1.Giuliano A.E., Jones R.C., Brennan M. Sentinel lymphadenectomy in breast cancer. J Clin Oncol. 1997;15:2345–2350. doi: 10.1200/JCO.1997.15.6.2345. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U., Paganelli G., Galimberti V. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]
- 3.Albertini J.J., Lyman G.H., Cox C. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996;276:1818–1822. [PubMed] [Google Scholar]
- 4.Hunt K.K., Yi M., Mittendorf E.A. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250:558–566. doi: 10.1097/SLA.0b013e3181b8fd5e. [DOI] [PubMed] [Google Scholar]
- 5.Boughey J.C., Suman V.J., Mittendorf E.A. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuehn T., Bauerfeind I., Fehm T. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 7.Ivens D., Hoe A.L., Podd T.J. Assessment of morbidity from complete axillary dissection. Br J Cancer. 1992;66:136–138. doi: 10.1038/bjc.1992.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larson D., Weinstein M., Goldberg I. Edema of the arm as a function of the extent of axillary surgery in patients with stage I-II carcinoma of the breast treated with primary radiotherapy. Int J Radiat Oncol Biol Phys. 1986;12:1575–1582. doi: 10.1016/0360-3016(86)90280-4. [DOI] [PubMed] [Google Scholar]
- 9.Keramopoulos A., Tsionou C., Minaretzis D. Arm morbidity following treatment of breast cancer with total axillary dissection: A multivariated approach. Oncology. 1993;50:445–449. doi: 10.1159/000227227. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano A.E., Ballman K.V., McCall L. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donker M., van Tienhoven G., Straver M.E. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamounas E.P., Anderson S.J., Dignam J.J. Predictors of locoregional recurrence after neoadjuvant chemotherapy: Results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30:3960–3966. doi: 10.1200/JCO.2011.40.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haffty B.G., McCall L.M., Ballman K.V. Patterns of local-regional management following neoadjuvant chemotherapy in breast cancer: Results from ACOSOG Z1071 (Alliance) Int J Radiat Oncol Biol Phys. 2016;94:493–502. doi: 10.1016/j.ijrobp.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebhardt B.J., Thomas J., Horne Z.D. Standardization of nodal radiation therapy through changes to a breast cancer clinical pathway throughout a large, integrated cancer center network. Pract Radiat Oncol. 2018;8:4–12. doi: 10.1016/j.prro.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Boileau J.F., Poirier B., Basik M. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J Clin Oncol. 2015;33:258–264. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]