Abstract
Purpose
Patients with large, high-grade soft tissue sarcomas are commonly treated with aggressive limb preservation regimens. This study aimed to assess cancer control outcomes of patients treated with neoadjuvant chemoradiation (CRT) compared with radiation therapy (RT) alone.
Methods
We reviewed records of patients with high-grade extremity or trunk soft tissue sarcomas ≥5 cm who were treated with neoadjuvant radiation with or without chemotherapy. Patient and disease characteristics were compared using t test and χ2 tests. Standardized mortality ratio weighted method was used to compare overall survival (OS), local control, and disease-free (DFS) survival. Acute radiation and surgical toxicity were reported.
Results
In the study, 64 patients (34 CRT and 30 RT) treated between 1997 and 2015 were analyzed. In the RT group compared with the CRT group, the patient population was older, with a median age of 65 versus 50 years (P < .001), and more likely to have cardiovascular disease (CVD; 30% vs 0%, P < .001). At a median follow-up of 41 months, after adjusting for propensity score of receiving RT, the 3-year LC was 87.3% versus 86.1%, DFS was 58.5% versus 56.6%, and OS was 75.6% versus 69.0% for the CRT and RT groups, respectively (P > .05). Acute dermatitis occurred in 18% versus 3% and surgical complications occurred in 32% versus 17% of CRT and RT patients, respectively.
Conclusions
In this study, patients receiving RT alone were more likely to be older and have comorbid cardiovascular disease. When controlling for baseline differences, neoadjuvant CRT and RT provided similar rates of LC, DFS, and OS.
Introduction
Soft tissue extremity sarcomas remain a difficult site to treat due to its relative rarity, various histologic subtypes, and aggressive nature. Limb-preserving treatment paradigms remain the standard of care with contemporary management including wide local excision plus radiation to achieve high rates of local control comparable to amputation.1 Risk factors associated with increased risk of local or metastatic recurrence include size >5 cm, high-grade, positive margins, and histologic subtype.2, 3, 4 More aggressive combined modality treatment regimens, with the addition of chemotherapy, aim to improve disease-free survival.
A 2003 Harvard retrospective series showed promising results using neoadjuvant chemoradiation for patients with large (>8 cm) soft tissue sarcomas. Patients were treated with interdigitated chemotherapy with 3 cycles of mesna, doxorubicin, ifosfamide, dacarbazine (MAID) and radiation (44 Gy in 2 cycles) followed by surgery and 3 additional cycles of adjuvant MAID.5 Five-year results showed local control, disease-free survival (DFS), and overall survival (OS) of 92%, 70%, and 87%, respectively. When these outcomes were compared with historic controls, there was a significant improvement in DFS and OS. The results of the Harvard retrospective series prompted a Radiation Therapy Oncology Group phase II trial (RTOG 9514), which enrolled 64 patients with large (≥8 cm), high-grade extremity or trunk soft tissue sarcomas (STS).6 Patients were treated with similar neoadjuvant interdigitated therapy with 3 cycles of MAID and 2 split courses of RT. The 5-year LC, DFS, and OS was 78%, 56%, and 71%, respectively. However, there were high rates of toxicity with 5% treatment related deaths and 84% of patients experiencing grade 4 toxicity.6,7 Our own institution has published our experience managing high-grade soft tissue sarcoma with interdigitated neoadjuvant chemotherapy and radiation before surgical resection, also showing promising 3-year rates of LC (100%), DFS (62.5%), and OS (73.4%).8
Although an aggressive combined modality approach using neoadjuvant chemoradiation has shown promising outcomes, its routine use remains controversial owing to increased risks of toxicity. This study aimed to assess cancer control outcomes and toxicity of patients treated with neoadjuvant chemotherapy and radiation compared with neoadjuvant radiation alone.
Methods and Materials
Patient selection
Records of patients with high-grade, large (≥5 cm) extremity or trunk soft tissue sarcomas treated at our institution between 1997 and 2015 were identified using institutional registries. Patients were included if they were 18 years and older and were treated with either neoadjuvant chemoradiation (CRT) or radiation therapy (RT) alone followed by surgery. All patients had imaging workup including computed tomography or magnetic resonance imaging of the primary tumor region along with a diagnostic core needle or incisional biopsy of their tumor with pathology reviewed at our institution. Patients were included if pathology reported grade 2 or 3 in the World Health Organization grading system or if they had a high-grade histology, including epithelioid, synovial, malignant peripheral nerve sheath tumor, malignant fibrous histiocytoma (MFH), or pleomorphic undifferentiated sarcoma (formerly diagnosed as MFH). Patients were excluded if they had known metastatic disease at diagnosis. Patient demographics, including age at diagnosis, sex, race, and comorbidities were all recorded. Patients were considered to have cardiovascular disease (CVD) if they had a history of coronary artery disease, myocardial infarction, or congestive heart failure.
Patient treatment
All patients included were planned for definitive treatment with preoperative radiation of 44 to 50 Gy with or without neoadjuvant chemotherapy before limb sparing surgery. Planned chemotherapy regimens included MAID (modified mesna, doxorubicin, ifosfamide, and dacarbazine) or MAI (mesna, doxorubicin, ifosfamide). Chemotherapy and radiation could either be delivered sequentially with chemotherapy followed by radiation to 50 Gy or chemotherapy interdigitated with radiation to a total dose of 44 Gy in split courses of 22 Gy delivered between the first and second cycle and between the second and third cycles of preoperative chemotherapy. Computed tomography or magnetic resonance imaging was used to define target volumes, and external beam radiation with photons was given in 1.8 to 2 Gy daily fractions. All patients were planned for R0 limb-sparing resections with incisional biopsy sites resected within the surgical specimen. Postoperative external beam radiation boost or intraoperative RT was permitted for patients in both groups with positive margins or if planned preoperative radiation was unable to be completed owing to toxicity.
Pathologic evaluation
All surgical specimens were reviewed at our institution by experienced musculoskeletal pathologists. Histology, margin status, and World Health Organization grade were reported. Tumor size and percentage of histologic necrosis were also recorded when present in the pathology report. Surgical resection was defined as R0 if margins were microscopically negative, R1 if all gross disease was removed with microscopically positive margins, and R2 if there was a grossly positive margin.
Study endpoints and toxicity
The primary cancer control outcomes included OS, LC, distant metastases-free survival (DMFS), and DFS. All time points for cancer control outcomes were calculated from the date of confirmed pathologic diagnosis and analyzed as time-to-event data. OS was calculated from date of diagnosis to the date of death owing to any cause or date of last contact if alive. When available, date of death was confirmed using the Social Security Death Index. LC was calculated from date of diagnosis to the date of confirmed local or regional progression or recurrence, confirmed by either pathology or imaging. Patients were censored at the date of death if no prior local or regional progression or recurrence was observed, or last contact if alive without local or regional progression. DMFS was calculated from date of diagnosis to the date of distant progression or recurrence, confirmed by either pathology or imaging, or the date of death if no prior distant progression or recurrence observed. DFS was calculated from date of diagnosis to the date of local, regional, or distant progression or recurrence or death without progression, whichever occurred first. For LC, DMFS and DFS, patients were censored at the date of last contact if alive without respective events.
Skin toxicity was scored using the RTOG acute radiation morbidity scoring criteria. Surgical complications were scored using the Clavien-Dindo Classification of Surgical Complications.9 Patients were considered to have acute chemotherapy-related complications if they were hospitalized for infection, required a transfusion, or developed febrile neutropenia or neurotoxicity associated with the chemotherapy.
Statistical analysis
Differences in baseline patient and disease characteristics between CRT and RT were compared using t test and χ2 tests. Univariate Cox regression analysis was used to assess the marginal effects of patient and disease characteristics on clinical endpoints. Given the retrospective study nature, to minimize the potential confounding, we used standardized mortality ratio weighted (SMRW) method, based on estimated propensity score of receiving RT, to estimate both the adjusted rates of clinical outcomes based on weighted Kaplan-Meier estimates10 and the average treatment effect on the treated based on multiple weighted Cox regression models.11 Propensity scores were estimated by generalized boosted regression models using all patient and disease characteristics.12
Results
Patient characteristics
A total of 64 patients (34 CRT and 30 RT) treated between 1997 and 2015 were analyzed. Patient and tumor characteristics are detailed in Table 1. In the RT group compared with the CRT group, the patient population was older, with a median age of 65 years versus 50 years (P < .001). Among the CRT group, 30% (n = 9) had comorbid cardiovascular disease compared with the RT alone group in which no patients had CVD (P < .001). There was no difference between the 2 groups when comparing race, sex, tumor size, histology, or margin status. MFH or pleomorphic undifferentiated sarcoma was the most common histology (n = 22 out of 64, 34%) followed by liposarcoma (n = 15 out of 64, 23%). Lower extremity, hip, or pelvis were the most common tumor location (n = 50 out of 64, 78%). Median pretreatment tumor size was 11.75 cm (range, 5-27.8 cm).
Table 1.
Patient and tumor characteristics
| Characteristics | Chemoradiation |
Radiation alone |
All patients |
P value |
|---|---|---|---|---|
| (n = 34) | (n = 30) | (N = 64) | ||
| Sex, no. (%) | ||||
| Male | 19 (55.9) | 12 (40) | 31 (48.4) | |
| Female | 15 (44.1) | 18 (60) | 33 (51.6) | .21 |
| Age, y | ||||
| Median | 50 | 64.5 | 55 | |
| Range | 18-68 | 24-90 | 18-90 | <.001 |
| Race, no. (%) | ||||
| White | 26 (76.5) | 20 (66.7) | 46 (71.9) | |
| Other | 8 (23.5) | 10 (33.3) | 18 (28.1) | .38 |
| Presence of CVD, no. (%) | ||||
| Yes | 0 (0) | 9 (30) | 9 (14.1) | |
| No | 34 (100) | 21 (70) | 55 (85.9) | <.001 |
| Tumor size, cm | ||||
| Median | 11.3 | 11.9 | 11.8 | |
| Range | 5-27.8 | 5-24 | 5-27.8 | .71 |
| Histology, no. (%) | ||||
| MFH/PUS | 8 (23.5) | 14 (46.7) | 22 (34.4) | |
| Liposarcoma | 10 (29.4) | 5 (17.7) | 15 (23.4) | |
| Other | 16 (47.1) | 11 (36.7) | 27 (42.2) | .14 |
| Time from diagnosis to surgery, mo | ||||
| Mean | 4.32 | 2.77 | 3 | |
| Range | 2-16 | 2-4 | 2-16 | <.001 |
| Margin status | ||||
| R0 | 31 (91.2) | 29 (93.3) | 59 (92.2) | |
| R1 | 3 (8.8) | 2 (6.7) | 5 (7.8) | .75 |
Abbreviations: CVD = cardiovascular disease; MFH = malignant fibrous histiocytoma; PUS = pleomorphic undifferentiated sarcoma; R0 = surgical margins microscopically negative; R1 = microscopically positive surgical margins.
Treatments received
Description of the chemotherapy regimens are outlined in Table 2. MAI was the most common chemotherapy regimen (n = 24 out of 34, 71%), followed by MAID (n = 8 out of 24, 24%). One patient received doxorubicin and dacarbazine and one patient had an unknown chemotherapy regimen received at an outside hospital. In the CRT group, 16 patients (47%) received at least 1 cycle of adjuvant chemotherapy after surgery, but only 9 patients (26%) completed a total of 6 cycles of chemotherapy. Median number of total chemotherapy cycles completed was 4 (range, 1-6). In the patient cohort, 68% (n = 23 out of 34) had interdigitated CRT and 29% (n = 10 out of 34) had sequential CRT; in 1 patient, the order was unknown because CRT was completed at an outside hospital. Two patients in the CRT group had an upfront resection with positive margins and were then treated with neoadjuvant CRT followed by reresection. There was one patient in the RT alone group that presented with recurrence after upfront resection alone and was then treated with preoperative RT followed by reresection. Patients receiving spit-course RT received a dose of 44 Gy and patients receiving RT in one course received 50 Gy. One patient was treated with chemotherapy followed by concurrent chemoradiation to 45 Gy in 25 fractions. Two patients in the CRT did not receive the full course of RT due to either progression or toxicity. Patients in the CRT group had a longer interval between time from diagnosis to surgery with a mean time of 4.32 months, compared with the RT alone group with a mean time of 2.77 months (P < .001). In addition, 92% (n = 60 out of 64) of all patients had an R0 resection. All patients with a R1 resection (n = 5) received postoperative adjuvant radiation. There was one additional patient in the RT alone group who had negative margins but received IORT boost to 10 Gy.
Table 2.
Chemoradiation regimens
| Regimen | N = 34 |
|---|---|
| n (%) | |
| Interdigitated CRT | 23 (67) |
| Sequential CRT | 10 (29) |
| Unknown order | 1 (3) |
| MAI chemo | 24 (71) |
| MAID chemo | 8 (24) |
| Other/unknown chemo | 2 (6) |
| Adjuvant chemo | 16 (47) |
| Completed total 6 cycles | 9 (26) |
Abbreviations: CRT = chemoradiation therapy; MAI = mesna, doxorubicin, ifosfamide; MAID = mesna, doxorubicin, ifosfamide, and dacarbazine.
Pathologic tumor necrosis was >75% in 65% (n = 22 out of 34) of CRT patients and 40% (n = 12 out of 30) of RT patients. In addition, 11% (n = 4) of the CRT and 0% of the RT group had no viable tumor at the time of surgery; 2.9% (n = 1) in the CRT and 20% (n = 6) in the RT group had >75% viable tumor at the time of surgery.
Toxicity and complications
There were no reported treatment-related deaths. Acute dermatitis (greater than grade 1) occurred in 18% (n = 6 out of 34) versus 3% (n = 1 out of 30) and surgical complications (greater than grade 1) occurred in 32% (n = 11 out of 34) versus 17% (n = 5 out of 30) of CRT and RT patients, respectively. Nine patients (26.5%) in the CRT group and 5 patients (16.7%) in the RT alone group required at least 1 additional surgery owing to complications, including surgery for wound dehiscence, wound debridement, hematoma evacuation, and one patient who required prophylactic stabilization of the femur. Acute toxicity related to chemotherapy, including infection, transfusion, or hematologic toxicity or neurotoxicity, leading to hospitalization occurred in 38% (n = 13 out of 34) of CRT patients.
Treatment outcomes
The median follow-up time was 58 months (range, 11-227 months) in the CRT group and 37 months (range, 9-117 months) in the RT group. In the CRT group, there were 7 patients (20.5%) who developed local recurrence (LR) at a median of 20 months (range, 3-68 months) after diagnosis, and 13 patients (38%) who developed metastatic disease at a median time of 16 months (range, 7-37 months) after diagnosis. One patient in the CRT group was planned for an R0 resection but had progressive loss of function during preoperative treatment and required an amputation. In the RT alone group, there were 3 patients (10%) who developed LR at a median of 24 months (range, 6-31 months) after diagnosis, and 11 patients (36.7%) who developed metastatic disease at a median of 13 months (range, 5-96 months) after diagnosis. Of the 5 patients with microscopically positive margins, 3 developed LR.
On univariate analysis using Cox models, age (hazard ratio [HR] 1.04; 95% confidence interval [CI], 1.02-1.07; P = .002) and CVD (HR 6.93; 95% CI, 2.27-21.16; P = .001) were associated with decreased overall survival. Presence of positive margins at time of surgery was associated with LR on univariate analysis (HR 4.6; 95% CI, 1.19-17.99; P = .027). Other baseline characteristics, such as tumor size and histology were not associated with LC, DMFS, or DFS.
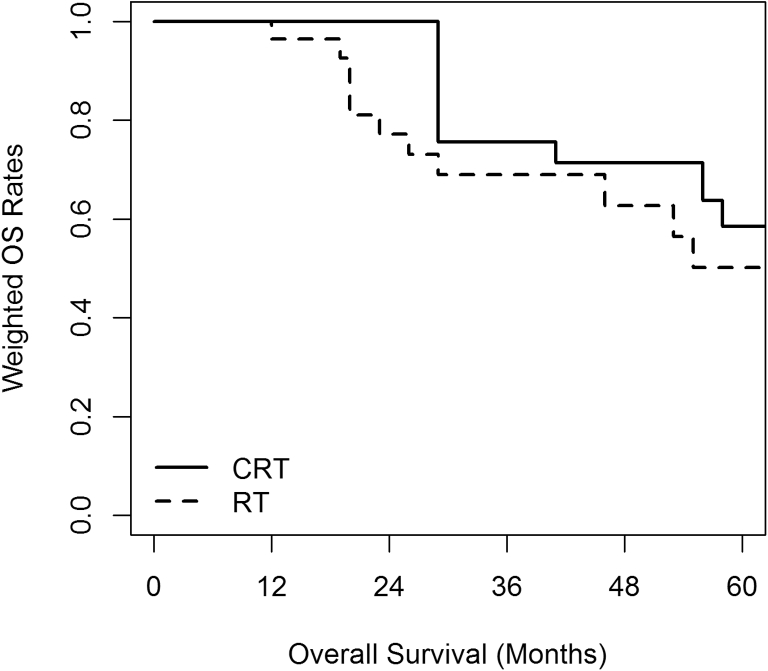
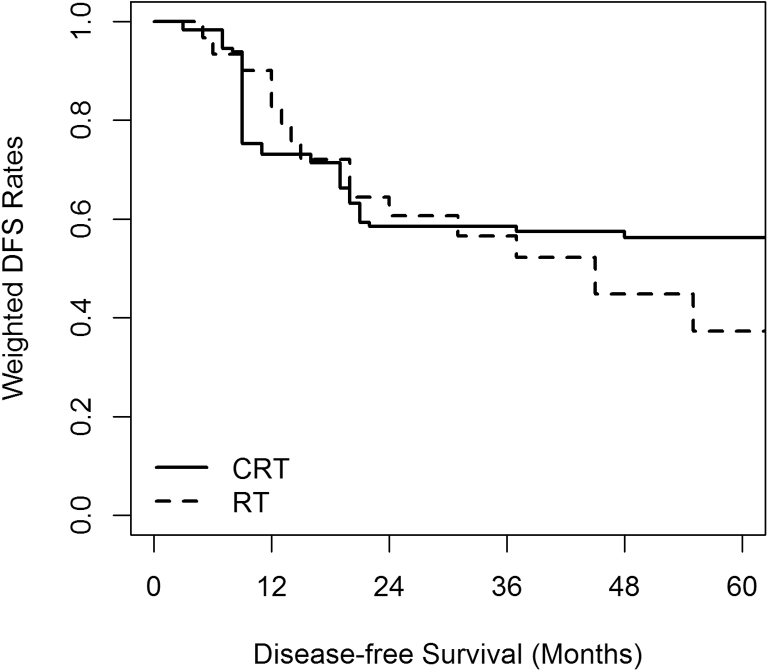
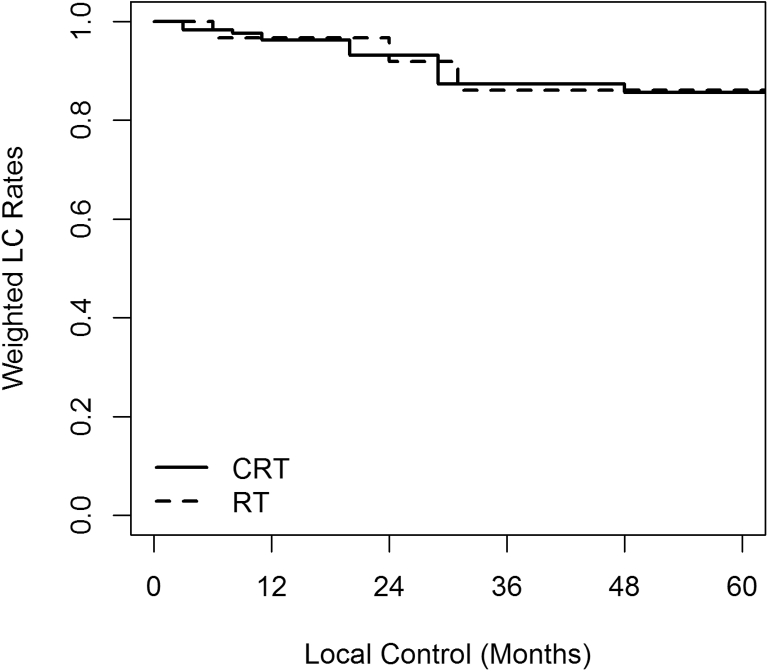
Three-year SMRW outcomes after adjusting for all baseline covariates using propensity scores were not statistically different between the 2 groups. At a median follow-up of 41 months, the 3-year LC was 87.3% versus 86.1% (HR 0.71; 95% CI, 0.13-3.84), DMFS was 58.4% versus 60.9% (HR 0.93; 95% CI, 0.26-3.36), DFS was 58.5% versus 56.6% (HR 0.98; 95% CI, 0.29-3.42), and OS was 75.6% versus 69.0% (HR 0.99; 95% CI, 0.20-4.87) for the CRT and RT groups, respectively. Propensity score SMRW Kaplan-Meier curves for OS, DFS, LC are shown in Figure 1, Figure 2, Figure 3.
Figure 1.
Overall survival (OS) standardized mortality ratio weighted Kaplan-Meier curve. Chemoradiation (CRT) 75.6% versus radiation (RT) 69.0% (hazard ratio 0.99; 95% confidence interval, 0.20-4.87).
Figure 2.
Disease-free survival (DFS) standardized mortality ratio weighted Kaplan-Meier curve. Chemoradiation (CRT) 58.5% versus radiation (RT) 56.6% (hazard ratio 0.98; 95% confidence interval, 0.29-3.42).
Figure 3.
Local control (LC) standardized mortality ratio weighted Kaplan-Meier curve. Chemoradiation (CRT) 87.3% versus radiation (RT) 86.1% (hazard ratio 0.71; 95% confidence interval, 0.13-3.84).
Discussion
The rate of local control and limb salvage are improved when surgery is combined with radiation in patients with large, high-grade soft tissue extremity sarcomas.1,13 However, intensive regimens with chemotherapy and radiation have remained controversial and have been slow to gain widespread acceptance owing to high rates of toxicity and study results showing conflicting outcomes.3,14,15 After the promising results of the Massachusetts General Hospital (MGH) experience using a combined modality interdigitated approach compared with historic controls,5 RTOG 9514 opened as the first prospective phase II trial incorporating this regimen. Its results were published in 2006 with longer follow-up published in 2010.6,7 However, there is still no level I evidence in support of this regimen. In a randomized EORTC phase II study aimed to assess the feasibility and outcome of neoadjuvant chemotherapy in adult patients with high-risk STS, there was no significant difference in DFS or OS at 7.3 years when comparing surgery alone versus surgery combined with neoadjuvant doxorubicin or ifosfamide.15
During the past 2 decades, our institution has been managing select sarcoma patients with neoadjuvant interdigitated chemotherapy and radiation, similar to the RTOG 9514 study, or with sequential neoadjuvant chemotherapy and radiation. A significant number of patients with large, high-grade STS are still treated with preoperative radiation alone though due to concerns regarding the toxicity associated with this aggressive regimen. This led to the question of whether clinical outcomes were compromised in the group of patients treated with neoadjuvant radiation alone.
At a median follow-up of 41 months, the 3-year cancer control outcomes in both groups of our study were not statistically different and were comparable to past published outcomes.5, 6, 7 In our cohort, the 3-year LC rate was 87.3% and 86.1% for the CRT and RT groups, respectively compared with 92% in the MGH MAID group and 78% in RTOG 9514. The 3-year DFS rate was 58.5% and 56.6% for the CRT and RT groups, respectively compared with 70% in the MGH MAID group and 56% in RTOG 9514; 3-year OS rate was 75.6% and 69.0% for the CRT and RT groups, respectively, compared with 87% in the MGH MAID group and 71% in RTOG 9514.
Many patients treated with radiation alone at our institution are likely selected for this regimen as they were deemed not suitable for aggressive chemotherapy owing to age and comorbidities. Patients in this group were older with a median age of 65 years, compared with 50 years, and more likely to have cardiovascular disease (30% vs 0%). When adjusting for these baseline covariates, however, the 2 groups had statistically similar 3-year outcomes, suggesting that withholding chemotherapy in certain populations did not negatively affect outcomes.
Potential benefits of administering chemotherapy before surgery include treating microscopic metastatic disease and facilitating an R0 resection. All patients in this cohort were planned for an R0 resection and limb sparing surgery was achieved in nearly all patients. Only one patient in our CRT group who was planned for an R0 resection required amputation owing to progressive loss of function during preoperative treatment. The quality of surgical resection and the presence of positive margins are independent adverse prognostic factors for both PFS and OS.16 The MGH study had an R0 resection rate of 81% in the control group and 85% in the MAID group; the RTOG study had an R0 rate of 91%. Similar rates of R0 resection were observed in both groups in our cohort with the CRT group achieving a 91.2% rate of R0 resection and the RT alone group achieving a 93.3% rate of R0 resection.
The pathologic response to neoadjuvant and adjuvant therapy may also influence or possibly predict outcomes. In an update of the MGH experience, more than half of the patients had greater than 75% pathologic necrosis documented in the resection specimen, but the extent of necrosis did not correlate with outcome.17 In our study, 8 patients did not have documentation of the extent of necrosis on pathology, and we did not statistically assess its importance. However, 5 of the 10 patients who developed local recurrence had ≥90% necrosis on their surgical pathology. Because there have been conflicting results regarding the prognostic value of pathologic necrosis after neoadjuvant therapy,18, 19, 20 additional studies are needed to evaluate other possible histologic findings, such as hyalinization or fibrosis, which may be better predictors of outcome.21,22
The major concern and hesitation in using an aggressive neoadjuvant chemotherapy regimen is due to toxicity. The RTOG 9514 study reported 3 treatment related deaths and an incidence of greater than or equal to grade 3 toxicity of 97%, and the MGH study had 1 treatment related death 53 months after the completion of treatment. Similarly, our study reports higher rates of acute dermatitis, surgical complications, and hospitalizations for chemotherapy-related infections, hematologic toxicity, or neurotoxicity. There were no treatment-related deaths among either group.
There are a number of limitations to this study that should be mentioned. Despite being a tertiary academic institution with sarcoma specialists, the relative rarity of high-grade STS, makes large retrospective studies comparing different neoadjuvant regimens challenging. As such, we were limited by the small sample size and retrospective nature of this study. Additionally, as patients may have received a portion of their therapy at an outside institution before referral, there is a degree of heterogeneity within the 2 cohorts in terms of chemotherapy regimens, radiation doses, and timing. There were few patients (n = 2) where the exact chemotherapy regimen or timing of radiation was unknown as outside records were unable to be obtained. Lastly, the difference in median follow up time between the 2 groups, 58 months for CRT versus 37 months for RT alone, does make interpretation of results more challenging. However, overall the median follow-up for the entire cohort was quite long at 41 months with median time to progression, either local or distant, at 32.5 months. Although the heterogeneity within each cohort creates some limitations in analysis and interpretation, it is a reflection of the diversity of treatment and lack of consensus in actual practice. In the end, this study was a comparison of patients who received both radiation and chemotherapy to radiation alone and thus we elected to include multiple regimens within these 2 groups.
Conclusions
The results of this study, in terms of clinical outcomes and toxicity, help confirm the potential that aggressive chemotherapy can be safely held in vulnerable populations without compromising clinic outcomes. When controlling for baseline differences, neoadjuvant CRT and RT alone provided similar rates of 3-year LC, DFS, and OS. This raises the question though of the added benefit of a toxic chemotherapy regimen. Additional prospective studies are still needed to solidify the role of aggressive chemotherapy in combination with radiation for the general population with large, high-grade soft tissue extremity sarcomas.
Footnotes
Sources of support: None.
Disclosures: Dr Alcorn reports grants from Elekta AB during the conduct of the study; others from Johns Hopkins Hospital, grants from NIH KL2 Mentored Career Development Grant, personal fees from Allegheny Health Network, outside the submitted work; and member of ASTRO Subcommittee on Research Grants and ASTRO Committee on Health, Equity, Diversity, and Inclusion. Dr Hu reports grants from National Cancer Institute during the conduct of the study; personal fees from Merck & Co., outside the submitted work. The remaining authors have no conflicts of interest related to this manuscript.
References
- 1.Rosenberg S.A., Tepper J., Glatstein E. Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisters P.W., Leung D.H., Woodruff J., Shi W., Brennan M.F. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 3.Italiano A., Le Cesne A., Mendiboure J. Prognostic factors and impact of adjuvant treatments on local and metastatic relapse of soft-tissue sarcoma patients in the competing risks setting. Cancer. 2014;120:3361–3369. doi: 10.1002/cncr.28885. [DOI] [PubMed] [Google Scholar]
- 4.Zagars G.K., Ballo M.T., Pisters P.W.T. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy. Cancer. 2003;97:2530–2543. doi: 10.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]
- 5.DeLaney T.F., Spiro I.J., Suit H.D. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–1127. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 6.Kraybill W.G., Harris J., Spiro I.J. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation therapy oncology group trial 9514. J Clin Oncol. 2006;24:619–625. doi: 10.1200/JCO.2005.02.5577. [DOI] [PubMed] [Google Scholar]
- 7.Kraybill W.G., Harris J., Spiro I.J. Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group trial 9514. Cancer. 2010;116:4613–4621. doi: 10.1002/cncr.25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raval R.R., Frassica D., Thornton K. Evaluating the role of interdigitated neoadjuvant chemotherapy and radiation in the management of high-grade soft-tissue sarcoma: The Johns Hopkins experience. Am J Clin Oncol Cancer Clin Trials. 2017;40:214–217. doi: 10.1097/COC.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clavien P.A., Barkun J., De Oliveira M.L. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 10.Williams R.L. Product-limit survival functions with correlated survival times. Lifetime Data Anal. 1995;1:171–186. doi: 10.1007/BF00985768. [DOI] [PubMed] [Google Scholar]
- 11.Lin D.Y. On fitting Cox’s proportional hazards models to survey data. Biometrika. 2000;87:37–47. [Google Scholar]
- 12.Hastie T., Tibshirani R., Friedman J. The elements of statistical learning. Math Intell. 2001;27:83–85. [Google Scholar]
- 13.Lindberg R.D., Martin R.G., Romsdahl M.M., Barkley H.T. Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer. 1981;47:2391–2397. doi: 10.1002/1097-0142(19810515)47:10<2391::aid-cncr2820471012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Tierney J.F., Mosseri V., Stewart L.A., Souhami R.L., Parmar M.K. Adjuvant chemotherapy for soft-tissue sarcoma: Review and meta-analysis of the published results of randomised clinical trials. Br J Cancer. 1995;72:469–475. doi: 10.1038/bjc.1995.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gortzak E., Azzarelli A., Buesa J. A randomised phase II study on neo-adjuvant chemotherapy for “high-risk” adult soft-tissue sarcoma. Eur J Cancer. 2001;37:1096–1103. doi: 10.1016/s0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 16.Le Cesne A., Ouali M., Leahy M.G. Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: Pooled analysis of two STBSG-EORTC phase III clinical trials. Ann Oncol. 2014;25:2425–2432. doi: 10.1093/annonc/mdu460. [DOI] [PubMed] [Google Scholar]
- 17.Hong N.J.L., Hornicek F.J., Harmon D.C. Neoadjuvant chemoradiotherapy for patients with high-risk extremity and truncal sarcomas: A 10-year single institution retrospective study. Eur J Cancer. 2013;49:875–883. doi: 10.1016/j.ejca.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullen J.T., Hornicek F.J., Harmon D.C. Prognostic significance of treatment-induced pathologic necrosis in extremity and truncal soft tissue sarcoma after neoadjuvant chemoradiotherapy. Cancer. 2014;120:3676–3682. doi: 10.1002/cncr.28945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menendez L.R., Ahlmann E.R., Savage K., Cluck M., Fedenko A.N. Tumor necrosis has no prognostic value in neoadjuvant chemotherapy for soft tissue sarcoma. Clin Orthop Relat Res. 2007;455:219–224. doi: 10.1097/01.blo.0000238864.69486.59. [DOI] [PubMed] [Google Scholar]
- 20.Gannon N.P., Stemm M.H., King D.M., Bedi M. Pathologic necrosis following neoadjuvant radiotherapy or chemoradiotherapy is prognostic of poor survival in soft tissue sarcoma. J Cancer Res Clin Oncol. 2019;145:1321–1330. doi: 10.1007/s00432-019-02885-4. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer I.M., Hornick J.L., Barysauskas C.M. Histologic appearance after preoperative radiation therapy for soft tissue sarcoma: Assessment of the European Organization for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group response score. Int J Radiat Oncol Biol Phys. 2017;98:375–383. doi: 10.1016/j.ijrobp.2017.02.087. [DOI] [PubMed] [Google Scholar]
- 22.Roberge D., Skamene T., Nahal A., Turcotte R.E., Powell T., Freeman C. Radiological and pathological response following pre-operative radiotherapy for soft-tissue sarcoma. Radiother Oncol. 2010;97:404–407. doi: 10.1016/j.radonc.2010.10.007. [DOI] [PubMed] [Google Scholar]