Abstract
Purpose
Previous studies have shown that stereotactic ablative radiation therapy (SABR) increases local control for cholangiocarcinoma, but gastrointestinal toxicity resulting from this treatment approach remains a concern. SABR using magnetic resonance–guided radiation therapy (MRgRT) may improve the therapeutic ratio of treatment for cholangiocarcinoma patients given the radiosensitivity of neighboring gastrointestinal organs.
Methods
Seventeen consecutive patients with unresectable locally advanced cholangiocarcinoma were treated with SABR using MRgRT between May 2015 and August 2017, subsequent to our previously reported series of patients treated using a standard Linac with cone beam computed tomography. Twelve patients presented with extrahepatic cholangiocarcinoma and 5 patients with intrahepatic tumors. MRgRT-based SABR was administered at a median dose of 40 Gy in 5 fractions.
Results
The median overall survival (OS) was 18.5 months, with a 1-year OS of 76% and 2-year OS of 46.1%. Three of the 17 patients progressed locally, yielding a 1-year local control of 85.6% and a 2-year local control of 73.3%. Although 12 of 17 patients experienced an acute grade 1 toxicity, none experienced acute grade 2 toxicities. One patient had an acute grade 3 duodenal ulcer with perforation (6%), and one patient had a late radiation-related toxicity grade 2 gastritis/colitis.
Conclusions
Our findings demonstrate diminished toxicity and excellent overall survival and local control. The clinical outcomes and safety profile of SABR delivered with MRgRT suggest that MRgRT is a promising treatment approach for treating cholangiocarcinoma.
Introduction
Cholangiocarcinoma is an aggressive hepatobilliary malignancy distinguished by predominantly advanced presentation and high mortality rates arising from locoregional patterns of failure. Radiation therapy has been demonstrated to prolong survival in both intrahepatic and extrahepatic disease, although local progression remains the primary mode of failure.1,2
Large-scale reviews of the National Cancer Database revealed that the addition of radiation therapy potentially offers significant survival advantage compared with chemotherapy treatment alone in patients with locally advanced disease.3, 4, 5 Progressive investigations have demonstrated the increasing role of radiation in definitive local treatment and value of radiation therapy followed by resection.6,7 Additional studies of cholangiocarcinoma have shown an association between radiation dose and improved overall survival (OS) and local control (LC), reporting 1-year LC rates of 90%8 and suggesting that a biologically effective dose (BED) >80.5 Gy yields outcomes similar to those achieved with resection.9
Stereotactic ablative radiation therapy (SABR) using volumetric image guided radiation therapy can be used to escalate dose, with increased radiation planning dose conformality and treatment delivery accuracy. Due to the intensive use of image guidance, SABR allows for steeper dose gradients between target and surrounding normal tissue compared with traditional techniques. Multiple single institution retrospective studies of Linac-based SABR for unresectable cholangiocarcinoma have achieved 1-year LC rates of 85% to 100% and median OS times of 10 to 15 months with median BEDs of 86 to 115 Gy.10, 11, 12, 13 However, although SABR yields improved survival outcomes and local control, the severe gastrointestinal toxicity that has plagued conventionally fractionated radiation therapy14,15 continues to represent an unmet challenge, with 10% to 26% of patients experiencing grade ≥3 gastrointestinal toxicity.16
One of the challenges of delivering SABR to abdominal tumors is the necessity of high-fidelity visualization of soft tissue structures and accurate tumor targeting, including accounting for both intrafractional respiratory, cardiac, and peristalsis-induced tumor motion and stochastic interfractional anatomic changes. It is particularly difficult to deliver high doses to cholangiocarcinoma owing to adjacent radiosensitive gastrointestinal organs, or organs at risk (OARs). Irradiation of these OARs leads to treatment toxicity and poses a critical obstacle to successful dose escalation. Their proximity to the tumor often necessitates an unfavorable tradeoff between tumor volume coverage and OAR avoidance.
Current image guided radiation delivery strategies, including the use of implanted fiducial markers and tumor surrogates; daily imaging using cone beam computed tomography, kilovoltage computed tomography, and CT on rails; and 4-dimensional computed tomography respiratory motion simulation, are compromised by poor soft tissue resolution, uncertainties in the use of surrogate-based treatments, inherent risks of fiducial marker implantation and possible migration, and, most importantly, challenges of imaging and visualizing OAR changes.17
MRI-guided radiation therapy (MRgRT) has garnered interest because it offers precise radiation delivery with excellent soft tissue contrast and direct tumor visualization, real-time synchronous tumor tracking and respiratory gating, and on-board daily adaptive planning capabilities.
This is the first dedicated series of cholangiocarcinoma patients treated with MRgRT, and we sought to evaluate the safety and efficacy of SABR treatment with this radiation therapy delivery modality. SABR using MRgRT may improve the therapeutic index of treatment for cholangiocarcinoma patients and achieve improved local control with minimal toxicity.
Methods and Materials
Patients and follow-up
Seventeen patients with cholangiocarcinoma were treated with SABR using MRgRT between May 2015 and August 2017 with a median follow-up time of 15.8 months (range, 4.5-29.9 months). Our previously reported series of cholangiocarcinoma patients included all patients before May 2015. Subsequent patients were treated with MRgRT-based SABR, with the exception of one patient in June 2015 who refused treatment with MRgRT and was treated using a standard Linac with CBCT. Our institutional review board approved the retrospective analysis of our department’s prospective patient registry, in which toxicity and cancer outcomes are coded in real time. All patients with cholangiocarcinoma (biopsy-proven malignancy, or both malignant stricture on cholangiography and cancer antigen 19-9 >100 U/mL) eligible for MRgRT treatment were included without patient selection criteria. MRgRT was delivered using a 0.35T tri-60Co magnetic resonance imaging (MRI)–guided radiation delivery system equipped with 3 rotating cobalt sources on a gantry ring (MRIdian, ViewRay Inc., Cleveland, OH), and SABR was administered at a median dose of 40 Gy in 5 fractions. Notably, one patient was treated with concurrent hyperthermia, and one patient received a SABR boost after intensity-modulated radiation therapy (IMRT) (54 Gy in 30 fractions). Twelve patients were evaluated on our institutional liver transplant protocol, which includes treatment with SABR at a dose of 40 Gy in 5 fractions, and 2 patients successfully underwent liver transplants subsequent to radiation therapy completion. Patients were followed clinically and radiographically after treatment as per standard of care and were routinely evaluated with computed tomography (CT) or positron emission tomography CT scans every 3 to 6 months.
SABR treatment planning
Treatment simulation scans were obtained on both the 0.35T split magnet MRI merged with a tri-60Co radiation source and standard CT (to obtain electron densities for tri-60Co plan dose calculations).18 Planning MRI scans of 3-mm thickness were obtained with an image acquisition of 17 seconds under breath-hold and were registered with diagnostic scans to demarcate gross tumor volume (GTV). The GTV was expanded by 3 mm to obtain the planning target volume (PTV), and radiation doses were prescribed to 95% of the PTV.
Normal tissue OAR constraints were set as follows: duodenal loop and stomach V35Gy < 0.5 mL; bowel bag V20Gy < 20 mL; spinal cord and left and right kidneys maximum 12.5 Gy; normal liver ≥1000 mL <15 Gy. The prescribed dose constraints were more stringent than reported objectives in similar studies and the constraints provided by the American Association of Physicists in Medicine Task Group 10119 because we had found that we could improve upon these recommended values.
The constraints were the same for all doses and fractionations, and the dose was escalated based on favorable tumor location. Tumors in close proximity to radiosensitive gastrointestinal structures were treated conservatively, with priority placed on limiting doses to the outlined OARs.
Respiratory gating
We used a PTV margin expansion of the GTV of 3 mm. To allow the use of this small margin, we used breath-hold respiratory gating. The commercial image guided radiation therapy system provided real-time 4 frames per second sagittal cine images and tracked the tumor as the patient breathed. The dose delivery was paused and resumed when the GTV moved outside and back inside of the prescribed location.
Adaptive planning
Adaptive planning was routinely implemented after treatment of the first few patients. Before each treatment, MRI imaging was obtained with the same parameters as the simulation scan. The simulation planning contours were transferred to the daily set-up MRI and manually adjusted, and the initial planned treatment dose was predicted onto the patient’s daily anatomy.20 Protection of surrounding radiosensitive OARs superseded PTV coverage, and reoptimization was necessitated if the new geometry predicted normal tissue overdose. Adaptive planning total dose constraints were not as conservative as the prescription constraints and were defined as follows: V35Gy <1 mL in the duodenum and stomach, and a maximum of 20 Gy to the spinal cord.
Statistical analysis
Survival curve analysis performed by the Kaplan-Meier method was used as a metric of clinical efficacy. Overall survival (OS), progression-free survival, and local control (LC) were defined as the time between completion of treatment to death, initial progression, and local failure, respectively, as determined by Response Evaluation Criteria in Solid Tumors, version 1.1. Treatment toxicities were graded according to the designations outlined in the Common Terminology Criteria for Adverse Events, version 4.0, and acute and late toxicities were defined using a cutoff of 90 days from completion of radiation therapy.
Results
Patient characteristics
Of the 17 cholangiocarcinoma patients treated with SABR using MRgRT between May 2015 and August 2017, 12 patients presented with hilar cholangiocarcinoma and 5 patients presented with intrahepatic tumors, with 2 patients treated for local recurrences after surgery. All of the treated cases were unresectable locally advanced cholangiocarcinoma, and all patients underwent a complete staging workup that consisted of positron emission tomography with CT and MRI. Sixteen out of 17 patients had biopsy-proven adenocarcinoma, and 1 patient had both malignant stricture on cholangiography and cancer antigen 19 to 9 >100 U/mL. Five cases had suspected regional disease in the form of locoregional nodes at the time of SABR. The median patient age was 57 for the 11 male patients (65%) and 6 female patients (35%), with a median Karnofosky Performance Scale score of 90. Fourteen patients had biliary stents at the time of treatment, and the median pretreatment bilirubin concentration was 1.7 mg/dL (range, 0.2-22.2 mg/dL). The median primary lesion size at consult was 25.5 mm (range, 14-37 mm), although 3 patients had ill-defined lesions. One patient had previously undergone a Whipple procedure for intraductal papillary mucinous neoplasm, and 3 patients had undergone prior surgery. Five patients had received previous courses of chemotherapy, most commonly gemcitabine/cisplatin, and 2 patients were treated with concurrent chemotherapy. The single patient treated with a SABR boost after IMRT received concurrent daily capecitabine, and the other patient received a cycle of cisplatin/gemcitabine chemotherapy during the course of radiation treatment at the discretion of the treating medical oncologist.
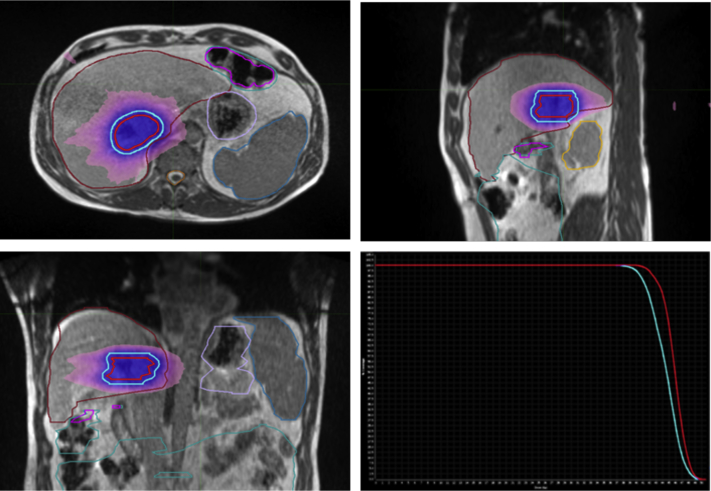
The median delivered SABR dose was 40 Gy in 5 fractions (Fig. 1), with only 4 exceptions: 2 patients were treated with 45 Gy in 3 fractions, one patient received 50 Gy in 5 fractions, and one patient was treated with a SABR boost of 12 Gy in 3 fractions. Tumor target volumes ranged from 9.72 mL for the patient receiving a SABR boost, to 31.48 to 169.72 mL for the definitively treated patients.
Figure 1.
Magnetic resonance–guided stereotactic ablative radiation therapy plan and accompanying dose-volume histogram for extrahepatic cholangiocarcinoma treated with 40 Gy in 5 fractions. Solid red line = gross tumor volume; solid blue line = planning target volume; blue color wash = 100% dose; pink color wash = 50% dose.
Clinical outcomes
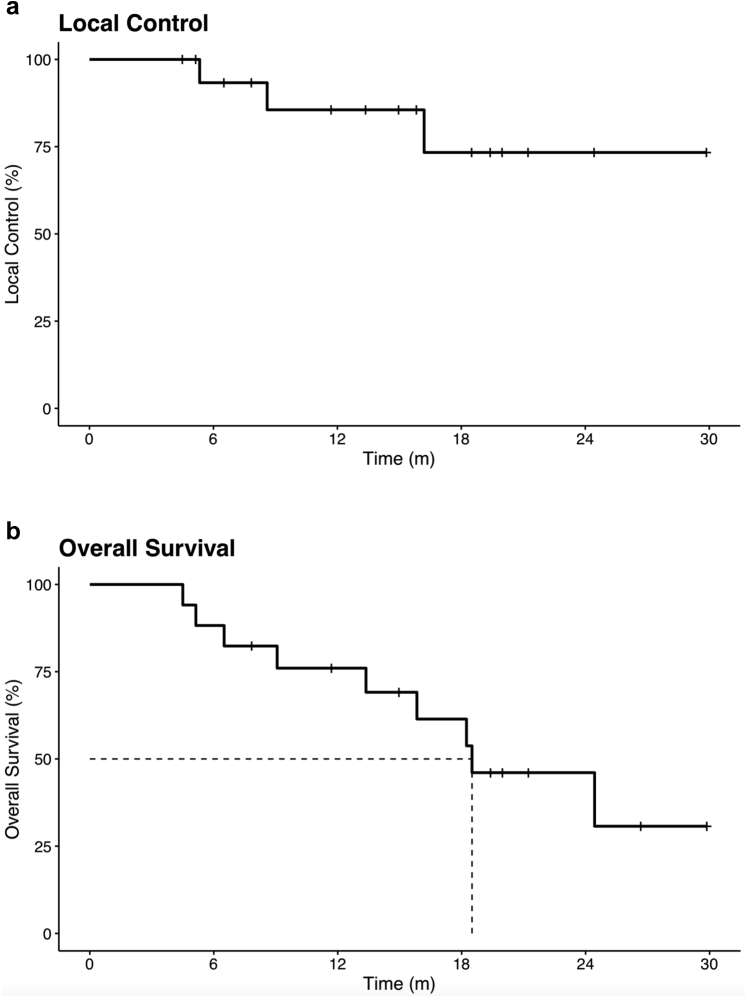
The median OS was 18.5 months, with a 1-year OS of 76% and 2-year OS of 46.1% (Fig. 2a). The median progression-free survival was 8.4 months, with a 1-year progression-free survival of 38.5%. Three patients had regional progression with intrahepatic failure outside of the radiated field, and 7 patients had distant failure. Three of the 17 patients progressed locally, yielding a 1-year local control of 85.6% and a 2-year local control of 73.3% (Fig. 2b). Twelve out of 17 patients were evaluated as orthotopic liver transplantation (OLT) candidates; of the 7 patients still alive at the time of the last analysis, 2 patients had successfully undergone OLT after radiation. One of the patients who received an OLT had no viable tumor cells, and the explant indicated no malignancy, consistent with pathologic complete response.
Figure 2.
Kaplan-Meier curves for (a) local control and (b) overall survival.
Two patients currently remain on the liver transplant list, although the patient who received a SABR boost after IMRT was not a transplant candidate and went on to undergo Y-90 hepatic radioembolization of 3 liver lesions.
Toxicity
Twelve of 17 patients experienced an acute grade 1 toxicity, with mild nausea and fatigue accounting for 80% of toxicities. No grade 2 acute toxicities were experienced; however, one patient had a grade 3 duodenal ulcer with perforation (6%). The same patient had a late radiation-related toxicity grade 2 gastritis/colitis; no other late toxicities occurred (Table 1).
Table 1.
Acute and late treatment-related toxicities
| Acute toxicity | Grade | No. (%) |
|---|---|---|
| Nausea | grade 1 | 8 (47) |
| Fatigue | grade 1 | 4 (24) |
| Abdominal pain | grade 1 | 2 (12) |
| Shoulder pain | grade 1 | 1 (6) |
| Duodenal ulcer | grade 3 | 1 (6) |
| Late toxicity | Grade | No. (%) |
|---|---|---|
| Gastritis/colitis | grade 2 | 1 (6) |
The patient who had a duodenal ulcer with perforation was one of the earliest patients treated—before the use of routine adaptive planning. Although the patient had an intrahepatic lesion, which is typically associated with less toxicity, the OAR dose constraint of the stomach was violated, with 3.83 mL of stomach volume receiving 35 Gy (stomach OAR constraint: V35Gy <.5 mL). Eight patients developed a cholangitis, but the episodes were deemed unrelated to treatment and were due to stent dysfunction.
Three patients were treated using reoptimized adaptive radiation plans. Two of the 3 patients adapted had intrahepatic tumors, and the highest grade of toxicity for patients treated with adaptive radiation therapy was grade 1. None of the 12 extrahepatic cholangiocarcinoma patients, including one patient treated with adaptive radiation therapy, experienced severe toxicity.
Discussion
Previous studies have shown that SABR increases local control for cholangiocarcinoma, but gastrointestinal toxicity resulting from treatment remains a major barrier for the adoption of SABR for abdominal tumors (including pancreatic and liver tumors and metastases, and cholangiocarcinoma). Our previous published experience of 31 cholangiocarcinoma patients definitively treated with SABR using Linac reported a median OS of 15.7 months, 1-year OS of 59%, and 1-year local control of 78%.21 Six patients (19%) in this series had severe toxicities; 5 of these patients experienced severe late gastrointestinal toxicities rather than acute injuries, which is characteristic of SABR treatment.16 The high prevalence of extrahepatic lesions (25 of 31 lesions), which are associated with higher risk of normal tissue injury owing to proximity to the radiosensitive gastrointestinal tract, was consistent with analogous studies with high rates of late gastrointestinal toxicities.10,11 More recently, a retrospective study of 28 patients with intrahepatic cholangiocarcinoma treated with CyberKnife (Accuray, Inc.) SABR using 3 to 6 implanted fiducials for synchronous respiratory tracking reported a median OS of 15 months, 1-year OS of 57.1%, and disease control rate of 89.3% with a median dose of 45 Gy in 3 fractions.13 The accompanying toxicity profile was substantial, with 5 grade 3 gastrointestinal toxicities (18%), and 3 grade 2 complications associated with fiducial implantation (11%). The survival outcomes are consistent with those of our prior study of Linac-based SABR; however, the proportional toxicity profile of a cohort of patients with exclusively intrahepatic disease emphasizes the gastrointestinal toxicity associated with dose escalation (BED = 85.5 Gy compared with BED = 72 Gy).13
MRgRT, although constrained by low-field MRI and a lower-energy cobalt radiation source, enables superior soft tissue visualization, 3-mm margin breath-hold respiratory gating, and daily online adaptive planning. The resultant smaller treatment margins and adaptive treatment plans tailored to daily anatomy conjointly minimize OAR dose and maximize intended target dose. Daily optimization enhances the overall therapeutic index of radiation therapy treatment through personalization of treatment delivery, and the unique capabilities of MRgRT potentiate improved local control and safety profile.
In our present study, radiation dose, fractionation, and dosimetric constraints matched those of our previous institutional review, and many patients in both cohorts were enrolled in an institutional liver transplant protocol, which generated a level of standardization between the studies. Survival outcomes were favorable, and we achieved a longer median follow-up time (15.8 months vs 11.5 months in our earlier study). Perhaps most significantly, the toxicity profile for our patient cohort was promising, with fewer complications than previous published series of cholangiocarcinoma treated with SABR. In contrast to prior studies,11,12 neither our current nor our older series had treatment-related hepatobiliary toxicities, and we previously postulated that the presence of pretreatment biliary stents in the majority of patients may have mitigated biliary fibrosis and stenosis.21 The patient demographic of the present study was similarly skewed toward extrahepatic lesions; notably, however, none of the 12 patients treated for extrahepatic cholangiocarcinoma experienced severe toxicity. The single grade 3 toxicity reported arose in a patient with intrahepatic cholangiocarcinoma, after radiation dose to the stomach exceeded OAR constraints. The stomach received a maximum dose (Dmax) of 49.05 Gy, and 3.83 mL of stomach volume received a dose of 35 Gy. V35Gy and Dmax are good dosimetric predictors of severe gastroduodenal toxicity, and Dmax >45 Gy and V35Gy >1 mL have been shown to correlate with 50% incidence of late grade ≥3 gastroduodenal toxicity (compared with 6% incidence below cutoff values; P = .0015).22 This was one of the earliest patients treated, spurring on efforts to routinely integrate adaptive plan reoptimization for patients with normal tissue dosimetry exceeding critical constraints. In the subsequent subset of 4 patients with intrahepatic lesions, 2 patients were treated using adaptive radiation therapy, neither of which experienced treatment-related toxicity. The reduction of toxicity with adaptive planning underscores the utility of MRgRT tissue visualization and the subsequent ability to more precisely target tumor and spare normal tissue, especially when the normal tissue positions fluctuate.
Several limitations of the study include its retrospective nature, heterogeneous patient population and treatment schemes, and small sample size, with a small number of patients treated with adaptive radiation therapy and a small number undergoing OLT. However, the study capitalized upon our department patient registry, in which both toxicity and outcomes are coded concurrently in real-time, and included all consecutive patients treated subsequent to our Linac series without patient selection criteria. Collectively, inclusion of all eligible cholangiocarcinoma patients and analysis of prospectively gathered patient data helps counterbalance the inherent constraints of a smaller retrospective study. Additionally, although the follow-up time exceeds that of our previous study, limited follow-up time could influence outcomes.
Despite these limitations, SABR using MRgRT shows promise as a means of improving the therapeutic ratio of treatment for cholangiocarcinoma and safely delivering doses high enough to maintain local control, which may translate to better survival and cancer specific outcomes, including control of regional metastases and improved quality of life measures.
Conclusions
Our findings using MRgRT for cholangiocarcinoma patients demonstrate favorable overall survival while maintaining rates of local control comparable with the outcomes of our previous retrospective study using Linac-based SABR. Treatment delivered through MRgRT resulted in one grade 3 toxicity (6%), although the Linac study determined that 6 patients (19%) had severe toxicities with the same median radiation dose and fractionation. Our clinical outcomes, coupled with a diminished toxicity profile, suggest SABR using MRgRT is a promising new treatment approach.
Footnotes
Sources of support: None.
Disclosures: Percy Lee declares grants, personal fees, and nonfinancial support from ViewRay Inc; personal fees and nonfinancial support from Varian Inc; and grants, personal fees, and nonfinancial support from AstraZeneca Inc. Michael L. Steinberg declares personal fees (honoraria) from ViewRay Inc and personal fees (consulting fees) from VisionRT. Minsong Cao declares personal fees (speaking honorarium) from ViewRay Inc. James M. Lamb declares personal fees from ViewRay Inc. Elaine Luterstein declares nonfinancial support (travel fees) from ViewRay Inc.
References
- 1.Shinohara E.T., Mitra N., Guo M. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2008;72:1495–1501. doi: 10.1016/j.ijrobp.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Shinohara E.T., Mitra N., Guo M. Radiotherapy is associated with improved survival in adjuvant and palliative treatment of extrahepatic cholangiocarcinomas. Int J Radiat Oncol Biol Phys. 2009;74:1191–1198. doi: 10.1016/j.ijrobp.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Jackson M.W., Amini A., Jones B.L. Treatment selection and survival outcomes with and without radiation for unresectable, localized intrahepatic cholangiocarcinoma. Cancer J. 2016;22:237–242. doi: 10.1097/PPO.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 4.Torgeson A., Lloyd S., Boothe D. Chemoradiation therapy for unresected extrahepatic cholangiocarcinoma: A propensity score-matched analysis. Ann Surg Oncol. 2017;24:4001–4008. doi: 10.1245/s10434-017-6131-9. [DOI] [PubMed] [Google Scholar]
- 5.Verma V., Appiah A.K., Lautenschlaeger T. Chemoradiotherapy versus chemotherapy alone for unresected intrahepatic cholangiocarcinoma: Practice patterns and outcomes from the national cancer data base. J Gastrointest Oncol. 2018;9:527. doi: 10.21037/jgo.2018.01.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamashita S., Koay E.J., Passot G. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: A comprehensive analysis of 362 consecutive patients. Cancer. 2017;123:1354–1362. doi: 10.1002/cncr.30488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang W.W., Hsiao P.K., Qin L. Treatment outcomes for unresectable intrahepatic cholangiocarcinoma: Nationwide, population-based, cohort study based on propensity score matching with the Mahalanobis metric. Radiother Oncol. 2018;129:284–292. doi: 10.1016/j.radonc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Weiner A.A., Olsen J., Ma D. Stereotactic body radiotherapy for primary hepatic malignancies–report of a phase I/II institutional study. Radiother Oncol. 2016;121:79–85. doi: 10.1016/j.radonc.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao R., Krishnan S., Bhosale P.R. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: A retrospective dose response analysis. J Clin Oncol. 2016;34:219. doi: 10.1200/JCO.2015.61.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopek N., Holt M.I., Hansen A.T. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol. 2010;94:47–52. doi: 10.1016/j.radonc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Barney B.M., Olivier K.R., Miller R.C. Clinical outcomes and toxicity using stereotactic body radiotherapy (SABR) for advanced cholangiocarcinoma. Radiat Oncol. 2012;7:67. doi: 10.1186/1748-717X-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Hoon Jung M.S., Cho C.K., Yoo H.J. Outcomes of stereotactic body radiotherapy for unresectable primary or recurrent cholangiocarcinoma. Radiat Oncol J. 2014;32:163. doi: 10.3857/roj.2014.32.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen Z.T., Zhou H., Li A.M. Clinical outcomes and prognostic factors of stereotactic body radiation therapy for intrahepatic cholangiocarcinoma. Oncotarget. 2017;8:93541. doi: 10.18632/oncotarget.19972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Josef E., Normolle D., Ensminger W.D. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 15.Elganainy D., Holliday E.B., Taniguchi C.M. Dose escalation of radiotherapy in unresectable extrahepatic cholangiocarcinoma. Cancer Med. 2018;7:4880–4892. doi: 10.1002/cam4.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J., Yoon W.S., Koom W.S. Efficacy of stereotactic body radiotherapy for unresectable or recurrent cholangiocarcinoma: A meta-analysis and systematic review. Strahlenther Onkol. 2019;195:93–102. doi: 10.1007/s00066-018-1367-2. [DOI] [PubMed] [Google Scholar]
- 17.Lim-Reinders S., Keller B.M., Al-Ward S. Online adaptive radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:994–1003. doi: 10.1016/j.ijrobp.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Mutic S., Dempsey J.F. The ViewRay system: Magnetic resonance–guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Benedict S.H., Yenice K.M., Followill D. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phy. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 20.Tyran M., Jiang N., Cao M. Retrospective evaluation of decision-making for pancreatic stereotactic MR-guided adaptive radiotherapy. Radiother Oncol. 2018;129:319–325. doi: 10.1016/j.radonc.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Sandler K.A., Veruttipong D., Agopian V.G. Stereotactic body radiotherapy (SBRT) for locally advanced extrahepatic and intrahepatic cholangiocarcinoma. Adv Radiat Oncol. 2016;1:237–243. doi: 10.1016/j.adro.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bae S.H., Kim M.S., Cho C.K. Predictor of severe gastroduodenal toxicity after stereotactic body radiotherapy for abdominopelvic malignancies. Int J Radiat Oncol Biol Phys. 2012;84:e469–e474. doi: 10.1016/j.ijrobp.2012.06.005. [DOI] [PubMed] [Google Scholar]