Abstract
A review of the literature indicated denosumab is gaining favorability in the oncology community, particularly with increasing frequency in GCTB. Will denosumab be the breakthrough GCTB treatment? Here, we provide a pertinent case example, a review of the literature regarding the history and basic science behind the use of denosumab for GCTB, highlight the newest insights into the dosing and duration of treatment, and note advancements in the field.
Keywords: Denosumab, RANKL, Giant cell tumor of the bone (GCTB)
1. Introduction
Our case involves a 36-year-old female with no pertinent past medical history who initially presented to an outside facility with right wrist pain and swelling. She was subsequently diagnosed with giant cell tumor of bone of the right distal radius in 2004. She underwent excision of a 1.6 × 3.1 cm tumor with hardware placement. Postoperatively she did well until 2006 when routine surveillance revealed multiple lung nodules. She underwent wedge resections by thoracotomy with pathology consistent with GCTB benign pulmonary implants. Three years later, computed tomography (CT) imaging of the chest revealed a new large 2.6 × 3.1 cm mass at the level of the left pulmonary hilum concerning for recurrent metastatic disease. This finding was determined to be resectable but would require pneumonectomy and a recommendation was made to initiate denosumab therapy prior to surgery. She began shortly thereafter a regiment of denosumab 120 mg subcutaneously every 4 weeks. Computed tomography scan taken at four months after initiation of therapy showed significant interval change in decreasing hilum tumor burden (Fig. 1). At the same follow-up appointment her interval of therapy was changed to every 6 weeks and her tumor burden has been stable for approximately 9 years as she continues to have a good pharmacologic response. As part of her surveillance she has had CT imaging of her chest and radiographs of her right wrist every six months for the past 9 years since beginning treatment. She has been without subjective complaint at each follow-up visit. After 9 years of denosumab therapy, she continues to tolerate the denosumab without any evidence of side effects or complications.
Fig. 1.
(A) Coronal CT scans of the chest showing initial 2.6 × 3.1 cm mass at the level of the left pulmonary hilum. (B) Interval 4 month follow-up CT scan of the chest showing significant interval change in decreasing hilum tumor burden.
This case supports the literature that indicates denosumab is gaining favorability in the oncology community in regards to certain GCTB treatment scenarios. Will denosumab be the breakthrough GCTB treatment? Here, we provide a review of the literature regarding the history and basic science behind the use of denosumab for GCTB, highlight the newest insights into the dosing and duration of treatment, and note advancements in the field.
2. Molecular biology of the bone microenvironment
Bone is a dual-purpose physiologically active connective tissue. While most well-known for structural support and locomotion, it is also the site of other integral functions such as, hematopoiesis, immunologic cell maturation and calcium/phosphorus reservoir. Bone is highly vascularized with multiple anastomotic channels aiding in its high metabolic turnover of intramedullary hemopoietic stems cells, bone matrix osteocytes, and periosteal progenitor cells.1 The high vascularity and cellular turnover make bone a potential area for abnormal growth patterns resulting in tumor or metabolic derangement.
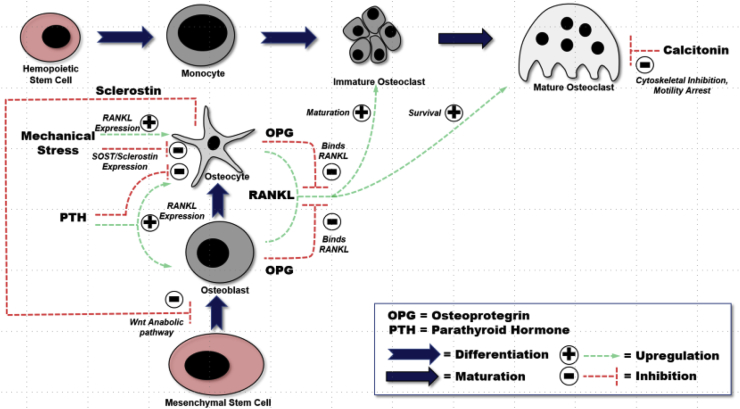
While bone itself is a matrix of mineralized hydroxyapatite (HA), this structure is in constant flux and maintained by osteoblasts, osteocytes, and osteoclasts together called a basic multicellular unit.2 Mesenchymal stem cells are precursors to these remodeling cells, which initially from a nidus of osteoprogenitor cells and differentiate into mature osteoblasts under influence of cytokines (Fig. 2). After differentiation, osteoblasts lay a scaffold of immature bone called osteoid, which combines with hydroxyapatite. Osteoblasts have a central role in bone metabolism by producing Receptor to Nuclear factor Kappa Ligand (RANKL) under influence of parathyroid hormone (PTH). RANKL binds a receptor located on osteoclasts (RANK) resulting in differentiation and maturation of osteoclasts.1,3, 4, 5 Osteoprotegerin (OPG) is also released by osteoblasts and involved in the regulation of bone, but is not well understood. However, it is known that OPG is a decoy receptor to RANKL, downregulating osteoclastic activity and decreasing bone resorption.1,3,4,6,7
Fig. 2.
Physiologic Signaling Pathway of the Basic Metabolic Unit (BMU) and precursors. Osteoblasts and Osteocytes are derived from Mesenchymal Stem Cells (MSC). Osteoblasts mineralize bone by forming Hydroxyapatite while Osteocytes support mature bone with nutrients and waste removal. Osteocytes are formed by differentiated Osteoblasts. Osteoclasts are of myeloid origin from the monocyte/macrophage lineage. Osteoclasts work to resorb bone by demineralizing the hydroxyapatite from the osteoid. The major cell responsible for osteological regulation of metabolism is the osteocyte by its regulation of osteoblasts through sclerostin; and the control of osteoclasts through RANKL/OPG expression.
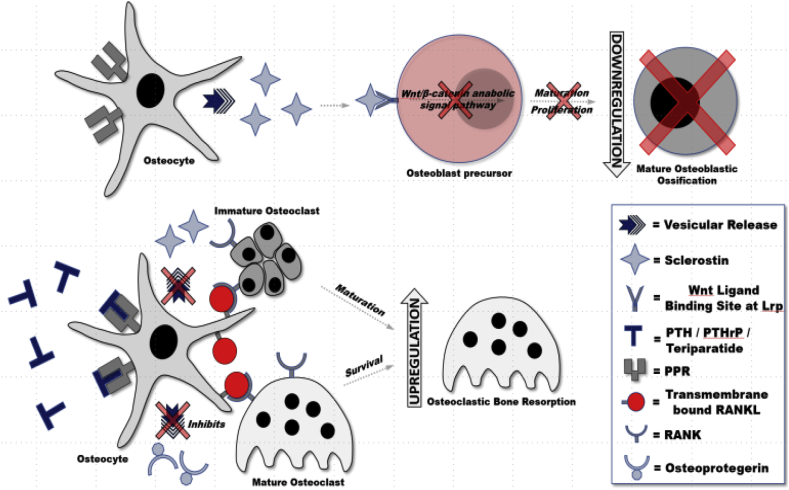
As osteoblasts become incorporated into osteoid, they are destined for differentiation into osteocytes. Osteocytes do not divide, but are metabolically active, providing support to surrounding bony matrix through nutrient supplementation and waste removal.4 Osteocytes are connected by cytoplasmic extensions which form a network between each osteocyte. Osteocytes also play an integral role in bone metabolism by upregulating and downregulating factors interacting with osteoblasts and osteoclasts. Osteocytes are responsive to a number of stimuli including inflammatory cytokines,1,4 but most importantly, PTH and mechanical loading. In response, osteocytes upregulate transmembrane RANKL located on its stellate arms upregulating osteoclastic activity necessary for bone remodeling (Fig. 3).1,4,8 Osteocytes have increased expression of RANKL compared to osteoblasts, and also has been shown to release OPG, arguably making osteocytes more important in the regulation of bone metabolism.4
Fig. 3.
Parathyroid Hormone (PTH), Parathyroid related Hormone (PTHrP), and Teriparatide actions on BMU physiology.
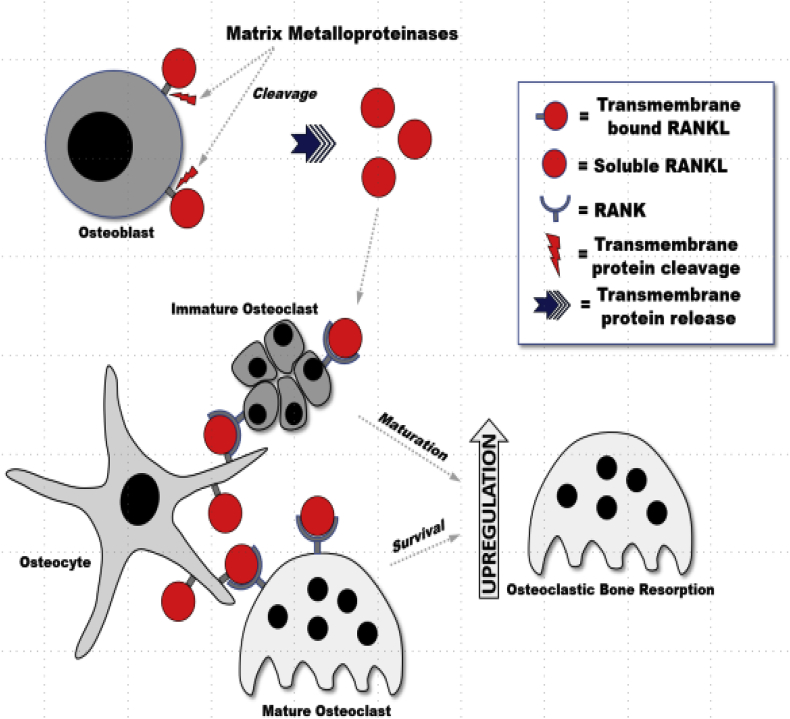
Osteoclasts are derived from a monocytic lineage and under the influence of RANKL, macrophage colony stimulating factor (M-CSF), and other cytokines, aggregate and form giant cells with multiple nuclei (Fig. 4). These giant cells eventually matures into osteoclasts.1 Osteoclasts function in bone resorption by demineralizing HA through a series of acid pumps and collagen digestion through enzymes in a resorptive pit at its ruffled border.14,15 Osteoclasts are one of the primary cells that induce the morbidities associated with bone pathology. Most osteological pharmacologic agents target osteoclasts in some way; whether acting directly on the osteoclast itself or indirectly through upstream components which regulate osteoclastic actions on bone. Upregulation of osteoclasts produces demineralized bones and osteoporotic-like features. Downregulation of osteoclasts may lead to hyper-mineralized bone which is stiff and brittle.
Fig. 4.
RANKL acts on RANK and promotes osteoclastogenesis and osteoclast survival. RANKL expression and signaling is one of the most important mechanisms for activating osteoclasts and causing subsequent bone remodeling through hydroxyapatite and collagen resorptive events.
3. Giant cell tumor of bone (GCTB)
GCTB is commonly a benign, osteolytic tumor that rarely metastasizes. A majority of patients affected are on average in the 3rd decade of life with female gender favored.16,17 Initially, the tumor expansion begins with local osseous structures, with subsequent incursion into neighboring soft tissues.17 GCTB comprises less than 5% of the total primary bone tumors in the United States, but accounts for 20% of benign tumors in the adult population.18 Radiographically, tumors appear as eccentric, lytic lesions without sclerosis, but a distinct identifiable border. GCTB occurs at several osseous sites, but typically is found in the meta-epiphyses of long bones. The majority of lesions develop around the knee, but also sites such as the proximal humerus, distal radius, spine and pelvis.16,19,20 A plain radiograph is the recommended initial diagnostic study for primary and recurrent lesions of GCTB.21,22
GCTB is associated with a category of giant cell bone tumors including giant cell granulomas, aneurysmal bone cysts, and chondroblastomas.23 These tumors are characterized by reactive multinucleated osteoclastic giant cells which, express receptor activator of nuclear factor kB ligand (RANKL).17,24 While GCTB is benign, it exhibits a wide-range of behaviors including local recurrence, multicentricity, distant metastatic lesion and invasiveness. The World Health Organization classifies these tumors as “aggressive, potentially malignant lesions”23 however, ~80% of diagnosed GCTB will follow a benign clinical course. Nonetheless, with a local recurrence rate reaching 50%, and 10% chance of malignant transformation, one can see how this would give GCTB its aggressive reputation.17 In addition, approximately 4% of benign and malignant GCTB cases can potentially generate pulmonary implants, which have benign histological characteristics similar to the original tumor. Patients with pulmonary lesions have been shown to survive for years, but display a blunted response to chemotherapy in some cases.16
In vivo, GCTB is a collection of highly vascularized collagenous bands with hemorrhages and associated hemosiderin deposits.25 Histologically, GCTB has three typical cell lineages including spindle mesenchymal stromal cells, mononuclear monocytes, and typical multinucleated osteoclastic giant cells.17 The primary neoplastic constituents are stromal cells with high expression of RANKL, which activate osteoclastic cell types resulting in overexpression of RANK receptors. Multiple endogenous factors are implicated in this upregulation of RANKL, and also downregulation of OPG, suggesting cell pathways as potential therapeutic targets.26 RANK-RANKL binding and M-CSF are primarily responsible for the osteoclastogenesis seen in GCTB.26,27 It has been theorized that an important role is played in metastasis by the stimulation of osteoblasts via factors excreted from tumor increasing RANKL expression.28 This in turn binds OPG leading to increase bone resorption which releases tumor cells to seed at distant sites. The upregulation of epidermal growth factor receptor in response to M-CSF was recently correlated to recurrent and metastatic disease.29 The exact mechanism for RANKL upregulation on stromal cells is unknown. Yet, bone resorption capabilities have been found to function via cathepsin-K, a protease functioning in GCTB giant cells.30,31 It has been proposed that RANK/RANKL interaction is the reason for aggressive osteoclast-type cells and pervasive bone resorption in this disease.17
Telomere fusion is a common chromosomal abnormality in GCTB, having been observed in 50–70% of cases.33 In the 1990s, GCTB was found to exhibit a protective telomeric capping process maintaining telomere length in osteoclastic and stromal cells.34 Genomic analyses have identified mutations in the H3F3-A gene on histone-3.3 of neoplastic stromal cells and not in osteoclastic cells.35 This aids not only for diagnosing GCTB,36 but differentiating between GCTB and chondroblastoma.37 Two particularly similar lesions, GCTB exhibits H3F3-A and chondroblastoma exhibits H3F3–B.37,38 Centrosome amplification and chromosome aneuploidy has been linked to recurrent and metastatic behaviors in GCTB. This revealed a possibility where disease prognosis regarding malignant degeneration can be identified by a readily available test.39 Other genes implicated in metastatic GCTB are DCN and LUM controlling cell membrane stability.40 Malignant transformation has been seen with mutations in H-ras and TP53, which are not present in benign GCTB, which may allow identification before progression of disease.41 Collectively, these findings suggest genetic entities may be future diagnostic targets for predicting the clinical behavior of GCTB.
4. Surgical management of GCTB
Current treatment of appendicular GCTB tumors consists primarily of surgery. The recurrence rates range between 27 and 65% and is usually seen within 2 years.17,20,21 Ideally, intralesional curettage with adjuncts is recommended in GCTB with emphasis on preserving joint integrity and function. Implementation of high-speed burrs and adjunctive cements to extend the intralesional curettage, successfully reduced recurrence rates to 12–27%.17,42 Studies regarding adjuvants such as cryotherapy, liquid nitrogen, polymethyl methacrylate (PMMA) and phenol have been advocated to decreased risk of recurrence.43, 44, 45, 46, 47, 48 Studies comparing liquid nitrogen and phenol demonstrated similar oncologic outcomes. However, a combination of phenol and PMMA showed minimal difference versus PMMA alone suggesting combing adjuncts has its limitations.49 Studies have shown local eradication of tumors about 75% of the time with one curettage treatment and even upwards of 85–100% after a second.50 These studies have demonstrated that intralesional surgery is a viable initial option for GCTB. Despite favorable responses in the appendicular skeleton, the axial skeleton presents a unique surgical challenge. Depending on location, tumors may reside in areas of complex anatomy and dense neurovascular structures making the use of adjuvants difficult. Magnetic resonance imaging has proven its practicality in predicting clinical behavior and is recommended for surgical planning in each case.51
Local tumor recurrence is strongly associated with soft tissue invasion if present.52,53 When these cases occur, the practicality of intralesional surgery hinges on whether soft tissue involvement could be improved with adjuvant systemic denosumab. Commonly, patients present with pathologic fractures, which has been shown to increase local recurrence rates as well.49,54 Curettage with adjuvant therapy remains a viable option, as are en bloc resections. As one would expect, the complication rate is higher for en bloc resections because of the often-complex reconstruction required afterwards. However, treatment in patients with recurrent GCTB includes either multiple intralesional curettage procedures or en bloc resection. The incidence of local recurrence widely varies depending on which treatment modality is selected. En bloc resection should be a last resort procedure, only after intralesional curettage has been performed, or if contraindications exist precluding curettage or pharmacologic therapy. However, while en bloc resection results in greater complications and worse functional results, it is associated with lower recurrence rates (0–12%).42 Therefore, en bloc resection should be considered in patients with intra-articular pathologic fractures needing prompt fixation. Overall, en bloc resection is a morbid procedure that may not definitively treat the patient. It also leaves physicians to weight options like reconstruction, adding levels of complexity to the surgical and postoperative planning.
Benign pulmonary implants, if present, can also be surgically treated. If possible, surgical resection is recommended, given a favorable prognosis in up to 80% of surgical cases.55 Approximately 20% of patients, regardless of the rate of distant proliferation, will succumb to the disease if left untreated. The use of radiotherapy has been shown to improve local control in a reported 84% of patients.56 However, the risk of radiotherapy includes potential sarcomatous transformation with the large doses needed for these lesions.57
5. Multidisciplinary medical management of GCTB
The use of pharmacologic therapy (Fig. 5) in GCTB was heralded as a breakthrough treatment as early as the 1990s. Developments in radiological, histological and pharmacological sciences continue to shift the approach to this disease process from entirely surgical to multidisciplinary. Systemic pharmacologic therapy for GCTB, including chemotherapy, has shown promise. Bisphosphonates (BP) were initially used due to their anti-osteoclastic properties and favorable side effect profiles. With one study noting a local recurrence rate of 4.2% using bisphosphonates versus 30% in a control, bisphosphonates appeared to be a promising therapy.46
Fig. 5.
The effects of Bisphosphonates, Denosumab, and PTH agonists on BMU physiology. PTHrP and Teriparatide acts in the same manner as PTH in inducing RANKL expression and sclerostin inhibition. Denosumab is a human monoclonal antibody against RANKL that inhibits osteoclastogenesis. Nitrogen containing bisphosphonates (N-BPs) primarily inhibit the mevalonate pathway in osteoclast precursors and mature osteoclasts. Non-Nitrogen containing bisphosphonates (NN-BPs) inhibit cellular metabolism of osteoclasts and their precursors by producing non-hydrolysable ATP.
There are two groups of BPs that act by naturally binding calcium and resisting hydrolysis, preventing calcification by alkaline phosphatase.15 Normally calcium in HA is taken up by osteoclasts during resorption. BPs use this calcium chelating ability to bind HA in bone with high affinity. During resorption osteoclasts cause a separation of the calcium-phosphate salt (Fig. 6). Nitrogen containing BPs are most commonly used and mechanistically block Farnesyl Diphosphate Synthase in a pathway similar to statins, but produce a different substrate which is toxic, promoting apoptosis of osteoclasts.15,58
Fig. 6.
Nitrogen Containing Bisphosphonates (N-BP) mechanism of action on osteoclasts.
In the early 1990s, BPs were implemented as gold standard pharmacological (and even primary) treatment for benign and malignant bone tumors.59 BPs were presumed to act in the osteolytic phase of bone resorption by inhibiting the production of osteoclasts and increasing apoptosis of osteoclasts.60 Zoledronic acid is the most widely used drug in these benign and malignant diseases. Early studies showed BPs demonstrate stabilization of lesions in both local and metastatic GCTB.46,61,62 Contrary to earlier studies, later studies failed to show any improvement in recurrence rates when using zoledronic acid as an adjuvant therapy, therefore a prospective randomized trial is still warranted.63 The discovery and mapping of cellular signaling pathways has allowed for formulation of drugs targeting these natural processes. The regulation of bone turnover and discover of the function of RANKL in bone resorption is a potential target by pharmacological agents modulating osteoclast activity.
6. Development of denosumab
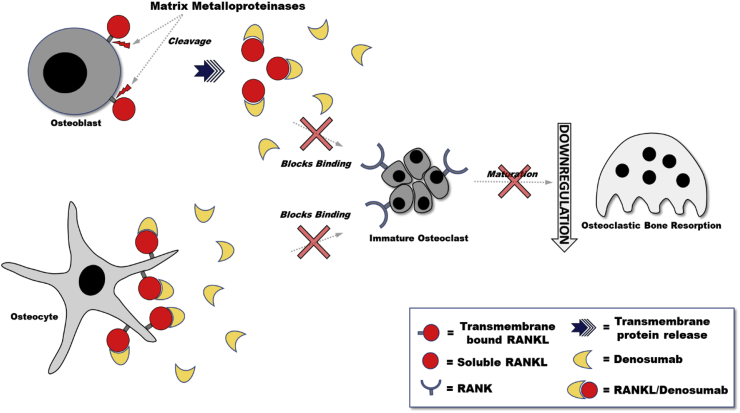
In 2003, a phase I clinical trial tested the therapeutic capability of RANKL modulation by studying a recombinant OPG molecule and its effect on osteoclast function.64 In early trials OPG had been reported to modulate tumor necrosis factor-related apoptosis inducing ligand, which showed an antiapoptotic task in preclinical models.65 Although the exact role in malignancy has yet to be proven, OPG demonstrated the worth of investigating RANKL modulation. Inhibiting RANKL effects bone turnover, particularly the resorption phase (Fig. 7). Realizing its effects on the bone metastases hypothesis may also decrease metastatic events. Previous animal studies determined the formulation of RANK-Fc by RANKL binding to a recombinant antibody, overwhelmingly resulted in functional inhibition of osteoclastogenesis.66 This binding has also been shown to decrease metastasis and seeding by osteosarcoma cells resulting in a reduction of lung lesions.67 Denosumab is a human monoclonal antibody with binding affinity for RANKL, which in turn blocks osteoclast surface protein RANK binding.19 This binding subsequently decreases osteoclast activity, formation and lifetime. It has been shown that denosumab blocks bone resorption (and bone turnover) in different diseases such as metastatic prostate and breast cancers, and multiple myeloma.68
Fig. 7.
Human monoclonal antibody against RANKL (Denosumab) mechanism of action. RANKL has been shown to take different forms. Recent studies have shown that the transmembrane form of RANKL on osteocytes is the most physiologically active from which promotes upregulation of osteoclast activity. The soluble form of RANKL (sRANKL) has been shown to be released from the membrane by metalloproteinases and are not as physiologically activating. Denosumab inhibits RANKL by binding RANKL and preventing RANKL binding to RANK on osteoclasts, downregulating osteoclastogenesis. Interestingly, studies show Denosumab action against osteoclastogenesis only, and not osteoclast survival.
Disruption of the relationship between RANK and RANKL could hypothetically decrease the formation of osteoclastic-like giant cells and the successive infiltrative mononuclear cells in GCTB. Recent studies have shown a dose-dependent reduction in bone turnover with an associated boost in bone mineral density after the administration of a single dose of denosumab.69,70 Initially developed for treatment of osteoporosis, a Phase I trial confirmed the proposed bone turnover effects and excellent safety profile.70 Moreover, a phase-III trial demonstrated a statistically significant decrease in fragile fractures as a result of denosumab treated patients with osteoporosis versus placebo.71 Subsequently, the effect of denosumab on bone metastasis in patients with breast malignancy. A dose of 120–180 mg denosumab was given every 4 weeks with treatment providing predictable and steady indication of suppressed bone turnover.72 Denosumab's optimal dose regimen was determined to be 120 mg every 4 weeks in subsequent studies examining the drug's optimum efficacy and safety profile. One analysis found that postmenopausal women with diagnosed breast cancer experienced a 7.6% increase in bone mineral density in lumbar spine measurements with the use of denusomab.73 Likewise, a study looked at the use of denosumab in men with known prostate cancer with no metastatic features receiving androgen-deprivation therapy, and found that men treated with denosumab compared to placebo after 36 months had decreased incidence of metastatic lumbar vertebral fractures.74
The original phase-II trial indicating the superiority of denosumab to bisphosphonates evaluated a population of patients with bone metastases from various tumors.68 Consecutively, double-blind studies were conducted to confirm efficacy over zoledronic acid showing a lower incidence of skeletal related events and decrease in detection of bone resorption markers.75, 76, 77 Denosumab was also found to increase survival without bone metastasis and time to initial bone metastasis in castration-resistant prostate cancer.76 Finally, meta-analyses have revealed denosumab to be superior to zoledronic acid and pamidronate in not only delaying the time to skeletal related events but the quantity of first and even sequential bone metastases from solid tumors.78,79 It is important to note hwoever, no significant dissimilarities were discovered between denosumab and zoledronic acid in morbidity and mortality.
7. Clinical trial of denosumab in GCTB
Reports surfaced in the early 2000's regarding denosumab, and its ability to modulate RANKL could prevent the destructive mechanism related to GCTB.24 Osteoclast activity correlates with RANKL expression on stromal cells, which hypothetically leads to the aggressive osteolytic nature of this tumor.80 With denosumab showing the ability to decrease osteoclast activity by way of the RANK pathway, it has been postulated that denosumab could significantly affect cells within GCTB.
A proof of principle study was conducted to ascertain the activity of denosumab in 37 patients with non-chemo-responsive or unresectable GCTB.81 Doses were administered subcutaneously, 120 mg every 28 days, with additional loading doses at 8 and 15 days in the first cycle. Tumor responsiveness was seen in 86% of patients, delineated by histologic complete eradication of giant cells or lack of progression of disease by radiographic evaluation. Radiographic responsiveness was defined as a calcified rim around a decreased tumor size. The study also documented improvement in pain while having increased functional activity with radiological verification of bone restoration. The treatment response was calculated by fluorodeoxyglucose positron emission tomography (PET) scan and urine telopeptides, which correlated with unbalanced bone turnover. PET scanning usually saw marked decreases in metabolic activity within four weeks of treatment initiation. Urine telopeptides were reduced immediately after the first dose of denosumab and remained reduced throughout the course of the entire study. A minority of patients included in the study went on to receive intralesional curettage after denosumab, but GCTB recurrence was not reported. Denosumab administration was tolerated well without significant side effects and no malignant transformation was noted. Branstetter et al.82 followed similar methodology, with greater than 90% of patients experiencing tumor response, and no major side effect or malignant transformation was identified.
Denosumab has also been studied in patients with planned operative tumors and patients with unresectable tumors (i.e., axial skeleton including sacrum and posterior elements of the spine).17 Patients reported minimal side effects and 99% of patients had no tumor progression at 13 months follow-up.83 Evaluation at nine months revealed 74% of these patients had yet to undergo surgery, and found 16% had a reduction in the morbidity of surgery. A follow-up, larger Phase-II clinical study in 2015 evaluated the use of denosumab in 222 patients who had resection planned but with a high anticipated morbidity.84 The authors reported a significant reduction in tumor soft tissue incursion after denosumab with operative GCTB lesions. A total of 86% of patients demonstrated clinical benefit of which 48% did not require surgical intervention. However, 38% of patients who did undergo surgical resection experienced less morbidity than initially anticipated.
Tumor necrosis is also reported by some studies, which makes the prospect of treatment with denosumab intriguing.21 Necrosis, while beneficial to tumor burden, could cause loss of scaffolding when intralesional curettage is conducted near a joint even when augmented with cement. To that fact, a prospective nonrandomized study looked at whether the addition of preoperative denosumab before curettage of resectable GCTB facilitated better joint preservation.85 Radiographs showed bone formation visible in 100% of patients, fractures associated with tumors were completely healed during treatment and joint preservation was successful in 90% of patients postoperatively. Yet, authors observed a local recurrence trend of 15% at 16 months, similar to previously reported numbers.84,85 Denosumab, they concluded, provided good clinical response with joint preservation surgery while also lessening morbidity and improving functional outcomes.
Preoperative evaluation of GCTB tumors can reveal potentially morbid resections, poor surgical candidates with comorbidities, or difficult location of tumor. Denosumab has been recommended for these patients for possible future resection if responsive, and as a cure for patients determined not to be surgical candidates. Overall, denosumab can be used in multiple cases of GCTB not treatable by gold standard surgical resection with good response and little to no adverse effects.
The primary indication, currently, for denosumab in GCTB is if complete surgical removal of the tumor is impossible.86 Denosumab protocols have also been used in patients with identified GCTB lung metastasis as well.87 Debates around length of treatment regarding long-term effects are being discussed but major consideration needs to been given to post-denosumab withdrawal malignancy. Currently, there is no quantifiable risk factors to identify when possible recurrence will occur, but many recurrences return about nine months after ceasing denosumab. Some authors have concluded this stems from the indirect inhibitory effects on GCTB stromal cells leading to a lack of apoptosis and continued proliferation of stromal cells.60,88 Overall, if GCTB recurrence is unavoidable once denosumab therapy is stopped, then long-term therapy is a must. Therefore, identifying risks of recurrence becomes imperative, which trials will most certainly be geared toward in the future.
Secondary uses for denosumab include difficult tumors needing neoadjuvant treatment prior to resection in order to decrease morbidity. More case reports in addition to one mentioned in the introduction of this paper suggests neoadjuvant Denosumab provided an improved surgical environment.89 Response to denosumab should be evaluated by radiographic means, but decreases in tumor size should not be the indicator of effectiveness.90 A bonus of denosumab some authors have postulated is the reduction of blood loss during resection.91 This effect is likely secondary to the conversion of stromal cells decreasing vascularity by reducing factors related to angiogenesis.
8. Adverse effects of denosumab
A total list of common adverse events revealed from studies has been osteonecrosis of the jaw, hypophosphatemia, hypocalcemia, pain in extremities, rash, diarrhea, constipation, cataracts, sciatica, and urinary tract infection.92 In all studies comparing denosumab to bisphosphonates, osteonecrosis of the jaw was linked to both zoledronic acid and denosumab (1.3% vs. 1.8%).75, 76, 77 Even though vitamin deficiencies were common, hypocalcemia was still observed in both groups as well, however, it was associated more frequently with denosumab (9.6% vs. 5%).93 Fever, myalgia, and bone pain were documented in a period of 3 days from initiation of treatment, indicating the potential for acute-phase reactions with both zoledronic acid (20%) and denosumab (8.7%). This is important for possible suppression of the immune system, luckily the rate of infection is similar whether patients were taking denosumab or zoledronic acid with no development of malignancies.75, 76, 77,93 Even with documented side effects available, a recent report detailed a case of critical rebound hypercalcemia in a young patient after discontinuing denosumab.94 The authors attributed this phenomenon to rebound osteoclast activity and possible osteoporotic bone, but soft bone in a young person is unlikely. More cases of rebound hypercalcemia have since surfaced in the younger populations upon withdrawing denosumab,95 therefore, cautious watch of electrolytes may be best when discontinuing denosumab in all populations. Singular reports of osteosclerosis at the epiphyses of long bones have been reported in off-label denosumab yet had no long-term sequelae.96 Prenatal effects of denosumab have not been elucidated so adequate contraception is recommended during treatment. Reports of malignant transformation into high-grade sarcomas have also been reported with denosumab use.57,97 The side effects, fertility restrictions, and possible malignant transformation with denosumab has sparked a conversation regarding dose and duration of therapy. Anecdotal evidence indicates GCTB appears 6–12 months after treatment discontinuation, but fortunately GCTB has analogous response rates in the reinitiating of denosumab. This would signify that discontinuation of denosumab therapy for females who wish to become pregnant is possible.
9. Future considerations
Undoubtedly, denosumab affords patients with late-stage or unresectable GCTB an option for treatment. One major advancement in the treatment of GCTB is the inclusion of patients that are not candidates for intralesional surgery. Offering improved functional outcomes and quality of life to all GCTB patients should be the ultimate target. However, the risk versus benefit profiles of pharmacologic options is convoluted and necessitating more data. The next step to achieving these goals may be neoadjuvant therapy utilizing denosumab while avoiding large morbid surgeries, which are underway.98 Yet, many questions still exist that require answering before absolute implementation into standards of care.
The efficacy of pharmacologic therapies in the RANKL pathway has made its strides over the years. However, as effective in inhibiting RANKL has been shown to be, it only modulates neoplastic stromal cells indirectly. Targeting stromal cells would be necessary for transforming adjunctive therapies into definitive systemic therapies for GCTB. Denosumab therapy thus far has been determined to be short lived, with GCTB found growing again after cessation of therapy in some cases.99,100 In situations where GCTB lesions are inoperable, continuous long-term denosumab has been postulated and possibly required, as an exact duration and dosage has yet to be determined. Studies are underway to examine the effects of long-term denosumab with data already becoming available.101 Frequently, cases are seen where large sacral GCTBs are diagnosed and surgical or radiotherapy would bear indelible repercussions, however, the use of denosumab has shown promise.102 Similar situations can be applied also to female patients making reproductive decisions while receiving denosumab therapy. Pregnancy, while never studied, remains a contraindication to denosumab use. However, radiotherapy especially around the pelvis is likely to affect reproductive organs in young females with inoperable tumors. Denosumab with its capability for drug holidays, with similar tumor response rates as initial denosumab administration, would be a viable option in reproductive patients. In spite of what is known about GCTB's response to denosumab, surgery remains the gold standard and removal should encompass the entire tumor to prevent recurrence. As previously mentioned, there has yet to be a prospective randomized trial suggesting the use of denosumab after surgery to reduce tumor recurrences. Based on current evidence available there is also no consensus on whether preoperative us increases or decrease local recurrence rates.103,104 Concerns of malignant transformation surrounding denosumab therapy have been raised from cases of patients developing high-grade sarcomas while on denosumab therapy.57,97 The question remains unanswered as to whether the cause was denosumab, diagnostic error, or whether transformation would have occurred despite systemic therapy. Intraoperative frozen section remains the gold standard to confirm final histological diagnosis. Malignancy identified in biopsies should be disclosed to patients on denosumab therapy for a possible relationship.105 With GCTB typically having an excellent response rate, lesions unresponsive to denosumab should be scheduled for repeat biopsy and histologic evaluation looking for malignancy. While the long-term effect profile of denosumab on the developing younger population has yet to be determined. If long-term drug safety studies are to be conducted the focus should include younger developing skeletons but also bone mineral density and skeletal related events.
Some experts recommend a shorter period of neoadjuvant therapy of around 3–4 months. This duration is theorized to be the time it takes to develop a sclerotic rim encircling the whole tumor. Some authors have said this creates a favorable situation when operating on previously inoperable GCTB. If the denosumab duration is longer, a soft, sticky residue is noticed consist with GCTB in vivo, instead of a sclerotic bony rim, making complete removal difficult.106 This promise of identifiability intraoperatively could allow previously unsalvageable tumors to be readily identified and completely removed.107
Denosumab has been shown to be a remarkably effective, high affinity antagonist in GCTB. Treatment with denosumab does inhibit bone turnover but may also offer greatly needed symptom and tumor control. In summary, 3–4 months of a 120 mg dose1 of neoadjuvant denosumab should be considered for inclusion in multidisciplinary treatment protocols of patients with late stage GCTB. The goal of this therapy should be to ease later surgeries, making curettage or resections straightforward. Unsalvageable GCTB, however, remains the topic of interest regarding chronic denosumab therapy. Questions of optimal dosage, extent of therapy and intervals between drug courses still remain on the horizon. Even though future studies are underway and will eventually elucidate important answers, denosumab will remain at the forefront of GCTB management.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Administered subcutaneously at day 1, 8, 15, 29 then every 4 weeks following.
References
- 1.Nakashima T., Hayashi M., Takayanagi H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab. 2012;23(11):582–590. doi: 10.1016/j.tem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Kenkre J.S., Bassett J.H.D. The bone remodelling cycle. Ann Clin Biochem. 2018;55(3):308–327. doi: 10.1177/0004563218759371. [DOI] [PubMed] [Google Scholar]
- 3.Kushlinskii N.E., Timofeev Y.S., Solov’ev Y.N., Gerstein E.S., Lyubimova N.V., Bulycheva I.V. Components of the RANK/RANKL/OPG system, IL-6, IL-8, IL-16, MMP-2, and calcitonin in the sera of patients with bone tumors. Bull Exp Biol Med. 2014;157(4):520–523. doi: 10.1007/s10517-014-2605-y. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien C.A., Nakashima T., Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013;54(2):258–263. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branstetter D., Rohrbach K., Huang L.Y. RANK and RANK ligand expression in primary human osteosarcoma. J Bone Oncol. 2015;4(3):59–68. doi: 10.1016/j.jbo.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rochette L., Meloux A., Rigal E. The role of osteoprotegerin in vascular calcification and bone metabolism: the basis for developing new therapeutics. Calcif Tissue Int. 2019;105(3):239–251. doi: 10.1007/s00223-019-00573-6. [DOI] [PubMed] [Google Scholar]
- 7.Marley K., Bracha S., Seguin B. Osteoprotegerin activates osteosarcoma cells that co-express RANK and RANKL. Exp Cell Res. 2015;338(1):32–38. doi: 10.1016/j.yexcr.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Silva B.C., Bilezikian J.P. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers M.J., Crockett J.C., Coxon F.P., Mönkkönen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49(1):34–41. doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Russell R.G.G. Bisphosphonates: the first 40 years. Bone. 2011;49(1):2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Raskin K.A., Schwab J.H., Mankin H.J., Springfield D.S., Hornicek F.J. Giant cell tumor of bone. J Am Acad Orthop Surg. 2013;21(2):118–126. doi: 10.5435/JAAOS-21-02-118. [DOI] [PubMed] [Google Scholar]
- 17.López-Pousa A., Broto J.M., Garrido T., Vázquez J. Giant cell tumour of bone: new treatments in development. Clin Transl Oncol. 2015;17(6):419–430. doi: 10.1007/s12094-014-1268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beebe-Dimmer J.L., Cetin K., Fryzek J.P., Schuetze S.M., Schwartz K. The epidemiology of malignant giant cell tumors of bone: an analysis of data from the Surveillance, Epidemiology and End Results Program. Rare Tumors. 2009;1:52–159. doi: 10.4081/rt.2009.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brodowicz T., Hemetsberger M., Windhager R. Denosumab for the treatment of giant cell tumor of the bone. Future Oncol. 2015;11(13):1881–1894. doi: 10.2217/fon.15.94. [DOI] [PubMed] [Google Scholar]
- 20.Ng V.Y., Davidson D.J., Kim E.Y., Pollack S.M., Conrad E.U., III, Jones R.L. The multidisciplinary management of giant cell tumor of bone. Expert Rev Anticancer Ther. 2014;14(7):783–790. doi: 10.1586/14737140.2014.901891. [DOI] [PubMed] [Google Scholar]
- 21.Chakarun C.J., Forrester D.M., Gottsegen C.J., Patel D.B., White E.A., Matcuk G.R. Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics. 2013;33(1):197–211. doi: 10.1148/rg.331125089. [DOI] [PubMed] [Google Scholar]
- 22.Yi J., Lee Y.H., Kim S.K. Response evaluation of giant-cell tumor of bone treated by denosumab: histogram and texture analysis of CT images [published online ahead of print] J Orthop Sci. 2018:1–8. doi: 10.1016/j.jos.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher CDM, World Health Organization . fourth ed. IARC Press; Lyon: 2013. International Agency for Research on Cancer. WHO Classification of Tumours of Soft Tissue and Bone. [Google Scholar]
- 24.Huang L., Xu J., Wood D.J., Zheng M.H. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-κB in giant cell tumor of bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol. 2000;156(3):761–767. doi: 10.1016/s0002-9440(10)64942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberghini M., Kliskey K., Krenacs T. Morphological and immunophenotypic features of primary and metastatic giant cell tumour of bone. Virchows Arch. 2010;456(1):97–103. doi: 10.1007/s00428-009-0863-2. [DOI] [PubMed] [Google Scholar]
- 26.Hemingway F., Taylor R., Knowles H.J., Athanasou N.A. RANKL-independent human osteoclast formation with APRIL, BAFF, NGF, IGF I and IGF II. Bone. 2011;48:938–944. doi: 10.1016/j.bone.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 27.Clézardin P. The role of RANK/RANKL/osteoprotegerin (OPG) triad in cancer-induced bone diseases: physiopathology and clinical implications. Bull Cancer. 2011;98(7):837–846. doi: 10.1684/bdc.2011.1398. [DOI] [PubMed] [Google Scholar]
- 28.Hiraga T. Bone metastasis: interaction between cancer cells and bone microenvironment. J Oral Biosci. 2019;61(2):95–98. doi: 10.1016/j.job.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Balla P., Moskovszky L., Sapi Z. Epidermal growth factor receptor signalling contributes to osteoblastic stromal cell proliferation, osteoclastogenesis and disease progression in giant cell tumour of bone. Histopathology. 2011;59(3):376–389. doi: 10.1111/j.1365-2559.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- 30.Lindeman J.H.N., Hanemaaijer R., Mulder A. Cathepsin K is the principal protease in giant cell tumor of bone. Am J Pathol. 2004;165(2):593–600. doi: 10.1016/S0002-9440(10)63323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J.K., Rosen A., Saffitz J.E. Expression of cathepsin K and tartrate-resistant acid phosphatase is not confined to osteoclasts but is a general feature of multinucleated giant cells: systematic analysis. Rheumatol (United Kingdom) 2013;52(8):1529–1533. doi: 10.1093/rheumatology/ket184. [DOI] [PubMed] [Google Scholar]
- 33.Gorunova L., Vult von Steyern F., Storlazzi C.T. Cytogenetic analysis of 101 giant cell tumors of bone: nonrandom patterns of telomeric associations and other structural aberrations. Gene Chromosome Canc. 2009;48(7):583–602. doi: 10.1002/gcc.20667. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz H.S., Allen G.A., Butler M.G. Telomeric associations. Appl Cytogenet J Assoc Cytogenet Technol. 1990;16(6):133. [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto H., Ishihara S., Toda Y., Oda Y. Histone H3.3 mutation in giant cell tumor of bone: an update in pathology. Med Mol Morphol. 2019 doi: 10.1007/s00795-019-00238-1. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto H., Iwasaki T., Yamada Y. Diagnostic utility of histone H3.3 G34W, G34R, and G34V mutant-specific antibodies for giant cell tumors of bone. Hum Pathol. 2018;73:41–50. doi: 10.1016/j.humpath.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Behjati S., Tarpey P.S., Presneau N. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet. 2013;45(12):1479–1482. doi: 10.1038/ng.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cleven A., Hocker S., Briaire-de Bruijn I. Mutation analysis of H3F3A and H3F3B as a diagnostic tool for giant cell tumor of bone and chondroblastoma. Am J Surg Pathol. 2015;39(11):1576–1583. doi: 10.1097/PAS.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 39.Moskovszky L., Szuhai K., Krenács T. Genomic instability in giant cell tumor of bone. A study of 52 cases using DNA ploidy, relocalization FISH, and array-CGH analysis. Gene Chromosome Canc. 2009;48(6):468–479. doi: 10.1002/gcc.20656. [DOI] [PubMed] [Google Scholar]
- 40.Lieveld M., Bodson E., De Boeck G. Gene expression profiling of giant cell tumor of bone reveals downregulation of extracellular matrix components decorin and lumican associated with lung metastasis. Virchows Arch. 2014;465(6):703–713. doi: 10.1007/s00428-014-1666-7. [DOI] [PubMed] [Google Scholar]
- 41.Oda Y., Sakamoto A., Saito T. Secondary malignant giant-cell tumour of bone: molecular abnormalities of p53 and H-ras gene correlated with malignant transformation. Histopathology. 2001;39(6):629–637. doi: 10.1046/j.1365-2559.2001.01275.x. [DOI] [PubMed] [Google Scholar]
- 42.Klenke F.M., Wenger D.E., Inwards C.Y., Rose P.S., Sim F.H. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591–599. doi: 10.1007/s11999-010-1501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kivioja A.H., Blomqvist C., Hietaniemi K. Cement is recommended in intralesional surgery of giant cell tumors A Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008;79(1):86–93. doi: 10.1080/17453670710014815. [DOI] [PubMed] [Google Scholar]
- 44.Jones K.B., DeYoung B.R., Morcuende J.A., Buckwalter J.A. Ethanol as a local adjuvant for giant cell tumor of bone. Iowa Orthop J. 2006;26:69–76. [PMC free article] [PubMed] [Google Scholar]
- 45.van der Heijden L., van der Geest I.C.M., Schreuder H.w.B., Sande MA j, van de, Dijkstra P.d.S. Liquid nitrogen or phenolization for giant cell tumor of bone?: a comparative cohort study of various standard treatments at two tertiary referral centers. J Bone Jt Surg. 2014;96(5):e35. doi: 10.2106/JBJS.M.00516. [DOI] [PubMed] [Google Scholar]
- 46.Tse L.F., Wong K.C., Kumta S.M., Huang L., Chow T.C., Griffith J.F. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case–control study. Bone. 2008;42(1):68–73. doi: 10.1016/j.bone.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 47.Gouin F., Rochwerger A.R., Marco A Di. Adjuvant treatment with zoledronic acid after extensive curettage for giant cell tumours of bone. Eur J Canc. 2014;50:2425–2431. doi: 10.1016/j.ejca.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Gaston C.L., Bhumbra R., Watanuki M. Does the addition of cement improve the rate of local recurrence after curettage of giant cell tumours in bone? J Bone Joint Surg Br. 2011;93-B(12):1665–1669. doi: 10.1302/0301-620X.93B12.27663. [DOI] [PubMed] [Google Scholar]
- 49.van der Heijden L., van de Sande M., Dijkstra P. Soft tissue extension increases the risk of local recurrence after curettage with adjuvants for giant-cell tumor of the long bones. Acta Orthop. 2012;83(4):401–405. doi: 10.3109/17453674.2012.711193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Heijden L., Dijkstra P.D.S., van de Sande M.A.J. The clinical approach toward giant cell tumor of bone. Oncol. 2014;19(5):550–561. doi: 10.1634/theoncologist.2013-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphey M.D., Nomikos G.C., Flemming D.J., Gannon F.H., Temple H.T., Kransdorf M.J. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radiographics. 2001;21(5):1283–1309. doi: 10.1148/radiographics.21.5.g01se251283. [DOI] [PubMed] [Google Scholar]
- 52.Balke M., Schremper L., Gebert C. Giant cell tumor of bone: treatment and outcome of 214 cases. J Canc Res Clin Oncol. 2008;134:969–978. doi: 10.1007/s00432-008-0370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thornley P., Habib A., Bozzo A., Evaniew N., Ghert M. The role of denosumab in the modern treatment of giant cell tumor of bone. J Bone Jt Surg Rev. 2017;5(4):1–7. doi: 10.2106/JBJS.RVW.16.00072. [DOI] [PubMed] [Google Scholar]
- 54.Salunke A.A., Chen Y., Chen X. Does pathological fracture affect the rate of local recurrence in patients with a giant cell tumour of bone? Bone Joint Lett J. 2015;97-B(11):1566–1571. doi: 10.1302/0301-620X.97B11.35326. [DOI] [PubMed] [Google Scholar]
- 55.Siebenrock K.A., Unni K.K., Rock M.G. Giant-cell tumour of bone metastasising to the lungs. A long-term follow-up. J Bone Joint Surg Br. 1998;80(1):43–47. doi: 10.1302/0301-620x.80b1.7875. [DOI] [PubMed] [Google Scholar]
- 56.Ruka W., Rutkowski P., Morysiński T. The megavoltage radiation therapy in treatment of patients with advanced or difficult giant cell tumors of bone. Int J Radiat Oncol. 2010;78(2):494–498. doi: 10.1016/j.ijrobp.2009.07.1704. [DOI] [PubMed] [Google Scholar]
- 57.Wu C.-C., Hsieh P.-P. Denosumab-treated giant cell tumor of the bone mimicking low-grade central osteosarcoma [published online ahead of print february 12 2018] J Pathol Transl Med. 2018;52 doi: 10.4132/jptm.2016.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitrofan L.M., Pelkonen J., Mönkkönen J. The level of ATP analog and isopentenyl pyrophosphate correlates with zoledronic acid-induced apoptosis in cancer cells in vitro. Bone. 2009;45(6):1153–1160. doi: 10.1016/j.bone.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Coleman R.E., McCloskey E.V. Bisphosphonates in oncology. Bone. 2011;49(1):71–76. doi: 10.1016/j.bone.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Lau C.P.Y., Huang L., Wong K.C., Kumta S.M. Comparison of the anti-tumor effects of denosumab and zoledronic acid on the neoplastic stromal cells of giant cell tumor of bone. Connect Tissue Res. 2013;54(6):439–449. doi: 10.3109/03008207.2013.848202. [DOI] [PubMed] [Google Scholar]
- 61.Balke M., Campanacci L., Gebert C. Bisphosphonate treatment of aggressive primary, recurrent and metastatic Giant Cell Tumour of Bone. BMC Canc. 2010;10(1):462. doi: 10.1186/1471-2407-10-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu X., Xu M., Xu S., Su Q. Clinical outcomes of giant cell tumor of bone treated with bone cement filling and internal fixation, and oral bisphosphonates. Oncol Lett. 2013;5(2):447–451. doi: 10.3892/ol.2012.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lipplaa A., Kroep J.R., van der Heijden L. Adjuvant zoledronic acid in high‐risk giant cell tumor of bone: a multicenter randomized phase II trial. Oncol. 2019;24(7) doi: 10.1634/theoncologist.2019-0280. 889-e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Body J.-J., Greipp P., Coleman R.E. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97(3 Suppl):887–892. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- 65.Holen I., Cross S.S., Neville-Webbe H.L. Osteoprotegerin (OPG) expression by breast cancer cells in vitro and breast tumours in vivo--a role in tumour cell survival? Breast Canc Res Treat. 2005;92(3):207–215. doi: 10.1007/s10549-005-2419-8. [DOI] [PubMed] [Google Scholar]
- 66.Kitazawa S., Kitazawa R. RANK ligand is a prerequisite for cancer-associated osteolytic lesions. J Pathol. 2002;198(2):228–236. doi: 10.1002/path.1199. [DOI] [PubMed] [Google Scholar]
- 67.Akiyama T., Choong P.F.M., Dass C.R. RANK-Fc inhibits malignancy via inhibiting ERK activation and evoking caspase-3-mediated anoikis in human osteosarcoma cells. Clin Exp Metastasis. 2010;27(4):207–215. doi: 10.1007/s10585-010-9319-y. [DOI] [PubMed] [Google Scholar]
- 68.Fizazi K., Lipton A., Mariette X. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27(10):1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 69.Dougall W.C., Chaisson M. The RANK/RANKL/OPG triad in cancer-induced bone diseases. Canc Metastasis Rev. 2007;25(4):541–549. doi: 10.1007/s10555-006-9021-3. [DOI] [PubMed] [Google Scholar]
- 70.Bekker P.J., Holloway D.L., Rasmussen A.S. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2005;20(12):2274–2282. doi: 10.1359/jbmr.2005.20.12.2274. [DOI] [PubMed] [Google Scholar]
- 71.Cummings S.R., Martin J.S., McClung M.R. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 72.Lipton A., Steger G.G., Figueroa J. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25(28):4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 73.Ellis G.K., Bone H.G., Chlebowski R. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26(30):4875–4882. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 74.Smith M.R., Egerdie B., Toriz N.H. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stopeck A.T., Lipton A., Body J.-J. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 76.Smith M.R., Saad F., Coleman R. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henry D.H., Costa L., Goldwasser F. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 78.Ford J.A., Jones R., Elders A. Denosumab for treatment of bone metastases secondary to solid tumours: systematic review and network meta-analysis. Eur J Canc. 2013;49(2):416–430. doi: 10.1016/j.ejca.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 79.Peddi P., Lopez-Olivo M.A., Pratt G.F., Suarez-Almazor M.E. Denosumab in patients with cancer and skeletal metastases: a systematic review and meta-analysis. Canc Treat Rev. 2013;39(1):97–104. doi: 10.1016/j.ctrv.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas D.M. RANKL, denosumab, and giant cell tumor of bone. Curr Opin Oncol. 2012;24(4):397–403. doi: 10.1097/CCO.0b013e328354c129. [DOI] [PubMed] [Google Scholar]
- 81.Thomas D., Henshaw R., Skubitz K. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 82.Branstetter D.G., Nelson S.D., Manivel J.C. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Canc Res. 2012;18(16):4415–4424. doi: 10.1158/1078-0432.CCR-12-0578. [DOI] [PubMed] [Google Scholar]
- 83.Chawla S., Blay J., Rutkowski P. Articles Denosumab in patients with giant-cell tumour of bone : a multicentre , open-label , phase 2 study. Lancet Oncol. 2019;2045(19):1–11. doi: 10.1016/S1470-2045(19)30663-1. [DOI] [PubMed] [Google Scholar]
- 84.Rutkowski P., Ferrari S., Grimer R.J. Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol. 2015;22(9):2860–2868. doi: 10.1245/s10434-015-4634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Traub F., Singh J., Dickson B.C. Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone ScienceDirect. Eur J Canc. 2016;59:1–12. doi: 10.1016/j.ejca.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Chawla S., Henshaw R., Seeger L. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901–908. doi: 10.1016/S1470-2045(13)70277-8. [DOI] [PubMed] [Google Scholar]
- 87.Demirsoy U., Karadogan M., Selek O. Golden bullet—denosumab: early rapid response of metastatic giant cell tumor of the bone. J Pediatr Hematol Oncol. 2014;36(2):156–158. doi: 10.1097/MPH.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 88.Mak IW.y., Evaniew N., Popovic S., Tozer R., Ghert M. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Jt Surg. 2014;96(15):e127. doi: 10.2106/JBJS.M.01332. [DOI] [PubMed] [Google Scholar]
- 89.Agarwal A., Larsen B.T., Buadu L.D. Denosumab chemotherapy for recurrent giant-cell tumor of bone: a case report of neoadjuvant use enabling complete surgical resection. Case Rep Oncol Med. 2013;2013 doi: 10.1155/2013/496351. 496-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oguro S., Okuda S., Sugiura H. Giant cell tumors of the bone: changes in image features after denosumab administration. Magn Reson Med Sci. 2018;17(2):1–6. doi: 10.2463/mrms.mp.2017-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Girolami I., Mancini I., Simoni A. Denosumab treated giant cell tumour of bone: a morphological, immunohistochemical and molecular analysis of a series. J Clin Pathol. 2016;69(3):240–247. doi: 10.1136/jclinpath-2015-203248. [DOI] [PubMed] [Google Scholar]
- 92.AMGEN Safety and side effects of prolia (denosumab) https://www.prolia.com/about/safety/ Accessed.
- 93.Lipton A., Fizazi K., Stopeck A.T. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Canc. 2012;48(16):3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 94.Gossai N., Hilgers M.V., Polgreen L.E., Greengard E.G. Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr Blood Canc. 2015;62(6):1078–1080. doi: 10.1002/pbc.25393. [DOI] [PubMed] [Google Scholar]
- 95.Uday S., Gaston C.L., Rogers L. Osteonecrosis of the jaw and rebound hypercalcemia in young people treated with denosumab for giant cell tumor of bone. J Clin Endocrinol Metab. 2018;103(2):596–603. doi: 10.1210/jc.2017-02025. [DOI] [PubMed] [Google Scholar]
- 96.Kobayashi E., Setsu N. Osteosclerosis induced by denosumab. Lancet. 2015;385(9967):539. doi: 10.1016/S0140-6736(14)61338-6. [DOI] [PubMed] [Google Scholar]
- 97.Aponte-Tinao L.A., Piuzzi N.S., Roitman P., Farfalli G.L. A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop Relat Res. 2015;473(9):3050–3055. doi: 10.1007/s11999-015-4249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Urakawa H., Mizusawa J., Tanaka K. A randomized phase III trial of denosumab before curettage for giant cell tumor of bone: Japan Clinical Oncology Group Study JCOG1610. Jpn J Clin Oncol. 2019;49(4):379–382. doi: 10.1093/jjco/hyz004. [DOI] [PubMed] [Google Scholar]
- 99.Gaston C.L., Grimer R.J., Parry M. Current status and unanswered questions on the use of Denosumab in giant cell tumor of bone. Clin Sarcoma Res. 2016;6(1):1–6. doi: 10.1186/s13569-016-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matcuk G.R., Patel D.B., Schein A.J., White E.A., Menendez L.R. Giant cell tumor: rapid recurrence after cessation of long-term denosumab therapy. Skeletal Radiol. 2015;44(7):1027–1031. doi: 10.1007/s00256-015-2117-5. [DOI] [PubMed] [Google Scholar]
- 101.Palmerini E., Chawla N.S., Ferrari S. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur J Canc. 2017;76:118–124. doi: 10.1016/j.ejca.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 102.Sambri A., Medellin M.R., Errani C. Denosumab in giant cell tumour of bone in the pelvis and sacrum: long-term therapy or bone resection? J Orthop Sci. 2019 doi: 10.1016/j.jos.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 103.Scoccianti G., Totti F., Scorianz M. Preoperative denosumab with curettage and cryotherapy in giant cell tumor of bone: is there an increased risk of local recurrence? Clin Orthop Relat Res. 2018;476(9):1783–1790. doi: 10.1007/s11999.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Errani C., Tsukamoto S., Leone G. Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J Bone Jt Surg - Am. 2018;100(6):496–504. doi: 10.2106/JBJS.17.00057. [DOI] [PubMed] [Google Scholar]
- 105.Biermann J.S. Updates in the treatment of bone cancer. J Natl Compr Canc Netw. 2013;11(5S):681–683. doi: 10.6004/jnccn.2013.0200. [DOI] [PubMed] [Google Scholar]
- 106.Van Der Heijden L., Dijkstra S., Blay J.-Y., Gelderblom H. Giant cell tumour of bone in the denosumab era ScienceDirect. Eur J Canc. 2017;77:75–83. doi: 10.1016/j.ejca.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 107.Puri A., Gulia A., Hegde P., Verma V., Rekhi B. Neoadjuvant denosumab: its role and results in operable cases of giant cell tumour of bone. Bone Joint Lett J. 2019;101-B(2):170–177. doi: 10.1302/0301-620X.101B2.BJJ-2018-0907.R2. [DOI] [PubMed] [Google Scholar]