Abstract
This report presents a 74-year-old renal transplant patient suffering of polymorphic-post-transplant-associated lymphoproliferative disease (P-PTLD) within an Eppstein-Barr Virus (EBV) associated mucocutaneous rectal ulcer (MCU). He was initially treated by stapled hemorrhoidopexy for a symptomatic grade III hemorrhoidal prolapse refractory to conservative treatment and rubber band ligations. This leads to severe urge, frequency and stool fragmentation. The symptoms were investigated with a number of interventions until a proctoscopy with biopsies finally revealed the diagnosis. The patient had triple therapy of tacrolimus, mycophenolate mofetil and prednisone initially after transplant several years ago with recent reduction to mycophenolate. The MCU was successfully treated with Retuximab and there was no sign of relaps after 6 months. As EBV-associated PTLD is a well known complication after renal transplant, rectum-MCU seems a rare and only recently described subform of this disease that should be excluded in case of ulcerating lesions in immunosuppressed patients.
INTRODUCTION
Eppstein-Barr Virus–post-transplant-associated lymphoproliferative disease (EBV-PTLD) is well known after kidney transplantation. The incidence varies between 1 and 5% [1, 2] polymorphic-post-transplant-associated lymphoproliferative disease (P-PTLD) regarding to EBV usually develops in the first year after transplantation [1]. Recently, EBV-associated mucocutaneous ulcer (EBVMCU) was reported the first time by Dojcinov et al. [3].
CASE REPORT
A 75-year-old male was referred to a proctologist with a symptomatic grade III hemorrhoidal prolapse refractory to conservative treatment and rubber band ligations, a stapled hemorrhoidopexy was performed, without specimen analysis [4]. Two weeks later, the patient presented with severe urge, frequency and stool fragmentation.
The personal history revealed a living donor human leucocyte antigens (HLA) identical renal transplant in 2003, status after coronary artery bypass and a curatively treated basal cell carcinoma on his back before transplantation. Immunosuppression after transplantation was established with a triple therapy of tacrolimus, mycophenolate-mofetil and prednisone, which was tapered within 3 months to zero. Due to HLA identical transplantation immunosuppression was reduce to mycophenolate mofetil in January 2006.
After the hemorrhoidopexy, he reported of up to 10 bowel movements per day. The symptoms were interpreted as dyssynergy of the pelvic floor and biofeedback therapy involving an anorectal electrostimulator was started. Following this, the patient developed severe anal pain with and after defecation. The complaints were interpreted as an acute anal fissure and again, conservative management involving local nifedipine application and stool regulation were started. However, these measures failed to improve symptoms.
Five months after the initial operation a flexible sigmoidoscopy revealed a large ulceration 3.5 cm from the anal verge (Fig. 1). The consecutively performed magnetic resonance imaging (MRI) suggested a subcutaneous fistula at 6 o’clock.
Figure 1.
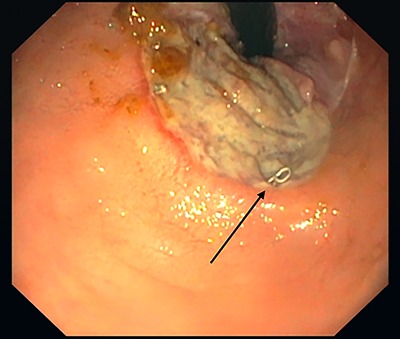
Rectal ulcer, 3.5 cm from the rear circumference. Stapler after hemorrhoidopexy (black arrow).
In the subsequent examination under anesthesia several ulcerations at 4–5, 6–7 and 10 o’clock were debrided and biopsies were taken and the remaining staples were removed (Fig. 2). The fistula was not detected, probably a localized mucosal intussuseption had mimicked this finding on MRI.
Figure 2.
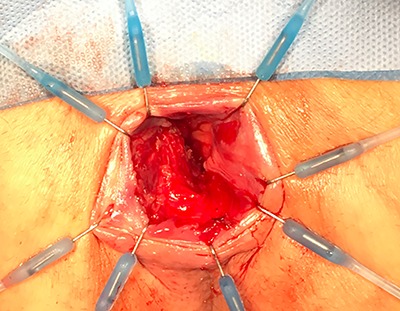
Examination under anaesthetic.
The biopsies showed an ulceration of the mucosa with an underlying polymorphic inflammatory infiltrate including several large atypical immunoblastic cells. The latter were positive for CD20, CD30 and EBV (shown by an EBV encode RNA (EBER), in situ hybridization). Stains for human papilloma virus, cytomegalovirus and fungi (PAS-staining) were negative.
In conclusion, the diagnosis of an EBV-associated, P-PTLD with the phenotype of a mucocutaneous ulcer (MCU) was established. A positron emission tomography-computed tomography for the staging of this lymphoproliferative disorder showed an uptake in the transverse colon. The following colonoscopy was inconspicuous. The patient was referred to the oncologist and treating transplant physician, which initiated a therapy with rituximab.
With this therapy, the MCU healed within the following 8 weeks while urge and high frequency only improved slowly. The hemorrhoidal prolapse did not recur and the anal sphincter function was good with no further episodes of incontinence or staining.
DISCUSSION
This is to our knowledge the first report of a MCU diagnosed following a stapled hemorrhoidopexy in an immunosuppressed patient. It remains unclear though whether the MCU was present at the time of the hemorrhoidopexy or if the intervention triggered the development of the condition as no histopathological examination of the resected mucosa at the time of the first operation was performed. Nevertheless, the treating proctologist reported no abnormal ulcerative lesions at the time of the first consultation.
Prieto-Torres et al. [5] described in a study of nine cases the spectrum of the EBVMCU. Every patient had either an immunosuppressive medication or immunosenescence, a rectal involvement was described in two cases, one related to an HIV infection and the other with immunosuppression due to inflammatory bowel disease, unfortunately, no further information was given about the underlying disease and the type of immunosuppressive therapy [5]. EBVMCU can be split up in two histopathological subtypes with skin and oral tumors or oropharyngeal, rectal and genital ulcers. The latter showed a polymorphic pattern with a Reed-Sternberg or Hodgkin-like appearance. In our case, CD20, CD30 and the EBER were positive (Fig. 3). A polymorphic presentation is typical for the EBVMCU [6].
Figure 3.
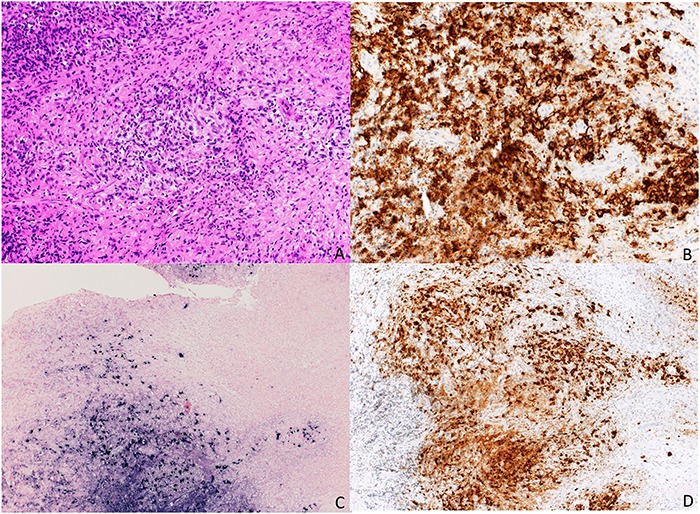
Staining (a) HE; (b) CD 20; (c) EBER; (d) CD 30.
Treatment options in patients with P-PTLD vary widely, depending on patients condition and extent of the disease and the affected organ site. Expectant management with symptomatic treatment as described by Prieto-Torres et al. was successful in two patients with rectal lesions. In one of them, the lesions disappeared after several continuous biopsies and the other patient was asymptomatic and had no further diagnostic or treatment [5]. TK Roberts et al. described the therapy options in patients with EBVMCU of two major groups, the iatrogenic immunosuppressed (31 patients) and the age-associated immunosenescence (20 patients). Seventeen patients (68%) of the first group showed a complete remission with reduction of the immunosuppressive therapy. Three patients (12%) received additional rituximab, one patient each (4%) was surgically resected, received R-CHOP chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) and dual antimetabolite and anti-TNFα therapy. All showed a complete clinical response. Nevertheless two patients (8%) died shortly after diagnosis [7]. This large variety of treatment options has mainly been adopted from the established approach to PTLD at other locations like small bowel. Primarily the reduction of immunosuppressive therapy in addition to rituximab show good results and can be escalated to more aggressive treatment involving R-CHOP or the surgical resection in localized lesions [8]. In the immunosenescence group radiation was also described, sometimes in combination with chemotherapy [7].
The most likely etiology of the EBVMCU in our patient was the low-dose immunosuppressive therapy after transplantation, already described by Hart et al. [9]. However, it is remarkable, a rejection reaction under a mycophenolate-mofetil monotherapy is not described in the literature so far. Furthermore, the EBV-associated PTLD arises mostly in the first year after transplantation and correlates with enhanced immunosuppression and rejection treatment, and again this has not been demonstrated in our patient [1, 10]. Our case report underlines the importance of active surveillance of immunocompromised patients with trivial mucocutaneous findings and their frequent clinical and histopathological follow-up. The treatment with rituximab was safe and well tolerated by the patient and lead to a quick recovery with no signs of recurrence after 6 months.
References
- 1. Morscio J, Dierickx D, Tousseyn T. Molecular pathogenesis of B-cell posttransplant lymphoproliferative disorder: what do we know so far? Clin Dev Immunol 2013;2013:150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu M, Husain S, Famure O, Li Y, Kim S. Incidence, risk factors, clinical management, and outcomes of Posttransplant Lymphoproliferative disorder in kidney transplant recipients. Prog Transplant 2019;29:185–93. [DOI] [PubMed] [Google Scholar]
- 3. Dojcinov S, Venkataraman G, Raffled M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer – a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol 2010;34:405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical Practice Committee, American Gastroenterological Association American Gastroenterological Association medical position statement: diagnosis and treatment of hemorrhoids. Gastroenterology 2004;126:1461–2. [DOI] [PubMed] [Google Scholar]
- 5. Prieto-Torres L, Eraña I, Gil-Redondo R, Gomez de la Riva I, Manso R, Pajares R, et al. The spectrum of EBV-positive mucocutaneous ulcer. Am J Surg Pathol 2019;43:201–10. [DOI] [PubMed] [Google Scholar]
- 6. Langerak AW, Groenen PJ, Brueggemann M, Beldjord K, Bellan C, Bonello L, et al. Euro- Clonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia 2012;26:2159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts T, Chen X, Liao J. Diagnostic and therapeutic challenges of EBV-positive mucocutaneous ulcer: a case report and systematic review of the literature. Exp Hematol Oncol 2016;5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heslop HE, Li C, Krance R, Loftin S, Rooney C. Epstein-Barr infection after bone marrow transplantation. Blood 1994;83:1706–8. [PubMed] [Google Scholar]
- 9. Hart M, Thakral B, Yohe S, Balfour H, Singh C, Spears M, et al. EBV-positive mucocutaneous ulcer in organ transplant recipients: a localized indolent posttransplant lympho-proliferative disorder. Am J Surg Pathol 2014;38:1522–9. [DOI] [PubMed] [Google Scholar]
- 10. Allen UD. Preiksaitis JK (2019) post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: guidelines from the American Society of Transplantation infectious diseases community of practice. Clin Transplant 2019;00:e13652. [DOI] [PubMed] [Google Scholar]


