Key Points
Question
Is the cost of chimeric antigen receptor T-cell therapy administration associated with the site of care and the safety profile of the therapy?
Findings
In this economic evaluation, a decision-tree model was used to document the clinical outcomes and costs of chimeric antigen receptor T-cell therapy for the treatment of adult patients with relapsed or refractory large B-cell lymphoma. After excluding the chimeric antigen receptor T-cell acquisition cost, hospitalization and office visits comprised 65.3% of the costs in inpatient settings and 48.4% of the costs in outpatient settings; the outpatient administration of chimeric antigen receptor T-cell therapy was associated with a $32 987 (40.4%) reduction in total costs.
Meaning
The administration of chimeric antigen receptor T-cell therapy in the outpatient setting of nonacademic specialty oncology networks was associated with lower estimated overall costs.
Abstract
Importance
Chimeric antigen receptor (CAR) T-cell therapies are currently administered at a limited number of cancer centers and are primarily delivered in an inpatient setting. However, variations in total costs associated with these therapies remain unknown.
Objective
To estimate the economic differences in the administration of CAR T-cell therapy by the site of care and the incidence of key adverse events.
Design, Setting, and Participants
A decision-tree model was designed to capture clinical outcomes and associated costs during a predefined period (from lymphodepletion to 30 days after the receipt of CAR T-cell infusion) to account for the potential incidence of acute adverse events and to evaluate variations in total costs for the administration of CAR T-cell therapy by site of care. Cost estimates were from the health care practitioner perspective and were based on data obtained from the literature and publicly available databases, including the Healthcare Cost and Utilization Project National Inpatient Sample, the Medicare Hospital Outpatient Prospective Payment System, the Medicare physician fee schedule, the Centers for Medicare and Medicaid Services Healthcare Common Procedure Coding System, and the IBM Micromedex RED BOOK. The model evaluated an average adult patient with relapsed or refractory large B-cell lymphoma who received CAR T-cell therapy in an academic inpatient hospital or nonacademic specialty oncology network.
Intervention
The administration of CAR T-cell therapy.
Main Outcomes and Measures
Total cost of the administration of CAR T-cell therapy by site of care. The costs associated with lymphodepletion, acquisition and infusion of CAR T cells, and management of acute adverse events were also examined.
Results
The estimated total cost of care associated with the administration of CAR T-cell therapy was $454 611 (95% CI, $452 466-$458 267) in the academic hospital inpatient setting compared with $421 624 (95% CI, $417 204-$422 325) in the nonacademic specialty oncology network setting, for a difference of $32 987. After excluding the CAR T-cell acquisition cost, hospitalization and office visit costs were $53 360 (65.3% of the total cost) in academic inpatient hospitals and $23 526 (48.4% of the total cost) in nonacademic specialty oncology networks. The administration of CAR T-cell therapy in nonacademic specialty oncology networks was associated with a $29 834 (55.9%) decrease in hospitalization and office visit costs and a $3154 (20.1%) decrease in procedure costs.
Conclusions and Relevance
The potential availability of CAR T-cell therapies that are associated with a lower incidence of adverse events and are suitable for outpatient administration may reduce the total costs of care by enabling the use of these therapies in nonacademic specialty oncology networks.
This economic evaluation uses a decision-tree model and cost data from publicly available databases to examine the economic differences associated with the administration of chimeric antigen receptor T-cell therapy by site of care among patients with relapsed or refractory large B-cell lymphoma.
Introduction
Chimeric antigen receptor (CAR) T-cell therapies are at the forefront of adoptive cell transfer therapies, in which patients’ own immune cells are used to treat their cancer. With autologous CAR T-cell therapy, the patient’s blood is collected, and T cells are then genetically modified and reinfused into the patient.1 The genetically engineered T cells contain CARs that specifically target the patient’s tumor.2,3,4 Chimeric antigen receptor T-cell therapies hold promise for patients with hematologic malignant neoplasms that are unresponsive or resistant to standard treatments.2,5,6
In 2017, the US Food and Drug Administration approved the first CAR T-cell therapy targeting B-lymphocyte antigen CD19 for the treatment of patients with relapsed or refractory large B-cell lymphoma (LBCL), a common type of non-Hodgkin lymphoma. Additional CAR T-cell therapies are in clinical development. Approximately one-third of patients with LBCL will develop relapsed or refractory disease.7,8,9 Before the approval of CAR T-cell therapy, the available treatments for patients with relapsed or refractory LBCL included high-dose chemotherapy, salvage chemotherapy, and autologous hematopoietic stem cell transplantation (auto-HSCT)10; however, the prognosis after these treatments is often poor.11,12,13,14,15 Although not directly compared in clinical trials, patients treated with CAR T-cell therapies had higher response rates when compared with current non–CAR T-cell standard-of-care treatments.11,16,17
Chimeric antigen receptor T-cell therapies are currently administered at a limited number of cancer centers and are primarily delivered in the inpatient setting.18,19,20 However, an ongoing clinical trial (the Study of Efficacy and Safety of CTL019 in Adult DLBCL Patients [JULIET] clinical trial [ClinicalTrials.gov Identifier NCT02445248], which evaluates tisagenlecleucel) is exploring the safety and feasibility of administering CAR T-cell therapy as an outpatient infusion.21 This transition would mirror the shift from the inpatient to the outpatient setting that was previously seen in auto-HSCT therapy administration.22
The site of care in which CAR T-cell therapy is administered may be associated with cost differences. The costs of other cancer therapies, particularly chemotherapy, are considerably higher in the inpatient setting than in the outpatient setting.23 Furthermore, it is important to include economic disparities across sites of care in the larger discussion regarding costs of new therapies that can be administered in both inpatient and outpatient settings. Studies have reported that the costs and fees between hospital inpatient and outpatient departments are less differentiated than those between inpatient and nonaffiliated outpatient centers.24 Because the cost structures differ most substantially between hospital inpatient departments and nonaffiliated outpatient centers, we intentionally simplified our analysis to examine the contrast between distinct sites of care: inpatient hospitals and outpatient facilities not wholly owned by hospitals.
This study was undertaken to investigate the economic differences in the administration of CAR T-cell therapy by site of care. The analysis focused on the differences between the costs associated with administering CAR T-cell therapy and managing adverse events (AEs) in academic inpatient hospitals compared with nonacademic specialty oncology networks (ie, outpatient clinics in which health care specialists treat and manage patients with conditions that are usually not included in general practice care). Although cancer therapies and CAR T-cell therapies are also provided in hospital outpatient departments, this analysis was intentionally simplified to indicate the contrast between inpatient hospitals and outpatient facilities not wholly owned by hospitals. In addition, a scenario analysis was used to evaluate outcomes based on the potential association between the incidence of key AEs associated with CAR T-cell therapy and overall costs.
Methods
This study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline for economic evaluations. The study comprised an economic model that used secondary literature to inform model inputs. Only hypothetical patients and facilities were assessed. Therefore, institutional review board approval and informed consent were not required per 45 CFR 46.101(b)(4), as no data from actual patients were collected or evaluated.
Cost Model
The cost model incorporated clinical and economic inputs after lymphodepletion and during a predefined 30-day period beginning 1 day after receipt of the CAR T-cell therapy infusion and ending 30 days after receipt of the infusion (ie, until the resolution of potential AEs that occurred immediately after the administration of the CAR T-cell infusion). The costs of lymphodepletion were added to the costs incurred during the 30-day period. This period accounted for the resolution of acute AEs to evaluate variations in the total costs of administering CAR T-cell therapy to patients with relapsed or refractory LBCL by site of care. We used a decision-tree model rather than a Markov model because of the short period studied and the assumption that clinical events and their consequences would occur only once. The decision-tree model was constructed through analysis of patient care guidelines and clinical trial reports to reflect the clinical pathways of a patient undergoing CAR T-cell therapy. The pathways comprised the course of treatment and follow-up, including administration procedures by site of care, risk of AEs, and AE management strategies. The model did not include apheresis because this procedure is primarily performed in an outpatient setting, regardless of the site in which the patient receives CAR T-cell therapy infusion.25 The decision-tree model, conceptual approach, and patient flow are presented in Figure 1.
Figure 1. Decision-Tree Model.
Adverse events (AEs) included in the model were cytokine release syndrome and neurological events. Costs incurred by patients who initiated treatment at an academic inpatient hospital or nonacademic specialty oncology network (NASON) were estimated by following the patient from the time of lymphodepletion to chimeric antigen receptor (CAR) T-cell infusion at the first decision-tree node, then following the patient through subsequent sites of care inclusive of AE management. The AE rates were applied to the probability nodes of the decision tree. Squares are decision nodes, indicating a decision to be made; circles are chance nodes, indicating multiple uncertain outcomes; and triangles are end point nodes, indicating a final outcome. ICU indicates intensive care unit; LBCL, large B-cell lymphoma.
The participant population included average adult patients with relapsed or refractory LBCL who received CAR T-cell therapy. The model structure followed a patient after the initial decision to administer CAR T-cell therapy at the initial site of care. After the initial decision, the patient received lymphodepletion, CAR T-cell therapy, and AE monitoring at the initial site of care. During CAR T-cell therapy, patients first underwent leukapheresis to collect T cells. After the T-cell product was manufactured, patients received lymphodepleting chemotherapy followed by an infusion of CAR T-cell therapy 2 to 7 days later. The patient may have subsequently experienced an AE and used health care resources that were dependent on AE management strategies and the initial site of care of CAR T-cell therapy administration. Throughout the course of treatment, a patient may have been admitted to the hospital, including the intensive care unit, for AE management.26 Because the model was focused on a short period, no discounting was applied to the cost inputs. In addition, no discounting was applied to treatment consequences because the economic evaluation was not a cost-effectiveness analysis and did not incorporate clinical outcomes.
The following assumptions were made regarding the administration, monitoring, and management protocols. The model assumed that patients underwent lymphodepletion at the same site of care in which they received CAR T-cell therapy. Patients who received CAR T-cell therapy in the hospital inpatient setting were assumed to have a 3-day stay for lymphodepletion that was followed by the administration of CAR T-cell therapy 2 to 7 days later. Patients were then assumed to have experienced the lesser of the following 2 scenarios: a 7-day stay for monitoring after the receipt of CAR T-cell therapy or a length of stay based on the days to AE onset26 plus the days required to manage the AE.16 Patients who received CAR T-cell therapy in nonacademic specialty oncology networks were assumed to have had 3 office visits for lymphodepletion and CAR T-cell administration followed by 2 office visits for monitoring.27,28 In the base-case analysis, AE management varied by the site of care of CAR T-cell administration. In contrast, the safety profile of the CAR T-cell therapy was assumed to be constant across the sites of administration. Both assumptions were tested in sensitivity and scenario analyses.
The model was developed using Microsoft Excel 2016 software (Microsoft Corp). The cost inputs were obtained from 2018 databases, such as the Medicare physician fee schedule29 and the Medicare Hospital Outpatient Prospective Payment System,30 or were adjusted to 2018 US dollars using the US Bureau of Labor Statistics consumer price index.31
Model Inputs
Health care resource utilization data from the period of lymphodepletion to AE resolution were aggregated. The model was developed using parameter values and inputs obtained from the literature or publicly available databases, including the Healthcare Cost and Utilization Project National Inpatient Sample for 2015,32 the Medicare Hospital Outpatient Prospective Payment System fee schedule for 2018,30 the Medicare physician fee schedule for 2018,29 the Centers for Medicare and Medicaid Services Healthcare Common Procedure Coding System,33 and IBM Micromedex RED BOOK.34 We included cytokine release syndrome and neurological events, which are AEs reported to be associated with CAR T-cell therapy, and categorized them into grades 1 (mild reaction), 2 (moderate reaction), 3 (severe reaction), and 4 or higher (life-threatening reaction) (Table 1).36,37 Although treatment-specific cytokine release syndrome and neurological event data were available, an AE scenario was created based on published clinical trial data, including AE rates, days to onset, and duration.16,17,38 These AE parameters were used in the model for base-case analysis (Table 1).
Table 1. Key Adverse Event Inputs.
| AE input | Base-case analysis AE rates, % |
Scenario analysis AE rates, % |
Both analysesa | |
|---|---|---|---|---|
| Time to AE onset, d | Duration of AE, d | |||
| Proportion with no AE | 2.5 | 50.0 | NA | NA |
| CRS grade | ||||
| 1 | 7.5 | 10.0 | 2 | 8 |
| 2 | 7.5 | 7.5 | 2 | 8 |
| 3 | 2.5 | 2.5 | 2 | 10.5 |
| ≥4 | 2.5 | 2.5 | 2 | 10.5 |
| NE grade | ||||
| 1 | 2.5 | 2.5 | 3 | 15 |
| 2 | 2.5 | 2.5 | 3 | 15 |
| 3 | 2.5 | 2.5 | 3 | 26 |
| ≥4 | 2.5 | 2.5 | 3 | 26 |
| CRS plus NE grade | ||||
| 1 | 25.0 | 5.0 | 2 | 15 |
| 2 | 25.0 | 2.5 | 2 | 15 |
| 3 | 15.0 | 7.5 | 2 | 26 |
| ≥4 | 2.5 | 2.5 | 2 | 26 |
The decision-tree model was a deterministic evaluation of a patient undergoing CAR T-cell therapy in an academic inpatient hospital setting compared with a nonacademic specialty oncology network setting. According to aggregate data published by CAR T-cell manufacturers, the onset of some AEs may occur after the initial monitoring period.16,17,39 In addition, differences may exist between the infused CAR T-cell products and the associated discharge day. Therefore, we included assumptions about the site of AE management in the base-case analysis that were then tested in the sensitivity analysis.
The model assumed that if a patient received a CAR T-cell infusion in the inpatient setting and experienced a grade 1 or 2 AE, the AE would be managed in the outpatient setting 50% of the time after the required initial monitoring period; otherwise, the patient would remain in the inpatient setting for AE management.40,41 Among patients who received CAR T-cell therapy in nonacademic specialty oncology networks and experienced grade 1 or 2 AEs, the model assumed that 100% would remain in the outpatient setting for AE management.36 If a patient experienced any grade 3 or higher AE, the model assumed that the patient’s AE would be managed in the inpatient setting, regardless of the administration site. These AE management assumptions were based on published recommended guidelines for AE type and grade.42,43 The differences in AE management by site of care were also consistent with findings from the literature regarding the outpatient and inpatient administration of auto-HSCT therapies.22,44
These assumptions regarding AE management were tested in sensitivity analyses to assess their association with total cost variation. The percentage of AEs that were managed in the inpatient setting varied from 0% to 100% in the inpatient and outpatient settings in the base-case and scenario analyses. If all AEs were managed in the inpatient setting, the cost difference would be associated with the cost of the inpatient stay for the administration and monitoring of CAR T-cell therapy before AE onset.
The monitoring period assumed for each site of care was based on the literature regarding auto-HSCT therapies.45,46,47 Current CAR T-cell therapies have distinct AE profiles that are associated with varying degrees of severe cytokine release syndrome and neurological events or their rapid onset. Future CAR-T cell therapies are also likely to have these distinct AE profiles. These events may also require a minimum number of days for additional postadministration monitoring of patient safety, especially given the possibility that patients may develop severe AEs.27,28 In addition, lower rates of AEs may have consequences for the site of care of AE management, such as fewer patients who require hospitalization, and for the feasibility of administering CAR T-cell therapy in nonacademic specialty oncology networks. Scenario analyses were used to investigate the association between the safety profiles of the CAR T-cell therapy and the therapy administration site.
For AE management costs, published management guidelines for cytokine release syndrome and neurological events were used to estimate health care resource utilization by AE type and grade (Table 1 and Table 2). General management guidelines for cytokine release syndrome include hospitalization, blood tests, seizure prophylaxis, antipyretics, antibiotics, corticosteroids, and tocilizumab.16,17,27,28,36,49,50 Recommendations for neurological event management include hospitalization, neurological diagnostic tests, anticonvulsants, antiepileptics, corticosteroids, and tocilizumab if a patient has concurrent cytokine release syndrome.27,28,36
Table 2. Key Health Care Inputs.
| Economic input | Value, $ | |
|---|---|---|
| Academic inpatient hospital | Nonacademic specialty oncology network | |
| Drug costs | ||
| CAR T cella | 373 000 | 373 000 |
| Lymphodepletionb | 1578 | 1578 |
| Tocilizumabb | 4368 | 4368 |
| Dexamethasoneb | 2.5 | 2.5 |
| Filgrastimb | 315 | 315 |
| Procedure costs (OPPS HCPCS code)c | ||
| Radiography (71045) | 76 | 22 |
| EEG (95812) | 445 | 330 |
| ECG (83351) | 656 | 239 |
| Lumbar puncture (62272) | 117 | 87 |
| MRI (70552) | 441 | 327 |
| 1-h chemotherapy administration (96413) | 364 | 144 |
| CAR T-cell administration (38241) | 313 | 176 |
| Facility costsd | ||
| ICU daye | 6546 | 6546 |
| Inpatient dayf | 2668 | 2668 |
| Nonacademic specialty oncology network visitg | NA | 74 |
Abbreviations: CAR, chimeric antigen receptor; ECG, electrocardiogram; EEG, electroencephalogram; HCPCS, Health Care Common Procedure Coding System; ICU, intensive care unit; MRI, magnetic resonance imaging; NA, not applicable; OPPS, Outpatient Prospective Payment System.
Based on assumption of cost.
Costs were obtained from IBM Micromedex RED BOOK34 wholesale acquisition costs: 1 vial of tocilizumab, 80 mg/4 mL, was $436.81; 1 vial of dexamethasone, 10 mg/mL, was $2.50; and 1 vial of filgrastim was $315 ($3148.33/10 vials of 300 μg each).
Inpatient values were estimated by adjusting the OPPS reimbursement values30 using methods from Neelapu et al43 and Meisenberg et al.48
Costs were the same regardless of the site of administration. The ICU and inpatient day inputs for nonacademic specialty oncology networks were only relevant when an adverse event occurred.
Costs were based on research performed by Dasta et al.37
Costs were based on the National Inpatient Sample from 2015.32
Costs were based on the Medicare physician fee schedule for 2018.29
Costs were applied to each resource using cost data from the literature and publicly available databases, such as the Healthcare Cost and Utilization Project National Inpatient Sample32 and the Medicare Hospital Outpatient Prospective Payment System30 (Table 2) (eMethods 1-3 and eTables 1-3 in the Supplement).29,34 Differential cost data were sourced for academic inpatient hospitals and nonacademic specialty oncology networks (with costs for ambulatory surgical centers used as proxies). The costs of CAR T-cell therapy were also held constant across sites. Costs were an estimated national average.
Model Outcomes
The model estimated the total costs of CAR T-cell administration and acute AE management for an average individual patient (ie, a patient aged ≥18 years with the expectation of experiencing an AE in accordance with the safety profiles of the given scenario) at each initial site of care as the primary outcome. Costs were assessed in 2 settings: academic inpatient hospitals and nonacademic specialty oncology networks. Although several possible scenarios existed between these options, these 2 scenarios were considered to be on opposite ends of the spectrum of possible scenarios, which would allow us to examine a range of differing costs.
The total cost of care was measured from the health care practitioner perspective, including the costs to all practitioners involved in a patient’s treatment. The practitioner perspective was chosen given the heterogeneity in the methods used for reimbursement of inpatient and outpatient services across both Medicare and private payers. Because most of the reimbursements for the Medicare and privately insured populations with LBCL who are treated within the inpatient setting are based on a bundle episode of care payment or case rate, and outpatient services are based on a fee-for-service method, the actual economic consequences to payers are more evaluable. In contrast, practitioners observe greater uncertainty for most of the costs, reflecting the need to assess the economic factors associated with the site of care from a practitioner perspective. Although a societal perspective is often recommended, this approach is typically preferred when conducting cost-effectiveness analyses and takes into consideration both direct and indirect costs.51,52 An analysis conducted from the practitioner perspective (or the health care system perspective), as was done in the analysis of CAR T-cell therapies performed by the Institute for Clinical and Economic Review,53 can allow a more generalizable and relevant outcome to be estimated.
We imputed costs by individual category of health care resource utilization, specifically drugs, procedures, and hospitalization and office visits, by CAR T-cell administration site of care. Procedure costs included diagnostic testing, monitoring, drug administration, and oxygen supplementation. We conducted a 1-way deterministic sensitivity analysis. We also performed probabilistic sensitivity analyses via a Monte Carlo simulation with 1000 iterations. All outcomes are presented in 2018 US dollars.
Results
Base-Case Analysis
The model estimated that the total cost of care for CAR T-cell therapy administration would be $454 611 (95% CI, $452 466-$458 267) in the inpatient setting compared with $421 624 (95% CI, $417 204-$422 325) in the nonacademic specialty oncology network setting, a difference of $32 987. When considering only costs ancillary to the acquisition of CAR T cells, including lymphodepletion and AE management, this difference reflected a 40.4% cost reduction (Figure 2).
Figure 2. Base-Case and Scenario Analyses.
A, In this analysis, 97.5% of patients had an adverse event. Costs in the inpatient hospital setting were $12 561 for drugs, $15 690 for procedures, and $53 360 for hospitalization and office visits, for a total cost of $81 611. Costs in the nonacademic specialty oncology network (NASON) setting were $12 561 for drugs, $12 537 for procedures, and $23 526 for hospitalization and office visits, for a total cost of $48 624. The total cost difference between the inpatient hospital and NASON settings was $32 987, reflecting a 40.4% cost reduction. The chimeric antigen receptor (CAR) T-cell acquisition cost, assumed to be $373 000, was excluded from the total costs. All costs are in US dollars. B, In this analysis, 50% of patients had an adverse event. Costs in the inpatient hospital setting were $6460 for drugs, $10 313 for procedures, and $42 003 for hospitalization and office visits, for a total cost of $58 776. Costs in the NASON setting were $6460 for drugs, $8718 for procedures, and $16 304 for hospitalization and office visits, for a total cost of $31 482. The total cost difference between the inpatient hospital and NASON settings was $27 294, reflecting a 46.4% cost reduction. The CAR T-cell acquisition cost, assumed to be $373 000, was excluded from the total costs. All costs are in US dollars.
After excluding the CAR T-cell acquisition cost, hospitalization and office visits comprised a substantial portion of costs (for academic inpatient hospitals, $53 360 [65.3%]; for nonacademic specialty oncology networks, $23 526 [48.4%]; Figure 2). The administration of CAR T-cell therapy in nonacademic specialty oncology networks was associated with a $29 834 (55.9%) reduction in hospitalization and office visit costs and a $3154 (20.1%) decrease in procedure costs. Because uniform costs for drug acquisition were applied to both sites of care, no cost differences in this category were estimated. Assuming that both CAR T-cell AE profiles could be observed at either site of care, the AE incidence was associated with a cost of $41 063 (95% CI, $417 204-$458 267) in the base-case analysis.
Scenario Analysis
A scenario analysis was performed for a patient receiving CAR T-cell therapy with a lower overall AE incidence burden to examine the consequences of safety with regard to model outcomes. A scenario was created with lower AE incidence, while all other AE and model parameters were held constant, including days to AE onset, AE duration, and AE management strategies. Detailed rates of cytokine release syndrome, neurological events, and AE grades for the scenario analysis are provided in Table 1.
The scenario analysis estimated that the total cost of care for CAR T-cell therapy administration would be $431 776 (95% CI, $423 443-$430 177) in the inpatient setting compared with $404 482 (95% CI, $402 772-$408 728) in the nonacademic specialty oncology network setting, a difference of $27 294. After excluding the CAR T-cell acquisition cost, this difference reflected a 46.4% cost reduction (Figure 2). A breakdown of health care resource utilization costs by category was performed to evaluate the consequences of a lower AE burden (Figure 2). Assuming that both CAR T-cell AE profiles could be observed at either site of care, the AE incidence was associated with a cost of $27 404 (95% CI, $402 772-$430 177) in the scenario analysis.
Sensitivity Analysis
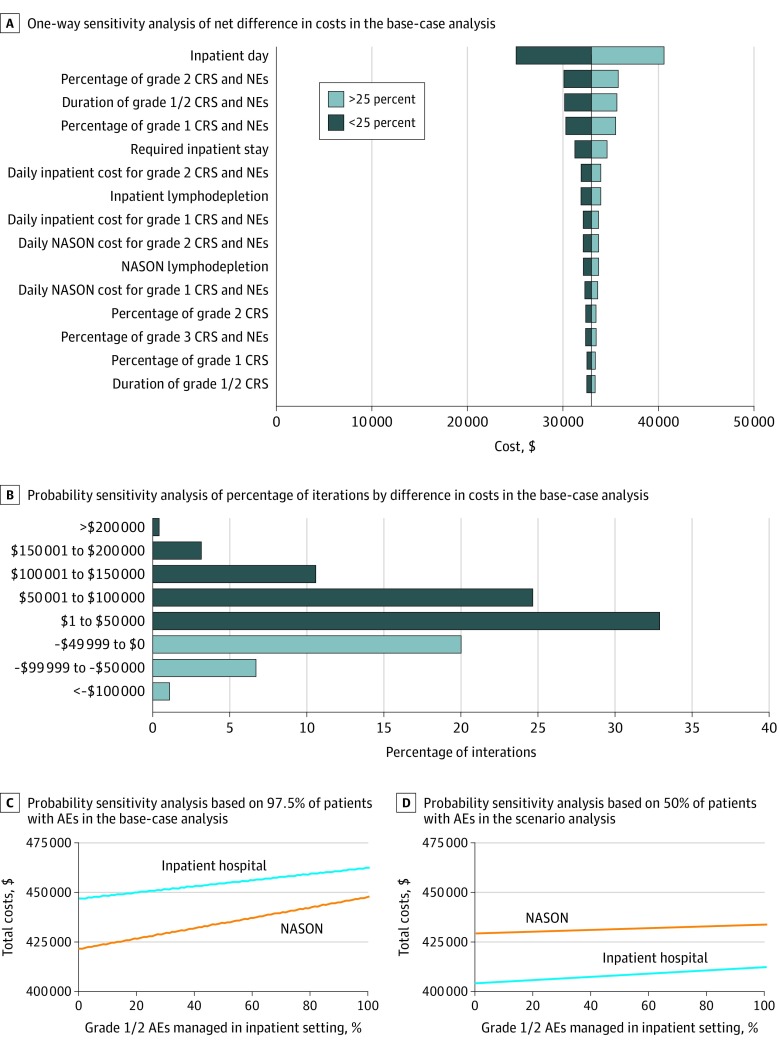
The deterministic 1-way sensitivity analysis varied all parameters by less than or greater than 25% by each site of care to assess the association with total costs of care (Figure 3A). Inpatient costs were an important factor. Overall, model outcomes were most sensitive to AE-related parameters at both sites of care.
Figure 3. Sensitivity Analyses.
A, One-way sensitivity analysis of net difference in costs in the base-case analysis. B, Probability sensitivity analysis of percentage of iterations by net difference in the base-case analysis. Dark blue bars indicate iterations in which the administration of CAR T-cell therapy in the nonacademic specialty oncology network (NASON) setting was associated with cost reduction (71.9% of total iterations). Light blue bars indicate iterations in which the administration of chimeric antigen receptor (CAR) T-cell therapy in the inpatient hospital setting was associated with cost reduction (28.1% of total iterations). Of 1000 iterations, 0.5% had costs greater than $200 000, 3.2% had costs between $150 001 and $200 000, 10.6% had costs between $100 001 and $150 000, 24.7% had costs between $50 001 and $100 000, 32.9% had costs between $1 and $50 000, 20.1% had costs between –$49 999 and $0, 6.8% had costs between –$99 999 and –$50 000, and 1.2% had costs less than –$100 000. C, Probability sensitivity analysis based on 97.5% of patients with adverse events (AEs) in the base-case analysis. The net difference in costs ranged from $14 654 to $25 251. D, Probability sensitivity analysis based on 50% of patients with AEs in the scenario analysis. The net difference in costs ranged from $21 199 to $24 935. CRS indicates cytokine release syndrome; NE, neurological event.
The probabilistic sensitivity analyses that used Monte Carlo simulation focused on AE parameters to address this parameter uncertainty in both AE profile scenarios. In the probabilistic sensitivity analyses, the mean total cost of care was substantially higher in the academic inpatient setting than in the nonacademic specialty oncology network setting. Across 1000 Monte Carlo iterations, the administration of CAR T-cell therapy in nonacademic specialty oncology networks indicated cost reductions in 719 simulations (71.9%) using the AE profile from the base-case analysis (Figure 3B) and in 628 simulations (62.8%) using the AE profile from the alternative scenario analysis (Table 1) compared with the administration of CAR T-cell therapy in academic inpatient hospitals.
An additional sensitivity analysis varied the percentage of grade 1 or 2 AEs that were managed in the inpatient setting (Figure 3C and D). In the base-case analysis, the range of cost difference decreased to $14 654 when all patients at both administration sites were managed in the inpatient setting (Figure 3D). The range was smaller in the scenario analysis, with lower AE rates. As the management of grade 1 or 2 AEs expanded into the outpatient setting, the net cost difference between the sites of care increased.
Discussion
The administration of CAR T-cell therapy in nonacademic specialty oncology networks was associated with cost reductions in both the base-case analysis (difference of $32 987) and the scenario analysis (difference of $27 294). Notably, both hospitalization and office visit costs and procedural costs were substantially reduced. The decrease in incremental cost reductions in the scenario analysis was associated with a lower overall incidence of AEs, which reduced the consequences of associated AE management costs, while the monitoring required at baseline was held constant.
The overall incidence of AEs in the probabilistic sensitivity analyses may be associated with economic factors when probabilistic uncertainty is considered. In the base-case analysis, the 95% CI estimated by the model was $452 466 to $458 267 in the academic inpatient setting and $417 204 to $422 325 in the nonacademic specialty oncology network setting. In the scenario analysis, the 95% CIs in the academic inpatient and nonacademic specialty oncology network settings were $423 443 to $430 177 and $402 772 to $408 728, respectively. Assuming that both CAR T-cell AE profiles could be observed at either site of care, the base-case AE profile was associated with a larger range of total costs ($41 063; 95% CI, $417 204-$458 267) compared with the lower AE incidence from the scenario analysis ($27 404; 95% CI, $402 772-$430 177). This result suggests that a CAR T-cell therapy with a better safety profile may be more economical and able to further leverage the outpatient site of care. Unique methods of reimbursement for novel therapies have been proposed by government and private payers. Our analysis can contribute to prescriptive strategies for these important developments. Therefore, CAR T-cell therapy products with lower AE rates may provide a more consistent estimate of the total costs of care.
Bundled payment programs, or case rates, are growing in prominence for cancer treatment.34,54,55 Because of the shift to these fixed payment rates per episode of care, it is necessary to explore the implications that costs may have for reimbursement. An evidence-based case rate is informed by data from studies of current CAR T-cell treatments.27,28 As the landscape of CAR T-cell therapy continues to change, particularly with the potential emergence of CAR T-cell therapies that may be appropriate for outpatient administration, the current data informing case rates may not be applicable in the future, which may result in a misaligned case rate for future cases of CAR T-cell treatment. For instance, a case rate based on inpatient CAR T-cell administration may be unsuitable for outpatient administration.
Given a fixed-payer environment, resource allocations for patient treatment should also be considered. The existing literature regarding this disease has reported that patients who received auto-HSCT therapy in the outpatient setting had comparable clinical outcomes, despite lower costs.22,56 Previous studies have also indicated that the administration of HSCT therapy in the outpatient setting is likely to have comparable or better patient-reported outcomes and quality of life compared with HSCT therapy administered in the inpatient setting.57 Similar benefits may exist with the administration of CAR T-cell therapy in the outpatient setting.
Because of potential capacity concerns at inpatient hospitals, extending the administration and monitoring of CAR T-cell therapy to the outpatient setting may allow more patients to be treated. A 2008 study indicated that the largest HSCT therapy centers were working at full capacity and would be unable to increase the number of HSCT treatments performed without an increase in staff resources.58 The administration of CAR T-cell therapies, in particular, is currently limited to a small number of cancer centers.18,19 In addition, given limited resources, reduced costs may allow additional patients to be treated. The availability of outpatient administration at nonacademic specialty oncology networks may also reduce travel difficulties for patients who live far from an academic inpatient transplantation center. As a consequence, CAR T-cell therapy that is administered in the outpatient setting may allow more patients to be treated because of the alleviation of inpatient hospital capacity and geographic concerns.
Limitations
This study has limitations. Several clinical and economic assumptions were made to build the model used in this study. The use of assumptions is not an uncommon approach in these types of analyses, and it exists in other CAR T-cell therapy economic modeling exercises, such as those conducted by the Institute for Clinical and Economic Review53 and Hernandez et al,59 which previously reported on the total costs of CAR T-cell therapy. In instances in which information was absent from the literature, expert opinion and anecdotal experience were used to inform our model structure and parameter values. The accuracy of the model was limited by the validity of the assumptions made; however, sensitivity analyses were conducted to address the uncertainty. The direction of the results was sustained despite the introduction of uncertainty. Although this study used assumptions, the direction of the difference in cost and the relative extent of difference was observed to be stable.
Although cost differentials by site of care are well recognized in the literature, elucidating the cost differences for novel cancer therapies highlights the importance of ensuring that patients can access these therapies in nonacademic specialty oncology networks (when clinically appropriate) at a lower cost to the hospital, given the possibility that payers may restrict access to therapies based on cost. In addition, other possible scenarios could have been included in the analysis. However, the 2 sites of care included provided the most contrast among the possible scenarios, with costs on the opposite ends of the spectrum compared with the other scenarios, which had costs that were between those ends.
In addition, cytokine release syndrome and neurological event rates were imprecisely based on those reported in clinical trials. Costs for these AEs were general estimates, given the anticipated severity of the AE and the site of AE management. Reimbursement rates were used as proxies owing to limited cost data. This study used approximated national average costs; however, actual total costs of care are dependent on geographic location owing to variation across localities.
Cytokine release syndrome and neurological events are not the only AEs associated with CAR T-cell therapy that may require hospitalization; other serious or severe AEs include tumor lysis syndrome, infection, and macrophage activation syndrome or hemophagocytic lymphohistiocytosis.60 These events were not considered in the analysis.
This study was also predicated on the assumption that a patient is equally eligible for outpatient and inpatient administration of CAR T-cell therapy, which represents a limitation of the analysis because outpatient administration may not be an option for all patients. Eligibility for the outpatient administration of CAR T-cell therapy is contingent on the patient’s health status and support system and the availability of housing near hospitals, among other factors. The results of this study may only be applicable to the subset of patients who are eligible for outpatient treatment and monitoring.
Conclusions
This study adds to the body of literature assessing differences in the costs of therapy between sites of care and contributes new information specific to CAR T-cell therapy. The potential availability of CAR T-cell therapies with lower AE rates that are suitable for outpatient administration may reduce the total costs of care.
eMethods 1. Model Inputs
eMethods 2. Costs per Day for Inpatient Stay, Intensive Care Unit, and Outpatient or Clinic Office Visit
eMethods 3. Adverse Events Management
eTable 1. Costs of Lymphodepletion and CAR T-Cells Used in the Model
eTable 2. Cost of Office Visits, Hospital Stays, and Drugs
eTable 3. Main Cost Considerations for Adverse Event Management
eReferences.
References
- 1.Wang X, Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:. doi: 10.1038/mto.2016.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol. 2018;15(1):31-. doi: 10.1038/nrclinonc.2017.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13(8):525-541. doi: 10.1038/nrc3565 [DOI] [PubMed] [Google Scholar]
- 4.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388-398. doi: 10.1158/2159-8290.CD-12-0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor–expressing T cells. Immunol Rev. 2014;257(1):107-126. doi: 10.1111/imr.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartmann J, Schubler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9(9):1183-1197. doi: 10.15252/emmm.201607485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin. 2010;60(6):393-408. doi: 10.3322/caac.20087 [DOI] [PubMed] [Google Scholar]
- 8.Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:498-505. doi: 10.1182/asheducation-2011.1.498 [DOI] [PubMed] [Google Scholar]
- 9.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107(1):265-276. doi: 10.1182/blood-2005-06-2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raut LS, Chakrabarti PP. Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J Cancer. 2014;3(1):66-70. doi: 10.4103/2278-330X.126531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800-1808. doi: 10.1182/blood-2017-03-769620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elstrom RL, Martin P, Ostrow K, et al. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clin Lymphoma Myeloma Leuk. 2010;10(3):192-196. doi: 10.3816/CLML.2010.n.030 [DOI] [PubMed] [Google Scholar]
- 13.Moore S, Kayani I, Peggs K, et al. Mini-BEAM is effective as a bridge to transplantation in patients with refractory or relapsed Hodgkin lymphoma who have failed to respond to previous lines of salvage chemotherapy but not in patients with salvage-refractory DLBCL. Br J Haematol. 2012;157(5):543-552. doi: 10.1111/j.1365-2141.2012.09096.x [DOI] [PubMed] [Google Scholar]
- 14.Nagle SJ, Woo K, Schuster SJ, et al. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol. 2013;88(10):890-894. doi: 10.1002/ajh.23524 [DOI] [PubMed] [Google Scholar]
- 15.Vose JM, Bierman PJ, Anderson JR, et al. Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: clinical course and patient follow-up. Blood. 1992;80(8):2142-2148. doi: 10.1182/blood.V80.8.2142.2142 [DOI] [PubMed] [Google Scholar]
- 16.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 18.Kuehn BM. The promise and challenges of CAR-T gene therapy. JAMA. 2017;318(22):2167-2169. doi: 10.1001/jama.2017.15605 [DOI] [PubMed] [Google Scholar]
- 19.The ASCO Post Staff . Treatment centers authorized to administer CAR T-cell therapy. Published May 25, 2018. Accessed March 5, 2019. https://www.ascopost.com/issues/may-25-2018/treatment-centers-authorized-to-administer-car-t-cell-therapy/
- 20.Smith S, Essell J. Evolving the delivery of CAR T-cell therapies to the outpatient setting. J Clin Pathw. 2018;4(8):42-47. doi: 10.25270/jcp.2018.10.00039 [DOI] [Google Scholar]
- 21.The ASCO Post Staff . ASH 2017: JULIET trial: 6-month analysis of tisagenlecleucel in relapsed/refractory DLBCL shows sustained responses. Published December 12, 2017. Updated December 13, 2017. Accessed March 14, 2019. https://www.ascopost.com/News/58349
- 22.Graff TM, Singavi AK, Schmidt W, et al. Safety of outpatient autologous hematopoietic cell transplantation for multiple myeloma and lymphoma. Bone Marrow Transplant. 2015;50(7):947-953. doi: 10.1038/bmt.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lion J, Henderson M, Malbon A, Bergman A, Come S. Case mix and charges for inpatient and outpatient chemotherapy. Health Care Financ Rev. 1987;8(4):65-71. [PMC free article] [PubMed] [Google Scholar]
- 24.Medicare Payment Advisory Commission . Hospital inpatient and outpatient services. In: Report to the Congress: Medicare Payment Policy. Medicare Payment Advisory Commission; March 2014:chap 3. Accessed January 10, 2020. http://www.medpac.gov/docs/default-source/reports/mar14_ch03.pdf?sfvrsn=0%20&%20https://www.mass.gov/files/documents/2018/03/28/Cost%20Trends%20Report%202017.pdf
- 25.McGuirk J, Waller EK, Qayed M, et al. Building blocks for institutional preparation of CTL019 delivery. Cytotherapy. 2017;19(9):1015-1024. doi: 10.1016/j.jcyt.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration . BLA clinical review memorandum. Submission tracking number 125643 (axicabtagene ciloleucel [Yescarta]). October 5, 2017. Accessed May 14, 2019. https://www.fda.gov/media/109149/download
- 27.Yescarta (axicabtagene ciloleucel). Prescribing information. Kite Pharma; October 2017. Accessed May 14, 2019. https://www.yescarta.com/files/yescarta-pi.pdf
- 28.Kymriah (tisagenlecleucel). Prescribing information. Novartis Pharmaceuticals; May 2018. Accessed May 14, 2019. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kymriah.pdf
- 29.Medicare physician fee schedule. Centers for Medicare and Medicaid Services; 2018. Updated 2019. Accessed March 5, 2019. https://www.cms.gov/apps/physician-fee-schedule/
- 30.Hospital Outpatient Prospective Payment System (OPPS) fee schedule database. Centers for Medicare and Medicaid Services; 2018. Accessed May 14, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/HospitalOPPS
- 31.US Bureau of Labor Statistics. Consumer price index data tables. 2018. Accessed March 5, 2019. https://www.bls.gov/cpi/
- 32.Healthcare Cost and Utilization Project (HCUP) National Inpatient Sample (NIS) database. Agency for Healthcare Research and Quality; 2015. Accessed May 14, 2019. https://www.hcup-us.ahrq.gov/nisoverview.jsp
- 33.Centers for Medicare & Medicaid Services. Healthcare Common Procedural Coding System (HCPCS) database. Accessed May 14, 2019. https://www.cms.gov/Medicare/Coding/MedHCPCSGenInfo
- 34.IBM Watson Health . IBM Micromedex RED BOOK database. Accessed March 5, 2019. https://www.ibm.com/watson/health/provider-client-training/micromedex-red-book/
- 35.Abramson JS, Gordon LI, Palomba ML, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL [abstract]. J Clin Oncol. 2018;36(suppl 15):7505. doi: 10.1200/JCO.2018.36.15_suppl.7505 [DOI] [Google Scholar]
- 36.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. doi: 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266-1271. doi: 10.1097/01.CCM.0000164543.14619.00 [DOI] [PubMed] [Google Scholar]
- 38.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. doi: 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20(2):119-122. doi: 10.1097/PPO.0000000000000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11(1):35. doi: 10.1186/s13045-018-0571-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor–modified T-cell therapy. Blood. 2017;130(21):2295-2306. doi: 10.1182/blood-2017-06-793141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teachey DT, Bishop MR, Maloney DG, Grupp SA. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘ALL’. Nat Rev Clin Oncol. 2018;15(4):218. doi: 10.1038/nrclinonc.2018.19 [DOI] [PubMed] [Google Scholar]
- 43.Neelapu SS, Tummala S, Kebriaei P, et al. Re: Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘ALL’. Nat Rev Clin Oncol. 2018;15(4):218. doi: 10.1038/nrclinonc.2018.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obiozor C, Subramaniam DP, Divine C, et al. Evaluation of performance status and hematopoietic cell transplantation specific comorbidity index on unplanned admission rates in patients with multiple myeloma undergoing outpatient autologous stem cell transplantation. Biol Blood Marrow Transplant. 2017;23(10):1641-1645. doi: 10.1016/j.bbmt.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 45.University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center . Autologous peripheral blood stem cell transplant. University of Maryland Medical System website. Accessed March 5, 2019. https://www.umms.org/umgccc/cancer-services/cancer-care/blood-marrow-transplant/patient-education/autologous-peripheral-blood-stem-cell-transplant
- 46.Barban A, Coracin FL, Musqueira PT, et al. Analysis of the feasibility of early hospital discharge after autologous hematopoietic stem cell transplantation and the implications to nursing care. Rev Bras Hematol Hemoter. 2014;36(4):264-268. doi: 10.1016/j.bjhh.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul TM, Liu SV, Chong EA, et al. Outpatient autologous stem cell transplantation for patients with myeloma. Clin Lymphoma Myeloma Leuk. 2015;15(9):536-540. doi: 10.1016/j.clml.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 48.Meisenberg BR, Ferran K, Hollenbach K, Brehm T, Jollon J, Piro LD. Reduced charges and costs associated with outpatient autologous stem cell transplantation. Bone Marrow Transplant. 1998;21(9):927-932. doi: 10.1038/sj.bmt.1701191 [DOI] [PubMed] [Google Scholar]
- 49.Maloney DG, Abramson JS, Palomba ML, et al. Preliminary safety profile of the CD19-directed defined composition CAR T cell product JCAR017 in relapsed/refractory aggressive B-NHL patients: potential for outpatient administration. Blood. 2017;130(suppl 1):1552. https://ashpublications.org/blood/article/130/Supplement%201/1552/79812/Preliminary-Safety-Profile-of-the-CD19-Directed [Google Scholar]
- 50.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188-195. Published correction appears in Blood. 2015;126(8):1048. doi: 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jönsson B. Ten arguments for a societal perspective in the economic evaluation of medical innovations. Eur J Health Econ. 2009;10(4):357-359. doi: 10.1007/s10198-009-0173-2 [DOI] [PubMed] [Google Scholar]
- 52.Garrison LP Jr, Mansley EC, Abbott TA III, Bresnahan BW, Hay JW, Smeeding J. Good research practices for measuring drug costs in cost-effectiveness analyses: a societal perspective: the ISPOR Drug Cost Task Force report–Part II. Value Health. 2010;13(1):8-13. doi: 10.1111/j.1524-4733.2009.00660.x [DOI] [PubMed] [Google Scholar]
- 53.Institute for Clinical and Economic Review . Chimeric antigen receptor T-cell therapy for B-cell cancers: effectiveness and value. Draft evidence report for the California Technology Assessment Forum. Published December 19, 2017. Accessed May 14, 2019. https://icer-review.org/wp-content/uploads/2017/07/ICER_CAR_T_Draft_Evidence_Report_121917.pdf
- 54.Dahl A. The new oncology bundle model: what we know and don't know. Published November 14, 2018. Accessed March 11, 2019. https://www.ajmc.com/contributor/darcie-hurteau/2018/11/the-new-oncology-bundle-model-what-we-know-and-dont-know
- 55.Press MJ, Rajkumar R, Conway PH. Medicare’s new bundled payments: design, strategy, and evolution. JAMA. 2016;315(2):131-132. doi: 10.1001/jama.2015.18161 [DOI] [PubMed] [Google Scholar]
- 56.Holbro A, Ahmad I, Cohen S, et al. Safety and cost-effectiveness of outpatient autologous stem cell transplantation in patients with multiple myeloma. Biol Blood Marrow Transplant. 2013;19(4):547-551. doi: 10.1016/j.bbmt.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 57.Summers N, Dawe U, Stewart DA. A comparison of inpatient and outpatient ASCT. Bone Marrow Transplant. 2000;26(4):389-395. doi: 10.1038/sj.bmt.1702534 [DOI] [PubMed] [Google Scholar]
- 58.Schriber JR, Anasetti C, Heslop HE, Leahigh AK. Preparing for growth: current capacity and challenges in hematopoietic stem cell transplantation programs. Biol Blood Marrow Transplant. 2010;16(5):595-597. doi: 10.1016/j.bbmt.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 59.Hernandez I, Prasad V, Gellad WF. Total costs of chimeric antigen receptor T-cell immunotherapy. JAMA Oncol. 2018;4(7):994-996. doi: 10.1001/jamaoncol.2018.0977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maus MV, Levine BL. Chimeric antigen receptor T-cell therapy for the community oncologist. Oncologist. 2016;21(5):608-617. doi: 10.1634/theoncologist.2015-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Model Inputs
eMethods 2. Costs per Day for Inpatient Stay, Intensive Care Unit, and Outpatient or Clinic Office Visit
eMethods 3. Adverse Events Management
eTable 1. Costs of Lymphodepletion and CAR T-Cells Used in the Model
eTable 2. Cost of Office Visits, Hospital Stays, and Drugs
eTable 3. Main Cost Considerations for Adverse Event Management
eReferences.