Abstract
Background
Pregnant women with symptoms of depression or anxiety often do not receive adequate treatment. In view of the high incidence of these symptoms in pregnancy and their impact on pregnancy outcomes, getting treatment is of the utmost importance. A guided internet self-help intervention may help to provide more women with appropriate treatment.
Objective
This study aimed to examine the effectiveness of a guided internet intervention (MamaKits online) for pregnant women with moderate to severe symptoms of anxiety or depression. Assessments took place before randomization (T0), post intervention (T1), at 36 weeks of pregnancy (T2), and 6 weeks postpartum (T3). We also explored effects on perinatal child outcomes 6 weeks postpartum.
Methods
This randomized controlled trial included pregnant women (<30 weeks) with depressive symptoms above threshold (ie, Center for Epidemiological Studies Depression scale [CES-D] >16) or anxiety above threshold (ie, Hospital Anxiety and Depression Scale-Anxiety subscale [HADS-A] >8) or both of them. Participants were recruited via general media and flyers in prenatal care waiting rooms or via obstetricians and midwives. After initial assessment, women were randomized to (1) MamaKits online in addition to treatment as usual or (2) treatment as usual (control condition). MamaKits online is a 5-week guided internet intervention based on problem solving treatment. Guidance was was provided by trained students pursuing a Master's in Psychology. Outcomes were based on a Web-based self-report. Women in the control condition were allowed to receive the intervention after the last assessment (6 weeks postpartum).
Results
Of the 159 included women, 79 were randomized to MamaKits online, 47% (79/37) of whom completed the intervention. Both groups showed a substantial decrease in affective symptoms on the CES-D, HADS-A, and Edinburgh Postnatal Depression Scale over time. In the intervention group, affective symptoms decreased more than that in the control group, but between-group effect sizes were small to medium (Cohen d at T3=0.45, 0.21, and 0.23 for the 3 questionnaires, respectively) and statistically not significant. Negative perinatal child outcomes did not differ between the 2 groups (χ21=0.1; P=.78). Completer analysis revealed no differences in outcome between the treatment completers and the control group. The trial was terminated early for reasons of futility based on the results of an interim analysis, which we performed because of inclusion problems.
Conclusions
Our study did show a significant reduction in affective symptoms in both groups, but the differences in reduction of affective symptoms between the intervention and control groups were not significant. There were also no differences in perinatal child outcomes. Future research should examine for which women these interventions might be effective or if changes in the internet intervention might make the intervention more effective.
Trial Registration
Netherlands Trial Register NL4162; https://tinyurl.com/sdckjek
Keywords: pregnancy, depression, anxiety, internet, pregnancy outcome, treatment
Introduction
Background
Depression and anxiety are common problems in women in the perinatal period. Major depressive disorder and anxiety disorders affect 7% to 15% of women during pregnancy [1-4]. The prevalence of symptoms of depression and anxiety is even higher, as they occur in 18% to 20% of the pregnant women [1,5,6]. Depression and anxiety are both associated with poor pregnancy outcomes [7], postpartum depression [4,8,9], and negative influences on child development [10-14]. Hence, effective treatment of these disorders is of the utmost importance.
Psychotherapeutic interventions such as cognitive behavioral therapy and interpersonal therapy have proven effective in treating perinatal depression and anxiety [15-17]. However, the implementation of effective treatment interventions is often hampered by factors relating to the pregnancy, for example, nonrecognition of the symptoms because of overlapping symptomatology with pregnancy itself [18], or by feelings of stigmatization, lack of time, problems with transportation, or difficulties arranging childcare [11,19,20]. Some of these barriers may be overcome by providing guided internet-based self-help interventions. Indeed, Web-based interventions are easier to access, have no waiting lists, allow anonymity, and can be carried out whenever and wherever the patient wants [19,21,22]. Moreover, because the therapeutic input is smaller than that in regular face-to-face treatments, internet-based interventions are likely to be less costly and more scalable. This is especially advantageous for disorders that are characterized by a combination of high prevalence and a low treatment-seeking rate, which is the case for pregnant women with depressive and anxiety symptoms. Although internet-based interventions proved effective in the general population [23,24] as well as postpartum [25], recent studies of internet-based interventions during pregnancy [26-28] showed varying success. Outcomes may have been influenced by differences in the methodology, content, and duration of these internet-based therapies.
Previously, we developed an internet-based problem solving treatment (PST) consisting of five modules and support provided by a trained coach, which proved effective for depressed and anxious people in general [29,30]. PST is a generic treatment that is used for different kinds of psychiatric problems, such as depression [31] and anxiety [32]. The core assumption of PST is that affective symptoms are generated when people become overwhelmed by practical problems they face in their daily lives. In PST, participants make a list of all their worries and problems and learn structured ways to resolve those problems. This approach makes them feel less overwhelmed, which in turn alleviates their mood.
Although the effectiveness of face-to-face PST has been firmly established [31], there is no evidence yet whether online guided PST might be effective in reducing symptoms of depression and anxiety in pregnant women.
Objectives
For this study, we adapted the Web-based guided PST to provide an effective, easily accessible intervention for above-threshold affective symptoms in pregnant women. We hypothesized that the intervention would be effective (1) in reducing depressive and anxiety symptoms post intervention during pregnancy, at the end of pregnancy, and at 6 weeks postpartum and (2) in improving perinatal child outcomes, such as preterm birth, growth restriction, and breastfeeding initiation.
Methods
Study Design
We performed a randomized controlled trial with an intervention condition (internet-based PST) and a control group (care-as-usual). For ethical reasons, the participants in the control condition were also offered access to the intervention, but only after the last follow-up (6 weeks postpartum). Both groups were allowed to use concurrent treatment (care-as-usual) as well. The use of additional care was monitored through self-report.
The study protocol, information brochure, and informed consent form were approved by the Medical Ethics Committee of the VU University Medical Center (registration number 2013.275) and registered with the Dutch Trial Registry (NL4162). The tenets of the Declaration of Helsinki were observed. An extensive description of the study protocol can be found elsewhere [33].
Participants
All participants were self-referred. They were recruited through articles and advertisement in national newspapers and magazines and through social media, pregnancy websites, and websites of patient’s associations. Information flyers and posters were also distributed in maternity clinics and in clinics for primary care nationwide. Pregnant women with symptoms of depression and/or anxiety were advised to visit our study website, where they could find more information about the study and were given the opportunity to register online. After registration, they received an informed consent letter by post. After returning the signed informed consent form, they were invited to complete the first Web-based questionnaire.
Inclusion and Exclusion Criteria
Women aged 18 years and older were eligible if they were pregnant for less than 30 weeks, showed symptoms of depression or anxiety or both, and had sufficient access to the internet. Symptoms of depression were measured with the initial Web-based questionnaire using the Center for Epidemiological Studies Depression scale (CES-D) [34], and symptoms of anxiety were assessed using the Hospital Anxiety and Depression Scale-Anxiety subscale (HADS-A) [35]. Women were eligible to participate if their score on the CES-D was at least 16 or the score on the HADS-A was 8 or more. Women with severe depressive or anxiety symptoms (CES-D ≥25 or HADS-A ≥12) were also allowed to participate. However, we advised them to contact their general practitioner as well to check if another treatment or additional treatment was needed. We did not exclude them because internet-based PST has also proven to be effective for severe depressive and anxiety symptoms [29,36,37]. However, women were excluded if they reported intentions to harm themselves or to attempt suicide (assessed by one question of the Web Screening Questionnaire) [38].
During the trial, participants in the intervention group were allowed to receive additional care-as-usual, such as psychiatric treatment including psychotherapy or psychopharmacological drugs. Any additional treatments were monitored through participants’ self-reports at every assessment.
Randomization
Women who were included in the study were randomized in a 1:1 ratio to the intervention condition versus the control condition. An independent researcher created a computer-generated randomization scheme based on blocks of 10 and provided the next randomization outcome to one of the coaches. This procedure ensured allocation concealment. The research assistant informed all the participants on the randomization outcome by email. The participants of the intervention group also received the name of the website and details on where and how they could log-in to start the intervention.
Intervention
An existing evidence-based internet version of PST [29] was used. We adapted this version for pregnant women by adding one session of psychoeducation on pregnancy and affective symptoms and adjusting all the existing case examples to pregnancy-related case examples. The adapted Web-based intervention for pregnant women was named MamaKits online. The course consists of 5 modules, and participants are advised to try to carry out one module each week. Each module consists of information, examples of other pregnant women with depressive or anxiety symptoms carrying out the intervention, and homework assignments.
The intervention consists of 3 steps: (1) participants describe what really matters to them, (2) participants write down all their current worries and problems, and (3) participants make a plan for the future, in which they describe how they will try to accomplish those things that matter most to them. After that, they categorize the problems into three types: unimportant problems (problems unrelated to the things that matter to them), problems that can potentially be solved, and problems that cannot be solved (eg, the loss of a loved one). The core of the intervention consists of a structured approach to solve the potentially solvable problems. This approach consists of 6 steps: (1) write down a clear definition of the problem, (2) generate multiple solutions to the problem, (3) select the best solution, (4) work out a systematic plan for this solution, (5) carry out the solution, and (6) evaluate whether the solution has resolved the problem.
After each module, trained coaches (students pursuing Master’s in Psychology) provided feedback on the assignments via secured email. All coaches were trained for 4 hours in PST and providing feedback via secured email. They were trained by an experienced psychotherapist, who also provided the coaches with regular supervision. On average, the coaches gave 20 min of feedback per patient per module. The feedback was directed to helping the patient work through the intervention; the coaches answered questions if something was not clear and provided feedback on homework assignments. If a participant was delayed in submitting the homework, the coach sent a reminder by email, with a maximum of three emails and one phone call after that.
Measures
Assessments
Assessments took place at baseline (T0), 10 weeks after baseline (T1), 4 weeks before the expected date of delivery (T2), and 6 weeks postpartum (T3). Participants who started the intervention after 24 weeks’ gestation were not assessed at T2, as the period between T1 and T2 would have been too short to expect any effects. All assessments were based on self-report and took place online. At baseline, we additionally collected demographic data and data on current mental treatment, parity, pregnancy duration, and previous and current pregnancy complications. At T1, we collected additional data about treatment satisfaction, and at T3, we additionally collected data on perinatal child outcomes.
Primary Outcomes
Primary outcomes were reduction in symptoms of depression and anxiety and perinatal child outcomes. Depression was measured with the Dutch version of the CES-D [34]. This scale has 20 self-rated items, each of which is scored from 0 to 3. The total score range is 0 (no depressive symptoms) to 60 (high number of depressive symptoms). The validity of the CES-D has been tested in different populations, including pregnant women [39,40] and also online [41]. Scores of 16 and higher represent a clinically significant level of depressive symptoms with a sensitivity of 0.82 to 1.00 and a specificity of 0.69 to 0.88 [37,38].
Anxiety was measured with the Dutch version of the HADS-A [35]. The HADS-A is a 7-item anxiety subscale of the HADS with item responses on a 0 to 3 scale. Total score range is 0 to 21. Higher scores indicate more anxiety. The questionnaire has been found to be reliable in the internet version [42]. The HADS-A has an optimal cut-off ≥8 with a sensitivity of 0.89 and a specificity of 0.75 [43].
Perinatal child outcomes were assessed through self-report and analyzed by calculating the differences between the percentages of women in the intervention and control condition who delivered preterm (gestational age <37 weeks), whose babies had a low birth weight for gestational age (weight ≤tenth percentile, according to the guidelines by the Dutch Association of Gynecologists and Obstetricians, based on data of the Dutch National Birth Register), who delivered with an emergency cesarean section or vacuum extraction, or who did not continue breastfeeding until 6 weeks postpartum.
Secondary Outcomes
Secondary outcomes were reduction in symptoms of depression as measured with the Edinburgh Postnatal Depression Scale (EPDS), additional psychological health care use, and treatment satisfaction. The EPDS [44] is a 10-item depression scale developed for women primarily in the postpartum period, but also in pregnancy. Depending on the trimester, the cut-off score varied worldwide from 6.5 to 14.5, and in the Netherlands, it varied from 10 to 11 [45,46]. Item response varies from 0 to 3, and the total score range is 0 to 30 [45]. Information about additional mental health care was obtained using the Trimbos/institute for Medical Technology Assessment, Erasmus University Rotterdam, questionnaire for Costs associated with Psychiatric Illness [47].
We also used the Client Satisfaction Questionnaire (CSQ-8). The CSQ-8, a questionnaire with 8 items measured on a 4-point scale, has good psychometric properties in the Dutch population [48]. We added several questions about the intervention, the website, and the feedback of the coach. These questions could be answered through visual analog scales (VASs).
Sample Size Considerations
The between-group effect size (Cohen d) at post test (T1) was assumed to be at least 0.40, as was demonstrated in previous studies using the same internet-based PST [29,30]. Using an alpha of .05 (2-tailed), a statistical power (1-beta) of 0.80, and an attrition rate of 30% (as seen in other internet-based therapies in depressed patients) [30], we calculated that we needed to enroll 143 respondents in each arm.
After reviewing the literature, we assumed that symptoms of major depressive disorder and any anxiety disorder affect 7% to 15% of women during pregnancy [1-4] and that about 17% of the pregnant women have mild affective symptoms in pregnancy [6]. On the basis of a yearly birth rate of 171,341 in the Netherlands [49], at least about 29,127 women would be eligible for screening. With an expected response rate of 1%, 291 women would be included. Therefore, inclusion was expected to be completed within 1 year.
Statistical Analysis
All data were analyzed according to intention-to-treat analysis (comprising all the participants who were randomized) as well as per-protocol analysis (focusing on the participants who completed the intervention, ie, a subset of the intention-to-treat sample).
Mean total scores (standard deviations) of the 3 questionnaires (CES-D, HADS-A, and EPDS) were computed for the intervention and control arms separately at different time points (T0, T1, T2, and T3). The internet-based PST intervention effect was tested with linear mixed model (LMM) analyses, while correcting for baseline differences in the depressive and anxiety symptoms. LMM analysis can handle missing data owing to dropout under the assumption that the data are missing-at-random. Adverse perinatal child outcomes were defined as having experienced any negative outcome and were also evaluated by means of chi-square tests. Statistical analyses were carried out with SPSS (version 24; IBM, Armonk, New York) and Stata (version 15; StataCorp, College Station, Texas) software.
Results
Inclusion, Study Flow, Study Termination, and Dropout
The inclusion period was extended from 1 to 3 years owing to a low inclusion rate (March 2014 until January 2017). After 3 years, we performed an interim analysis, which had not been planned in the study protocol, to decide if inclusion of additional participants (and applying for additional funding) would be worthwhile or not. We developed an interim analysis protocol, which was approved by the ethical board. We evaluated the intervention effect on the first primary outcome CES-D at posttest (T1) when 153 participants had been randomized. According to the interim analysis protocol, the trial would be stopped for efficacy if the estimated intervention effect (in terms of standardized mean difference) exceeded 0.54 (in other words, extra patients would not be needed because the power was enough to establish the effect with significance). Inclusion would also be stopped, for futility, if the intervention effect was below 0.29 (in other words, continuing with our previously planned number of patients would not be useful because even if this number was reached, the power would be insufficient to demonstrate the effect with significance). As the interim analysis provided an estimated effect size of 0.035, the inclusion of participants was terminated prematurely. Although the inclusion of new participants stopped, all measurements continued as scheduled for the participants already included.
At the time of closure, a total of 349 women had expressed interest in the intervention. Of those women, 99 were excluded because they did not fulfill the inclusion criteria (eg, due to being pregnant beyond 30 weeks or due to not exceeding the required threshold for depression or anxiety scores). Of the remaining 250 women, 91 did not want to participate because of several reasons (eg, they already felt better or started another type of therapy). Of the originally planned 286 women, 159 were included in the study, as another 6 women were in the process of inclusion during the interim analysis. Of these 159 women, 79 were randomly allocated to the intervention group and 80 to the control group (Figure 1). Study dropout was 14% (11/80) in the control arm versus 22% (17/79) in the experimental arm at T1 (P=.20), 15% (12/80) versus 27% (21/79) at T2 (P=.07), and 19% (15/80) versus 32% (25/79) at T3 (P=.06). Overall, 60% (48/80) of the control group versus 43% (34/79) of the intervention group responded in all waves (T0, T1, T2, and T3) and 21% (17/80) of the control group versus 25% (20/79) of the intervention group missed either T1 or T2 (or both).
Figure 1.
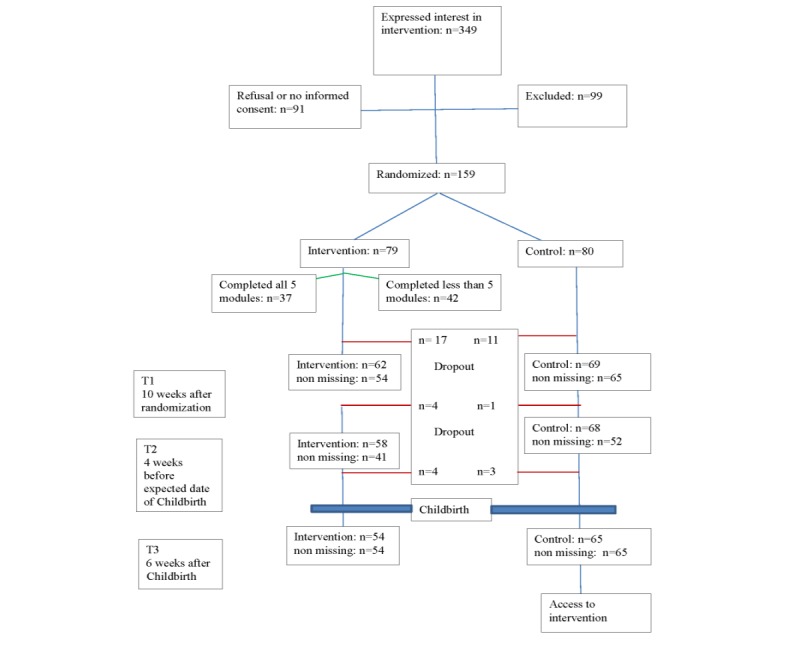
Flowchart of participants throughout the trial. Intervention: still in intervention group, but not everyone participated in this assessment; Control: still in control group, but not everyone participated in this assessment; Nonmissing: did participate in this assessment; Dropout: not in study anymore.
Of the 79 participants who were randomized to the intervention group, 37 (47%) completed all 5 modules of the intervention, 39 (49%) women completed at least four modules, 50 (63%) women completed at least three modules, 67 (72%) women completed at least two modules, 70 (89%) women completed at least one module, and 9 (11%) women did not even complete the first module. Reasons for nonadherence included being too busy (n=7), feeling better (n=4), need for other treatment (too sick; n=5), not being motivated (n=8), difficulties in confessing to the computer (n=1), intervention not meeting the expectations (n=3), and other reason/no reason given (n=14). There were no statistically significant differences between the baseline scores of treatment completers (having done all five modules) and noncompleters (having done less than five modules). The number of women using additional therapy was similar in both groups (P=.68, P=.82, and P=.73 at T0, T1, and T3, respectively).
Description of Participants
In total, 159 women were randomized. Differences in baseline demographics between the internet-based PST group and the control group were small (Table 1). Most women were of native Dutch origin (134/159, 84.2%), highly educated (120/159, 75.4%), and employed (111/159, 69.8%). Differences between the intervention and the control group with respect to baseline severity scores of depression and anxiety (primary and secondary outcomes) were also small and nonsignificant.
Table 1.
Sociodemographic and clinical characteristics at baseline for the intervention group and the control group (primary and secondary outcomes).
| Demographic factors | Intervention, n=79 | Control, n=80 | ||
| Age (years), mean (SD) | 32.08 (4.61) | 31.94 (4.83) | ||
| Background, n (%) | ||||
|
|
Dutch | 72 (91) | 62 (78) | |
|
|
Other | 7 (9) | 18 (23) | |
| Educationª, n (%) | ||||
|
|
Low | 4 (5) | 0 (0.0) | |
|
|
Middle | 14 (18) | 21 (26) | |
|
|
High | 61 (77) | 59 (74) | |
| Marital status, n (%) | ||||
|
|
In a relationship | 76 (96) | 76 (95) | |
|
|
Living together | 71 (90) | 73 (91) | |
| Employed, n (%) | 57 (72) | 54 (68) | ||
| Pregnancy, n (%) | ||||
|
|
Duration by study entrance | |||
|
|
|
<12 weeks | 5 (6) | 11 (14) |
|
|
|
>12 and <26 weeks | 48 (61) | 44 (55.0) |
|
|
|
> 26 weeks | 26 (33) | 25 (31) |
| Nulliparous | 42 (53) | 36 (45) | ||
| Complications in previous pregnancyb | 29 (60) | 39 (72) | ||
| Complications in this pregnancy | 9 (11) | 7 (9) | ||
| Previous mental healthc, n (%) | ||||
|
|
Depressive disorder | 24 (30) | 29 (36) | |
|
|
Anxiety disorder | 20 (25) | 25 (31) | |
|
|
Other mental problems | 9 (11) | 2 (3) | |
|
|
No diagnosis | 31 (39) | 30 (38) | |
| Current treatment, n (%) | ||||
|
|
Psychological treatment | 31 (39) | 34 (43) | |
|
|
Psychotropic medication | 12 (15) | 14 (18) | |
| Affective symptoms, mean (SD) | ||||
|
|
Primary outcomes | |||
|
|
|
Center for Epidemiological Studies Depression | 28.84 (7.54) | 27.94 (9.04) |
|
|
|
Hospital Anxiety and Depression Scale-Anxiety | 11.44 (3.50) | 11.89 (3.38) |
|
|
Secondary outcome | |||
|
|
|
Edinburgh Postnatal Depression Scale | 14.27 (4.91) | 13.96 (4.94) |
aDutch Standard Classification of Education: 2006–Edition 2016/’17, CBS, Statistics Netherlands.
bFirst pregnancies excluded.
cNote that women can be both in the category “depressive disorder” and in the category “anxiety disorder.”
Effects on Mood Within the Intention-to-Treat Sample
In the intervention group, large within-group effect sizes in primary and secondary outcomes were found between T0 and T1, T2 and T3 (Table 2). However, within-group effect sizes in the control group were also large (Figure 2). Differences in effects, as measured in the between-group effect sizes, were small and statistically insignificant (Table 3). The only exception to this finding was the medium effect size of the CES-D outcome on T3, but this was not significant either (d=0.45; P=.06).
Table 2.
Mean scores (standard deviations) for affective symptoms (primary and secondary outcomes) considering the intervention group and the control group at baseline, 10 weeks after randomization, 4 weeks before expected birth date, and 6 weeks after child birth.
| Condition | Baseline | 10 weeks after randomization | 4 weeks before expected birth date | 6 weeks after child birth | |||||||||||||||||
|
|
n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |||||||||||||
| Primary outcomes | |||||||||||||||||||||
|
|
Center for Epidemiological Studies Depression | ||||||||||||||||||||
|
|
|
Intervention | 79 | 28.8 (7.5) | 54 | 19.5 (10.2) | 41 | 19.7 (11.1) | 54 | 13.8 (10.3) | |||||||||||
|
|
|
Waitlist control | 80 | 27.9 (9.0) | 65 | 18.6 (9.4) | 52 | 18.6 (10.0) | 65 | 16.8 (11.9) | |||||||||||
|
|
Hospital Anxiety and Depression Scale-Anxiety | ||||||||||||||||||||
|
|
|
Intervention | 79 | 11.4 (3.5) | 54 | 8.4 (4.2) | 41 | 7.9 (4.4) | 54 | 7.1 (4.4) | |||||||||||
|
|
|
Waitlist control | 80 | 11.9 (3.4) | 65 | 8.6 (3.7) | 52 | 7.9 (4.1) | 65 | 7.9 (4.5) | |||||||||||
| Secondary outcomes | |||||||||||||||||||||
|
|
Edinburgh Postnatal Depression Scale | ||||||||||||||||||||
|
|
|
Intervention | 79 | 14.3 (4.9) | 54 | 9.5 (5.6) | 41 | 9.0 (5.5) | 54 | 8.0 (5.2) | |||||||||||
|
|
|
Waitlist control | 80 | 14.0 (4.9) | 65 | 8.9 (5.5) | 52 | 8.2 (5.2) | 65 | 8.7 (5.9) | |||||||||||
Figure 2.
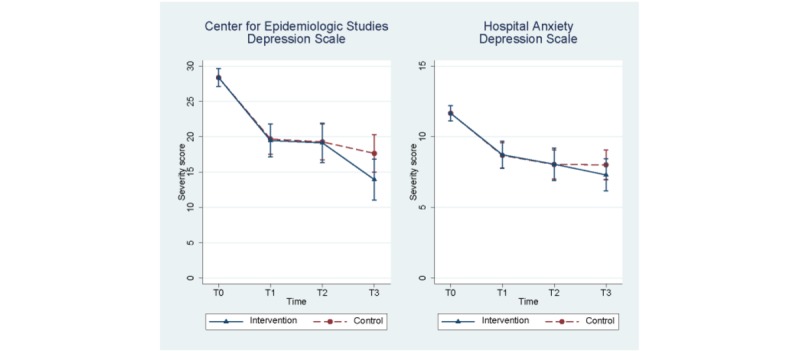
Predicted Center for Epidemiological Studies Depression and Hospital Anxiety and Depression Scale severity scores (primary outcomes) estimated using linear mixed models correcting for baseline differences. Measurements taken at T0: baseline; T1: 10 weeks after baseline; T2: 4 weeks before the expected date of delivery; T3: 6 weeks postpartum.
Table 3.
Estimated effects (unstandardized), test results, and effect sizes of the differences in primary and secondary outcomes (Center for Epidemiological Studies Depression, Hospital Anxiety and Depression Scale-Anxiety, and Edinburgh Postnatal Depression Scale) within groups and between the intervention group and the control group, using linear mixed model analysis at baseline, 10 weeks after randomization, 4 weeks before expected birth date, and 6 weeks after child birth after correction for scores at baseline.
| Conditiona | Estimated effectb | Test results | Effect sizec (Cohen d) | ||||||
|
|
|
Test statistic, z | P value | Within control condition | Within intervention condition | Between groups | |||
| Primary outcomes | |||||||||
|
|
Center for Epidemiological Studies Depression | ||||||||
|
|
|
Intervention×T1d | −0.21 | −0.14 | .89 | −1.05 | −1.07 | −0.03 | |
|
|
|
Intervention×T2e | −0.14 | −0.08 | .94 | −1.10 | −1.11 | −0.02 | |
|
|
|
Intervention×T3f | −3.71 | −1.87 | .06 | −1.29 | −1.74 | −0.45 | |
|
|
Hospital Anxiety and Depression Scale-Anxiety | ||||||||
|
|
|
Intervention×T1 | 0.04 | 0.07 | .95 | −0.87 | −0.86 | 0.01 | |
|
|
|
Intervention×T2 | 0.02 | 0.02 | .98 | −1.06 | −1.05 | 0.01 | |
|
|
|
Intervention×T3 | −0.71 | −0.92 | .36 | −1.06 | −1.27 | −0.21 | |
| Secondary outcomes | |||||||||
|
|
Edinburgh Postnatal Depression Scale | ||||||||
|
|
|
Intervention×T1 | 0.00 | 0.01 | .10 | −0.91 | −0.91 | 0.00 | |
|
|
|
Intervention×T2 | 0.10 | 0.11 | .91 | −1.09 | −1.07 | 0.02 | |
|
|
|
Intervention×T3 | −1.12 | −1.13 | .26 | −1.02 | −1.25 | −0.23 | |
aThe test on all three parameters tests the null hypothesis that all three intervention-by-timepoint interaction terms are zero, meaning that the course of the outcome variable within the intervention group is identical to the course of the outcome variable within the waitlist control group.
bEstimated effects (unstandardized) are the parameter estimates of the intervention-by-timepoint interaction terms and reflect the additional increase (or decrease) within the intervention group compared with the increase (or decrease) in the waitlist control group.
cEffect sizes (Cohen d) are standardized effects, obtained by dividing the unstandardized estimated effects by the standard deviation of the primary outcomes.
dT1: 10 weeks after randomization.
eT2: 4 weeks before expected birth date.
fT3: 6 weeks after child birth.
Effects on Perinatal Child Outcomes Within the Intention-to-Treat Sample
The analyses of perinatal child outcomes revealed that 50.4% (60/119) of the women experienced one or more negative perinatal child outcomes or early cessation of breastfeeding. There was no statistically significant difference in these perinatal outcomes between the intervention group and the control group (Table 4).
Table 4.
Perinatal child outcomes in the intervention group compared with the control group.
| Perinatal child outcomes | Intervention, n (%) | Control, n (%) | P value |
| Preterm birth | 4 (7) | 1 (2) | .12 |
| Small for gestational age | 1 (2) | 4 (6) | .24 |
| Emergency cesarean section | 7 (13) | 9 (14) | .89 |
| Vacuum extraction | 7 (13) | 9 (14) | .89 |
| No breastfeeding initiation | 9 (17) | 5 (8) | .13 |
| Stopped breastfeeding early | 9 (17) | 11 (17) | .31 |
| At least one negative perinatal child outcome (including no or early cessation of breastfeeding) | 28 (52) | 32 (49) | .78 |
Per-Protocol Analysis of Treatment Completers (Mood and Perinatal Outcomes)
Of all 79 intervention patients, 37 (47%) completed the whole intervention. We examined the effects for those patients compared with the controls and found no significant differences in any of the outcome measures. LMM analyses revealed predominantly small nonsignificant differences between group effect sizes, with the exception of the CES-D on T3, which had a significant, medium to high effect size (CES-D T1: d=−0.25 and P=.21, CES-D T3: d=−0.53 and P=.04, HADS T1: d=−0.04 and P=.85, HADS T3: d=−0.41 and P=.09, EPDS T1: d=−0.09 and P=.66, and EPDS T3: d=−0.27 and P=.25).
Client Satisfaction
The CSQ-8 was completed at T1 by 53 intervention participants. The majority of the participants 87% (46/53) were satisfied with the help they received and 74% (39/53) would recommend the intervention to others. The total intervention was rated 7.1 (SD 1.6) on a 10-point VAS. The website was rated as fairly good to excellent by 83% (44/53) of the participants, and the feedback of coaches was also rated as fairly good to excellent by 83% (44/53) of the participants.
Additional Psychological Health Care
Both groups used additional psychological health care interventions, and in all cases, these interventions consisted of outpatient care. There were no statistically significant differences between the groups in the use of additional psychological health care. This was 42% (25/54) in the intervention group and 46% (27/65) in the control group (P=.60).
Discussion
Principal Findings
To the best of our knowledge, this randomized controlled trial is the first to investigate the effects of offering Web-based guided PST to pregnant women with symptoms of depression and anxiety, with the purpose of reducing barriers for effective therapy. In both the intervention group and the control group, symptoms decreased significantly over time, till 6 weeks postpartum. Although this difference was more pronounced in the intervention group than in the control group, the between-group differences were small and not statistically significant. The only statistically significant difference was shown in the per-protocol analysis at T3 on depression. We consider this result a lucky finding, and therefore, we do not think that this result is clinically meaningful. Except for this outlier, the outcomes of the questionnaires did not differ much. The differences in outcomes on the CES-D were larger than those on the EPDS. This might be explained by the fact that the EPDS also contains anxiety items (question 4 and 5), assuming that the intervention had a smaller effect on anxiety than on depression. There were also no differences between the groups in perinatal child outcomes. Attrition was high, with 47% (37/79) women completing the whole intervention and 63% (50/79) women completing more than three modules. In both groups, many women (52/119, 43.6%) used additional psychological treatment.
Our results are not in line with those of other studies on the effects of face-to-face PST in pregnant women, or with those of studies on the effects of internet-based PST in general, or with those of other Web-based therapies for pregnant women. Studies on face-to-face PST delivered perinatally did show medium to high effect [16] on depression, and studies on internet PST among people recruited in the general population showed moderate effects on both depression and anxiety [29,30]. Of 2 other studies on Web-based cognitive behavioral treatment (CBT) for depression during pregnancy [26,50], one showed favorable effects on the follow-up of anxiety but not on depression [50], whereas the other showed a large effect on depression [26] and only a small nonsignificant effect on anxiety.
There might be several reasons why our findings are not in line with those of previous studies. One possible explanation could be the intervention itself. Although the pregnant women who were included were generally satisfied with the intervention, a considerable proportion of these women dropped out. This proportion was larger than that in the intervention of Loughnan [50]. Although we do not know the reasons for the high dropout rate, one reason might have been that the women had sufficiently recovered and did not need more therapy. However, the dropout rate might also indicate that the treatment was not optimal or not sufficiently adapted to the population. The participants might have preferred additional modules (eg, with psychoeducation about changing relationships and role transition) and more supplementary resources, or they might have preferred another type of treatment (eg, CBT), one more like the treatments offered in the above-mentioned studies [26,50]. Another possibility is that the women in our study might have preferred face-to-face therapy, which is the default treatment in the Netherlands. The other 2 trials on Web-based CBT for depression during pregnancy were performed in Australia and Sweden, where people might be more familiar with electronic health because of their inability to commute to health care facilities if they live in remote areas [26,50]. Nevertheless, the fact that almost half of all participants in this study did complete the whole intervention indicates that an internet treatment might be a useful addition to the existing mental health services in the Netherlands.
The second possible reason why our findings differ from those of previous studies is the difference in measuring techniques. In the study that found a significant treatment effect for depression, symptoms were measured with the Montgomery Åsberg Depression Rating Scale Self-report version [26]. This instrument might be more sensitive to picking up relevant changes, but as far as we know, it has not been validated in pregnancy, and the changes could also be related to the improvement of symptoms of pregnancy itself. Besides, both studies on Web-based CBT for depression during pregnancy also used the EPDS as secondary outcome, resulting in small nonsignificant treatment effects.
The third possible reason for the lack of effect in this study is the remarkable improvement in the control group. This suggests that the improvement in both groups might rather be explained by spontaneous recovery. In general, people seek treatment when they are feeling at their worst. It is not unusual that symptoms improve spontaneously afterward [51]. This improvement might also be explained by the use of additional psychological services. A considerable part of the intervention group as well as the control group used other psychological treatments, and the majority of them started treatment before the intervention and continued after starting the intervention.
The fourth possible reason is that the patients in our study were relatively healthy. They had less severe depressive symptoms than those in the studies on Web-based interventions that showed greater effects [26,27]. Studies with patients with more severe symptoms often demonstrate higher effects than studies with patients with less severe symptoms [37].
The fifth possible reason is that the intervention might have been offered at the wrong moment during pregnancy. Most participants (92/159, 57.8%) were in the second trimester of their pregnancy, and several systematic reviews concluded that interventions carried out toward the end of pregnancy or in the postpartum period might be more effective [16,52]. However, in view of the negative consequences of anxiety and depression in pregnancy, an early intervention is of the utmost importance. Although we did not offer our intervention later in pregnancy or in the postpartum period, as recommended, we did meet the other 2 mentioned requirements of a successful treatment, which are an individual approach and an approach targeted at an at-risk population [52].
Strengths
Our study has several strengths. First, we created and tested the first internet version of evidence-based PST in a perinatal setting. Second, we had a relatively long follow-up of 20 weeks. Third, we used an array of different outcome measures, including perinatal child outcomes. Fourth, we allowed women of both groups to use concurrent treatment, including treatment as usual, which makes the results of our study compatible with clinical practice.
Limitations
Despite all our efforts to increase the number of women included in the study (by seeking publicity and prolonging the study period by 2 years), the required number of participants was not obtained. Second, adherence to the intervention was limited. Third, perinatal child outcomes were self-reported, which makes them less objective. The fourth limitation is that there was a sampling bias of mostly native Dutch, employed, and highly educated women, which makes the results less representative of the general population. The fifth limitation is that because of trial reasons, and to keep the population more homogeneous, women in the last 10 weeks of pregnancy were excluded from starting the intervention because they might not be able to finish the treatment before delivery. This is a limitation because by setting this limit, we excluded a group of women who could have benefited from the intervention, and we also possibly reduced the inclusion rate. Furthermore, as we mentioned earlier, interventions carried out toward the end of pregnancy or in the postpartum period might have been more effective [16,52]. We, therefore, might have been able to demonstrate larger effects if the intervention had been offered during this period.
The sixth limitation is that due to the small inclusion sample, the prevalence of negative perinatal child outcomes is probably less reliable.
Clinical Implications
The aim of our study was to improve the care for pregnant women with symptoms of depression or anxiety or both by offering a Web-based intervention with the intention to overcome perceived barriers to treatment. Although inclusion was low, attrition was high, and outcome differences between the intervention group and the control group were mostly nonsignificant, we still recommend investigating how adherence and the effectiveness might be improved by adjusting the Web-based intervention, as satisfaction with the offered modules was high and the intervention is easily applicable at low cost.
Conclusions
To the best of our knowledge, this is the first study to examine a Web-based PST intervention in pregnant women. Although this study did not show a significant reduction in depression and anxiety in comparison with a control condition, Web-based interventions remain a practical, cost-effective, complementary, or alternative therapy modality for face-to-face treatment. Future research is needed to see if the intervention might be more successful if it is offered later in pregnancy or if it is better adapted to the pregnant population or both.
Acknowledgments
The authors disclosed receipt of the following financial support: the randomized controlled trial was supported by the Stichting tot SteunVCVGZ.
Abbreviations
- CBT
cognitive behavioral treatment
- CES-D
Center for Epidemiological Studies Depression scale
- CSQ-8
Client Satisfaction Questionnaire
- EPDS
Edinburgh Postnatal Depression Scale
- HADS-A
Hospital Anxiety and Depression Scale-Anxiety
- PST
problem solving treatment
- LMM
linear mixed model
- VAS
visual analog scale
Appendix
CONSORT-EHEALTH checklist. (V 1.6.1).
Footnotes
Conflicts of Interest: None declared.
References
- 1.Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017 May;210(5):315–23. doi: 10.1192/bjp.bp.116.187179. [DOI] [PubMed] [Google Scholar]
- 2.Falah-Hassani K, Shiri R, Dennis C. The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. Psychol Med. 2017 Sep;47(12):2041–53. doi: 10.1017/S0033291717000617. [DOI] [PubMed] [Google Scholar]
- 3.Fisher J, de Mello MC, Patel V, Rahman A, Tran T, Holton S, Holmes W. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ. 2012 Feb 1;90(2):139G–49G. doi: 10.2471/BLT.11.091850. http://europepmc.org/abstract/MED/22423165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelaye B, Rondon MB, Araya R, Williams MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry. 2016 Oct;3(10):973–82. doi: 10.1016/S2215-0366(16)30284-X. http://europepmc.org/abstract/MED/27650773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 6.Josefsson A, Berg G, Nordin C, Sydsjö G. Prevalence of depressive symptoms in late pregnancy and postpartum. Acta Obstet Gynecol Scand. 2001 Mar;80(3):251–5. doi: 10.1034/j.1600-0412.2001.080003251.x. [DOI] [PubMed] [Google Scholar]
- 7.Jarde A, Morais M, Kingston D, Giallo R, MacQueen GM, Giglia L, Beyene J, Wang Y, McDonald SD. Neonatal outcomes in women with untreated antenatal depression compared with women without depression: a systematic review and meta-analysis. JAMA Psychiatry. 2016 Aug 1;73(8):826–37. doi: 10.1001/jamapsychiatry.2016.0934. [DOI] [PubMed] [Google Scholar]
- 8.Postpartum Depression: Action Towards Causes Treatment (PACT) Consortium Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiatry. 2015 Jan;2(1):59–67. doi: 10.1016/S2215-0366(14)00055-8. http://europepmc.org/abstract/MED/26359613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26(4):289–95. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Barker ED, Jaffee SR, Uher R, Maughan B. The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depress Anxiety. 2011 Aug;28(8):696–702. doi: 10.1002/da.20856. [DOI] [PubMed] [Google Scholar]
- 11.Goodman JH. Women's attitudes, preferences, and perceived barriers to treatment for perinatal depression. Birth. 2009 Mar;36(1):60–9. doi: 10.1111/j.1523-536X.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- 12.Grigoriadis S, Graves L, Peer M, Mamisashvili L, Tomlinson G, Vigod SN, Dennis C, Steiner M, Brown C, Cheung A, Dawson H, Rector NA, Guenette M, Richter M. Maternal anxiety during pregnancy and the association with adverse perinatal outcomes: systematic review and meta-analysis. J Clin Psychiatry. 2018 Sep 4;79(5):pii: 17r12011. doi: 10.4088/JCP.17r12011. http://www.psychiatrist.com/JCP/article/Pages/2018/v79/17r12011.aspx. [DOI] [PubMed] [Google Scholar]
- 13.Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis C, Koren G, Steiner M, Mousmanis P, Cheung A, Radford K, Martinovic J, Ross LE. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013 Apr;74(4):e321–41. doi: 10.4088/JCP.12r07968. [DOI] [PubMed] [Google Scholar]
- 14.Verbeek T, Bockting CL, van Pampus MG, Ormel J, Meijer JL, Hartman CA, Burger H. Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. J Affect Disord. 2012 Feb;136(3):948–54. doi: 10.1016/j.jad.2011.08.035. https://linkinghub.elsevier.com/retrieve/pii/S0165-0327(11)00521-0. [DOI] [PubMed] [Google Scholar]
- 15.US Preventive Services Task Force. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW, Grossman DC, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Tseng C, Wong JB. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendation statement. J Am Med Assoc. 2019 Feb 12;321(6):580–7. doi: 10.1001/jama.2019.0007. [DOI] [PubMed] [Google Scholar]
- 16.Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affect Disord. 2015 May 15;177:7–21. doi: 10.1016/j.jad.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 17.van Ravesteyn LM, den Berg MP, Hoogendijk WJ, Kamperman AM. Interventions to treat mental disorders during pregnancy: a systematic review and multiple treatment meta-analysis. PLoS One. 2017;12(3):e0173397. doi: 10.1371/journal.pone.0173397. http://dx.plos.org/10.1371/journal.pone.0173397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004 Apr;103(4):698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 19.Kim DR, Hantsoo L, Thase ME, Sammel M, Epperson CN. Computer-assisted cognitive behavioral therapy for pregnant women with major depressive disorder. J Womens Health (Larchmt) 2014 Oct;23(10):842–8. doi: 10.1089/jwh.2014.4867. http://europepmc.org/abstract/MED/25268672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Mahen HA, Flynn HA. Preferences and perceived barriers to treatment for depression during the perinatal period. J Womens Health (Larchmt) 2008 Oct;17(8):1301–9. doi: 10.1089/jwh.2007.0631. [DOI] [PubMed] [Google Scholar]
- 21.Maloni JA, Przeworski A, Damato EG. Web recruitment and internet use and preferences reported by women with postpartum depression after pregnancy complications. Arch Psychiatr Nurs. 2013 Apr;27(2):90–5. doi: 10.1016/j.apnu.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 22.van den Heuvel JF, Groenhof TK, Veerbeek JH, van Solinge WW, Lely AT, Franx A, Bekker MN. eHealth as the next-generation perinatal care: an overview of the literature. J Med Internet Res. 2018 Jun 5;20(6):e202. doi: 10.2196/jmir.9262. https://www.jmir.org/2018/6/e202/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson G, Cuijpers P. Internet-based and other computerized psychological treatments for adult depression: a meta-analysis. Cogn Behav Ther. 2009;38(4):196–205. doi: 10.1080/16506070903318960. [DOI] [PubMed] [Google Scholar]
- 24.Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev. 2012 Jun;32(4):329–42. doi: 10.1016/j.cpr.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Lee EW, Denison FC, Hor K, Reynolds RM. Web-based interventions for prevention and treatment of perinatal mood disorders: a systematic review. BMC Pregnancy Childbirth. 2016 Feb 29;16:38. doi: 10.1186/s12884-016-0831-1. https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-016-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsell E, Bendix M, Holländare F, von Schultz BS, Nasiell J, Blomdahl-Wetterholm M, Eriksson C, Kvarned S, van der Linden JL, Söderberg E, Jokinen J, Wide K, Kaldo V. Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord. 2017 Oct 15;221:56–64. doi: 10.1016/j.jad.2017.06.013. https://linkinghub.elsevier.com/retrieve/pii/S0165-0327(17)30129-5. [DOI] [PubMed] [Google Scholar]
- 27.Haga SM, Drozd F, Lisøy C, Wentzel-Larsen T, Slinning K. Mamma Mia - a randomized controlled trial of an internet-based intervention for perinatal depression. Psychol Med. 2019 Aug;49(11):1850–8. doi: 10.1017/S0033291718002544. http://europepmc.org/abstract/MED/30191779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krusche A, Dymond M, Murphy SE, Crane C. Mindfulness for pregnancy: a randomised controlled study of online mindfulness during pregnancy. Midwifery. 2018 Oct;65:51–7. doi: 10.1016/j.midw.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 29.van Straten A, Cuijpers P, Smits N. Effectiveness of a web-based self-help intervention for symptoms of depression, anxiety, and stress: randomized controlled trial. J Med Internet Res. 2008 Mar 25;10(1):e7. doi: 10.2196/jmir.954. https://www.jmir.org/2008/1/e7/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warmerdam L, van Straten A, Twisk J, Riper H, Cuijpers P. Internet-based treatment for adults with depressive symptoms: randomized controlled trial. J Med Internet Res. 2008 Nov 20;10(4):e44. doi: 10.2196/jmir.1094. https://www.jmir.org/2008/4/e44/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuijpers P, de Wit L, Kleiboer A, Karyotaki E, Ebert DD. Problem-solving therapy for adult depression: an updated meta-analysis. Eur Psychiatry. 2018 Feb;48:27–37. doi: 10.1016/j.eurpsy.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Kleiboer A, Donker T, Seekles W, van Straten A, Riper H, Cuijpers P. A randomized controlled trial on the role of support in Internet-based problem solving therapy for depression and anxiety. Behav Res Ther. 2015 Sep;72:63–71. doi: 10.1016/j.brat.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Heller HM, van Straten A, de Groot CJM, Honig A. The (cost) effectiveness of an online intervention for pregnant women with affective symptoms: protocol of a randomised controlled trial. BMC Pregnancy Childbirth. 2014 Aug 14;14:273. doi: 10.1186/1471-2393-14-273. https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/1471-2393-14-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouma J, Ranchor VA, Sanderman R, van Sonderen E. Measuring Symptoms of Depression With the CES-D: A Manual. Groningen: University of Groningen; 1995. [Google Scholar]
- 35.Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997 Mar;27(2):363–70. doi: 10.1017/s0033291796004382. [DOI] [PubMed] [Google Scholar]
- 36.Boeschoten RE, Dekker J, Uitdehaag BM, Polman CH, Collette EH, Cuijpers P, Beekman AT, van Oppen P. Internet-based self-help treatment for depression in multiple sclerosis: study protocol of a randomized controlled trial. BMC Psychiatry. 2012 Sep 11;12:137. doi: 10.1186/1471-244X-12-137. https://bmcpsychiatry.biomedcentral.com/articles/10.1186/1471-244X-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bower P, Kontopantelis E, Sutton A, Kendrick T, Richards DA, Gilbody S, Knowles S, Cuijpers P, Andersson G, Christensen H, Meyer B, Huibers M, Smit F, van Straten A, Warmerdam L, Barkham M, Bilich L, Lovell K, Liu ET. Influence of initial severity of depression on effectiveness of low intensity interventions: meta-analysis of individual patient data. Br Med J. 2013 Feb 26;346:f540. doi: 10.1136/bmj.f540. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=23444423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gega L, Kenwright M, Mataix-Cols D, Cameron R, Marks IM. Screening people with anxiety/depression for suitability for guided self-help. Cogn Behav Ther. 2005;34(1):16–21. doi: 10.1080/16506070410015031. [DOI] [PubMed] [Google Scholar]
- 39.Breedlove G, Fryzelka D. Depression screening during pregnancy. J Midwifery Womens Health. 2011;56(1):18–25. doi: 10.1111/j.1542-2011.2010.00002.x. [DOI] [PubMed] [Google Scholar]
- 40.Tandon SD, Cluxton-Keller F, Leis J, Le H, Perry DF. A comparison of three screening tools to identify perinatal depression among low-income African American women. J Affect Disord. 2012 Jan;136(1-2):155–62. doi: 10.1016/j.jad.2011.07.014. http://europepmc.org/abstract/MED/21864914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Ballegooijen W, Riper H, Cuijpers P, van Oppen P, Smit JH. Validation of online psychometric instruments for common mental health disorders: a systematic review. BMC Psychiatry. 2016 Feb 25;16:45. doi: 10.1186/s12888-016-0735-7. https://bmcpsychiatry.biomedcentral.com/articles/10.1186/s12888-016-0735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitehead L. Methodological issues in internet-mediated research: a randomized comparison of internet versus mailed questionnaires. J Med Internet Res. 2011 Dec 4;13(4):e109. doi: 10.2196/jmir.1593. https://www.jmir.org/2011/4/e109/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olssøn I, Mykletun A, Dahl AA. The Hospital Anxiety and Depression Rating Scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry. 2005 Dec 14;5:46. doi: 10.1186/1471-244X-5-46. https://bmcpsychiatry.biomedcentral.com/articles/10.1186/1471-244X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 45.Bergink V, Kooistra L, den Berg MP, Wijnen H, Bunevicius R, van Baar A, Pop V. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res. 2011 Apr;70(4):385–9. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Kozinszky Z, Dudas RB. Validation studies of the Edinburgh Postnatal Depression Scale for the antenatal period. J Affect Disord. 2015 May 1;176:95–105. doi: 10.1016/j.jad.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 47.Bouwmans C, Hakkaart-van Royen L. Research Gate. Rotterdam: Erasmus School of Health Policy & Management; 2002. [2020-01-21]. Manual Trimbos/iMTA Questionnaire for Costs Associated with Psychiatric Illness (in Dutch) https://www.researchgate.net/publication/254758194_Manual_TrimbosiMTA_Questionnaire_for_Costs_Associated_with_Psychiatric_Illness_TIC-P_in_Dutch. [Google Scholar]
- 48.de Brey H. A cross-national validation of the client satisfaction questionnaire: the Dutch experience. Eval Program Plann. 1983;6(3-4):395–400. doi: 10.1016/0149-7189(83)90018-6. [DOI] [PubMed] [Google Scholar]
- 49.StatLine. The Hague, The Netherlands: CBS StatLine; 2019. [2020-01-19]. Birth; key figures https://opendata.cbs.nl/statline/#/CBS/en/dataset/37422eng/table?ts=1579432841643. [Google Scholar]
- 50.Loughnan SA, Sie A, Hobbs MJ, Joubert AE, Smith J, Haskelberg H, Mahoney AE, Kladnitski N, Holt CJ, Milgrom J, Austin M, Andrews G, Newby JM. A randomized controlled trial of 'MUMentum Pregnancy': Internet-delivered cognitive behavioral therapy program for antenatal anxiety and depression. J Affect Disord. 2019 Jan 15;243:381–90. doi: 10.1016/j.jad.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 51.Spijker J, de Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS) Br J Psychiatry. 2002 Sep;181:208–13. doi: 10.1192/bjp.181.3.208. [DOI] [PubMed] [Google Scholar]
- 52.Dennis C, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev. 2013 Feb 28;(2):CD001134. doi: 10.1002/14651858.CD001134.pub3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT-EHEALTH checklist. (V 1.6.1).


