Abstract
Purpose
To assess the longitudinal evolution of radiographic edema using chest X-rays (CXR) in patients with Acute Respiratory Distress Syndrome (ARDS) and to examine its association with prognostic biomarkers, ARDS subphenotypes and outcomes.
Materials and methods
We quantified radiographic edema on CXRs from patients with ARDS or cardiogenic pulmonary edema (controls) using the Radiographic Assessment of Lung Edema (RALE) score on day of intubation and up to 10 days after. We measured baseline plasma biomarkers and recorded clinical variables.
Results
The RALE score had good inter-rater agreement (r = 0.83, p < 0.0001) applied on 488 CXRs from 129 patients, with higher RALE scores in patients with ARDS (n = 108) compared to controls (n = 21, p = 0.01). Baseline RALE scores were positively correlated with levels of the receptor for end-glycation end products (RAGE) in ARDS patients (p < 0.05). Baseline RALE scores were not predictive of 30- or 90-day survival. Persistently elevated RALE scores were associated with prolonged need for mechanical ventilation (p = 0.002).
Conclusions
The RALE score is easily implementable with high inter-rater reliability. Longitudinal RALE scoring appears to be a reproducible approach to track the evolution of radiographic edema in patients with ARDS and can potentially predict prolonged need for mechanical ventilation.
Keywords: Respiratory distress syndrome, Chest X-ray, Pulmonary Edema, Phenotype, Heterogeneity, Extubation
1. Introduction
The underlying biological and clinical heterogeneity of the Acute Respiratory Distress Syndrome (ARDS) has impaired our ability to identify efficacious pharmacological therapies [1,2]. Recent efforts to disentangle ARDS heterogeneity have identified subphenotypes of patients with potentially differential responses to therapies based on plasma biomarker profiles [3–5]. Nonetheless, subphenotyping approaches require timely measurements of plasma biomarkers, which are currently unavailable at the point-of-care, thus making clinical translation and implementation challenging.
Among the readily available clinical data in the intensive care unit (ICU), chest radiographs (CXR) represent an underutilized source of potentially useful information for real-time ARDS subphenotyping. Although characteristic CXR abnormalities are essential for the diagnosis of ARDS, i.e. presence of bilateral opacities consistent with pulmonary edema and not fully explained by effusions, atelectasis or nodules [6], the extent or density of radiographic pulmonary edema are not otherwise factored into the assessment of clinical severity. A new CXR scoring system (termed the radiographic assessment of lung edema (RALE) score) has been proposed, which has been shown to correlate with gravimetric edema in lung explants and with the severity of hypoxemia and survival in a retrospective analysis of a clinical trial population with ARDS [7,8]. However, it remains unknown whether the RALE score is associated with established clinical indicators of ARDS severity, emerging biomarker-based subphenotypes, or clinical outcomes in ARDS patients outside of clinical trials.
Therefore, we assessed the evolution of radiographic edema as quantified by the RALE score in patients with ARDS, and we examined the relationship of RALE scores with the severity of ARDS, subphenotypic classification of ARDS, inflammatory biomarkers, and clinical outcomes in a prospective observational cohort study.
2. Materials and methods
2.1. Patient population
From October 2011 – January 2018, we enrolled a convenience sample from consecutive, mechanically-ventilated patients from the Medical Intensive Care Unit (ICU) at University of Pittsburgh Medical Center (UPMC) to the Pittsburgh Acute Lung Injury Registry and Biospecimen Repository (ALIR) study [5,9]. Eligible patients were 18 years or older with acute respiratory failure (of any cause) requiring mechanical ventilation via endotracheal intubation. Exclusion criteria included the inability to obtain informed consent, presence of tracheostomy, or mechanical ventilation for >72 h. The University of Pittsburgh Institutional Review Board approved the study (PRO10110387) and written informed consent was provided by all participants or their surrogates in accordance with the Declaration of Helsinki.
We prospectively collected baseline clinical data obtained at the time of enrollment (including demographics, comorbidities, physiologic and laboratory variables) and quantified baseline severity of illness with modified sequential organ failure assessment (SOFA) scores (i.e. excluding the neurologic component). We followed patients prospectively for the following clinical outcomes: 30- and 90-day mortality, duration of ICU stay, incidence of acute kidney injury [10] or shock (defined as need for vasopressors) within 7-days from intubation and ventilator-free days at day 28 from enrollment (VFD) [11].
We reviewed the etiology and severity of acute respiratory failure following consensus committee examination of clinical and radiographic data and we retrospectively classified subjects into four distinct clinical categories (without knowledge of biomarkers): a. ARDS per Berlin criteria [6], b. patients at risk for ARDS, based on presence of an identifiable lung injury risk factor on enrollment, but not fulfilling ARDS criteria, and c. patients not at-risk for ARDS, including patients intubated for airway protection or for cardiogenic pulmonary edema for whom no lung injury risk factor was identified, and d. other, capturing all other critically-ill patients that could not be classified in one of the categories above [5]. For the purposes of quantifying radiographic edema for this study, we compared patients who were diagnosed with ARDS to patients with cardiogenic pulmonary edema (diagnosis of congestive heart failure without an identifiable risk-factor for ARDS).
From enrolled patients, plasma samples were collected and processed as previously described [5] in order to measure biomarkers belonging to different biologic pathway categories: a. innate immune responses (interleukin [IL]-6, IL-8, IL-10, tumor necrosis factor receptor 1 [TNFR1], suppression of tumorigenicity-2 [ST-2], fractalkine) [12–16], b. epithelial injury (receptor of advanced glycation end-products [RAGE]) [17], c. endothelial injury (Angiopoietin-2) [18] and d. host-response to bacterial infections (procalcitonin and pentraxin-3) [19,20]. We performed subphenotypic classifications in ARDS patients by utilizing the predicted probabilities for subphenotype classifications (hyperinflammatory vs. hypoinflammatory) from a parsimonious, previously validated logistic regression model that uses baseline values of three variables: Subphenotype = 2.25–1.97*(IL-8) + 1.71*(Bicarbonate) −1.71*(TNFR1) [21].
2.2. RALE score calculation
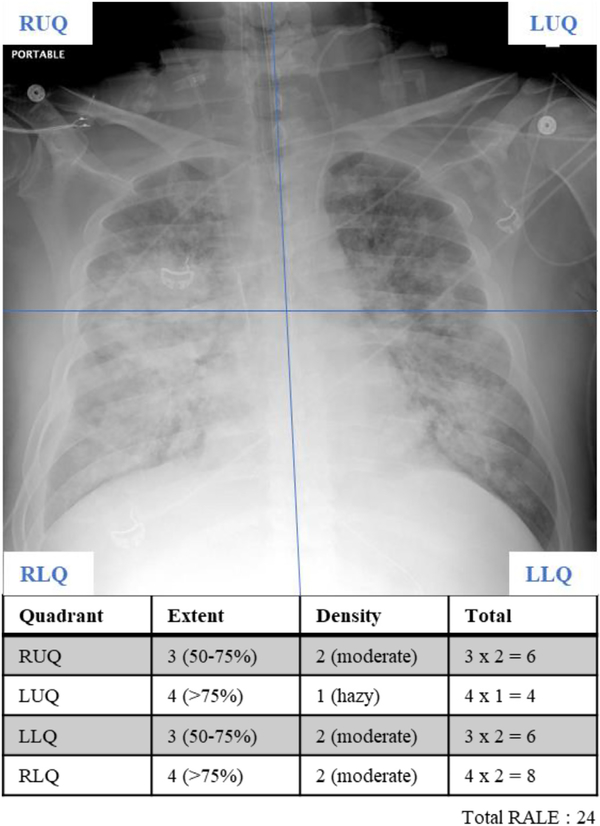
We quantified the radiographic edema by calculating the RALE score as described by Warren and colleagues [7]. Briefly, the RALE score assesses two aspects of pulmonary edema in four quadrants divided horizontally by a line through the first branch of the left main bronchus and vertically by the spinal column: consolidation extent (quantified as percent of CXR quadrant involved; 0% = 0; <25% = 1; 25–50% = 2; 50–75% = 3; >75% = 4) and density (1 = hazy; 2 = moderate; 3 = dense). Edema extent and density are multiplied for each quadrant and the total RALE score is obtained by addition of all quadrant scores (0–48) (Fig. 1). Higher RALE scores correlate with increased burden of radiographic edema. Two independent reviewers (DK and GDK) scored CXRs obtained on the day of intubation (day 0), and then on days 3, 5, 7 and 10 following intubation when available. In cases where reviewers disagreed on RALE scores (defined as difference in RALE score greater or equal than one standard deviation minus one [RALE ≥7]), a third, blinded reviewer (JWE, WB, DD) scored the CXR in question, and the final RALE score was then calculated as the mean of the three reviewer scores.
Fig. 1.
RALE score calculation from a chest radiograph (CXR) of an intubated patient with ARDS. First, the CXR is divided in four quadrants defined by drawing a horizontal line from the first branch of the left main bronchus with the spinal column separating the left and right lungs. The consolidation extent and density of each quadrant is scored separately, which are then multiplied for each quadrant and the total RALE score is obtained by simple addition of all quadrant scores (0–48).
2.3. Statistical analysis
We assessed interrater agreement (DK, GDK) using the interclass correlation coefficient (ICC) with a two-way mixed agreement model [22,23] and visualized it with a scatter plot. Given that CXR acquisition was not protocolized for our study but CXRs were obtained for clinical reasons, we attempted to minimize the interval between timing of CXRs and baseline research sample acquisition by using the RALE scores from the CXRs (day 0 vs. day 3) obtained more closely to the timing of enrollment (Fig. S1). For ARDS cases in which ARDS diagnostic criteria were not met on the day of intubation (day 0) but did so on day 3 post-intubation (i.e. incident ARDS, n = 4/108 [4%]), we used day 3 CXR for examining baseline associations (Fig. S2). We compared baseline characteristics using Wilcoxon signed-rank and Fisher’s exact tests for continuous and categorical variables, respectively. To assess the longitudinal evolution of RALE scores, we used repeated measures analysis of variance (ANOVA) of RALE scores over time, stratified by early liberation vs. prolonged mechanical ventilation requirement. Prolonged mechanical ventilation was defined as the presence of an endotracheal tube on the CXR obtained on day 7 post-intubation. Patients who were extubated but had CXRs available after extubation were also included in the analysis. In order to examine the prognostic impact of evolving RALE scores over time on the outcome of early liberation, we used extended Cox models adjusted for age and baseline SOFA scores with the RALE score as a time-dependent covariate. We applied Pearson’s correlation test to examine for baseline associations between the RALE score and indices of hypoxemia, i.e. P:F ratio (ratio of partial pressure of arterial oxygen [P] and fraction of inspired oxygen [F]) and S:F ratio (ratio of pulse oximetric saturation [SpO2] and fraction of inspired oxygen [F]) and to examine for associations between the RALE score and biomarkers. Multiple testing adjustments were implemented with the Benjamini-Hochberg method.
We compared median RALE scores across ARDS severity categories defined by P:F ratios [6] and other categorical clinical outcomes with a Wilcoxon signed-rank test. We further divided the distribution of baseline RALE score observations in patients with ARDS into four quartiles (Q1: 0–15, Q2: 16–20, Q3: 21–25, Q4: 26–48), and then examined associations of RALE quartiles with mechanical ventilation parameters (positive end-expiratory pressure [PEEP], tidal volume [expressed in ml/kg of ideal body weight], driving pressure [defined as plateau pressure – PEEP]) and lung compliance (tidal volume/driving pressure) using a random effects ANOVA. We examined for associations between VFDs and RALE score using a zero-inflated binomial negative regression model. We used Cox proportional hazard models adjusting for age and baseline SOFA score to examine the effects of baseline RALE scores on 30- and 90-day survival. To assess for the impact of radiographic edema improvement on outcomes, we considered different thresholds of RALE score reduction from baseline CXR (day of intubation) to day 3 and 7 CXR (i.e. ≤25%, 50% or 75% reduction) and then compared outcomes for patients with vs. without improvement of edema. All statistical analyses were performed with the R statistical software, version 3.5 [24].
3. Results
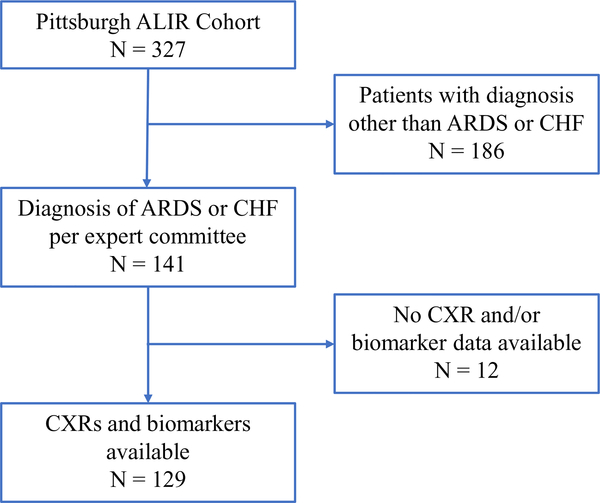
We included 129 mechanically-ventilated patients (108 with ARDS and 21 CHF controls) comprising a total of 488 CXRs (Day 0, N = 124; Day 3, N = 119; Day 5, N = 95; Day 7, N = 80; Day 10, N = 70) (Fig. 2). Patients with ARDS had more severe hypoxemia compared to CHF controls (p = 0.01) but otherwise covariates were similarly distributed (Table 1).
Fig. 2.
Selection process of patients from the ALIR cohort. 129 patients of the 327 enrolled were diagnosed with ARDS or CHF and had both CXRs and data required for analysis. Of these, we were able to retrieve baseline (day of intubation) CXRs for 124 patients (104 ARDS and 20 CHF). ALIR: Acute Lung Injury Repository; ARDS: Acute respiratory distress syndrome; CHF: Congestive heart failure; CXR: Chest x-ray.
Table 1.
Baseline characteristics and clinical outcomes in mechanically-ventilated patients with ARDS or CHF from the ALIR cohort.
| Group classification | ARDS | CHF | p-value |
|---|---|---|---|
| n | 108 | 21 | |
| Male (%) | 57 (52.8) | 15 (71.4) | 0.18 |
| BMI (median [IQR]) | 30.1 [25.2, 35.2] | 31.3 [26.6, 37.6] | 0.28 |
| Age (median [IQR]) | 55.6 [43.2, 64.2] | 59.3 [47.4, 69.4] | 0.12 |
| Sepsis (%) | 93 (86.9) | 0 (0) | <0.001 |
| Shock (%) | 71 (65.7) | 13 (61.9) | 0.93 |
| P:F Ratio1 | 130 [84, 178] | 178 [126, 223] | 0.01 |
| SOFA Score2 | 7 [5, 9] | 7 [5, 10] | 0.89 |
| RALE Score (median [IQR])3 | 20 [15, 26] | 15 [13, 21] | 0.01 |
| 90-Day Mortality (%) | 37 (34.3) | 7 (33.3) | 1.00 |
Comparison of baseline characteristics of patients with ARDS and controls with cardiogenic pulmonary edema. P-values from nonparametric tests are shown in bold when significant (p < 0.05). ALIR: Acute Lung Injury Registry; ARDS: Acute respiratory distress syndrome; CHF: Congestive heart failure; BMI: Body mass index; P:F Ratio: Ratio of partial pressure of arterial oxygen and fraction of inspired oxygen; SOFA Score: Sequential organ failure assessment score; RALE Score: Radiographic assessment of lung edema score; IQR: interquartile range. Superscripts denote numbers of patients available for analysis of specified variable, ARDS/CHF:
96/19
92/18
104/20.
3.1. Inter-reviewer agreement for RALE score
Strong inter-reviewer agreement was demonstrated by an ICC of 0.83 (95% CI: 0.80–0.85) for the 488 CXRs examined across the study period (Fig. S3). A total of 82 (17%) CXRs required reconciliation by a third reviewer due to significant differences in RALE scores between the two original reviewers. Agreement between third reviewer and original reviewers for cases requiring reconciliation was moderate with ICC of 0.62. (95% CI: 0.42–0.76).
3.2. Associations with baseline clinical variables and parameters of mechanical ventilation
At baseline, patients with ARDS had higher median RALE scores (20, interquartile range [IQR:15–26]) compared to CHF controls (14, [13–21], p = 0.01). Patients with ARDS in the lowest RALE score quartile (Q1, i.e. less radiographic edema) had higher incidence of shock (82.8% vs 58.7%, p = 0.04) but lower incidence of direct lung injury (51.7% vs 77.3%, p = 0.02) compared to all other patients (Table 2). Among patients with ARDS, baseline RALE scores were not significantly associated with indices of hypoxemia, i.e. P:F ratio (r = −0.005, p = 0.96), S:F ratio (r = −0.11, p = 0.31) or ARDS severity groups by P:F ratio (Fig. S4). With regards to the relationship of radiographic edema with parameters of mechanical ventilation, we found no significant associations of RALE score quartiles with delivered tidal volumes, driving pressure, lung compliance or PEEP (Fig. S5).
Table 2.
Baseline characteristics of patients stratified by RALE score qualifies.
| RALE Quartile | 1st | 2nd | 3rd | 4th | p |
|---|---|---|---|---|---|
| n | 29 | 25 | 25 | 25 | |
| Direct Injury (%) | 15 (51.7) | 18 (72.0) | 21 (84.0) | 19 (76.0) | 0.06 |
| Incident Shock (%) | 24 (82.8) | 18 (72.0) | 13 (52.0) | 13 (52.0) | 0.04 |
| SOFA Score (median [IQR]) | 9.00 [5, 10] | 8.00 [5, 8] | 7.00 [6, 9] | 7.00 [5, 9] | 0.32 |
Incidence of shock (vasopressor requirement) was significantly different between quartiles (p = 0.04). P-values from nonparametric tests are shown in bold when significant (p < 0.05). SOFA Score: Sequential organ failure assessment score, IQR: interquartile range.
3.3. Associations with biomarkers and ARDS subphenotypes
Forty-two (46%) patients with ARDS were classified into the hyper-inflammatory subphenotype using the parsimonious model as previously described [5]. We found no significant differences in median RALE scores between hyper-inflammatory and hypoinflammatory subphenotype groups (Fig. S6); both the hypoinflammatory and hyper-inflammatory ARDS subphenotypes had significantly higher median RALE scores compared to CHF controls (p = 0.001 and p = 0.005, respectively). For individual biomarkers, we detected significant correlations of baseline RALE scores with procalcitonin (r = −0.26 [95% CI: −0.44, −0.06], p = 0.01), RAGE (r = 0.27 [95% CI: 0.08, 0.46], p = 0.007) and ST-2 (r = −0.22 [95% CI: −0.41, −0.02], p = 0.03). After adjustment for multiple comparisons, these associations did not reach statistical significance (procalcitonin, adjusted p-value = 0.06; RAGE, adjusted p-value = 0.06; ST-2, adjusted p-value = 0.1) (Table S1).
3.4. Association with clinical outcomes
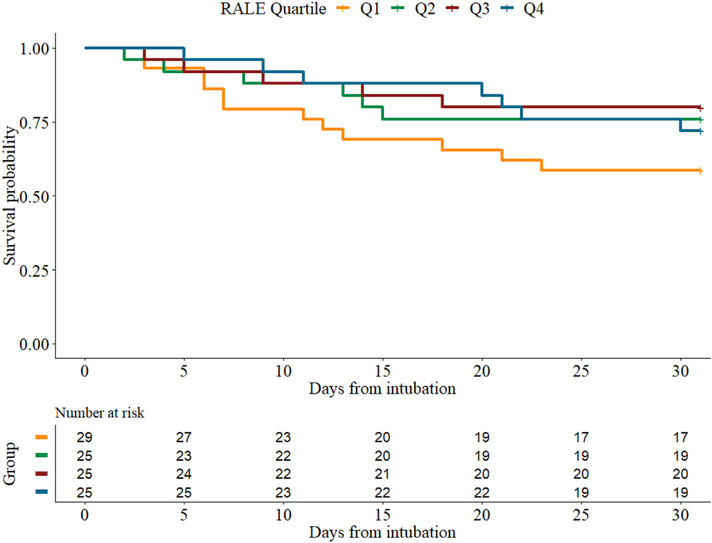
In univariate analyses, we did not find significant associations of baseline RALE scores with 30-day (p = 0.19) or 90-day mortality (p = 0.11), duration of ICU stay (p = 0.26), acute kidney injury (p = 0.66) or VFDs (p = 0.09). In Cox-proportional hazards models, baseline RALE scores were not associated with 30- (Fig. 3) or 90-day survival after adjustment for SOFA score and age (HR: 0.65, 95% CI: 0.24–1.76, p-value = 0.4 and HR: 0.91, 95% CI: 0.4–2.11, p-value = 0.83, respectively).
Fig. 3.
Kaplan-Meier estimates of 30-day survival in patients with ARDS stratified by baseline RALE score quartiles. There was no statistically significant difference in survival between the quartiles after adjusting for age and SOFA score (HR: 0.65, 95% CI: 0.24–1.76, p-value = 0.4). Q: Quartile.
3.5. Longitudinal evolution of the RALE score
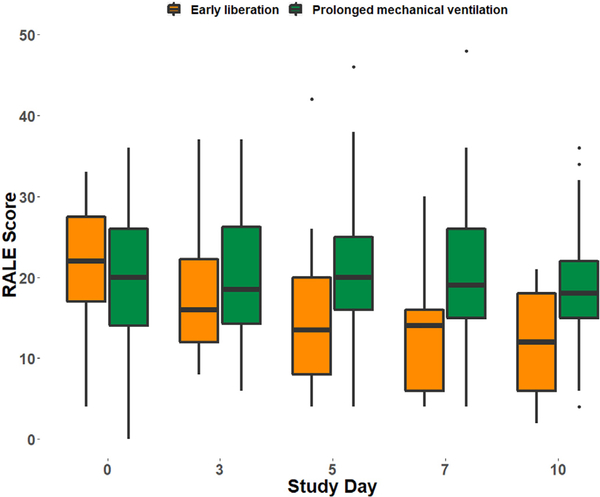
In patients with early liberation from mechanical ventilation, the RALE score significantly decreased over the study period (p < 0.0001) while in those with prolonged mechanical ventilation there was no significant change over time (p = 0.16) (Fig. 4). Similar results were obtained in patients with CHF (p = 0.002 and p = 0.2 for change over time in those with early resolution and prolonged need for mechanical ventilation, respectively) (Fig. S7). Time-dependent RALE scores were associated with a higher risk of prolonged need for mechanical ventilation after adjustment for age and baseline SOFA score (HR: 1.02, 95% CI: 1.01–1.04, p = 0.002). Similar results were also observed in CHF controls (9% increase in the hazards of requiring prolonged mechanical ventilation, HR: 1.09, 95% CI: 1.04–1.14, p = 0.0002). Early mild improvement of radiographic edema, defined as a decrease of 25% or more of the baseline RALE score at day 3, was not associated with improved 30-day survival (HR: 1.53, 95% CI: 0.71–3.31, p = 0.28). (Fig. S8A). However, more substantial resolution of edema by day 7 (defined as >50% reduction of baseline RALE score) was associated with significantly improved 90-day survival (unadjusted log-rank test, p = 0.03) but not 30-day survival (unadjusted log-rank test, p = 0.07) (Fig. S8B).
Fig. 4.
Longitudinal evolution of the RALE score in patients with ARDS over the study period. When separated into groups based on need for prolonged mechanical ventilation (still intubated day 7 post-intubation), gradual decline in the RALE score was only observed in patients with early liberation from mechanical ventilation (p < 0.0001).
4. Discussion
In this observational study, we have characterized the longitudinal evolution of radiographic pulmonary edema in a cohort of patients with ARDS or CHF using the RALE score [7]. We demonstrated that RALE scoring is reproducible among independent reviewers and that RALE scores declined over time in patients that were liberated from mechanical ventilation within the first week in contrast to patients who required prolonged mechanical ventilation. We did not find any significant associations of baseline RALE scores with important clinical outcomes or inflammatory subphenotypes, indicating that baseline radiographic assessment of pulmonary edema may not be able to capture important patient heterogeneity and extrapulmonary organ damage in ARDS.
The RALE score is reproducible across different cohorts and independent reviewers. This is evidenced by the high ICC observed in both the original study describing the RALE score [7] as well as this investigation. A recent study assessing a simplified version of the RALE was able to show similar agreement [25]. The reproducibility of the RALE scoring system, combined with its reliance only on physician interpretation of routine, portable CXRs make it a user-friendly tool for quantifying radiographic edema.
The progressive decline in RALE score observed in patients liberated from mechanical ventilation before day 7 and its persistent elevation in patients with the need for prolonged mechanical ventilation provide proof-of-concept evidence that reduction of radiographic edema tracks with clinical/physiologic improvements leading to extubation. While these data are unable to disentangle the mechanisms underlying the decline of serial RALE scores (e.g. resolution of the underlying pathologic process that lead to ARDS vs. differences in ventilatory or fluid management strategies [26,27]), the differential trajectory of RALE scoring in patients with clinical improvement highlights its potential use as a marker of treatment response. Serial calculation of CXR RALE scores in patients enrolled in clinical trials may thus allows for objective quantification of the effects of investigational therapies on radiographic edema.
The relationship between radiographic edema and the measured physiologic derangements in ARDS is not clear. With regards to hypoxemia severity, we found no significant differences in baseline RALE scores across ARDS severity groups or association with the P:F ratio. These negative findings may reflect the low sensitivity of CXR abnormalities for predicting gas exchange deficits compared to computed tomography or lung ultrasound [28] or could also be attributed to the small size of our cohort and variation in time of sampling in relation to chest imaging. With regards to lung mechanics, the lack of association of RALE score with PEEP, tidal volume, driving pressure or lung compliance is likely the result of the variable effects of these parameters on chest radiographic findings. While higher tidal volume can potentially promote inflation of under-aerated lung areas, it can also promote volume- and pressure- mediated lung injury resulting in further edema formation [29]. Attenuation of radiographic edema due to lung recruitment has been observed with incremental increases in PEEP within subjects at predefined time intervals [30,31], but the relationship between PEEP and edema cannot be predicted in cross-sectional assessments. Taken together, these findings suggest that the ability of plain radiography to capture the physiologic gas exchange and lung mechanical derangements in ARDS is limited.
Whether previously described ARDS subphenotypes can be distinguished on the basis of radiographic edema has not been previously studied. As subphenotype designation relies on measurement of biomarkers that were not associated with radiographic edema in our study (sTNFR1, IL-8 and serum bicarbonate), it is not surprising that baseline RALE scores were not different between the two subphenotypes. Nonetheless, we discovered a significant positive correlation with RAGE, a marker of alveolar epithelial injury that has been associated with diffuse patterns of radiographic edema (“nonfocal ARDS”) [32] and worse outcomes in ARDS [17]. On the other hand, RALE was negatively correlated with procalcitonin, a biomarker of bacterial infections [19]. Although procalcitonin had not been previously considered in biomarker-based subphenotyping approaches in ARDS, we recently demonstrated that procalcitonin is strongly associated with the hyperinflammatory ARDS subphenotype and worse outcomes in ARDS [5]. Thus, we identified significant, but opposing associations of RALE scores with biomarkers of both direct (RAGE) and indirect lung injury (extra-pulmonary sepsis by procalcitonin), which may explain the overall null associations of RALE with established inflammatory subphenotypes.
We found no association between baseline radiographic edema and outcomes in patients with ARDS. This can be explained by similar outcomes reported in patients with extrapulmonary and pulmonary ARDS [33]. Whereas direct lung injury (from aspiration or pneumonia) may result in numerically higher RALE scores, this does not necessarily translate into different outcomes compared to those with other etiologies for ARDS. This was true in our study where patients in the 2nd to 4th RALE quartiles had higher incidence of direct lung injury but lower incidence of shock compared to those in the 1st (lowest) quartile, suggesting that physiologic derangements not otherwise captured by plain chest radiography may be contributing to outcomes. Such differences in indices of severity of illness by RALE scores may also result from the inherent subjectivity in reaching a consensus diagnosis of ARDS according to the Berlin definition [6]. Whereas clinicians often reach agreement on ARDS diagnosis for patients with typical bilateral infiltrates consistent with pulmonary edema, less classic radiographic presentations are challenging for classification. As the low reliability of CXR interpretation is a major driver of low reliability of ARDS diagnosis [34], clinicians implicitly take into consideration additional clinical factors that are not part of the ARDS diagnostic criteria. As shown in a recent retrospective analysis of patients in whom physicians agree or disagree about the diagnosis of ARDS, physicians were more likely to reach ARDS diagnosis in the presence of pneumonia (direct lung injury risk factor), shock and more severe hypoxemia (P/F ratio <120) [35]. Such implicit factors may have influenced our consensus committee diagnostic evaluation, i.e. reaching ARDS diagnosis in patients with worse clinical parameters (shock and SOFA scores) but with less extensive radiographic infiltrates. Such challenges inherent to using CXRs in the diagnosis of ARDS may have led to the observation that patients in the lowest RALE quartile had higher severity of illness compared to other patients with more extensive radiographic edema. Thus, quantification of radiographic edema alone at baseline is not sufficient to account for the clinical heterogeneity underlying ARDS.
The discrepancy in outcome associations observed between our study and the original study describing the RALE score [7] could potentially be explained by differences in the cohorts examined. Our observational study had very inclusive eligibility criteria resulting in a heterogenous population, unlike the FACTT cohort which has likely excluded moribund patients and those with several comorbidities that could confound the radiographic appearance of pulmonary edema (the presence of severe chronic lung disease, requirement of renal replacement therapy, morbid obesity and history of lung transplant). A recent study attempting to validate a simplified version of the RALE score in a more inclusive observational cohort of patients in a medical ICU was unable to replicate the original study’s results in the subgroup of patients with ARDS [25], although the lack of statistical significance in outcomes associations was attributed to sample size. The external validity of RALE score thus needs to be examined in additional observational ARDS cohorts.
Our study has several limitations. Most importantly, our cohort was obtained from a single ICU and represents a small sample size. As we did not protocolize CXR or arterial blood gas acquisition for the purposes of this study but relied on clinical tests obtained by the treating physicians, variability in time intervals between available RALE scores and indices of hypoxemia, biomarkers or other clinical variables may have limited our ability to detect significant associations. We nonetheless made every effort to analyze observations obtained temporally close to each other. Fluid management was also not protocolized as done in the FACTT trial, thus the longitudinal evolution of RALE scores described in our study reflects the common final effects of underlying pathology evolvement and impact of supportive care, and we cannot draw any inferences on the effects of specific interventions. Additionally, while PEEP can affect the radiographic appearance of pulmonary edema, we did not protocolize PEEP titration in our observational cohort and adjustments were made by clinicians according to patients’ clinical status in accordance with principles of lung protective ventilation.
5. Conclusion
In conclusion, the RALE score is a highly reproducible tool that can be easily implemented at the bedside for quantification of radiographic edema. In our cohort, baseline, cross-sectional RALE measurements were not found to be predictive of biological variability or clinical outcomes in ARDS. We demonstrated though that the longitudinal evolution of RALE scores tracks with the important clinical outcome of early liberation from mechanical ventilation, making it an appealing tool for prospective assessment of radiographic pulmonary edema. Consequently, the RALE score can be considered in clinical trials where the quantification of baseline radiographic edema and its evolution over time are of interest.
Supplementary Material
Acknowledgments
Funding
National Institutes of Health grants: [K23 HL139987 (GDK); U01 HL098962 (AM); P01 HL114453 (BJM, JSL, YZ); R01 HL097376 (BJM); K24 HL123342 (AM); U01 HL137159 (DVM, PVB); R01 LM012087 (DVM, PVB); R01 HL142084 (JSL); R01 HL136143 (JSL); F32 HL137258 (JWE); F32 HL142172 (WB); K08 HS025455 (IJB); K23 GM122069 (FS)].
Abbreviations
- ALIR
Acute Lung Injury and Biospecimen Repository
- ARDS
Acute Respiratory Distress Syndrome
- CHF
Congestive heart failure
- CXR
Chest X-ray
- ICC
Interclass correlation coefficient
- ICU
Intensive care unit
- IL
Interleukin
- P:F ratio
Ratio of partial pressure of arterial oxygen [P] and fraction of inspired oxygen [F]
- PEEP
Positive end expiratory pressure
- Q
Quartile
- RAGE
Receptor of advanced glycation end-products
- RALE
Radiographic Assessment of Lung Edema
- S:F ratio
Ratio of pulse oximetric saturation [SpO2, S] to fraction of inspired oxygen [F]
- SOFA
Sequential Organ Failure Assessment
- ST-2
Suppression of tumorigenicity-2
- TNFR1
Tumor necrosis factor receptor-1
- UPMC
University of Pittsburgh Medical Center
- VFD
Ventilator-free days
Footnotes
Declaration of Competing Interest
Dr. Georgios Kitsios receives research funding from Karius, Inc. The other authors have no competing interests to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2020.01.012.
References
- [1].Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med 2017;5:524–34. 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- [2].Bos LD, Martin-Loeches I, Schultz MJ. ARDS: challenges in patient care and frontiers in research. Eur Respir Rev 2018;27:170107 10.1183/16000617.0107-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 2015;147:1539–48. 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sinha P, Delucchi KL, Thompson BT, McAuley DF, Matthay MA, Calfee CS, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med 2018;44:1859–69. 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kitsios GD, Yang L, Manatakis DV, Nouraie M, Evankovich J, Bain W, et al. Hostresponse subphenotypes offer prognostic enrichment in patients with or at risk for acute respiratory distress syndrome. Crit Care Med 2019;47(12):1724–34. 10.1097/CCM.0000000000004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].ARDS Definition Task Force V, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- [7].Warren MA, Zhao Z, Koyama T, Bastarache JA, Shaver CM, Semler MW, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax 2018;73:840–6. 10.1136/thoraxjnl-2017-211280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ware LB, Neyrinck A, O’Neal HR, Lee JW, Landeck M, Johnson E, et al. Comparison of chest radiograph scoring to lung weight as a quantitative index of pulmonary edema in organ donors. Clin Transplant 2012;26:665–71. 10.1111/j.1399-0012.2011.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kitsios GD, Fitch A, Manatakis DV, Rapport SF, Li K, Qin S, et al. Respiratory microbiome profiling for etiologic diagnosis of pneumonia in mechanically ventilated patients. Front Microbiol 2018;9:1413 10.3389/fmicb.2018.01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].KDIGO. Clinical Practice Guideline for Acute Kidney Injury. Kidney International; Supplements 2012;2:19–36. 10.1038/kisup.2011.32. [DOI] [Google Scholar]
- [11].Huang DT, Angus DC, Moss M, Thompson BT, Ferguson ND, Ginde A, et al. Design and rationale of the reevaluation of systemic early neuromuscular blockade trial for acute respiratory distress syndrome. Ann Am Thorac Soc 2017;14:124–33. 10.1513/AnnalsATS.201608-629OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu C-H, Kuo S-W, Ko W-J, Tsai P-R, Wu S-W, Lai C-H, et al. Early measurement of IL-10 predicts the outcomes of patients with acute respiratory distress syndrome receiving extracorporeal membrane oxygenation. Sci Rep 2017;7:1021 10.1038/s41598-017-01225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2:611–20. 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bajwa EK, Volk JA, Christiani DC, Harris RS, Matthay MA, Thompson BT, et al. Prognostic and diagnostic value of plasma soluble suppression of tumorigenicity-2 concentrations in acute respiratory distress syndrome. Crit Care Med 2013;41: 2521–31. 10.1097/CCM.0b013e3182978f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327–36. 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- [16].Hoogendijk AJ, Wiewel MA, van Vught LA, Scicluna BP, Belkasim-Bohoudi H, Horn J, et al. Plasma fractalkine is a sustained marker of disease severity and outcome in sepsis patients. Crit Care 2015;19:412 10.1186/s13054-015-1125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jabaudon M, Blondonnet R, Pereira B, Cartin-Ceba R, Lichtenstern C, Mauri T, et al. Plasma sRAGE is independently associated with increased mortality in ARDS: a meta-analysis of individual patient data. Intensive Care Med 2018;44:1388–99. 10.1007/s00134-018-5327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA, NHLBI ARDS Network. Plasma angiopoietin-2 in clinical acute lung injury. Crit Care Med 2012;40:1731–7. 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu D, Su L, Guan W, Xiao K, Xie L. Prognostic value of procalcitonin in pneumonia: a systematic review and meta-analysis. Respirology 2016;21:280–8. 10.1111/resp.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mauri T, Coppadoro A, Bellani G, Bombino M, Patroniti N, Peri G, et al. Pentraxin 3 in acute respiratory distress syndrome: an early marker of severity*. Crit Care Med 2008;36:2302–8. 10.1097/CCM.0b013e3181809aaf. [DOI] [PubMed] [Google Scholar]
- [21].Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy; 2017. 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods 1996;1:30–46. 10.1037/1082-989X.1.1.30. [DOI] [Google Scholar]
- [23].Bartko JJ. The Intraclass correlation coefficient as a measure of reliability. Psychol Rep 1966;19:3–11. 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- [24].R Core Team. R: A Language and Environment for Statistical Computing; 2018.
- [25].Mason SE, Dieffenbach PB, Englert JA, Rogers AA, Massaro AF, Fredenburgh LE, et al. Semi-quantitative visual assessment of chest radiography is associated with clinical outcomes in critically ill patients. Respir Res 2019;20:218 10.1186/s12931-019-1201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–8. 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- [27].The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564–75. 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- [28].Xirouchaki N, Magkanas E, Vaporidi K, Kondili E, Plataki M, Patrianakos A, et al. Lung ultrasound in critically ill patients: comparison with bedside chest radiography. Intensive Care Med 2011;37:1488–93. 10.1007/s00134-011-2317-y. [DOI] [PubMed] [Google Scholar]
- [29].Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369: 2126–36. 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- [30].Wallet F, Delannoy B, Haquin A, Debord S, Leray V, Bourdin G, et al. Evaluation of recruited lung volume at inspiratory plateau pressure with PEEP using bedside digital chest X-ray in patients with acute lung injury/ARDS. Respir Care 2013;58:416–23. 10.4187/respcare.01893. [DOI] [PubMed] [Google Scholar]
- [31].Zimmerman JE, Goodman LR, Shahvari MB. Effect of mechanical ventilation and positive end-expiratory pressure (PEEP) on chest radiograph. AJR Am J Roentgenol 1979;133:811–5. 10.2214/ajr.133.5.811. [DOI] [PubMed] [Google Scholar]
- [32].Mrozek S, Jabaudon M, Jaber S, Paugam-Burtz C, Lefrant J-Y, Rouby J-J, et al. Elevated plasma levels of sRAGE are associated with nonfocal CT-based lung imaging in patients with ARDS: a prospective multicenter study. Chest 2016;150:998–1007. 10.1016/j.chest.2016.03.016. [DOI] [PubMed] [Google Scholar]
- [33].Agarwal R, Srinivas R, Nath A, Jindal SK. Is the mortality higher in the pulmonary vs the extrapulmonary ARDS? Chest 2008;133:1463–73. 10.1378/chest.07-2182. [DOI] [PubMed] [Google Scholar]
- [34].Sjoding MW, Hofer TP, Co I, Courey A, Cooke CR, Iwashyna TJ. Interobserver reliability of the Berlin ARDS definition and strategies to improve the reliability of ARDS diagnosis. Chest 2018;153:361–7. 10.1016/j.chest.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sjoding MW, Hofer TP, Co I, McSparron JI, Iwashyna TJ. Differences between patients in whom physicians agree and disagree about the diagnosis of acute respiratory distress syndrome. Ann Am Thorac Soc 2019;16:258–64. 10.1513/AnnalsATS.201806-434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.