This cohort study examines the association of a care bundle for early sepsis management with mortality and organ dysfunction in adult hospitalized patients with community-onset or hospital-onset sepsis.
Key Points
Question
What is the association of a care bundle for early sepsis management, the Early Management Bundle for Severe Sepsis/Septic Shock (SEP-1), and its components with mortality and organ dysfunction in hospitalized patients with community-onset or hospital-onset sepsis?
Findings
In this cohort study of 6404 patients, SEP-1–adherent care was not associated with reduced mortality or decreased vasopressor support among patients with sepsis in the emergency department (community-onset sepsis), patients first meeting sepsis criteria after arrival in an inpatient unit (hospital-onset sepsis), or the total sample. Multiple components of SEP-1 were associated with improved outcomes among patients with sepsis in the emergency department, a statistically significant finding.
Meaning
The SEP-1–adherent care was not associated with improved outcomes, but individual SEP-1 components were associated with benefit in patients with community-onset sepsis.
Abstract
Importance
The Early Management Bundle for Severe Sepsis/Septic Shock (SEP-1) is a quality metric based on a care bundle for early sepsis management. Published evidence on the association of SEP-1 with mortality is mixed and largely excludes cases of hospital-onset sepsis.
Objective
To assess the association of the SEP-1 bundle with mortality and organ dysfunction in cohorts with hospital-onset or community-onset sepsis.
Design, Setting, and Participants
This retrospective cohort study used data from 4 University of California hospitals from October 1, 2014, to October 1, 2017. Adult inpatients with a diagnosis consistent with sepsis or disseminated infection and laboratory or vital signs meeting the Sepsis-3 (Third International Consensus Definitions for Sepsis and Septic Shock) criteria were divided into community-onset sepsis and hospital-onset sepsis cohorts based on whether time 0 of sepsis occurred after arrival in the emergency department or an inpatient area. Data were analyzed from April to October 2019. Additional analyses were performed from December 2019 to January 2020.
Exposures
Administration of SEP-1 and 4 individual bundle components (serum lactate level testing, blood culture, broad-spectrum intravenous antibiotic treatment, and intravenous fluid treatment).
Main Outcomes and Measures
The primary outcome was in-hospital mortality. The secondary outcome was days requiring vasopressor support, measured as vasopressor days.
Results
Among the 6404 patient encounters identified (3535 men [55.2%]; mean [SD] age, 64.0 [18.2] years), 2296 patients (35.9%) had hospital-onset sepsis. Among 4108 patients (64.1%) with community-onset sepsis, serum lactate level testing within 3 hours of time 0 was associated with reduced mortality (absolute difference, –7.61%; 95% CI, –14.70% to –0.54%). Blood culture (absolute difference, –1.10 days; 95% CI, –1.85 to –0.34 days) and broad-spectrum intravenous antibiotic treatment (absolute difference, –0.62 days; 95% CI, –1.02 to –0.22 days) were associated with fewer vasopressor days. Among patients with hospital-onset sepsis, broad-spectrum intravenous antibiotic treatment was the only bundle component significantly associated with any improved outcome (mortality difference, –5.20%; 95% CI, –9.84% to –0.56%). Care that was adherent to the complete SEP-1 bundle was associated with increased vasopressor days in patients with community-onset sepsis (absolute difference, 0.31 days; 95% CI, 0.11-0.51 days) but was not significantly associated with reduced mortality in either cohort (absolute difference, –0.07%; 95% CI, –3.02% to 2.88% in community-onset; absolute difference, –0.42%; 95% CI, –6.77% to 5.93% in hospital-onset).
Conclusions and Relevance
SEP-1–adherent care was not associated with improved outcomes of sepsis. Although multiple components of SEP-1 were associated with reduced mortality or decreased days of vasopressor therapy for patients who presented with sepsis in the emergency department, only broad-spectrum intravenous antibiotic treatment was associated with reduced mortality when time 0 occurred in an inpatient unit. Current sepsis quality metrics may need refinement.
Introduction
Most patients with sepsis exhibit the signs and symptoms of a dysregulated host response to infection at the time of admission to the hospital (ie, community-onset sepsis).1,2,3 Only 10% to 20% of cases are hospital onset, meaning that the signs and symptoms develop after hospital admission. The level of risk varies markedly among individuals with sepsis, with mortality ranging from 5% to 50%.4,5,6 On average, in-hospital mortality is more common among patients with hospital-onset sepsis than those with community-onset sepsis.2,7,8,9
Protocols that combine multiple interventions for early sepsis care, or sepsis bundles, have mainly been studied in the context of community-onset sepsis (including the major trials related to early goal-directed therapy).10,11,12,13,14,15 For management of hospital-onset sepsis, the effectiveness of sepsis bundles remains unproven, to our knowledge. Nonetheless, the Early Management Bundle for Severe Sepsis/Septic Shock (SEP-1) is recommended for all patients with sepsis, including those with community-onset and hospital-onset sepsis.16,17 The purpose of this study was to assess the association of SEP-1 with mortality and organ dysfunction in patients with community-onset or hospital-onset sepsis and, thus, to evaluate the use of SEP-1 as a quality metric.
Methods
Study Design and Data Source
For this retrospective cohort study, clinical data were obtained from the electronic medical records of 4 academic teaching hospitals (Ronald Reagan Medical Center, Los Angeles, California; Santa Monica Hospital, Santa Monica, California; Jacobs Medical Center, La Jolla, California; and University of San Diego Medical Center, Hillcrest, San Diego, California) offering diverse clinical services. All data were collected as part of routine clinical care. The UCLA (University of California, Los Angeles) institutional review board approved the protocol and waived requirement of informed consent. Data from University of California, San Diego were deidentified. Data from UCLA were linked by the study identifier to medical record numbers that could be used to access charts for data validation.
Inclusion and Exclusion Criteria
All hospital admissions for patients 18 years or older who had a diagnosis of sepsis or disseminated infection at 2 hospitals between October 1, 2014, and October 1, 2017, and at another 2 hospitals between October 1, 2014, and October 1, 2016, were screened for inclusion. The following exclusion criteria were used as specified by SEP-1: hospitalization for more than 120 days or fewer than 6 hours, admission by transfer from another acute care facility, or receipt of broad-spectrum intravenous antibiotic treatment for 24 hours or longer at time 0 of sepsis. Only patients with vital signs and laboratory values consistent with the Sepsis-3 (Third International Consensus Definitions for Sepsis and Septic Shock)18 definition of sepsis (formerly severe sepsis or septic shock) were included in the analysis.
For encounters before October 1, 2015, sepsis encounters were identified using diagnosis codes from Martin et al.19,20,21,22 For hospitalizations after October 1, 2015, diagnosis codes provided by the Center for Medicare & Medicaid Services (CMS) were paired with diagnosis codes for organ dysfunction in a process analogous to the Martin method. If an explicit diagnosis of severe sepsis or septic shock was present, a concurrent diagnosis of organ dysfunction was not required.
Definitions
We defined sepsis as suspected infection with organ dysfunction, including syndromes previously called severe sepsis and septic shock, based on Sepsis-3.18 Other definitions were based on the core measure SEP-1.16
Time 0 of sepsis required 2 signs of a systemic inflammatory response and 1 sign of organ dysfunction in the context of suspected or confirmed infection. We determined time 0 using an automated algorithm that identified the first instance in a given encounter when a patient’s laboratory values and vital signs met these criteria instead of using a medical record review.
To proxy for clinician suspicion of infection, time 0 was required to occur within 48 hours of an order for an antibiotic, antifungal, or clinical culture. A 48-hour window was chosen to capture situations in which morning laboratories showed a new abnormality, such as leukocytosis, that did not provoke a response until found to be persistent the following day. Two-day window periods have been used for similar purposes in other electronic measures for identifying episodes of sepsis, such as the adult sepsis event.23,24
To capture differences between hospitalized and ambulatory patients, hospital-onset sepsis was defined by occurrence of time 0 after patient arrival in an inpatient unit, and community-onset sepsis was defined by time 0 after arrival in the emergency department. If a patient showed signs of infection or instability in the emergency department but did not meet the criteria for sepsis until arriving in an inpatient unit, they were categorized as having hospital-onset sepsis.
Variables
The primary outcome was in-hospital mortality, expressed as an absolute probability difference comparing those who did and did not receive SEP-1–adherent care. Negative values represent a mortality reduction (ie, increased likelihood of survival) associated with treatment.
The secondary outcome was requirement of blood pressure support with a vasopressor medication in the 10 days after time 0, expressed as vasopressor days. Vasopressor days are a marker of organ dysfunction that may reflect improved physiological characteristics of sepsis in the absence of a mortality difference.25 When counting vasopressor days, death was given equal weight to vasopressor administration; thus, patients who died within the 10-day window were counted as receiving vasopressor medication from time of death until the end of day 10. Individuals who died more than 10 days after sepsis onset were not assigned any additional vasopressor days. This method of accounting for deaths is typical when counting organ support–free days.25,26 Vasopressor days were reported as an absolute difference in the number of vasopressor days, and this difference was used instead of vasopressor-free days26 so that the direction of the association (plus or minus sign) matched the primary outcome (ie, lower mortality and fewer vasopressor days represented benefit from treatment). A 10-day window was chosen to limit bias from lack of follow-up after discharge. Because calendar dates were not available, vasopressor days were calculated in hours from the medication administration record.
Treatments evaluated included the SEP-1 bundle and the 4 components required within 3 hours of time 0: (1) blood culture results, (2) broad-spectrum intravenous antibiotic treatment, (3) serum lactate level testing, and (4) intravenous fluid treatment if the blood pressure was low or if the lactate level was elevated. The SEP-1 bundle required those 4 components plus (5) follow-up serum lactate level testing and (6) initiation of vasopressor treatment, if indicated. Patients who received some but not all components of SEP-1 were counted as not having received SEP-1–adherent care per the CMS manual.27 A seventh component of SEP-1—reassessment of tissue perfusion within 6 hours of time 0—was excluded from this evaluation because of inherent flexibility and lack of a relevant field in the electronic medical record.
Covariates included year of admission, patient age, sex, baseline comorbidity, source of infection, immunosuppression, postoperative status, features of sepsis such as fever and shock, and the admitting hospital. Baseline comorbidity was represented by count of conditions present at admission from the Elixhauser Comorbidity Index.28,29 Elixhauser comorbidities included HIV infection, congestive heart failure, and malignant tumor, among others. Source of infection was determined by International Classification of Diseases diagnosis codes for pneumonia, urinary tract infection, and skin and/or soft-tissue infection that were recorded as present at admission (International Classification of Diseases, Ninth Revision was used for the period before October 1, 2015. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision was used for the period after October 1, 2015). Immunosuppression was determined by the receipt of chemotherapy, corticosteroids, or other immunosuppressive medication. The postoperative period included days 1 through 90 after a major diagnostic or therapeutic procedure, as defined by the Healthcare Cost and Utilization Project’s procedure classes.30 Septic shock was defined by either hypotension after intravenous fluid treatment or a lactate level of at least 4 mmol/L (to convert plasma lactate level to mg/dL, divide by 0.111). New bacteremia was defined by positive blood culture results within 7 days of sepsis onset in the absence of a previous positive culture result (excluding possible skin contaminants).
Statistical Analysis
Data analyses were performed from April through October 2019. Additional analyses were performed from December 2019 through January 2020. The mean association of treatment with outcomes among the participants was estimated by comparing outcomes between patients who did and did not receive SEP-1–adherent care. To address nonrandom treatment assignment, we used doubly robust models that incorporated propensity scores, Mahalanobis distance matching,31 and regression adjustment.
Propensity scores were estimated by multivariate probit regression with treatment as the dependent variable. Numerous clinical variables were factored into the propensity model in addition to the covariates described above, including whether the patient was admitted from home, the specialty of the admitting service, presence of each Elixhauser comorbidity, laboratory values, vital signs, and whether samples from other body sites were obtained for culture.
Treated and untreated patients were matched based on Mahalanobis distance between numerical propensity scores and select covariates (kmatch Stata [StataCorp LLC]). Patients who could not be paired within the bandwidth of the matching function (typically because of very high or very low probability of receiving treatment) were excluded.32 Pairs were assigned weights based on closeness of the match that were then used to rebalance the treated and untreated participants within each cohort on observable characteristics.33 Covariate distributions within rebalanced samples were evaluated by comparing standardized differences of the means.34,35,36 Presence of hypotension or shock was included in the matching function for all treatments except serum lactate level testing because serum lactate levels can be used to define shock in the context of a normal blood pressure.
The associations of SEP-1 and its components with outcomes in the rebalanced samples were estimated by regression adjustment including the propensity score as a covariate (teffects ra in Stata). The 95% CIs for outcomes were bootstrapped. Analyses were performed using Stata/IC, version 14.1. Statistical significance was 2-sided and set at P < .05.
On the basis of a predicted ratio of 2:1 between cases of SEP-1 nonadherence and cases of adherence, we estimated that an overall sample size of 6000 patients would provide more than 80% power to detect an absolute decrease in mortality of 3% (the difference between 20% and 17%, equivalent to a relative decrease of 15%). Sensitivity analyses included cohorts who were 65 years or older, pneumonia or no pneumonia, immunosuppression or no immunosuppression, bacteremia after sepsis onset or no bacteremia after sepsis onset, fever or no fever, septic shock or no septic shock, malignant tumor or no malignant tumor, and admission source from home or other than from home.
Results
Among the 6404 sepsis-related encounters in patients meeting the inclusion and exclusion criteria, inpatient mortality was 19.0% (1216 deaths). In the total sample, the mean (SD) age was 64.0 (18.2) years, 3535 (55.2%) were men, and 2436 (38.0%) met the criteria for septic shock. Patients presenting to the emergency department spent a median of 7.0 hours there (interquartile range, 4.9-12.6 hours); 1928 patients (30.1%) received SEP-1–adherent care, and 1856 patients (29.0%) received vasopressor medication within 10 days of sepsis onset. The mean (SD) number of vasopressor days was 2.0 (3.2) days.
A total of 2296 patients (35.9%) first met the clinical criteria for sepsis while admitted to an inpatient unit (hospital-onset sepsis). Patients with hospital-onset and those with community-onset sepsis (n = 4108) had similar rates of cultures positive for vancomycin-resistant enterococci (hospital-onset sepsis: 2.6%; community-onset sepsis: 1.9%) and for multidrug-resistant gram-negative bacteria (hospital-onset sepsis: 4.9%; community-onset sepsis: 4.6%), but cultures positive for methicillin-resistant Staphylococcus aureus were more common among patients with community-onset sepsis (11.7% vs 8.3%; P < .001). In-hospital mortality was 20.3% among patients with hospital-onset sepsis and 18.3% among patients with community-onset sepsis. Mean (SD) vasopressor days were 2.4 (3.3) days among patients with hospital-onset sepsis and 2.0 (3.2) days among patients with community-onset sepsis.
Cohort With Community-Onset Sepsis
A total of 1654 patients (39.9%) with sepsis on arrival in the emergency department received SEP-1–adherent care. Within 3 hours of time zero, 3702 patients (90.1%) in this cohort had samples obtained for blood culture before receiving broad-spectrum intravenous antibiotic treatment, 3516 (85.6%) had measurement of serum lactate level, and 3210 (78.1%) had initiated broad-spectrum intravenous antibiotic treatment. Intravenous fluid treatment was administered to 807 of 1173 eligible patients (40.8%). Among patients for whom care was considered to be SEP-1 nonadherent, 1609 (65.4%) received 5 of the 6 evaluated SEP-1 components (eTable 1 in the Supplement gives pairwise correlations between SEP-1 components).
Patients with community-onset sepsis who received SEP-1–adherent care differed significantly on patient-level clinical variables (age, hours from arrival to time 0, number of Elixhauser comorbidities, proportion of patients who were postoperative status, and proportion of patients who had fever, septic shock, and pneumonia present on admission) from patients with community-onset sepsis who did not receive SEP-1–adherent care (Table 1). After being rebalanced, recipients and nonrecipients of SEP-1–adherent care from the cohort with community-onset sepsis had similar observable characteristics except that recipients were less likely to have an elevated serum lactate level (466 of 1524 [30.6%] untreated vs 701 of 2051 [34.2%] treated).
Table 1. Characteristics of the Cohort With Community-Onset Sepsis Before and After Being Rebalanced for Treatment and Observable Characteristicsa.
| Characteristic | Before rebalance | After rebalanceb | ||||
|---|---|---|---|---|---|---|
| SEP-1 | Standardized difference | SEP-1 | Standardized difference | |||
| Received (n = 1647) | Did not receive (n = 2461) | Received (n = 1524) | Did not receive (n = 2051) | |||
| Time 0 of sepsis, mean (SD), hc | 1.8 (1.9) | 2.8 (4.6) | −0.27d | 1.7 (1.7) | 1.7 (1.6) | 0.01 |
| Age, mean (SD). y | 66.2 (18.0) | 64.6 (18.2) | 0.09d | 67.2 (17.6) | 67.5 (16.8) | −0.02 |
| Male sex, No. (%) | 923 (56.0) | 1333 (54.2) | −0.04 | 852 (55.9) | 1106 (53.9) | −0.04 |
| Elixhauser comorbidities, mean (SD), No. | 4.9 (3.0) | 5.2 (3.0) | −0.11d | 4.7 (2.7) | 4.8 (2.5) | −0.01 |
| Postoperative status, No. (%) | 28 (1.7) | 70 (2.8) | −0.08d | 7 (0.5) | 9 (0.5) | 0 |
| Present at admission, No. (%) | ||||||
| Pneumoniae | 582 (35.3) | 736 (29.9) | 0.12d | 517 (33.9) | 696 (33.9) | 0 |
| Urinary tract infection | 434 (26.4) | 617 (25.1) | 0.03 | 411 (27.0) | 558 (27.2) | −0.01 |
| Skin or soft-tissue infection | 131 (8.0) | 163 (6.6) | 0.05 | 118 (7.7) | 131 (6.4) | 0.05 |
| New bacteremia, No. (%)f | 256 (15.5) | 413 (16.8) | −0.03 | 240 (15.7) | 344 (16.8) | −0.03 |
| Immunosuppression, No. (%) | 305 (18.5) | 486 (19.7) | −0.03 | 244 (16.0) | 328 (16.0) | 0 |
| Fever, No. (%) | 615 (37.3) | 661 (26.9) | 0.23d | 552 (36.2) | 743 (36.2) | 0 |
| Septic shock, No. (%) | 436 (26.5) | 1147 (46.6) | −0.43d | 402 (26.4) | 541 (26.4) | 0 |
| Hypotension, No. (%) | 578 (35.1) | 1295 (52.6) | −0.36d | 530 (34.8) | 713 (34.8) | 0 |
| Elevated serum lactate level, No. (%)g | 491 (29.8) | 633 (25.7) | 0.09d | 467 (30.6) | 702 (34.2) | −0.08d |
| Hospital, No. (%) | ||||||
| 1 | 163 (9.9) | 264 (10.7) | −0.03d | 137 (9.0) | 184 (9.0) | 0 |
| 2 | 193 (11.7) | 363 (14.8) | −0.09d | 162 (10.6) | 218 (10.6) | 0 |
| 3 | 547 (33.2) | 1003 (40.8) | −0.16d | 511 (33.5) | 688 (33.5) | 0 |
| 4 | 744 (45.2) | 831 (33.8) | 0.23d | 714 (46.9) | 961 (46.9) | 0 |
| Outcomes | ||||||
| In-hospital mortality, No. (%) | 267 (16.2) | 484 (19.7) | −0.09d | 240 (15.7) | 343 (16.7) | −0.02 |
| Vasopressor days, mean (SD), d | 1.64 (3.0) | 2.3 (3.3) | −0.21d | 1.6 (2.9) | 1.5 (2.8) | 0.03 |
| Length of stay, mean (SD), dh | 11.7 (13.2) | 13.5 (15.3) | −0.12d | 11.6 (13.2) | 12.1 (13.8) | −0.04 |
Abbreviation: SEP-1, Early Management Bundle for Severe Sepsis/Septic Shock.
SI conversion factor: To convert plasma lactate level to mg/dL, divide by 0.111.
Community-onset sepsis was defined by time 0 in the emergency department.
In the rebalanced cohort, SEP-1 recipients and nonrecipients were matched and reweighted on observable characteristics using propensity scores and Mahalanobis distance between covariates.
Hours from arrival to the hospital.
Difference was significant at α = .05.
Source of infection present at admission was determined by International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes as appropriate per time period.
New bacteremia was defined by positive blood culture results after time 0 of sepsis in a patient who did not have a positive blood culture result in the preceding week. Possible skin contaminants were excluded.
Elevated serum lactate level was defined as a value of more than 20 mmol/L at onset of sepsis or in the preceding 6 hours. To meet the definition of shock, serum lactate level was 4 mmol/L or greater.
Length of stay was measured from time of onset of sepsis until hospital discharge.
Cohort With Hospital-Onset Sepsis
Overall SEP-1–adherent care among 2296 patients with time 0 of sepsis in an inpatient unit was administered to 281 patients (12.2%). With regard to individual SEP-1 components, 1405 patients (61.2%) in the cohort with hospital-onset sepsis had blood cultures performed, 897 (39.1%) had serum lactate levels measured, and 993 (43.3%) received broad-spectrum intravenous antibiotic treatment. Intravenous fluid treatment was administered to only 162 of 918 eligible patients (17.7%). Among 2015 patients for whom care was considered to be nonadherent, 610 (30.3%) received 5 of 6 SEP-1 components.
Completion of SEP-1 in the cohort with hospital-onset sepsis was associated with patient factors (Table 2). After being rebalanced, recipients and nonrecipients of SEP-1–adherent care who had hospital-onset sepsis had similar observable characteristics except recipients were more likely to have an elevated serum lactate level (63 [23.0%] treated vs 167 [9.32%] untreated).
Table 2. Characteristics of the Cohort With Hospital-Onset Sepsis Before and After Being Rebalanced for Treatment and Observable Characteristicsa.
| Characteristic | Before rebalancing | After rebalancingb | ||||
|---|---|---|---|---|---|---|
| SEP-1 | Standardized difference | SEP-1 | Standardized difference | |||
| Received (n = 281) | Did not receive (n = 2015) | Received (n = 274) | Did not receive (n = 1790) | |||
| Time 0 of sepsis, mean (SD), hc | 51.0 (105.2) | 73.3 (137.2) | −0.18d | 52.0 (106.4) | 51.2 (91.4) | 0.01 |
| Age, mean (SD), y | 61.9 (17.4) | 61.7 (18.1) | 0.01 | 62.1 (17.2) | 62.7 (16.7) | −0.03 |
| Male sex, No. (%) | 163 (58.0) | 1116 (55.4) | −0.05 | 159 (58.0) | 1035 (57.8) | −0.004 |
| Elixhauser comorbidities, mean (SD), No. | 5.0 (2.9) | 5.0 (3.1) | −0.01 | 5.0 (2.8) | 4.8 (2.5) | 0.04 |
| Postoperative status, No. (%) | 30 (10.6) | 363 (18.1) | −0.21d | 30 (10.9) | 231 (12.9) | −0.06 |
| Present at admission, No. (%) | ||||||
| Pneumoniae | 74 (26.3) | 394 (19.5) | 0.16d | 72 (26.2) | 470 (26.3) | 0 |
| Urinary tract infection | 55 (19.6) | 330 (16.3) | 0.08 | 54 (19.7) | 353 (19.7) | 0 |
| Skin or soft-tissue infection | 23 (8.2) | 109 (5.3) | 0.12d | 22 (8.0) | 110 (6.1) | 0.08 |
| New bacteremia, No. (%)f | 26 (9.3) | 233 (11.7) | −0.08 | 26 (9.5) | 200 (11.2) | −0.06 |
| Immunosuppression, No. (%) | 66 (23.5) | 618 (30.7) | −0.16d | 66 (24.1) | 618 (27.0) | −0.07 |
| Fever, No. (%) | 95 (33.8) | 351 (17.6) | 0.38d | 90 (32.8) | 588 (32.8) | 0 |
| Septic shock, No. (%) | 94 (33.5) | 759 (37.9) | −0.09 | 91 (33.2) | 584 (32.6) | 0.01 |
| Hypotension, No. (%) | 106 (37.7) | 1169 (57.9) | −0.41d | 102 (37.2) | 703 (39.3) | −0.04 |
| Elevated serum lactate level, No. (%)g | 63 (22.4) | 135 (6.7) | 0.45d | 63 (23.0) | 167 (9.3) | 0.40d |
| Hospital, No. (%) | ||||||
| 1 | 27 (9.6) | 251 (12.6) | −0.09 | 24 (8.8) | 157 (8.8) | 0 |
| 2 | 67 (23.8) | 412 (20.4) | 0.08 | 67 (24.5) | 372 (20.8) | 0.09 |
| 3 | 84 (29.9) | 848 (42.0) | −0.25d | 83 (30.3) | 608 (33.9) | −0.08 |
| 4 | 103 (36.7) | 504 (25.1) | 0.25d | 100 (36.5) | 653 (36.5) | 0 |
| Outcomes | ||||||
| In-hospital mortality, No. (%) | 54 (19.2) | 411 (20.6) | −0.04 | 53 (19.7) | 339 (19.5) | 0.01 |
| Vasopressor days, mean (SD), d | 2.3 (3.3) | 2.4 (3.3) | −0.03 | 2.3 (3.3) | 2.1 (3.1) | 0.06 |
| Length of stay, mean (SD), dh | 15.7 (14.4) | 18.9 (18.9) | −0.19d | 15.7 (14.8) | 17.0 (17.3) | −0.08 |
Abbreviation: SEP-1, Early Management Bundle for Severe Sepsis/Septic Shock.
SI conversion factor: To convert plasma lactate level to mg/dL, divide by 0.111.
Community-onset sepsis was defined by time 0 in the emergency department.
In the rebalanced cohort, SEP-1 recipients and nonrecipients were matched and reweighted on observable characteristics using propensity scores and Mahalanobis distance between covariates.
Hours from arrival to the hospital.
Difference was significant at α = .05.
Source of infection present at admission was determined by International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnosis codes as appropriate per time period.
New bacteremia was defined by positive blood culture results after time 0 of sepsis in a patient who did not have a positive blood culture result in the preceding week. Possible skin contaminants were excluded.
Elevated serum lactate level was defined as a value of more than 20 mmol/L at onset of sepsis or in the preceding 6 hours. To meet the definition of shock, the level serum lactate was 4 mmol/L or greater.
Length of stay was measured from time of onset of sepsis until hospital discharge.
Complete SEP-1
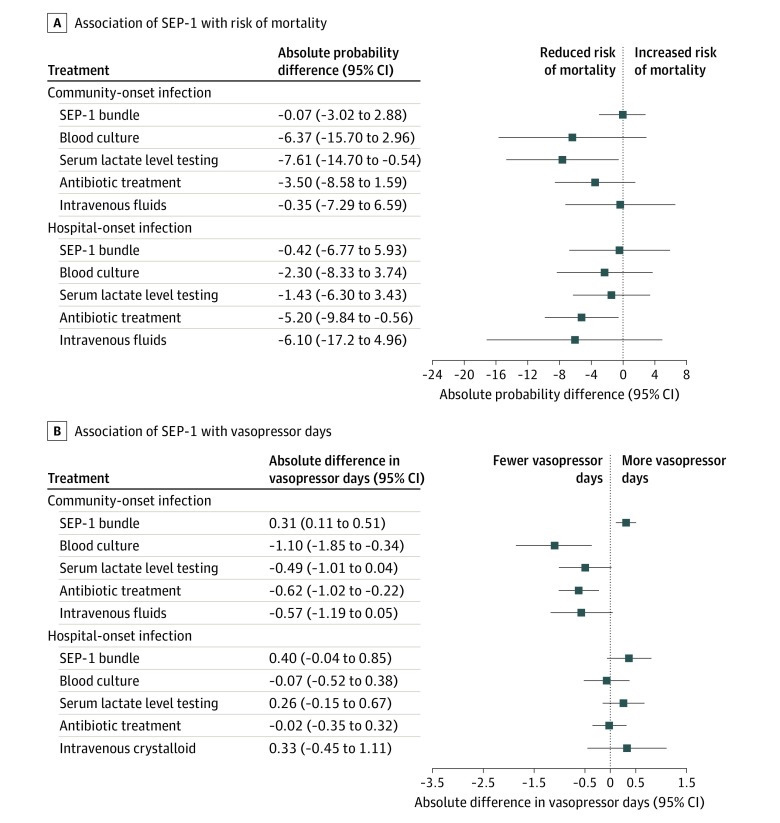
Receipt of the SEP-1 was not associated with reduced mortality or decreased vasopressor days in the cohort with community-onset sepsis (absolute mortality difference, –0.07%; 95% CI, –3.02% to 2.88%; absolute difference of 0.31 vasopressor days; 95% CI, 0.11 to 0.51 vasopressor days), the cohort with hospital-onset sepsis (absolute mortality difference, –0.42%; 95% CI, –6.77% to 5.93%; absolute difference of 0.40 vasopressor days; 95% CI, –0.04 to 0.85 vasopressor days), or the total overall sample (absolute mortality difference, 0.10%; 95% CI, –2.41% to 2.61%; absolute difference of 0.26 vasopressor days; 95% CI, 0.09 to 0.43 vasopressor days) (Table 3 and Table 4).
Table 3. Associations of SEP-1 and Components With Risk of Mortality and Vasopressor Days in Rebalanced Cohorts With Community-Onset and Hospital-Onset Sepsis.
| Component | Sepsisa | |
|---|---|---|
| Community-onset | Hospital-onset | |
| Risk of mortality difference, percentage points (95% CI) | ||
| SEP-1 bundle | −0.07 (−3.02 to 2.88) | −0.42 (−6.77 to 5.93) |
| Blood cultures | −6.37 (−15.70 to 2.96) | −2.30 (−8.33 to 3.74) |
| Serum lactate level testing | −7.61 (−14.70 to −0.54)b | −1.43 (−6.30 to 3.43) |
| Broad-spectrum intravenous antibiotic treatment | −3.50 (−8.58 to 1.59) | −5.20 (−9.84 to −0.56)b |
| Intravenous fluid treatment | −0.35 (−7.29 to 6.59) | −6.10 (−17.20 to 4.96) |
| Vasopressor days, mean absolute difference (95% CI) | ||
| SEP-1 bundle | 0.31 (0.11 to 0.51)b | 0.40 (−0.04 to 0.85) |
| Blood cultures | −1.10 (−1.85 to −0.34) | −0.07 (−0.52 to 0.38) |
| Serum lactate level testing | −0.49 (−1.01 to 0.04) | 0.26 (−0.15 to 0.67) |
| Broad-spectrum intravenous antibiotic treatment | −0.62 (−1.02 to −0.22)b | −0.02 (−0.35 to 0.32) |
| Intravenous fluid treatment | −0.57 (−1.19 to 0.05) | 0.33 (−0.45 to 1.11) |
Abbreviation: SEP-1, Early Management Bundle for Severe Sepsis/Septic Shock.
Outcomes are reported as the differences between treated and untreated. Risk of mortality difference is expressed in terms of percentage points.
Statistical significance defined as 2-sided P < .05.
Table 4. Treatment Association of the SEP-1 Bundle and Components With Mortality and Vasopressor Days in the Rebalanced Total Sample.
| Treatment | Mortality difference (95% CI)a | Vasopressor days (95% CI)a |
|---|---|---|
| SEP-1 bundle | 0.10 (−2.41 to 2.61) | 0.26 (0.09 to 0.43)b |
| Blood cultures | −5.01 (−10.9 to 0.91) | −0.44 (−0.65 to −0.02)b |
| Serum lactate level testing | −2.68 (−6.46 to 1.09) | −0.004 (−0.31 to 0.30) |
| Broad-spectrum intravenous antibiotic treatment | −3.83 (−7.62 to −0.04)b | −0.45 (−0.78 to −0.12)b |
| Intravenous fluid treatment | −0.57 (−6.10 to 4.95) | −0.23 (−0.71 to 0.24) |
Abbreviation: SEP-1, Early Management Bundle for Severe Sepsis/Septic Shock.
Outcomes are reported as the differences between treated and untreated. Risk of mortality difference is expressed in terms of percentage points.
Statistical significance defined as 2-sided P < .05.
SEP-1 Components and Community-Onset Sepsis
In the rebalanced cohort with community-onset sepsis, multiple SEP-1 components were associated with improved outcomes (Table 3 and Figure). Measurement of serum lactate level was associated with a reduction in risk of mortality (absolute difference, –7.61%; 95% CI, –14.70% to –0.54%). Blood cultures and broad-spectrum intravenous antibiotic treatment were associated with fewer vasopressor days.
Figure. Associations of Early Management Bundle for Severe Sepsis/Septic Shock (SEP-1) and Components With Risk of Mortality and Vasopressor Days in Cohorts With Community-Onset and Hospital-Onset Sepsis.
Community-onset sepsis was defined by occurrence of time 0 of sepsis in the emergency department; hospital-onset, time 0 in an inpatient unit. Patients with signs of infection or clinical instability in the emergency department who did not meet the criteria for sepsis until arrival in an inpatient unit were counted as having hospital-onset sepsis. Vasopressor days indicates the requirement of blood pressure support with a vasopressor medication in the 10 days after time 0.
SEP-1 Components and Hospital-Onset Sepsis
In the rebalanced cohort with hospital-onset sepsis, use of broad-spectrum intravenous antibiotic treatment was associated with a reduction in risk of mortality (absolute difference, –5.20%; 95% CI, –9.84% to –0.56%) (Figure). No other SEP-1 component showed an association with outcomes.
SEP-1 Components Overall
In the rebalanced total sample, broad-spectrum intravenous antibiotic treatment was associated with a reduction in risk of mortality (absolute difference, –3.83%; 95% CI, –7.62% to –0.04%) and fewer vasopressor days (Table 4). Collection of blood cultures was also associated with decreased vasopressor days (absolute difference, –0.44; 95% CI, –0.65 to –0.02). Serum lactate level testing and intravenous fluid treatment were not associated with either outcome.
Sensitivity Analyses
Multiple sensitivity analyses were performed, including evaluations of the association of SEP-1 and components with hospital length of stay, death or discharge to hospice, and 30-day in-hospital mortality, as well as analyses stratified by other risk factors (eTables 2-13 in the Supplement).
Discussion
In this retrospective propensity score–matched cohort study, the CMS core quality measure (SEP-1) for early sepsis care was not associated with a reduction in mortality or a decreased requirement for vasopressor treatment among eligible patients. This work is in line with other studies37,38 which have suggested that SEP-1–adherent care may not be associated with improved outcomes, although we observed associations between multiple individual components of SEP-1 and reduced mortality or decreased need for organ support among patients with community-onset sepsis. These findings suggest a path forward in which SEP-1 could be reconstructed to reflect quality more accurately in early sepsis care.
To our knowledge, this is the first study to estimate the association of SEP-1–adherent care with outcomes in a large cohort with hospital-onset sepsis. Previous studies,10,11,12,39 including the landmark observational study of the New York state sepsis mandate39 and randomized clinical trials of early, goal-directed therapy,10,11,12 involved only the emergency department. Of note, in this study, hospital-onset was defined based on whether time 0 occurred after admission to an inpatient unit instead of at a time cutoff (ie, 48 hours after arrival) or by a diagnosis code indicating presence at admission. We chose this definition to isolate differences in systems and processes of care for ambulatory and hospitalized patients. For instance, serum lactate level measurement has prognostic value that may guide admission to the intensive care unit from the emergency department.40,41,42 Observational data suggest that delayed transfer to the intensive care unit may be associated with increased sepsis-related mortality.43,44 Among patients who have already been triaged to an inpatient unit (either the general ward or the intensive care unit), serum lactate level may have prognostic value, but it can no longer serve the same function in early triage.
Among patients meeting our definition for hospital-onset sepsis, completion of the SEP-1 protocol was not associated with improved outcomes compared with routine care. The 2 potential reasons for this finding are that SEP-1 is a poor metric for use with hospital-onset sepsis or that routine care is adequate. In the present sample, only 43.3% of patients with hospital-onset sepsis received early broad-spectrum intravenous antibiotic treatment, suggesting the presence of a quality gap. However, SEP-1 may not be the best metric to represent that gap.
We suspect that SEP-1–adherent care is less valid and less effective in hospital-onset sepsis because of discrepancies related to the ascertainment of time 0, which has not been validated in this population, and because context is not considered in the protocol. For ambulatory patients, SEP-1 may alter the subsequent hospital course by guiding the initial workup. For instance, for an undifferentiated patient, emergency and outpatient clinicians may not otherwise routinely order blood cultures or commit to broad-spectrum intravenous antibiotic treatment. However, among hospital inpatients, sepsis management should consider context. At time 0, results from an admission workup may already be available and intravenous treatments may have already started. Measuring a lactate level is still important, but it may be less important than responding to other problems that have arisen since the patient arrived in the unit.
SEP-1 may also be less beneficial for patients with hospital-onset sepsis because of the characteristics of that patient population. A large proportion of sepsis-related mortality may not be preventable,38,45 and more patients with hospital-onset sepsis may have underlying terminal illness. In those cases, care bundles may not be expected to provide benefit and may encourage inappropriate care.
In our analysis, multiple components of SEP-1 were associated with improved outcomes in patients with community-onset sepsis. Although these findings suggest that SEP-1–adherent care is associated with improved outcomes in patients with community-onset sepsis, it is also possible that SEP-1–adherent care among ambulatory patients may be associated with earlier recognition of sepsis. Among patients with septic shock, infectious symptoms at the time of presentation have been associated with lower mortality.46 We attempted to control for localizing infectious symptoms by including site-specific cultures in our propensity model (ie, cerebrospinal fluid cultures, synovial fluid cultures), but symptoms were otherwise unobservable in the data. Regardless, the association between better outcomes and adherence to components of SEP-1 appears to support the use of these components in a measure of quality for treatment of community-onset sepsis.
At present, SEP-1 is an “all-or-nothing” measure in which each component is given equal weight. Our analysis suggests that this approach may be misguided. In the total sample, the cohort with community-onset sepsis and the cohort with hospital-onset sepsis, early administration of broad-spectrum intravenous antibiotic treatment was associated with improved outcomes. Thus, if SEP-1 is to be reconstructed to reflect quality more accurately, consideration should be given to prioritizing antibiotics. The other components of SEP-1 (serum lactate level testing, blood culture, intravenous fluid treatment, and vasopressor medication) are important in the management of early sepsis; however, we believe that a case in which broad-spectrum intravenous antibiotic treatment is delayed or not administered should not be considered the same as a case in which a second serum lactate level measurement is not taken.
Limitations
This study has limitations. Historically, observational studies using propensity scores to balance routinely collected data may estimate the association of treatment with outcomes inaccurately.47 Although we collected substantial amounts of clinical data, we were nonetheless unable to control completely for confounding from unobservable variables related to severity of illness, likelihood of treatment, and mortality. Prospective studies are needed to assess the association between SEP-1 and outcomes.
This study was also limited by the relative insensitivity of mortality as an outcome. As a secondary outcome, we used days of vasopressor treatment, which have been proposed as an alternative to mortality when studying septic shock.25,26 Because initiation of vasopressor treatment for persistent hypotension is a component of SEP-1, increased vasopressor days among SEP-1 recipients may reflect adherent care rather than increased organ dysfunction. Other outcomes related to organ support, such as days requiring mechanical ventilation or renal replacement therapy, would likely have been preferable for the evaluation of SEP-1 but were not available.
We were unable to obtain postdischarge follow-up data. To account for this, we shortened the period in which we evaluated patients for vasopressor treatment from 4 weeks, which is commonly used, to 10 days.26 Nonetheless, our estimates of vasopressor days may be biased by patients who were discharged from the hospital and died within 10 days of time 0.
We did not perform a medical record review. Administration of SEP-1 components was determined using structured data from the electronic medical record. Thus, we were unable to account for cases with an administrative contraindication to treatment, such as patient refusal. However, this limitation should not interfere with interpretation of our results because we explicitly planned to estimate the association between treatment and outcomes rather than the intention to treat.
Our results may not be generalizable. Our sample was identified using diagnosis codes and therefore did not capture individuals in whom sepsis was missed or whose diagnosis was never coded. Because we identified patients in this manner, our results may be sensitive to specific coding practices at the participating hospitals and may be susceptible to bias from changes in coding practices over time.2,8
Furthermore, we compared associations between treatment and outcomes in different cohorts qualitatively instead of using interaction terms, and we performed multiple comparisons without statistical correction. The relatively small counts of patients in the cohort with hospital-onset sepsis who received the complete SEP-1 (n = 281) or intravenous fluid treatment when indicated (n = 162) likely decreased the power of this study to detect an association between those interventions and outcomes. However, the point estimate for the association of SEP-1 with mortality in cases of hospital-onset sepsis did not suggest a benefit of treatment. The results from sensitivity analyses were exploratory.
Conclusions
The use of SEP-1–adherent care was not associated with improved outcomes of sepsis in patients with hospital-onset or community-onset sepsis. Although multiple components of SEP-1 were associated with reduced mortality or decreased vasopressor days for patients who presented with sepsis in the emergency department, only broad-spectrum intravenous antibiotic treatment was associated with reduced mortality when time 0 occurred in an inpatient unit. Current sepsis quality metrics may need refinement.
eTable 1. Pairwise Correlation Between Individual SEP-1 Sepsis Bundle Components for Patients with Community-onset Sepsis and Hospital-onset Sepsis
eTable 2. Relative Risk of In-hospital Mortality Associated with Clinical Variables in the Raw Cohorts with Community-onset and Hospital-onset Sepsis
eTable 3. Characteristics of the Total Sample Before and After Being Rebalanced on Propensity for Treatment and Observable Characteristics
eTable 4. Relative Risk of In-hospital Mortality Associated with Clinical Variables in the Raw Total Sample
eTable 5. Results from Endogenous Treatment Effects Models Compared with Unweighted/Unbalanced Regression
eTable 6. Prespecified Sensitivity Analyses Evaluating Treatment Effect of the Complete SEP-1 Bundle in Different Cohorts
eTable 7. Treatment Effects of SEP-1 Bundle Components in Different Cohorts
eTable 8. Treatment Effects of SEP-1 Sepsis Bundle and Components on 30-Day In-hospital Mortality in Total Sample and Rebalanced Cohorts with Community-onset and Hospital-onset Sepsis
eTable 9. Treatment Effects of SEP-1 Sepsis Bundle and Components on Hospital Length of Stay (Measured from Time Zero of Sepsis until Discharge)
eTable 10. Treatment Effects of SEP-1 Sepsis Bundle and Components on Composite Outcome of In-hospital Mortality or Discharge to Hospice
eTable 11. Treatment Effects of SEP-1 Sepsis Bundle and Components in Rebalanced Cohorts with Sepsis Presenting on the Inpatient Ward or Intensive Care Unit
eTable 12. Treatment Effects of SEP-1 Sepsis Bundle and Components in Rebalanced Cohorts with Time Zero of Sepsis <48 or >48 Hours After Arrival
eTable 13. Post Hoc Sensitivity Analyses
References
- 1.Novosad SA, Sapiano MR, Grigg C, et al. . Vital signs: epidemiology of sepsis: prevalence of health care factors and opportunities for prevention. MMWR Morb Mortal Wkly Rep. 2016;65(33):864-869. doi: 10.15585/mmwr.mm6533e1 [DOI] [PubMed] [Google Scholar]
- 2.Rhee C, Dantes R, Epstein L, et al. ; CDC Prevention Epicenter Program . Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241-1249. doi: 10.1001/jama.2017.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones SL, Ashton CM, Kiehne LB, et al. . Outcomes and resource use of sepsis-associated stays by presence on admission, severity, and hospital type. Med Care. 2016;54(3):303-310. doi: 10.1097/MLR.0000000000000481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seymour CW, Kennedy JN, Wang S, et al. . Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003-2017. doi: 10.1001/jama.2019.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankar-Hari M, Harrison DA, Rowan KM. Differences in impact of definitional elements on mortality precludes international comparisons of sepsis epidemiology—a cohort study illustrating the need for standardized reporting. Crit Care Med. 2016;44(12):2223-2230. doi: 10.1097/CCM.0000000000001876 [DOI] [PubMed] [Google Scholar]
- 6.Kempker JA, Martin GS. Does sepsis case mix heterogeneity prevent outcome comparisons? Crit Care Med. 2016;44(12):2288-2289. doi: 10.1097/CCM.0000000000001933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad PA, Shea ER, Shiboski S, Sullivan MC, Gonzales R, Shimabukuro D. Relationship between a sepsis intervention bundle and in-hospital mortality among hospitalized patients: a retrospective analysis of real-world data. Anesth Analg. 2017;125(2):507-513. doi: 10.1213/ANE.0000000000002085 [DOI] [PubMed] [Google Scholar]
- 8.Gohil SK, Cao C, Phelan M, et al. . Impact of policies on the rise in sepsis incidence, 2000-2010. Clin Infect Dis. 2016;62(6):695-703. doi: 10.1093/cid/civ1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States—an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46(12):1889-1897. doi: 10.1097/CCM.0000000000003342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouncey PR, Osborn TM, Power GS, et al. ; ProMISe Trial Investigators . Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-1311. doi: 10.1056/NEJMoa1500896 [DOI] [PubMed] [Google Scholar]
- 11.Peake SL, Delaney A, Bailey M; ARISE Investigators; ANZICS Clinical Trials Group . Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-1506. doi: 10.1056/NEJMoa1404380 [DOI] [PubMed] [Google Scholar]
- 12.Angus DC, Yealy DM, Kellum JA; ProCESS Investigators . Protocol-based care for early septic shock. N Engl J Med. 2014;371(4):386. [DOI] [PubMed] [Google Scholar]
- 13.Pepper DJ, Jaswal D, Sun J, Welsh J, Natanson C, Eichacker PQ. Evidence underpinning the Centers for Medicare & Medicaid Services’ Severe Sepsis and Septic Shock Management Bundle (SEP-1): a systematic review. Ann Intern Med. 2018;168(8):558-568. doi: 10.7326/M17-2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pepper DJ, Sun J, Cui X, Welsh J, Natanson C, Eichacker PQ. Antibiotic- and fluid-focused bundles potentially improve sepsis management, but high-quality evidence is lacking for the specificity required in the Centers for Medicare and Medicaid Service’s Sepsis Bundle (SEP-1). Crit Care Med. 2019;47(10):1290-1300. doi: 10.1097/CCM.0000000000003892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odden AJ, Govindan S, Sheth J, Iwashyna TJ. A systematic assessment of the Surviving Sepsis Campaign’s evidence supporting the care of patients with severe sepsis on the wards. Ann Am Thorac Soc. 2015;12(6):956-958. doi: 10.1513/AnnalsATS.201502-096LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services. Hospital inpatient specifications manuals. https://www.qualitynet.org/inpatient/specifications-manuals. Accessed December 1, 2019.
- 17.Rhodes A, Evans LE, Alhazzani W, et al. . Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 18.Singer M, Deutschman CS, Seymour CW, et al. ; The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554. doi: 10.1056/NEJMoa022139 [DOI] [PubMed] [Google Scholar]
- 20.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. What is the best method for estimating the burden of severe sepsis in the United States? J Crit Care. 2012;27(4):414.e1-414.e9. doi: 10.1016/j.jcrc.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 21.Kumar G, Kumar N, Taneja A, et al. ; Milwaukee Initiative in Critical Care Outcomes Research (MICCOR) Group of Investigators . Nationwide trends of severe sepsis in the 21st century (2000-2007). Chest. 2011;140(5):1223-1231. doi: 10.1378/chest.11-0352 [DOI] [PubMed] [Google Scholar]
- 22.Stoller J, Halpin L, Weis M, et al. . Epidemiology of severe sepsis: 2008-2012. J Crit Care. 2016;31(1):58-62. doi: 10.1016/j.jcrc.2015.09.034 [DOI] [PubMed] [Google Scholar]
- 23.Rhee C, Kadri S, Huang SS, et al. . Objective sepsis surveillance using electronic clinical data. Infect Control Hosp Epidemiol. 2016;37(2):163-171. doi: 10.1017/ice.2015.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee C, Dantes RB, Epstein L, Klompas M. Using objective clinical data to track progress on preventing and treating sepsis: CDC’s new “Adult Sepsis Event” surveillance strategy. BMJ Qual Saf. 2019;28(4):305-309. doi: 10.1136/bmjqs-2018-008331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourcier S, Hindlet P, Guidet B, Dechartres A. reporting of organ support outcomes in septic shock randomized controlled trials: a methodologic review—the Sepsis Organ Support Study. Crit Care Med. 2019;47(7):984-992. doi: 10.1097/CCM.0000000000003746 [DOI] [PubMed] [Google Scholar]
- 26.Russell JA, Lee T, Singer J, De Backer D, Annane D. Days alive and free as an alternative to a mortality outcome in pivotal vasopressor and septic shock trials. J Crit Care. 2018;47:333-337. doi: 10.1016/j.jcrc.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 27.CMS.gov Hospital inpatient specifications manuals. Accessed February 24, 2020. https://www.qualitynet.org/inpatient/specifications-manuals
- 28.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 29.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698-705. doi: 10.1097/MLR.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 30.Healthcare Cost and Utilization Project Procedure classes 2015. https://www.hcup-us.ahrq.gov/toolssoftware/procedure/procedure.jsp. Accessed December 1, 2019.
- 31.Rubin DB. Bias reduction using Mahalanobis-metric matching. Biometrics. 1980;36(2):293-298. doi: 10.2307/2529981 [DOI] [Google Scholar]
- 32.Frölich M. Matching estimators and optimal bandwidth choice. Stat Comput. 2005;15(3):197-215. doi: 10.1007/s11222-005-1309-6 [DOI] [Google Scholar]
- 33.Heckman JJ, Ichimura H, Todd P. Matching as an econometric evaluation estimator. Rev Econ Stud. 1998;65(2):261-294. doi: 10.1111/1467-937X.00044 [DOI] [Google Scholar]
- 34.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26(4):734-753. doi: 10.1002/sim.2580 [DOI] [PubMed] [Google Scholar]
- 37.Rhee C, Filbin MR, Massaro AF, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program . Compliance with the national SEP-1 quality measure and association with sepsis outcomes: a multicenter retrospective cohort study. Crit Care Med. 2018;46(10):1585-1591. doi: 10.1097/CCM.0000000000003261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhee C, Jones TM, Hamad Y, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program . Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open. 2019;2(2):e187571. doi: 10.1001/jamanetworkopen.2018.7571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seymour CW, Gesten F, Prescott HC, et al. . Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. doi: 10.1056/NEJMoa1703058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Meng Z, Li Y, et al. . Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with sepsis. Scand J Trauma Resusc Emerg Med. 2019;27(1):51. doi: 10.1186/s13049-019-0609-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frenzen FS, Kutschan U, Meiswinkel N, Schulte-Hubbert B, Ewig S, Kolditz M. Admission lactate predicts poor prognosis independently of the CRB/CURB-65 scores in community-acquired pneumonia. Clin Microbiol Infect. 2018;24(3):306.e1-306.e6. doi: 10.1016/j.cmi.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 42.Chen Y-X, Li C-S. Lactate on emergency department arrival as a predictor of mortality and site-of-care in pneumonia patients: a cohort study. Thorax. 2015;70(5):404-410. doi: 10.1136/thoraxjnl-2014-206461 [DOI] [PubMed] [Google Scholar]
- 43.Fernando SM, Rochwerg B, Reardon PM, et al. . Emergency department disposition decisions and associated mortality and costs in ICU patients with suspected infection. Crit Care. 2018;22(1):172. doi: 10.1186/s13054-018-2096-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wardi G, Wali AR, Villar J, et al. . Unexpected intensive care transfer of admitted patients with severe sepsis. J Intensive Care. 2017;5(1):43. doi: 10.1186/s40560-017-0239-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans L. A closer look at sepsis-associated mortality. JAMA Netw Open. 2019;2(2):e187565. doi: 10.1001/jamanetworkopen.2018.7565 [DOI] [PubMed] [Google Scholar]
- 46.Filbin MR, Lynch J, Gillingham TD, et al. . Presenting symptoms independently predict mortality in septic shock: importance of a previously unmeasured confounder. Crit Care Med. 2018;46(10):1592-1599. doi: 10.1097/CCM.0000000000003260 [DOI] [PubMed] [Google Scholar]
- 47.Hemkens LG, Contopoulos-Ioannidis DG, Ioannidis JPA. Agreement of treatment effects for mortality from routinely collected data and subsequent randomized trials: meta-epidemiological survey. BMJ. 2016;352:i493. doi: 10.1136/bmj.i493 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Pairwise Correlation Between Individual SEP-1 Sepsis Bundle Components for Patients with Community-onset Sepsis and Hospital-onset Sepsis
eTable 2. Relative Risk of In-hospital Mortality Associated with Clinical Variables in the Raw Cohorts with Community-onset and Hospital-onset Sepsis
eTable 3. Characteristics of the Total Sample Before and After Being Rebalanced on Propensity for Treatment and Observable Characteristics
eTable 4. Relative Risk of In-hospital Mortality Associated with Clinical Variables in the Raw Total Sample
eTable 5. Results from Endogenous Treatment Effects Models Compared with Unweighted/Unbalanced Regression
eTable 6. Prespecified Sensitivity Analyses Evaluating Treatment Effect of the Complete SEP-1 Bundle in Different Cohorts
eTable 7. Treatment Effects of SEP-1 Bundle Components in Different Cohorts
eTable 8. Treatment Effects of SEP-1 Sepsis Bundle and Components on 30-Day In-hospital Mortality in Total Sample and Rebalanced Cohorts with Community-onset and Hospital-onset Sepsis
eTable 9. Treatment Effects of SEP-1 Sepsis Bundle and Components on Hospital Length of Stay (Measured from Time Zero of Sepsis until Discharge)
eTable 10. Treatment Effects of SEP-1 Sepsis Bundle and Components on Composite Outcome of In-hospital Mortality or Discharge to Hospice
eTable 11. Treatment Effects of SEP-1 Sepsis Bundle and Components in Rebalanced Cohorts with Sepsis Presenting on the Inpatient Ward or Intensive Care Unit
eTable 12. Treatment Effects of SEP-1 Sepsis Bundle and Components in Rebalanced Cohorts with Time Zero of Sepsis <48 or >48 Hours After Arrival
eTable 13. Post Hoc Sensitivity Analyses