Key Points
Question
Fine particles (particulate matter 2.5 μm) are capable of reaching and being deposited in the small airways; can the use of a particle-removing device be associated with improvements in airflow and inflammation in children with asthma?
Findings
In this randomized, double-blind, crossover study of 43 children with mild or moderate asthma, using a particle-removing device in bedrooms for 2 weeks was significantly associated with improved small airway mechanics, increased peak expiratory flow, and reduced pulmonary inflammation.
Meaning
Indoor air filtration was associated with improved airflow in the small airways and reduced respiratory inflammation, and it thereby may serve as an important preventive measure in asthma management.
This observational study examines the pathophysiological changes in the small airways associated with using a fine particle (PM2.5)–removing device in bedrooms of children with asthma.
Abstract
Importance
Fine particles (particulate matter 2.5 μm [PM2.5]), a ubiquitous air pollutant, can deposit in the small airways that play a vital role in asthma. It appears to be unknown whether the use of a PM2.5 filtration device can improve small airway physiology and respiratory inflammation in children with asthma.
Objective
To discover what pathophysiological changes in the small airways are associated with using a PM2.5-removing device in the bedrooms of children with asthma.
Design, Setting, and Participants
Children with mild or moderate asthma were enrolled in this double-blind, crossover study. The participants used a true filtration device and a sham filtration device in their bedrooms in a random order for 2 weeks each with a 2-week washout interval. The study was conducted in a suburb of Shanghai, China, during a low-ozone season.
Exposures
Ozone and PM2.5 were measured inside bedrooms and outside a window.
Main Outcomes and Measures
Impulse oscillometry, spirometry, and fractional exhaled nitric oxide were measured at the beginning and the end of each intervention. Peak expiratory flow was measured twice daily at home.
Results
Forty-three children (5-13 years old; 26 boys [60%]) participated. Outdoor 24-hour mean PM2.5 concentrations were moderately high, ranging from 28.6 to 69.8 μg/m3 (median, 53 μg/m3). During true filtration, bedroom PM2.5 concentrations were a mean (SD) of 63.4% (35.9%) lower than during sham filtration. Compared with sham filtration, true filtration was significantly associated with improved airway mechanics, reflected in a 24.4% (95% CI, 11.8%-37.1%) reduction in total airway resistance, a 43.5% (95% CI, 13.7%-73.3%) reduction in small airway resistance, a 22.2% (95% CI, 2.2%-42.2%) reduction in resonant frequency, and a 73.1% (95% CI, 0.3%-145.8%) increase in airway reactance. True filtration was also associated with significant improvements in fractional exhaled nitric oxide (a 27.6% [95% CI, 8.9%-42.4%] reduction) and peak expiratory flow (a 1.6% [95% CI, 0.8%-2.5%] increase). These improvements were significantly associated with bedroom PM2.5 reduction. Improvements in small airway function were nonsignificant (8.4% [95% CI, −1.4% to 18.3%]) in all participants but significant (13.2% [95% CI, 1.2%-25.1%]) in participants without eosinophilic airway inflammation at baseline. No improvements were observed for forced vital capacity, forced expiratory volume during the first second, and the ratio of these in all participants or subgroups.
Conclusions and Relevance
Per these results, indoor PM2.5 filtration can be a practical method to improve air flow in an asthmatic lung through improved airway mechanics and function as well as reduced inflammation. This warrants a clinical trial to confirm.
Trial Registration
ClinicalTrials.gov Identifier: NCT03282864
Introduction
Air pollution, particularly fine particles of 2.5 μm or smaller in diameter (PM2.5), is a known risk factor for asthma exacerbation.1 Elevated PM2.5 levels are found in polluted atmospheres throughout the world and numerous indoor environments where tobacco smoke is present or solid fuels are used for cooking or heating2,3; PM2.5 can penetrate the lung deeply to deposit in small airways4,5,6 that have a physiology very important in asthma.7 Anatomically defined as the airways with inner diameters smaller than 2 mm, the small airways cumulatively represent a substantially greater cross-sectional area than the large airways.8 Compromised small airways contribute substantially to airflow obstruction9,10,11 and airway inflammation12,13,14 in asthma. Although PM2.5 exposure has been associated with increased oxidative stress and pulmonary inflammation,1,15,16 to our knowledge, no studies have examined whether reducing PM2.5 exposure can be associated with improvements in small airway physiology in individuals with asthma.
High-efficiency particulate air (HEPA) filters have proven to be effective in capturing airborne particles including PM2.5.17,18,19 However, previous studies have generated inconsistent findings regarding the effectiveness of HEPA filtration of indoor air in reducing symptoms and improving lung function of children with asthma.18,19,20,21,22 Our previous study in healthy adults demonstrated that a single night of bedroom-based HEPA intervention was associated with improvements in small airway mechanics but not respiratory inflammation.23 However, it is uncertain whether such improvements can be achieved in children with asthma over a longer period, because individuals with asthma are more responsive to dynamic levels (eg, day-to-day changes) of air pollution24 and their exposures outside of homes may negate the potential health benefits.
We think that PM2.5 deposited deep in the lungs would have a direct association with changes in the small airways and therefore hypothesized that the use of a PM2.5 filtration device is associated with improvements in small airway mechanics, airway inflammation, and possibly pulmonary function. To test this hypothesis, we conducted a HEPA intervention study in suburban Shanghai, China, where daily outdoor PM2.5 concentrations overlapped with high levels reported in cities in high-income countries.25,26,27 Because asthma is a heterogeneous disease,28,29 we further explored whether the presence or absence of baseline eosinophilic airway inflammation would be associated with children’s responses to the intervention.
Methods
Study Participants
We recruited children (5-13 years old) from the outpatient clinic of the Shanghai General Hospital. According to the Global Initiative for Asthma,30 all participants had mild or moderate asthma. Participants had had at least 1 episode of asthma exacerbation during the past 12 months. All participants had at least a 1-week run-in period to stabilize themselves and adjust medications before starting their participation in the study. At the screening visit, children and parents were trained and evaluated for their ability to perform the self-administrated peak expiratory flow (PEF) measurement by a physician. The study protocol was approved by the ethics committee of Shanghai General Hospital and Duke University Campus institutional review board. All participants provided oral assent, and their parents gave written consent.
Study Design and Sample Size
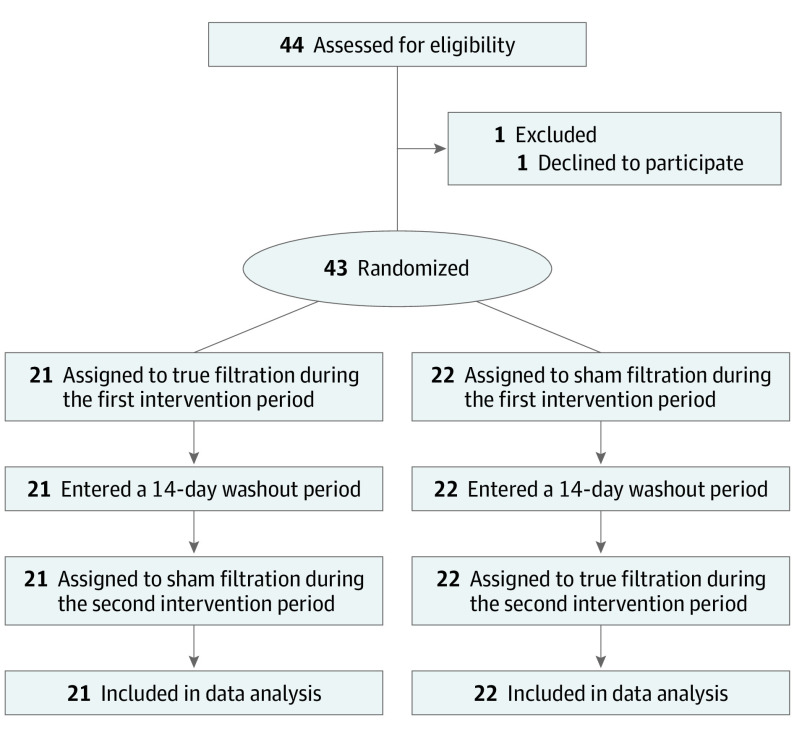
We designed this randomized, double-blind, crossover study with fractional exhaled nitric oxide (FeNO) as the primary outcome. Spirometry and impulse oscillometry (IOS) and PEF indicators were designed as secondary outcomes. Based on FeNO, which has been better recognized and established than airway mechanics, we performed a sample-size calculation using statistical significance at .05 and power at 90%. The between-participant and within-participant variance of FeNO were estimated based on the literature31,32 (Supplement 1). We generated a needed sample size of 40 individuals and added an additional 10% to account for potential participant dropout (to 44 individuals). During enrollment, 1 participant declined to participate after the screening visit, and thus we enrolled 43 participants (Figure 1). Now with data obtained from this study, we estimated the effect size detectable with 43 participants (eTable 1 in Supplement 2).
Figure 1. CONSORT Diagram.
Filtration Intervention
Each participant underwent 2 intervention sessions between February 14, 2017, and April 24, 2017. During the true filtration session, a commercially available portable air purifier (Atmosphere; Amway), was used intact. As listed in the direction of airflow, this device consisted of a prefilter (removing coarse particles), a HEPA filter (removing fine particles), and an activated carbon filter (removing certain gases). The air purifiers had 5 different fan settings, and we set the fan to level 2, which was designed to deliver filtered air at 2.8 m3/min, adequately to remove PM2.5 for the room size without generating a substantial level of noise. During a sham filtration session, the air purifier was modified by removing the HEPA filter and the activated carbon filter while keeping the external appearance identical. The noise levels generated by the true and sham filtration device were not easily discernible by human ears. In both sessions, the devices were operated in children’s bedrooms with the doors and windows closed whenever the children were home. The sequence of receiving the true and sham filtration devices was randomized. Because of the identical appearance of the true and sham filtration devices, the study participants, their parents, and research staff who evaluated health outcomes were blinded to this sequence. A 2-week washout period was used to separate the 2 sessions.
Baseline and Outcome Measurements
On enrollment, the children received venipuncture for a blood test and IgE-mediated allergy testing. They were measured for baseline spirometry results, height, and weight. Their parents also responded to a questionnaire survey of sociodemographics and home characteristics. Once within 24 hours prior to the start of a filtration intervention and again within 24 hours after the intervention ended, the children were measured for FeNO (NIOX VERO; Circassia), airway mechanics by impulse oscillometry (MasterScreen IOS; Jaeger), and lung function by spirometry (MasterScreen PFT system; Jaeger). The main parameters of airway mechanics included airway resistance at 5 Hz (R5; total airway resistance), airway resistance at 20 Hz (R20; large airway resistance), R5-R20 (small airway resistance), airway reactance at 5 Hz (X5), and resonant frequency (Fres). The impulse oscillometry instrument used in this study did not provide data on the area of reactance (Ax). Spirometry parameters included forced vital capacity (FVC), forced expiratory volume during the first second (FEV1), FEV1/FVC ratio, and forced expiratory flow during 25% to 75% of FVC (FEF25-75).
During each intervention session, the participants measured peak expiratory flow (PEF) at 7 am and 9 pm daily at home using a handheld mini-Wright PEF meter (Koka) provided by our pediatric asthma clinic, with assistance from their parents. The PEF values were written down by their parents in a paper-based diary.
Exposure Measurements
We measured PM2.5 and ozone (O3; with a known effect in asthma exacerbation1) in the bedrooms (>1 m away from the filtration device, 1.0-1.5 m above the floor) of all the participants. These pollutants were also measured outside a window at a subset of the homes. For the homes without outdoor monitoring, we used concentrations measured at a nearby home. The pollutants were measured continuously throughout each 2-week intervention period using a monitor containing a PMS3003 PM2.5 sensor (Plantower) and an O3 sensor (Model Ox-A4; Alphasense). These monitors, validated in Beijing and Shanghai, China, and other cities,33,34 were field calibrated in a location near the hospital. Hourly mean values were calculated from the mean data calculated by the minute and recorded by the monitors.
Statistical Analysis
First, we summarized PEF and the changes in other outcomes under sham and true filtration conditions and tested within-person differences in mean values between treatment conditions without involving any covariates (eTable 2 and eTable 3 in Supplement 2). Second, we conducted within-person comparisons between a physiological response to true filtration and sham filtration using linear mixed-effects models in which a random intercept was specified to account for multiple measurements from the same participant. A physiological response was defined as the difference between the value of an outcome (or ln[FeNO] because of highly skewed distribution) measured after an intervention session and that measured before the session. These models had adjustments for the number of hours that windows were open, the duration of time spent outdoors, sleep duration, outdoor O3 concentration, filtration duration, and medication use. We performed within-person comparisons of PEF values measured twice daily during true filtration and sham filtration, using linear mixed-effect models in which random intercepts were specified to account for measurements performed at the same points in the day and measurements from the same participants. The adjustments were the same, with the exception of filtration duration (this variable was not included). Second, we assessed changes in health outcome levels associated with a 10-μg/m3 reduction in bedroom PM2.5 levels across both true and sham sessions using linear mixed-effects models. The same covariates were included, with the exception of the number of hours that windows were open (this variable was not included). Third, we conducted post hoc stratified analyses using the same statistical model structure with respect to the presence or absence of eosinophilic airway inflammation, as indicated by baseline FeNO and blood eosinophil count. Fourth, we conducted sensitivity analyses excluding participants who had fever, asthma exacerbation, changes in inhaled corticosteroid use, or passive smoking at home during the study. We also conducted sensitivity analyses by adjusting for outdoor conditions (2-week mean values of temperature, relative humidity, and PM2.5 concentration). The FeNO data have been converted back to untransformed data in all reported results. Statistical analyses were performed using the lme4 and lmeTest packages of R software version 3.3.1 (R Foundation for Statistical Computing). A detailed description of statistical models is provided in Supplement 1.
Results
Participant Characteristics
Forty-three children (17 girls and 26 boys) were included. The baseline characteristics of the participants are shown in the Table. At baseline, 31 participants (72%) took long-term asthma control medications daily, while 12 (28%) took none. All participants had mild or moderate asthma. Most of the participants (n = 40 [93%]) had baseline FEV1 levels greater than 80% of the predicted value. Nine participants had FeNO values greater than 35 ppb, suggesting that eosinophilic airway inflammation was likely in these children per an American Thoracic Society guideline.35 Thirteen had blood eosinophil counts greater than 450 cells per μL (to convert to cells × 109, multiply by 0.001), another suggested indicator of eosinophilic airway inflammation.36 Based on blood IgE tests, 35 participants had atopic asthma, 27 were allergic to dust mites, and 9 were allergic to mold. During the study period, 14 children (32%) reported that they were exposed to secondhand smoke at home, and 11 children (26%) experienced a fever or physician-ascertained asthma exacerbation. The occurrence of these was not statistically different during true filtration and sham filtration (eTable 5 in Supplement 2).
Table. Baseline Characteristics of the 43 Study Participants.
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
| All | Received true filtration followed by the washout period and sham filtration | Received sham filtration followed by the washout period and true filtration | |
| Sample size, No. | 43 | 21 | 22 |
| Female | 17 (40) | 8 (38) | 9 (41) |
| Male | 26 (60) | 13 (62) | 13 (59) |
| Age, median (range), y | 7 (5-13) | 7 (5-13) | 8 (5-13) |
| Height, mean (SD), cm | 132 (13) | 131 (13) | 134 (14) |
| Weight, mean (SD), kg | 31 (10) | 30 (10) | 32 (11) |
| Mother with a college degree or more | 36 (84) | 17 (81) | 19 (86) |
| FEV1, % predicted value, mean (SD)a | 103.0 (16.5) | 104.8 (18.8) | 101.2 (14.2) |
| Participants with % predicted FEV1 <80% | 3 (7) | 2 (10) | 1 (4) |
| FEV1/FVC ratio, % predicted value, mean (SD) | 97.9 (9.4) | 97.5 (8.9) | 98.3 (9.9) |
| Fractional exhaled nitric oxide | |||
| Median (range), ppb | 15 (5-120) | 17 (5-82) | 14 (5-120) |
| Participants with >35 ppb | 9 (21) | 5 (24) | 4 (18) |
| Blood eosinophil count | |||
| Median (range) cells per μL | 360 (80-1260) | 390 (140-1260) | 290 (80-1100) |
| Participants with >450 cells/μL | 13 (30) | 8 (38) | 5 (23) |
| Long-term asthma control medication | |||
| None | 12 (28) | 6 (28) | 6 (27) |
| Inhaled corticosteroids alone | 10 (23) | 2 (10) | 4 (18) |
| Long-acting β agonist alone | 0 | 0 | 0 |
| Both drugs | 25 (58) | 13 (62) | 12 (54) |
| H1 receptor antagonist | 7 (16) | 4 (19) | 3 (14) |
| Leukotriene receptor antagonists | 2 (5) | 2 (10) | 0 |
| Positive blood IgE test resultb | 35 (82) | 15 (71) | 20 (91) |
| Dust-mite allergy | 27 (63) | 12 (57) | 15 (68) |
| Mold allergy | 9 (21) | 5 (24) | 6 (18) |
Abbreviations: FEV1, forced expiratory volume during the first second; FVC, forced vital capacity.
SI conversion factor: To convert the eosinophil count to cells × 109, multiply by 0.001.
Percentage of predicted values for the spirometry values were calculated using the built-in proprietary formula of the spirometry machine (MasterScreen PFT system; Jaeger) developed by Zapletal for children aged 2 to 18 years.
On enrollment, blood samples from each participant were analyzed using an allergen-specific IgE test (AllergyScreen; Mediwiss Analytic GmbH). A positive result indicated testing positive for any of the 19 allergens evaluated by this test. These 19 allergens included dust mites, mold, cat dander, dog dander, cockroaches, tree pollens, and foods (ie, eggs, milk, beef, shrimp). Blood IgE levels greater than 0.35 kU/L were considered positive results.
Filtration Interventions
The duration of filtration sessions was a mean of 14 days, with within-participant and between-participant variance of 1.28 days and 1.82 days, respectively. During the true and sham filtration periods, children spent a mean (SD) of 12.3 (2.8) and 12.2 (2.3) hours per day in their bedrooms, and 15.8 (1.4) and 15.8 (1.4) hours per day at home (in any room), respectively. The days of the week were highly similar across true and sham filtration interventions. The clinical visits before and after the same filtration intervention, as well as the clinical visits within the same participant after true and sham filtration, were scheduled almost on the same day of the week (mean [SD]: difference, 0.6 [0.7] days and 0.6 [0.8] days, respectively). Overall, 143 of 172 clinical visits (83.1%) were scheduled on either Fridays or Saturdays.
Pollutant Exposure
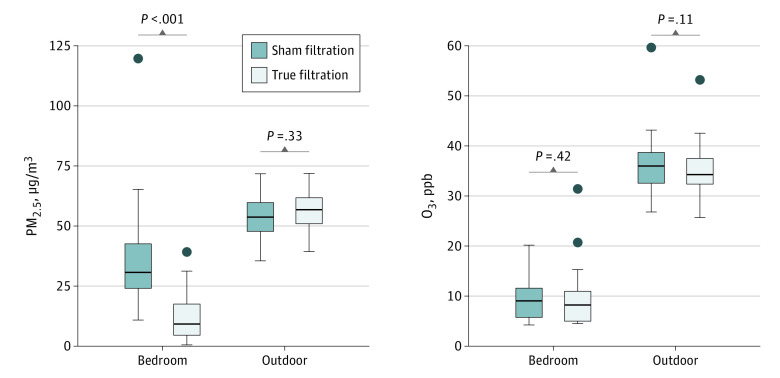
During the study period, daily mean concentrations of outdoor PM2.5 ranged from 28.6 to 69.8 μg/m3; daily 8-hour maximum O3 concentrations ranged from 7.7 to 98.3 ppb, according to measurements made at a nearby monitoring station. Figure 2 shows the intervention-period mean values of the 2 pollutants. Outdoor concentrations were not significantly different between the 2 periods. As expected, bedroom PM2.5 concentrations were significantly lower during true filtration. True filtration and sham filtration resulted in mean reductions of 79.6% and 35.7% in PM2.5 concentration from their corresponding outdoor levels, respectively. Compared with levels during sham filtration, true filtration resulted in a 63.4% (35.9%) mean (SD) reduction in bedroom PM2.5 concentrations. Bedroom O3 concentrations did not show a significant difference between the 2 periods. However, O3 concentrations during both periods were low and near the detection limit (6 ppb) of monitors for most of the time.
Figure 2. Pollutant Concentrations in Bedrooms and Outdoors by Filtration Status.
Concentrations graphed were 2-week mean values. Statistical significance in median concentrations was determined using a Wilcoxon signed rank test. PM2.5 indicates particulate matter smaller than 2.5 μm.
Outcomes Associated With Filtration
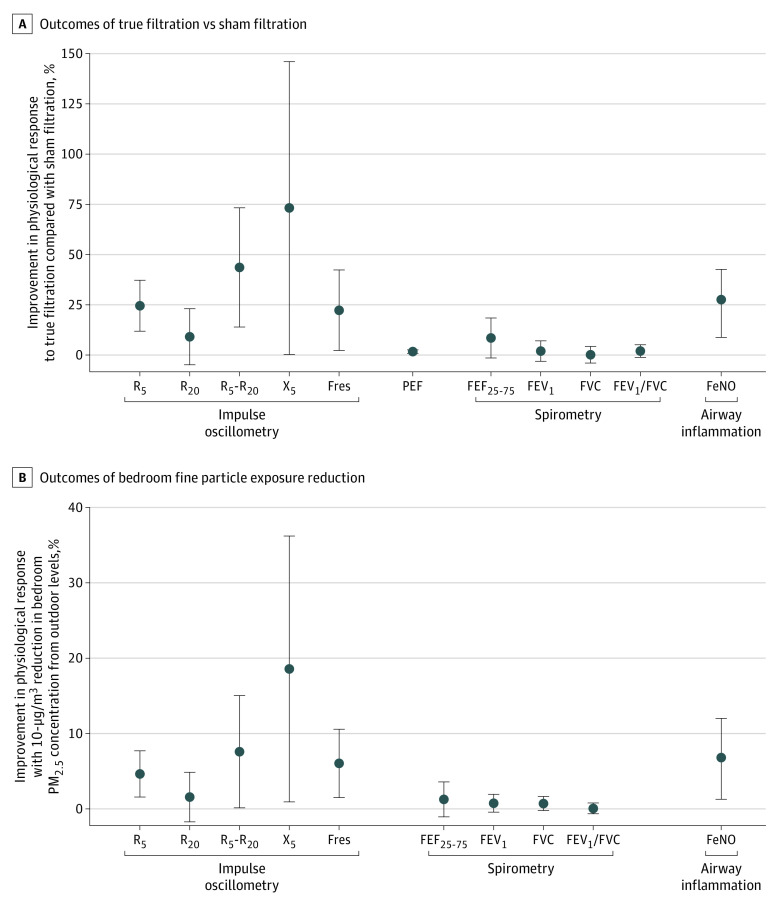
As shown in Figure 3, compared with sham filtration, true filtration was significantly associated with improvements in IOS outcomes of airway mechanics and respiratory inflammation (FeNO): a 24.4% (95% CI, 11.8%-37.1%) reduction in R5, a 43.5% (95% CI, 13.7%-73.3%) reduction in R5-R20, a 73.1% (95% CI, 0.3%-145.8%) increase in X5, a 22.2% (95% CI, 2.2%-42.2%) reduction in Fres, and a 27.6% (95% CI, 8.9%-42.4%) reduction in FeNO. The mean PEF value during the true filtration period was significantly higher (by 1.6% [95% CI, 0.8%-2.5%] or 4.1 [95% CI, 1.9-6.3] L/min) than during the sham filtration period. There was a statistically nonsignificant 8.4% (95% CI, −1.4% to 18.3%) improvement in FEF25-75 (P = .10), whereas no significant differences were observed for the changes in spirometry outcomes of FEV1, FVC, and FEV1/FVC between true filtration and sham filtration.
Figure 3. Outcomes Associated With True Filtration (vs Sham Filtration) and Bedroom Fine Particle (PM2.5) Exposure Reduction.
A, Outcomes of true filtration compared with sham filtration. For all outcomes except peak expiratory flow (PEF), the points and bars show mean and 95% CIs for outcome improvements when comparing filtration changes in outcome levels between true filtration and sham filtration. For PEF, the point and the bar show the mean and 95% CIs when comparing PEF values measured during the true filtration period with PEF values measured during the sham filtration period. For all outcomes, positive values indicate improvements and negative values indicate deterioration. For fractional exhaled nitric oxide (FeNO) data, the analysis was performed on log-transformed FeNO data and the result was converted back to untransformed data for presentation in this figure. B, outcomes associated with bedroom fine particle (PM2.5) exposure reduction. Points and bars show mean and 95% CIs for outcome improvements associated with a 10-μg/m3 reduction in bedroom PM2.5 concentration from outdoor levels. For all indicators, positive values indicate improvements and negative values indicate deterioration. For fractional exhaled nitric oxide (FeNO) data, the analysis was performed on log-transformed FeNO data, and the result was converted back to untransformed data for presentation in this figure. FEF25-75 indicates forced expiratory flow during 25% to 75% of forced vital capacity; FEV1, forced expiratory volume during the first second; Fres, resonant frequency; FVC, forced vital capacity; PM2.5, particulate matter smaller than 2.5 μm; R5, airway resistance measured at 5 Hz; R20, airway resistance measured at 20 Hz; R5-R20, the difference between R5 and R20, reflecting small airway resistance; X5, airway reactance measured at 5 Hz.
Response to PM2.5 Exposure Reduction
The air purifier used in this study was mainly designed to remove particulate pollutants. As shown in Figure 2, there was a considerable range of bedroom PM2.5 concentrations both within and across the 2 intervention periods. To further explore whether PM2.5 reduction played a role in the improvements associated with true filtration, we analyzed the exposure reduction–response association, showing results in Figure 3. We found that every 10-μg/m3 reduction in bedroom PM2.5 concentration was significantly associated with a 4.6% reduction in R5, a 7.6% reduction in R5-R20, an 18.6% increase in X5, a 6.0% reduction in Fres, and a 6.8% reduction in FeNO.
Stratified Analyses
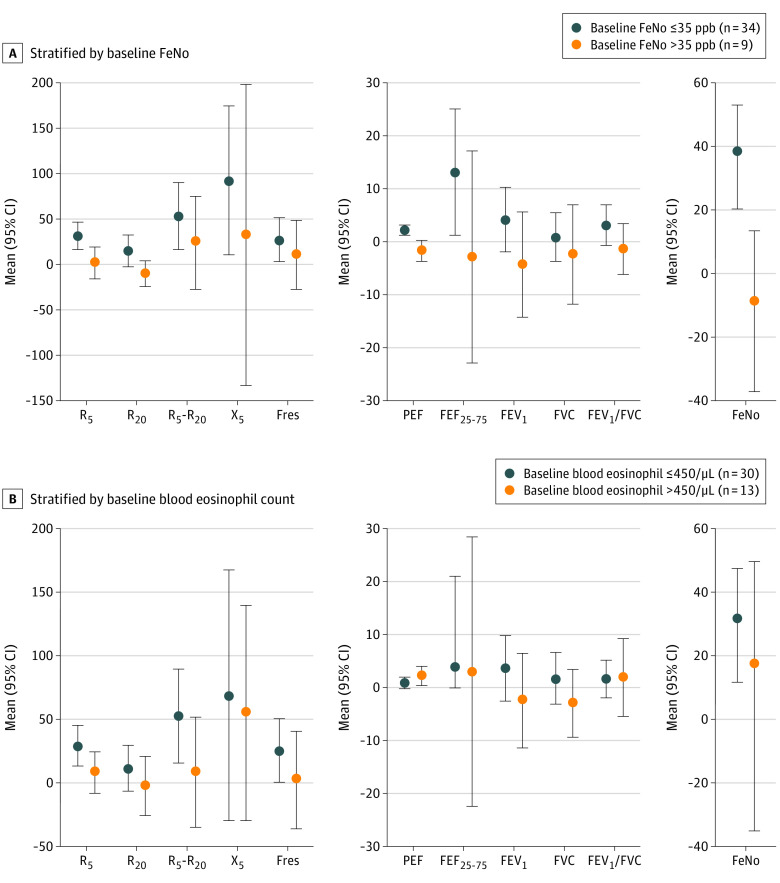
We considered baseline eosinophilic airway inflammation for stratified analyses in comparing changes in outcomes between true filtration and sham filtration. As shown in Figure 4A, children with lower baseline FeNO levels (≤35 ppb) showed significant improvements in almost all the IOS indicators of airway mechanics (R5, 32.0% [95% CI, 16.9%-47.1%]; R5-R20, 53.5% [95% CI, 16.3%-90.7%]; X5, 92.6% [95% CI, 10.4%-174.8%]; Fres, 27.2% [95% CI, 3.4%-51.1%]; but not R20), PEF (2.2% [95% CI, 1.2%-3.2%]), FEF25-75 (13.2% [95% CI, 1.2%-25.1%]), and FeNO (38.8% [95% CI, 20.3%-53.1%]). However, children with higher baseline FeNO levels showed significant improvements in none of these outcomes. As shown in Figure 4B, children with lower baseline eosinophil counts showed significant improvements in FeNO (31.8% [95% CI, 11.6%-47.4%]), as well as almost all the IOS indicators (including R5, 29.5% [95% CI, 13.6%-45.4%]; R5-R20, 52.7% [95% CI, 16.2%-89.3%]; Fres, 25.5% [95% CI, 0.6%-50.4%]; but not R20 and X5), whereas children with higher baseline eosinophil counts showed significant improvement only in PEF (2.2% [95% CI, 0.4%-4.0%]).
Figure 4. Outcomes of True Filtration vs Sham Filtration, Stratified by Baseline Eosinophilic Airway Inflammation, as Indicated by Baseline Fractional Exhaled Nitric Oxide (FeNO) or Blood Eosinophil Count.
The FeNO cut point was 35 ppb. The blood eosinophil count cut point was 450 cells per μL (to convert to cells × 109, multiply by 0.001). For all outcomes except peak expiratory flow (PEF), the points and bars show mean and 95% CIs for outcome improvements when comparing the before-and-after filtration changes in outcome levels between true filtration and sham filtration. For PEF, the point and the bar show the means and 95% CIs when comparing PEF values measured during the true filtration period with PEF values measured during the sham filtration period. For all outcomes, positive values indicate improvements and negative values indicate deterioration. For FeNO data, the analysis was performed on log-transformed FeNO data, and the result was converted back to untransformed data for presentation in this figure. FEF25-75 indicates forced expiratory flow during 25% to 75% of forced vital capacity; FEV1, forced expiratory volume during the first second; Fres, resonant frequency; FVC, forced vital capacity; PEF, peak expiratory flow; R5, airway resistance measured at 5 Hz; R20, airway resistance measured at 20 Hz; R5-R20, the difference between R5 and R20, reflecting small airway resistance; X5, airway reactance measured at 5 Hz.
Sensitivity Analyses
Adjusting for ambient temperature, humidity, and PM2.5 in the sensitivity analyses did not affect the results (eFigure 1 in Supplement 2). Sensitivity analyses excluding participants who reported changes in inhaled corticosteroid use, exposure to secondhand smoke at home, or a fever or asthma exacerbation event during the study did not change the main findings (eTable 4, eTable 5, and eFigure 2 in Supplement 2).
Discussion
Our observations from the present study suggest that reducing PM2.5 exposure was associated with improved air flow in the airways (especially the small airways) as reflected in decreased resistance and increased reactance (ie, reduced airway stiffness). These outcomes are important in people with asthma, because deterioration in the small airways is among the earliest and the most fundamental processes of asthma progression and aggravation.37,38 Changes in impulse oscillometry indicators by 8.6%39 to 20%40,41 are generally regarded as clinically important in children with asthma. The magnitude of the improvement in these indicators observed in this study appeared to be at or exceeding the threshold for clinical importance.
To our knowledge, this study is the first to discover these outcomes in children with asthma using a randomized, double-blind crossover study design of a filtration intervention. The intervention was completed within 70 days to minimize the potential influence of seasonal variation. The study was carried out in months with low concentrations of O3, a known environmental trigger of asthma exacerbation. We confirmed that the device, using a HEPA filter and an activated carbon filter, was able to reduce bedroom PM2.5 levels substantially. We observed significant associations of improvements in small airway physiology with bedroom use of this particulate filtration device (Figure 3A) and PM2.5 exposure reduction (Figure 3B).
There seemed to be fewer improvements on the large airways, in contrast with the improvements seen in the small airways. We did not observe any significant improvements in FEV1 and FVC. We observed a statistically significant but small in effect size (1.6%) improvement in daily mean PEF values. Because FEF25-75 is an indicator for small airway function, the 8.4% (95% CI, −1.4% to 18.3%) increase in FEF25-75, although not statistically significant, may reflect the peripheral airway or time-constant heterogeneity improvements seen in the impulse oscillometry indicators. This suggests that improvements in small airway mechanics associated with filtration intervention can potentially bleed into improvements of small airway function and airflow limitation. Our findings are consistent with literature reporting that IOS parameters are more sensitive at detecting lung pathophysiologic changes than spirometry.40,42
Our findings on the improvement of FeNO associated with HEPA filtration were consistent with previous studies,22,43 which reported that the delivery of HEPA-filtered laminar air to the breathing zone during sleep significantly decreased FeNO concentrations. In our study, the improvements in small airway mechanics (R5-R20), small airway function (FEF25-75), and airflow limitation (PEF) were significantly associated with improvement in FeNO concentrations, suggesting that respiratory inflammation may play an intermediate role in the improvement of small airway introduced by reduction of PM2.5 levels.44
In the present study, we used FeNO greater than 35 ppb per the American Thoracic Society guideline35 and blood eosinophils greater than 450 cells per μL39 as surrogates for the presence of eosinophilic airway inflammation at baseline. Our findings suggest that the baseline eosinophilic inflammatory status can be an important determinant for whether the filtration intervention can improve the airway physiology of children with asthma. Different from participants with eosinophilic airway inflammation, participants without eosinophilic airway inflammation at baseline also experienced improvements in small airway function in addition to improvements in small airway mechanics, airflow limitation, and respiratory inflammation. This is consistent with previous studies that demonstrated that individuals with eosinophilic inflammation were less responsive to air pollution.29,45,46
Clinical Implications
In 43 children with asthma, we found that reducing PM2.5 exposure through bedroom air filtration was associated with improved airway mechanics and airflow limitation, along with reduced airway inflammation. The use of a PM2.5-removing device may provide a measure for intervening on the small airways, in addition to existing medications targeting the same segment of the respiratory tract. Considering the lack of prior data on the association of indoor air filtration on airway mechanics in children with asthma, this research was designed mainly as an observational study, albeit using a crossover interventional study design, to examine pathophysiologic responses in a relatively small sample size. This interventional (observational) study provides compelling data to support a larger-scale clinical trial to evaluate the efficacy and effectiveness of residential air filtration in improving airway mechanics, inflammatory status, and lung function in children (and potentially adults) with asthma, especially in those without eosinophilic airway inflammation.
Our intervention lasted 2 weeks and only filtered the air in children’s bedrooms. If maintained longer and/or expanded to other microenvironments, such as classrooms, the intervention might have shown greater improvements. For example, a previous study has shown a significant improvement in FEV1 levels in asthmatic children in a similar age range following 12 months of indoor air filtration.47 Our intervention may not be long enough to have an association with FEV1, despite significant effects on airway mechanics. Future studies are needed for evaluating the outcomes of long-term intervention, with assessments performed at multiple points after the end of filtration.
The present study was carried out in the suburbs of Shanghai, China, where daily outdoor PM2.5 concentrations ranged from less than the US National Ambient Air Quality Standard of 35 μg/m3 to nearly double this level. These levels represent the lower end of PM2.5 ranges in Shanghai, China, and other cities in lower- and middle-income nations and overlap with PM2.5 concentrations measured in cities in high-income nations. In the presence of an indoor source (eg, wood burning, cigarette smoking), PM2.5 exposure can be substantially higher. Hence, a wide range of PM2.5 exposure should be considered in a large clinical trial.
Despite small sample sizes, we showed that baseline eosinophilic airway inflammation should be considered in children’s responses to air filtration. Possible effect modifiers, including pathophysiologic and molecular phenotypes, should be considered in future clinical trials to formulate a more targeted or precision medicine approach to preventative asthma management.
Limitations
Our study has several limitations. First, we did not measure aeroallergens and certain gases. Indoor air filtration devices have been shown to remove airborne allergens in addition to PM2.5.19,48 The activated carbon filter was intended for removing odorous gases. It is possible that the air cleaners might also have reduced concentrations of other pollutants in addition to PM2.5. However, we found significant associations of bedroom PM2.5 reduction with almost all airway mechanics indicators (except R20, with a marginal significance) and FeNO, suggesting that PM2.5 may play a role in airway, especially small airway mechanics and pulmonary inflammation in children with asthma. We would like to note that this is suggestive evidence, and causality should be further elucidated in future studies with suitable designs. Second, our intervention was conducted without selecting dates for the intervention based on outdoor conditions. However, adjusting for ambient temperature, humidity, and PM2.5 in the sensitivity analyses did not affect the results (eFigure 1 in Supplement 2). Third, our study design allowed the children to use asthma medication as needed and live their regular lifestyles. Sensitivity analyses excluding participants who reported changes in inhaled corticosteroid use, participants who were exposed to secondhand smoke at home, and participants who had a fever or an asthma exacerbation event during the study did not change our main findings (eTable 4, eTable 5, and eFigure 2 in Supplement 2).
Conclusions
Reducing exposure to fine particles in living spaces via air filtration intervention was associated with improved airway mechanics, airflow limitation, and respiratory inflammation in children with asthma. Children without eosinophilic airway inflammation at baseline also experienced improved small airway function. These observations support a future clinical trial to assess the efficacy and effectiveness of indoor air filtration in improving small airway pathophysiology that plays a vital role in asthma. We feel that the novelty of using airway mechanics as reflected in this study may provide important implications for future studies.
Research protocol and statistical analyses.
eTable 1. Effects detectable for primary and selected secondary outcomes.
eTable 2. The change in biomarker before and after filtration intervention (ΔBiomarker) for true filtration and sham filtration.
eTable 3. Peak expiratory flow during true filtration and sham filtration.
eTable 4. Participants that switched on and off inhaled corticosteroid (ICS) during the study.
eTable 5. Participants who had fever, asthma exacerbation, or self-reported environmental tobacco smoke (ETS) at home during the two weeks before the four clinical visits.
eFigure 1. Sensitivity analyses for the effects of true filtration compared to sham filtration, controlling for (a) outdoor PM2.5; (b) outdoor temperature; (c) outdoor relative humidity.
eFigure 2. Sensitivity analyses for filtration effect, excluding participants who had (a) fever; (b) asthma aggravation; (c) switched on or off inhaled corticosteroid (ICS) medication; (d) self-reported environmental tobacco smoke at home and (e) baseline FEV1 <80% during the study.
References
- 1.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581-1592. doi: 10.1016/S0140-6736(14)60617-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367-371. doi: 10.1038/nature15371 [DOI] [PubMed] [Google Scholar]
- 3.Gordon SB, Bruce NG, Grigg J, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2(10):823-860. doi: 10.1016/S2213-2600(14)70168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churg A, Brauer M, del Carmen Avila-Casado M, Fortoul TI, Wright JL. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ Health Perspect. 2003;111(5):714-718. doi: 10.1289/ehp.6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly FJ, Fussell JC. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ. 2012;60:504-526. doi: 10.1016/j.atmosenv.2012.06.039 [DOI] [Google Scholar]
- 6.Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1(4):315-320. doi: 10.1513/pats.200409-046TA [DOI] [PubMed] [Google Scholar]
- 7.Lipworth B, Manoharan A, Anderson W. Unlocking the quiet zone: the small airway asthma phenotype. Lancet Respir Med. 2014;2(6):497-506. doi: 10.1016/S2213-2600(14)70103-1 [DOI] [PubMed] [Google Scholar]
- 8.Macklem PT. The physiology of small airways. Am J Respir Crit Care Med. 1998;157(5 pt 2):S181-S183. doi: 10.1164/ajrccm.157.5.rsaa-2 [DOI] [PubMed] [Google Scholar]
- 9.Ingram RH., Jr Physiological assessment of inflammation in the peripheral lung of asthmatic patients. Lung. 1990;168(5):237-247. doi: 10.1007/BF02719700 [DOI] [PubMed] [Google Scholar]
- 10.Wagner EM, Bleecker ER, Permutt S, Liu MC. Direct assessment of small airways reactivity in human subjects. Am J Respir Crit Care Med. 1998;157(2):447-452. doi: 10.1164/ajrccm.157.2.9611043 [DOI] [PubMed] [Google Scholar]
- 11.Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol. 1992;72(3):1016-1023. [DOI] [PubMed] [Google Scholar]
- 12.Hamid QA. Peripheral inflammation is more important than central inflammation. Respir Med. 1997;91:11-12. [DOI] [PubMed] [Google Scholar]
- 13.de Magalhães Simões S, dos Santos MA, da Silva Oliveira M, et al. Inflammatory cell mapping of the respiratory tract in fatal asthma. Clin Exp Allergy. 2005;35(5):602-611. doi: 10.1111/j.1365-2222.2005.02235.x [DOI] [PubMed] [Google Scholar]
- 14.Hamid Q, Song Y, Kotsimbos TC, et al. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997;100(1):44-51. doi: 10.1016/S0091-6749(97)70193-3 [DOI] [PubMed] [Google Scholar]
- 15.O’Connor GT, Neas L, Vaughn B, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol. 2008;121(5):1133-1139. [DOI] [PubMed] [Google Scholar]
- 16.Delfino RJ, Staimer N, Gillen D, et al. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environ Health Perspect. 2006;114(11):1736-1743. doi: 10.1289/ehp.9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batterman S, Du L, Mentz G, et al. Particulate matter concentrations in residences: an intervention study evaluating stand-alone filters and air conditioners. Indoor Air. 2012;22(3):235-252. doi: 10.1111/j.1600-0668.2011.00761.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park HK, Cheng KC, Tetteh AO, Hildemann LM, Nadeau KC. Effectiveness of air purifier on health outcomes and indoor particles in homes of children with allergic diseases in Fresno, California: a pilot study. J Asthma. 2017;54(4):341-346. doi: 10.1080/02770903.2016.1218011 [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Raja S, Ferro AR, et al. Effectiveness of heating, ventilation and air conditioning system with HEPA filter unit on indoor air quality and asthmatic children’s health. Build Environ. 2010;45(2):330-337. doi: 10.1016/j.buildenv.2009.06.010 [DOI] [Google Scholar]
- 20.Morgan WJ, Crain EF, Gruchalla RS, et al. ; Inner-City Asthma Study Group . Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068-1080. doi: 10.1056/NEJMoa032097 [DOI] [PubMed] [Google Scholar]
- 21.Lanphear BP, Hornung RW, Khoury J, Yolton K, Lierl M, Kalkbrenner A. Effects of HEPA air cleaners on unscheduled asthma visits and asthma symptoms for children exposed to secondhand tobacco smoke. Pediatrics. 2011;127(1):93-101. doi: 10.1542/peds.2009-2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle RJ, Pedroletti C, Wickman M, et al. ; 4A Study Group . Nocturnal temperature controlled laminar airflow for treating atopic asthma: a randomised controlled trial. Thorax. 2012;67(3):215-221. doi: 10.1136/thoraxjnl-2011-200665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui X, Li F, Xiang J, et al. Cardiopulmonary effects of overnight indoor air filtration in healthy non-smoking adults: a double-blind randomized crossover study. Environ Int. 2018;114:27-36. doi: 10.1016/j.envint.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 24.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357(23):2348-2358. doi: 10.1056/NEJMoa071535 [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization WHO Global Urban Ambient Air Pollution Database. Published 2016. Accessed November 11, 2017. https://www.who.int/phe/health_topics/outdoorair/databases/cities/en/
- 26.American Lung Association State of the air 2016. Published 2016. https://www.lung.org/local-content/california/our-initiatives/state-of-the-air/2016/state-of-the-air-2016.html
- 27.European Environment Agency Particulate Matter (PM2.5): annual mean concentrations in Europe. Published 2017. Accessed November 21, 2017. https://www.eea.europa.eu/themes/air/interactive/pm2_5
- 28.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009;6(3):256-259. doi: 10.1513/pats.200808-087RM [DOI] [PubMed] [Google Scholar]
- 29.Berry M, Morgan A, Shaw DE, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62(12):1043-1049. doi: 10.1136/thx.2006.073429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. Published 2017. Accessed February 27, 2019. https://ginasthma.org/wp-content/uploads/2017/02/wmsGINA-2017-main-report-final_V2.pdf
- 31.Chen R, Zhao A, Chen H, et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. J Am Coll Cardiol. 2015;65(21):2279-2287. doi: 10.1016/j.jacc.2015.03.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barreto M, Rennerova Z, Montesano M, et al. Variations in exhaled nitric oxide in children with asthma during a 1-week stay in a mountain village sanatorium. J Asthma. 2008;45(6):453-458. doi: 10.1080/02770900802040035 [DOI] [PubMed] [Google Scholar]
- 33.Zheng T, Bergin MH, Johnson KK, et al. Field evaluation of low-cost particulate matter sensors in high- and low-concentration environments. Atmos Meas Tech. 2018;11(8):4823-4846. doi: 10.5194/amt-11-4823-2018 [DOI] [Google Scholar]
- 34.Johnson KK, Bergin MH, Russell AG, Hagler GSW. Field test of several low-cost particulate matter sensors in high and low concentration urban environments. Aerosol Air Qual Res. 2018;18(3):565-578. doi: 10.4209/aaqr.2017.10.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dweik RA, Boggs PB, Erzurum SC, et al. ; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications . An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fowler SJ, Tavernier G, Niven R. High blood eosinophil counts predict sputum eosinophilia in patients with severe asthma. J Allergy Clin Immunol. 2015;135(3):822-4.e2. doi: 10.1016/j.jaci.2014.09.034 [DOI] [PubMed] [Google Scholar]
- 37.McNulty W, Usmani OS. Techniques of assessing small airways dysfunction. Eur Clin Respir J. 2014;1. doi: 10.3402/ecrj.v1.25898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgel PR. The role of small airways in obstructive airway diseases. Eur Respir Rev. 2011;20(119):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komarow HD, Skinner J, Young M, et al. A study of the use of impulse oscillometry in the evaluation of children with asthma: analysis of lung parameters, order effect, and utility compared with spirometry. Pediatr Pulmonol. 2012;47(1):18-26. doi: 10.1002/ppul.21507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112(2):317-322. doi: 10.1067/mai.2003.1627 [DOI] [PubMed] [Google Scholar]
- 41.Song TW, Kim KW, Kim ES, Park JW, Sohn MH, Kim KE. Utility of impulse oscillometry in young children with asthma. Pediatr Allergy Immunol. 2008;19(8):763-768. doi: 10.1111/j.1399-3038.2008.00734.x [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Aledia AS, Tatavoosian AV, Vijayalakshmi S, Galant SP, George SC. Relating small airways to asthma control by using impulse oscillometry in children. J Allergy Clin Immunol. 2012;129(3):671-678. doi: 10.1016/j.jaci.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedroletti C, Millinger E, Dahlén B, Söderman P, Zetterström O. Clinical effects of purified air administered to the breathing zone in allergic asthma: a double-blind randomized cross-over trial. Respir Med. 2009;103(9):1313-1319. doi: 10.1016/j.rmed.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 44.Chalupa DC, Morrow PE, Oberdörster G, Utell MJ, Frampton MW. Ultrafine particle deposition in subjects with asthma. Environ Health Perspect. 2004;112(8):879-882. doi: 10.1289/ehp.6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57(7):643-648. doi: 10.1136/thorax.57.7.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelaia G, Vatrella A, Busceti MT, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015;2015:879783. doi: 10.1155/2015/879783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sulser C, Schulz G, Wagner P, et al. Can the use of HEPA cleaners in homes of asthmatic children and adolescents sensitized to cat and dog allergens decrease bronchial hyperresponsiveness and allergen contents in solid dust? Int Arch Allergy Immunol. 2009;148(1):23-30. doi: 10.1159/000151502 [DOI] [PubMed] [Google Scholar]
- 48.Francis H, Fletcher G, Anthony C, et al. Clinical effects of air filters in homes of asthmatic adults sensitized and exposed to pet allergens. Clin Exp Allergy. 2003;33(1):101-105. doi: 10.1046/j.1365-2222.2003.01570.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Research protocol and statistical analyses.
eTable 1. Effects detectable for primary and selected secondary outcomes.
eTable 2. The change in biomarker before and after filtration intervention (ΔBiomarker) for true filtration and sham filtration.
eTable 3. Peak expiratory flow during true filtration and sham filtration.
eTable 4. Participants that switched on and off inhaled corticosteroid (ICS) during the study.
eTable 5. Participants who had fever, asthma exacerbation, or self-reported environmental tobacco smoke (ETS) at home during the two weeks before the four clinical visits.
eFigure 1. Sensitivity analyses for the effects of true filtration compared to sham filtration, controlling for (a) outdoor PM2.5; (b) outdoor temperature; (c) outdoor relative humidity.
eFigure 2. Sensitivity analyses for filtration effect, excluding participants who had (a) fever; (b) asthma aggravation; (c) switched on or off inhaled corticosteroid (ICS) medication; (d) self-reported environmental tobacco smoke at home and (e) baseline FEV1 <80% during the study.