Key Points
Question
What was the predictive performance for large artery occlusion stroke and the association with onset-to-delivery times for endovascular and intravenous recanalization therapies during the first year following implementation of the prehospital Stockholm Stroke Triage System that combined motor symptom severity with ambulance-to-hospital teleconsultation?
Findings
In this cohort study of 2905 patients undergoing code-stroke ambulance transport within the Stockholm region (Sweden), the new triage system had an overall accuracy in predicting large-artery occlusion stroke of 87% (positive predictive value, 41%; negative predictive value, 93%). The median onset-to-puncture time for thrombectomy was 137 minutes vs 206 minutes in the previous year, while onset-to-needle time for intravenous thrombolysis was unchanged at a median of 115 minutes.
Meaning
Combining a symptom-based prehospital triage algorithm with ambulance-to-hospital teleconsultation may result in markedly reduced delivery times for thrombectomy without delaying intravenous thrombolysis.
Abstract
Importance
To our knowledge, it is unknown whether a prehospital stroke triage system combining symptom severity and teleconsultation could accurately select patients for primary stroke center bypass and hasten delivery of endovascular thrombectomy (EVT) without delaying intravenous thrombolysis (IVT).
Objective
To evaluate the predictive performance of the newly implemented Stockholm Stroke Triage System (SSTS) for large-artery occlusion (LAO) stroke and EVT initiation. Secondary objectives included evaluating whether the Stockholm Stroke Triage System shortened onset-to-puncture time for EVT and onset-to-needle time (ONT) for IVT.
Design, Setting, and Participants
This population-based prospective cohort study conducted from October 2017 to October 2018 across the Stockholm region (Sweden) included patients transported by first-priority (“code stroke”) ambulance to the hospital for acute stroke suspected by an ambulance nurse and historical controls (October 2016-October 2017). Exclusion criteria were in-hospital stroke and helicopter or private transport. Of 2909 eligible patients, 4 (0.14%) declined participation.
Exposures
Patients were assessed by ambulance nurses with positive the face-arm-speech-time test or other stroke suspicion and were evaluated for moderate-to-severe hemiparesis (≥2 National Institutes of Health stroke scale points each on the ipsilateral arm and leg [A2L2 test]). If present, the comprehensive stroke center (CSC) stroke physician was teleconsulted by phone for confirmation of stroke suspicion, assessment of EVT eligibility, and direction to CSC or the nearest primary stroke center. If absent, the nearest hospital was prenotified.
Main Outcomes and Measures
Primary outcome: LAO stroke. Secondary outcomes: EVT initiation, onset-to-puncture time, and ONT. Predictive performance measures included sensitivity, specificity, positive and negative predictive values, the overall accuracy for LAO stroke, and EVT initiation.
Results
We recorded 2905 patients with code-stroke transports (1420 women [49%]), and of these, 323 (11%) had A2L2+ teleconsultation positive results and were triaged for direct transport to CSC (median age, 73 years [interquartile range (IQR), 64-82 years]; 55 women [48%]). Accuracy for LAO stroke was 87% (positive predictive value, 41%; negative predictive value, 93%) and 91% for EVT initiation (positive predictive value, 26%; negative predictive value, 99%). Endovascular thrombectomy was performed for 84 of 323 patients (26%) with triage-positive results and 35 of 2582 patients (1.4%) with triage-negative results. In EVT cases with a known onset time (77 [3%]), the median OPT was 137 minutes (IQR, 118-180; previous year, 206 minutes [IQR, 160-280]; n = 75) (P < .001). The regional median ONT (337 [12%]) was unchanged at 115 minutes (IQR, 83-164; previous year, 115 minutes [IQR, 85-161]; n = 360) (P = .79). The median CSC IVT door-to-needle time was 13 minutes (IQR, 10-18; 116 [4%]) (previous year, 31 minutes [IQR, 19-38]; n = 45) (P < .001).
Conclusions and Relevance
The Stockholm Stroke Triage System, which combines symptom severity and teleconsultation, results in markedly faster EVT delivery without delaying IVT.
This cohort study examines a system for prehospital triage for Swedish patients with suspected acute stroke.
Introduction
The major positive effect of endovascular thrombectomy (EVT) and intravenous thrombolysis (IVT), as well as their time-dependent decay, has been established through several randomized clinical trials.1,2,3 Studies have shown shorter times (median difference, >90 minutes) from stroke onset to the start of EVT (onset-to-puncture time [OPT]) for patients arriving directly at a comprehensive stroke center (CSC), compared with those transferred to a CSC from a primary stroke center (PSC) following receiving an imaging proof of a large artery occlusion (LAO).4,5 This emphasizes the need for improved decision-making in prehospital stroke triage to identify patients with a high probability of requiring EVT.6 While numerous symptom-based scales have been designed for predicting LAO stroke, validation and implementation in the prehospital setting are still at an early stage.7 Importantly, studies are lacking on a combined triage approach using a symptom-based scale and teleconsultation between ambulance staff and a specialist stroke physician.
The Stockholm region (Sweden; 2.3 million population; 6519 km2) is served by 1 CSC and 6 PSCs. The CSC (Karolinska University Hospital) is the sole performer of neuroendovascular procedures, the first endovascular thrombectomy (EVT) done in 2005.8 The CSC and all PSCs have stroke units and treat patients with acute ischemic stroke (AIS) with IVT. Until October 2017, routine practice in Stockholm was to transport patients with prehospital suspicion of stroke (positive modified face-arm-speech-time [FAST] test results, including any of face, arm, or leg weakness, or speech impairment or other focal neurological symptoms or signs) and onset within 6 hours by first-priority code-stroke ambulance to the nearest stroke center.9 First-priority transport was also done for patients with an onset beyond 6 hours or an unknown onset time if their vital signs, including the level of consciousness, were critically affected. On hospital arrival, plain computed tomography (CT) was performed and IVT administered to eligible patients. At PSCs, if CT angiography (CTA) results showed LAO in the anterior or posterior circulation, images were digitally transferred to the CSC, where the regional stroke consultant was queried by phone on secondary transfer for EVT. Patients were accepted for transfer regardless of age and time since onset or last known well if plain CT results did not show an infarct of more than one-third of the middle cerebral artery or more than half of another territory, the patient had a prestroke modified Rankin Scale (mRS) score of 0 to 3, there were no severe comorbidities limiting prestroke life expectancy to fewer than 3 months, and the CTA results and medical history did not indicate severe catheter access difficulties. Secondary transfers represented 75% of patients who underwent EVT originating within Stockholm. Local quality registry data indicated that the OPT for EVT was 60 to 120 minutes longer for secondary transfer patients compared with those arriving directly at a CSC.
On October 10, 2017, the novel Stockholm Stroke Triage System (SSTS) was implemented in the entire region. The SSTS used a hemiparalysis severity rule to guide ambulance staff to either teleconsult the CSC stroke physician regarding PSC bypass eligibility or to prenotify the nearest PSC. Choosing hemiparalysis as a tool to guide which hospital would be contacted happened for several reasons. There was a request from prehospital services to limit the complexity of assessments. Evidence available at the time indicated only minor differences between existing LAO prediction scales.10 Hemiparalysis in stroke has a strong association with poor outcomes.11 The motor items of the National Institutes of Health Stroke Scale (NIHSS) are strongly associated with LAO, with high interrater reliability.12,13 Furthermore, hemiparalysis as a triage instrument has been tested for helicopter transport triage of patients with suspected stroke with promising results.14
The primary aim of this study was to evaluate the precision of the SSTS for LAO stroke identification. The secondary aims were to evaluate (1) the precision of the SSTS for correct triage destination, defined as initiation of EVT treatment, and (2) whether the SSTS leads to changes in OPT time in patients receiving EVT and in onset-to-needle time in patients receiving IVT compared with the year before implementation.
Methods
Ethics and Informed Consent
Ethical approval was obtained from the Stockholm Regional research ethics committee (approval 2017/374). The need for active consent was waived as the triage system was adopted by the region as routine clinical practice and the research part of the project was only the gathering of clinical data from health records and registries. Patients received written information regarding data collection from electronic health records and could decline participation.
Triage System
Patients assessed on scene by ambulance nurses with a positive FAST test result or other suspicion of stroke were evaluated for moderate-to-severe unilateral hemiparesis. This was defined as the simultaneous presence of ipsilateral arm and leg paresis corresponding to 2 or more NIHSS points in each of the affected extremities, termed the A2L2 test. The predictive capability of the A2L2 for LAO was comparable with other combinations of NIHSS items in a large registry study of patients treated with IVT.15 In A2L2-positive cases, the ambulance teleconsulted the CSC stroke physician (neurologist or purpose-trained resident) by phone for confirmation of stroke suspicion, assessment of EVT eligibility, and direction to the CSC or nearest PSC. In A2L2-negative cases, or if inapplicable (ie, unconscious patient, bilateral paresis, or seizures), the ambulance prenotified a stroke physician (eg, neurologist, internist, or purpose-trained resident) at the nearest hospital. In A2L2-positive patients, reasons for the CSC stroke consultant to decline PSC bypass during the teleconsultation included a low suspicion of stroke, prestroke mRS score of 4 to 5, prestroke life expectancy of fewer than 3 months, and life-threatening vital signs necessitating stabilization at the nearest hospital. When the CSC directed the ambulance to a PSC, they routinely telephoned the PSC to explain the decision and provide a preliminary assessment if the patient was a potential IVT candidate. The time since the last known well was not incorporated into the triage algorithm. However, standard practice among CSC stroke consultants was to decline PSC bypass for cases with longer than 24 hours since the last known well. All CSC and PSC stroke physicians had immediate access to the region-wide electronic health record system for in-hospital, outpatient, primary care, and rehabilitation services, which allowed for rapid assessment of prestroke mRS scores and comorbidities during the teleconsultation. The SSTS guideline and protocol, including a patient flow chart (Figure 1), was published on the Stockholm Healthcare Region website and programmed into portable tablet computers routinely used by ambulance staff.16 Implementation was preceded by web-based training and live lectures for ambulance nurses and live lectures and group training for hospital staff. All ambulances had a crew of 2, with at least 1 specialist ambulance nurse (3-year university degree plus 1 year of specialist ambulance or anesthesia training), the second staff member being an ambulance technician (nursing high school diploma plus professional training). To minimize IVT treatment delay due to longer transports in triage-positive patients approved for PSC bypass, an effort was made at the CSC in conjunction with SSTS implementation to optimize known factors associated with the door-to-needle time.17
Figure 1. Flow Chart for the Stockholm Stroke Triage System.
AIS indicates acute ischemic stroke; CSC, comprehensive stroke center; CT, computed tomography; EVT, endovascular thrombectomy; FAST, face-arm-speech-time; IVT, intravenous thrombolysis; LAO, large-artery occlusion; PSC, primary stroke center.
Eligibility Criteria
Data were prospectively collected for all patients eligible for participation, who included those undergoing first-priority ground ambulance transport to hospital and those treated for ambulance nurse suspicion of acute stroke within the Stockholm region. Data collection started on October 10, 2017, the first day of SSTS implementation, and continued for 1 year. Patients with in-hospital stroke, delivered to the hospital by helicopter or private transport, or transported from outside the Stockholm region were excluded.
Triage Status Definitions
Patients were categorized as triage positive if they had A2L2-positive results and were accepted by teleconsultation for direct transport to the CSC. They were triage negative if their A2L2 result was negative or inapplicable or if A2L2 positive but the CSC physician declined PSC bypass because of EVT contraindications or a low suspicion of stroke. Patients for whom the CSC was the nearest hospital were classified the same way (ie, triage-positive) if A2L2 positive and teleconsultation confirmed stroke suspicion and no EVT contraindications and triage negative otherwise.
Data Collection
We collected data on baseline demographic information, clinical and imaging parameters, treatments, time logistics, and final diagnosis. Data sources included the regionwide electronic health record system for emergency health services; the regionwide electronic health record system for in-hospital, outpatient, and primary care services; and the CSC radiological picture archiving and communication system.
Outcomes
The primary outcome, AIS with LAO, was defined as AIS with CTA evidence of occlusion or subocclusion in arteries accessible by stent retriever EVT in routine practice at the CSC: intracranial internal carotid artery, middle cerebral artery M1 or M2, anterior cerebral artery A1 or A2, posterior cortical artery P1, basilar artery, or intracranial vertebral artery. Patients not undergoing CTA because of severe renal failure were considered LAO positive if CT results showed a dense cerebral artery. For analyses of triage precision, patients with AIS not undergoing CTA because of contraindications to recanalization treatments as assessed by the responsible clinician were grouped with patients with a diagnosis of AIS without LAO. Imaging scans were routinely assessed by 2 radiologists. Secondary outcomes were EVT initiation by arterial puncture, OPT for EVT, and onset-to-needle time for IVT in patients with a known stroke onset time.
Statistical Analysis
We assessed sensitivity, specificity, positive and negative predictive values, likelihood ratios, and the overall accuracy of the combined SSTS procedure (A2L2 status and teleconsultation) for LAO stroke and initiated EVT, respectively. For continuous and ordinal variables, the differences in median and interquartile range (IQR) values were compared using the Mann-Whitney U test. For categorical variables, we calculated percentages by dividing the number of events by the total number of patients, excluding missing or unknown values. Pearson χ2 was used for calculating the significance of the difference between proportions. Two-sided P values of <.05 were considered significant. Analyses were performed using Statistica, version 13.1 (Dell). Results are reported according to STARD and Strengthening the Reporting of Observational Studies in Epidemiology guidelines for diagnostic accuracy and observational studies, respectively.18,19
Results
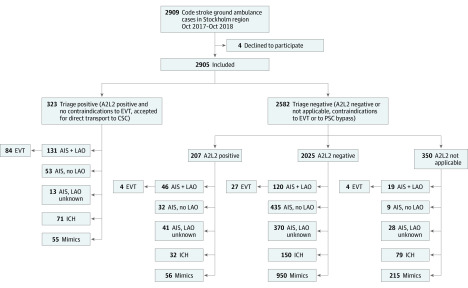
Between October 10, 2017, and October 9, 2018, 2909 first-priority code-stroke ambulance transports from the scene to the hospital were conducted in the Stockholm region. Four patients declined participation, leaving 2905 patients eligible for analysis. Of these, 323 (11.1%) were triage positive, ie, A2L2-positive, and by teleconsultation were deemed eligible for direct transport to the CSC. Figure 2 shows a flow chart of patient recruitment and distribution.
Figure 2. Flow Chart of Patient Recruitment and Distribution by Triage Status, A2L2 Test Status, Diagnostic Category, and Endovascular Thrombectomy (EVT) Treatment.
AIS indicates acute ischemic stroke; CSC, comprehensive stroke center; ICH, intracerebral hemorrhage; LAO, large-artery occlusion; PSC, primary stroke center.
Table 1 shows baseline characteristics, diagnostic categories, and logistic metrics for triage-positive and negative-patients. Triage-positive patients were slightly younger (median age 73 [interquartile range (IQR), 64-82] vs 75 years [IQR, 65-84]; P = .01), had markedly higher stroke severity (median NIHSS score 13 [IQR, 7-18] vs 3 [IQR, 1-8]; P < .001), and markedly lower onset-to-first-hospital-door times (ODT; median time, 86 [IQR, 58-245 minutes] vs 167 minutes [IQR, 73-557 minutes]; P < .001) compared with triage-negative patients. Among 323 triage-positive patients who were taken directly to the CSC, 291 (90%) had PSC bypass (ie, were closest to a PSC at onset). There were large differences in diagnostic categories; LAO stroke was seen in 131 triage-positive patients (41%) vs 185 triage-negative patients (7%). Intracerebral hemorrhage was more common (22% vs 10%) and stroke mimics less common (17% vs 47%) in the triage-positive group. While all triage-positive patients were by definition A2L2 positive, this was also the case in 207 of 2582 triage-negative patients (8%). This subgroup had been declined PSC bypass because of EVT contraindications as assessed through CSC teleconsultation. In this group, 46 of 207 (22%) had LAO stroke.
Table 1. Comparison of Clinical Characteristics, Triage and Diagnostic Categories, and Treatment Between Triage-Positive and Negative Patients.
| Characteristic | Triage-positive (n = 323) | Triage-negative (n = 2582) | P value | ||
|---|---|---|---|---|---|
| No./total No. (%) | Median (IQR) | No./total No. (%) | Median (IQR) | ||
| Age | 323/323 (100.0) | 73 (64-82) | 2582/2582 (100.0) | 75 (65-84) | .01 |
| Female sex | 155/323 (48.0) | NA | 1265/2582 (49.0) | NA | .73 |
| NIHSS score | 320/323 (99.4) | 13 (7-18) | 2199/2582 (85.2) | 3 (1-8) | <.001 |
| ODT | 317/323 (98.1) | 86 (58-245) | 2515/2582 (97.4) | 167 (73-557) | <.001 |
| ODT strata | |||||
| 0-4.5 h | 247/323 (76.5) | NA | 1576/2582 (61.0) | NA | <.001 |
| 4.5-8 h | 24/323 (7.4) | 233/2582 (9.0) | |||
| 8-24 h | 46/323 (14.2) | 706/2582 (27.3) | |||
| Unknown | 6/323 (1.9) | 67/2582 (2.6) | |||
| A2L2 status, % | |||||
| Positive | 323/323 (100.0) | NA | 207/2582 (8.0) | NA | <.001 |
| Negative | 0 | 0 | 2025/2582 (78.0) | ||
| NA | 0 | 0 | 350/2582 (13.6) | ||
| Diagnostic category | No./total No. | Median (IQR) or % | No./total No. | Median (IQR) or % | |
| AIS + LAO | 131/323 | 40.6 | 185/2582 | 7.2 | <.001 |
| AIS, no LAO | 53/323 | 16.4 | 476/2582 | 18.4 | |
| AIS, LAO status unknown | 13/323 | 4.0 | 439/2582 | 17.0 | |
| ICH, SAH or SDH | 71/323 | 22.0 | 261/2582 | 10.1 | |
| Mimic | 55/323 | 17.0 | 1221/2582 | 47.3 | |
| Treatment and logistics | |||||
| IVT initiated | 100/323 | 31.0 | 237/2582 | 9.2 | <.001 |
| NIHSS score (IVT cases) | 100/100 | 14 (9-20) | 235/237 | 5 (4-9) | <.001 |
| ONT, min (IVT) | 99/100 | 84 (64-110) | 228/237 | 132 (97-173) | <.001 |
| DNT, min (IVT) | 100/100 | 13 (10-18) | 236/237 | 39 (25-60) | <.001 |
| EVT initiated | 84/323 | 26.0 | 35/2582 | 1.4 | <.001 |
| NIHSS score (EVT cases) | 84/84 | 18 (14-21) | 35/35 | 10 (6-14) | <.001 |
| OPT min (EVT, known onset) | 60/84 | 127 (101-154) | 17/35 | 180 (440) | <.001 |
| OPT, min (EVT, including LKW time) | 84/84 | 145 (119-240) | 35/35 | 338 (192-490) | <.001 |
| DPT, min (EVT) | 84/84 | 62 (43-77) | 35/35 | 41 (32-58) | <.001 |
Abbreviations: AIS, acute ischemic stroke; A2L2, arm 2 or more points and leg 2 or more points (moderate to severe hemiparesis); CTA, computed tomographic angiography; DNT, door-to-needle time for IVT; DPT, CSC door to puncture time for EVT; EVT, endovascular thrombectomy; IQR, interquartile range; IVT, intravenous thrombolysis; LAO, large artery occlusion; LKW time, last known well time, cases without known onset time; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; ODT, onset-to-first-hospital-door time; ONT, onset-to-needle time for IVT; OPT, onset-to-puncture time for EVT.
Endovascular thrombectomy treatment was initiated in 84 of 323 triage-positive patients (26%) and 35 of 2582 triage-negative patients (1.4%). Intravenous thrombolysis was given to 100 of 323 triage-positive patients (31%) and 237 of 2582 triage-negative patients (9%). Table 2 shows the predictive performance metrics of the SSTS. The overall SSTS accuracy for identifying LAO stroke was 87% (95% CI, 86%-88%; 2528/2905) and 91% (95% CI, 90%-92%; 2631/2905) for identifying patients who received EVT.
Table 2. Performance of the Stockholm Stroke Triage System for Identification of Cases With AIS+LAO and EVT Treatment.
| Measure | AIS + LAO, % (95% CI) | EVT, % (95% CI) |
|---|---|---|
| Sensitivity | 41.5 (36.0-47.1) | 70.6 (61.5-78.6) |
| Specificity | 92.6 (91.5-93.6) | 91.4 (90.3-92.4) |
| PPV | 40.6 (36.1-45.2) | 26.0 (22.9-29.4) |
| NPV | 92.8 (92.2-93.4) | 98.6 (98.2-99.0) |
| Overall accuracy | 87.0 (85.8-88.2) | 90.6 (89.5-91.6) |
| PLR | Ratio, 5.6 (4.6-6.8) | Ratio, 8.2 (7.0-9.7) |
| NLR | Ratio, 0.6 (0.6-0.7) | Ratio, 0.3 (0.2-0.4) |
| LAO +, No.; LAO − or unknown, No. | EVT done, No.; EVT not done, No. | |
| Triage-positive | 131; 192 | 84; 239 |
| Triage-negative | 185; 2397 | 35; 2547 |
Abbreviations: −, negative; +, positive; AIS, acute ischemic stroke; EVT, endovascular thrombectomy; LAO, large-artery occlusion; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value.
Table 3 shows the characteristics and logistics in patients who received EVT and IVT during the SSTS year (EVT, 119 [4%]; IVT, 337 [12%]) and the year before its implementation (EVT, 105 [4%]; IVT, 360 [12%]). Between the 2 periods, there were no significant differences in age, sex, stroke severity, and proportion of patients with a known symptom onset time.
Table 3. Comparison of Clinical Characteristics and Time Logistics in Patients Treated With EVT and IVT Before and After the Stockholm Stroke Triage System.
| Characteristic | SSTS | Pre-SSTS | P value | ||
|---|---|---|---|---|---|
| No./total No. (%) | Median (IQR) | No./total No. (%) | Median (IQR) | ||
| EVT | |||||
| Age, y | 119/119 (100.0) | 74 (62-81) | 105/105 (100.0) | 71 (59-78) | .17 |
| Female sex | 55/119 (46.2) | NA | 44/105 (41.9) | NA | .52 |
| NIHSS score | 119/119 (100.0) | 16 (12-21) | 101/105 (96.2) | 17 (12-20) | .74 |
| Known exact time of onset, min | 77/119 (64.7) | NA | 75/105 (71.4) | NA | .28 |
| IVT prior to EVT | 60/119 (50.4) | NA | 53/10 (50.5)5 | NA | .99 |
| OTD, min (known onset) | 77/119 (64.7) | 66 (51-87) | 75/105 (71.4) | 56 (40-104) | .05 |
| OPT, min (known onset) | 77/119 (64.7) | 137 (118-180) | 75/105 (71.4) | 206 (160-280) | <.001 |
| DPT, min | 119/119 (100.0) | 57 (40-72) | 105/105 (100.0) | 45 (37-60) | .01 |
| Direct to CSC | 87/119 (73.1) | NA | 25/105 (23.8) | NA | <.001 |
| Secondary transport | 32/119 (26.9) | NA | 80/105 (76.2) | NA | <.001 |
| IVT | |||||
| Age | 337/337 (100.0) | 75 (64-82) | 360/360 (100.0) | 75 (66-84) | .13 |
| Female sex | 158/337 (46.9) | NA | 183/360 (50.8) | NA | .30 |
| NIHSS score | 335/337 (99.4) | 7 (4-13) | 357/360 (99.2) | 7 (4-13) | .39 |
| ODT, min. | 336/337 (99.4) | 73 (54-115) | 360/360 (100.0) | 70 (48-107) | .03 |
| ONT, min | 327/337 (97.0) | 115 (83-164) | 358/360 (99.4) | 115 (85-161) | .79 |
| DNT, min | 330/337 (97.9) | 29 (16-49) | 358/360 (99.4) | 38 (26-54) | <.001 |
| ODT, min (CSC) | 116/337 (34.4) | 69 (51-97) | 45/360 (12.5) | 67 (43-114) | .32 |
| DNT, min (CSC) | 116/337 (34.4) | 13 (10-18) | 45/360 (12.5) | 31 (19-38) | <.001 |
Abbreviations: AIS, acute ischemic stroke; A2L2, arm 2 or more points and leg 2 or more points (moderate to severe hemiparesis); CSC, comprehensive stroke center; CTA, computed tomographic angiography; DNT, door-to-needle time for IVT; DPT, CSC door-to-puncture time for EVT; EVT, endovascular thrombectomy; IQR, interquartile range; IVT, intravenous thrombolysis; LAO, large-artery occlusion; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; ODT, onset-to-first-hospital-door time; ONT, onset-to-needle time for IVT; OPT, onset-to-puncture time for EVT; SSTS, Stockholm Stroke Triage System.
During SSTS, 87 patients undergoing EVT (73%) arrived directly to the CSC compared with 25 (24%) in the previous year,(P < .001). In patients undergoing EVT with a known onset time, the median OPT was 2 hours and 17 minutes (IQR, 1 hour and 58 minutes to 3 hours) with the SSTS vs 3 hours and 26 minutes (IQR, 2 hours and 20 minutes to 4 hours and 40 minutes) the year before, a reduction of 69 minutes (P < .001). Meanwhile, the median ODT in patients undergoing EVT patients was higher during SSTS at 66 minutes (IQR, 51-87) vs 56 minutes (IQR, 40-104) the previous year (P = .05), and the CSC door-to-puncture time was longer at 57 minutes (IQR, 40-72) vs 45 minutes (IQR, 37-60) the previous year (P = .01). In patients undergoing IVT, the median ODT increased to 73 minutes from 70 minutes the previous year (P = .03). Meanwhile, the median regional DNT was lower during SSTS at 29 minutes (IQR, 16-49) vs 38 minutes (IQR, 26-54) (P < .001). The median CSC DNT was 13 minutes (IQR, 10-18) during the SSTS year compared with 31 minutes (IQR, 19-38) the previous year (P < .001).
Discussion
We conducted a large prospective observational study of a system for prehospital triage for patients with suspected acute stroke aimed toward predicting LAO and accelerating EVT delivery without delaying IVT. It was conducted in an urban region of 2.3 million inhabitants with 1 CSC and 6 PSCs. Our main findings indicate that the SSTS, based on the presence of moderate-to-severe hemiparesis and teleconsultation between the ambulance and a stroke physician in unselected code-stroke patients, can have high predictive accuracy for LAO stroke and EVT delivery. These findings are reinforced by a comparison of total in-hospital NIHSS scores between triage-positive and triage-negative patients (13 vs 3, respectively, with the NIHSS having a well-known association with LAO).20,21 The system increased the proportion of patients undergoing EVT arriving directly at the CSC to 73% from 24% during the previous year. In keeping with the many patients receiving EVT undergoing PSC bypass, the median OPT dropped by 69 minutes to 2 hours and 17 minutes from 3 hours and 26 minutes the previous year. This can be compared with a pooled median OPT in the pivotal EVT trials of 3 hours and 59 minutes.22 This major reduction occurred despite a 10-minute increase in time from the onset to first hospital door (likely attributable to longer ambulance drive times for PSC bypass cases) and a 12-minute higher CSC door-to-puncture time. The latter is explained by the need to perform a pre-EVT workup at the CSC in most EVT cases during the study year compared with the previous year, when most patients underwent some workup at the initial PSC.23
The reduction in OPT is similar to (approximately 1 hour) what was reported in recent PSC bypass studies using other prehospital triage algorithms. In Providence, Rhode Island, using a prehospital Los Angeles Motor Scale score of 4 or more for PSC bypass resulted in 59-minute shorter scene-departure-to-arterial-puncture time.24 Similarly, a study from Toledo, Ohio, using the prehospital rapid arterial occlusion evaluation (RACE) scale cutoff of more than 5 points for PSC bypass showed a 60-minute shorter time from first hospital arrival to EVT.25 A revalidation study of the RACE scale in 1822 patients in Catalonia, Spain, estimated that PSC bypass could lead to a 70-minute shorter OPT.26
Prior to SSTS implementation in 2017, there were concerns that longer transport times in PSC bypass cases could delay IVT treatment. However, the median ONT for IVT across the region was identical at 1 hour and 55 minutes during both periods. A slightly higher median onset-to-first-hospital-door time, 73 minutes vs 70 minutes the previous year, was offset by shorter median regional DNT, 29 minutes vs 38 minutes the previous year. Importantly, efforts to reduce DNT at the CSC were successful, with a median DNT of 13 minutes with the SSTS compared with 31 minutes the previous year. This was accomplished by implementing all measures described in 2012 as the Helsinki model,17 in addition to the fact that CSC patients undergoing IVT had relatively severe symptoms, facilitating rapid management.
At the SSTS design stage, limited CSC bed capacity and ambulance resources put a high priority on minimizing futile PSC bypass because such patients would, after the initial CSC workup, require ambulance transfer away to their local PSC. Thus, there was an a priori need for a high specificity and high negative predictive value (NPV) system. This was achieved by requiring a severe symptom (hemiparalysis) and PSC bypass approval by a stroke physician, which resulted in a relatively low proportion (323 [11%]) of ambulance code-stroke patients being triage positive. This can be compared with RACE scores of 5 or more, a commonly used triage-positive cutoff that classifies 48% as triage positive.26 The high SSTS NPV for LAO stroke (93%) and EVT initiation (NPV 99%) may be valuable in regional systems with a limited CSC bed capacity, mandating low rates of futile PSC bypass. A relevant comparison can be made with prehospital LAO triage using a mobile stroke unit (with onboard CTA). A recent study from Saarland, Germany, showed that this was capable of 100% triage accuracy for large-vessel occlusion, with the added benefit of rapid IVT initiation.27 The mobile stroke unit field has been developing rapidly, albeit amid an ongoing cost-efficacy debate, and the results of ongoing studies are anticipated.28,29 Another approach that has demonstrated reasonable time logistics in pilot studies is to transport the patient to the nearest PSC for potential IVT treatment and shipping the neurointerventionalist there from the CSC once an EVT treatment decision is made, the so-called drip and drive or drip and fly.30,31 The need for innovative approaches for stroke triage workflows is reinforced by published estimates that a pure mothership approach of taking all patients to hospitals performing EVT would result in a several-fold case load increase, likely exhausting the current capacity.32
Our approach using symptom severity and teleconsultation predicted LAO similarly to the recently published Ambulance Clinical Triage For Acute Stroke Treatment (ACT-FAST) algorithm from Melbourne, Australia.33 Like the SSTS, the ACT-FAST incorporates symptom severity assessment, a mimic screen, and some EVT eligibility questions, all administered by ambulance staff without teleconsultation. We believe the SSTS teleconsultation may allow more fine-grained prehospital diagnostic and contraindication assessment, improving transport decisions and facilitating in-hospital workflows through built-in prenotification.34
Limitations
Our study has several limitations. It was performed within a health care system with specific geographic, logistic, and organizational circumstances, which may limit generalizability. Meanwhile, the triage steps involved—a FAST test, evaluation of hemiparalysis, and teleconsultation with a stroke physician—are not complex and could be executed in most settings. Additionally, issues exist that could affect the interpretation of SSTS diagnostic accuracy. Of 981 patients classified as having AIS without proven LAO, 457 (47%) had no emergent CTA. The choice to abstain from CTA was made on clinical grounds or following local hospital guidelines, which included minor stroke symptoms with more than 8 hours since the last known well, manifest infarction on plain CT images matching the patient’s symptoms, severe renal failure, an allergy to intravenous contrast, or clear contraindications to EVT. It is likely that some patients without emergent CTA did have LAO. We cannot exclude that if all patients had undergone emergent vessel imaging that some might have been deemed eligible for EVT in regions or centers with different treatment criteria. We recommend validation of our system’s predictive accuracy for LAO and EVT also in settings with other criteria for routine vessel imaging and EVT treatment. Graphically examining quarterly medians of pre-hospital and intrahospital logistic metrics, we found no indication of major confounding associations from gradual improvement over time, within, or between individual study years (eFigures 1 and 2 in the Supplement). Meanwhile, improvement in CSC DNT was an inherent part of SSTS implementation to offset expected increases in transportation times. Overall, the study had a high degree of data completeness. However, NIHSS scores were unavailable for 383 (15%) of the triage-negative group, explained mainly by nonperformance of the NIHSS in patients deemed by the receiving physician to have low likelihood of acute stroke on hospital arrival. Lastly, we did not report comparisons of clinical outcomes. Power calculations have indicated the need for more patients undergoing EVT. Following 2 years of prospective SSTS data collection, we plan to analyze short-term neurological improvement and 3-month mRS scores compared with a 2-year retrospective period.
Conclusions
The SSTS, combining symptom severity and ambulance-to-hospital teleconsultation, has a high triage accuracy for LAO stroke and markedly reduces the time from stroke onset to EVT without delaying IVT.
eFigure 1. Quarterly evolution of medians of logistic time metrics from stroke onset
eFigure 2. Quarterly evolution of medians of logistic time metrics from hospital arrival
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 3.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 5.Froehler MT, Saver JL, Zaidat OO, et al. . Interhospital transfer prior to thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry. Circulation. 2017;136:2311–-2321.. doi: 10.1161/CIRCULATIONAHA.117.028920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwamm LH. Optimizing prehospital triage for patients with stroke involving large vessel occlusion: the road less traveled. JAMA Neurol. 2018;75(12):1467-1469. doi: 10.1001/jamaneurol.2018.2323 [DOI] [PubMed] [Google Scholar]
- 7.Almekhlafi MA, Holodinsky JK, Hill MD, Kamal N, Goyal M. Organizing stroke systems in the field for patients with suspected large vessel occlusion acute stroke. Expert Rev Cardiovasc Ther. 2019;17(1):3-9. [DOI] [PubMed] [Google Scholar]
- 8.Kuntze Söderqvist A, Kaijser M, Söderman M, Holmin S, Wahlgren N, Andersson T. Mechanical thrombectomy in acute ischemic stroke-experience from 6 years of practice. Neuroradiology. 2014;56(6):477-486. doi: 10.1007/s00234-014-1353-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berglund A, Svensson L, Sjöstrand C, et al. ; HASTA Collaborators . Higher prehospital priority level of stroke improves thrombolysis frequency and time to stroke unit: the Hyper Acute Stroke Alarm (HASTA) study. Stroke. 2012;43(10):2666-2670. doi: 10.1161/STROKEAHA.112.652644 [DOI] [PubMed] [Google Scholar]
- 10.Heldner MR, Hsieh K, Broeg-Morvay A, et al. . Clinical prediction of large vessel occlusion in anterior circulation stroke: mission impossible? J Neurol. 2016;263(8):1633-1640. doi: 10.1007/s00415-016-8180-6 [DOI] [PubMed] [Google Scholar]
- 11.Adams GF, Merrett JD. Prognosis and survival in the aftermath of hemiplegia. Br Med J. 1961;1(5222):309-314. doi: 10.1136/bmj.1.5222.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer U, Arnold M, Nedeltchev K, et al. . NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36(10):2121-2125. doi: 10.1161/01.STR.0000182099.04994.fc [DOI] [PubMed] [Google Scholar]
- 13.Brott T, Adams HP Jr, Olinger CP, et al. . Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864-870. doi: 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 14.Gupta R, Manuel M, Owada K, et al. . Severe hemiparesis as a prehospital tool to triage stroke severity: a pilot study to assess diagnostic accuracy and treatment times. J Neurointerv Surg. 2016;8(8):775-777. [DOI] [PubMed] [Google Scholar]
- 15.Cooray C, Mazya MV, Bottai M, et al. . Are you suffering from a large arterial occlusion? please raise your arm! Stroke Vasc Neurol. 2018;3(4):215-221. doi: 10.1136/svn-2018-000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Council SC. Prehospital triagering av patienter med stroke—Komplettering till det regionala vårdprogrammet om stroke i SLL. Accessed December 14, 2019. https://vardgivarguiden.se/globalassets/kunskapsstod/vardprogram/prehospitala-riktlinjer-for-stroke.pdf
- 17.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79(4):306-313. doi: 10.1212/WNL.0b013e31825d6011 [DOI] [PubMed] [Google Scholar]
- 18.Bossuyt PM, Reitsma JB, Bruns DE, et al. ; STARD Group . STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 20.Cooray C, Fekete K, Mikulik R, Lees KR, Wahlgren N, Ahmed N. Threshold for NIH stroke scale in predicting vessel occlusion and functional outcome after stroke thrombolysis. Int J Stroke. 2015;10(6):822-829. doi: 10.1111/ijs.12451 [DOI] [PubMed] [Google Scholar]
- 21.Heldner MR, Zubler C, Mattle HP, et al. . National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157. doi: 10.1161/STROKEAHA.111.000604 [DOI] [PubMed] [Google Scholar]
- 22.Bourcier R, Goyal M, Liebeskind DS, et al. ; HERMES Trialists Collaboration . Association of time from stroke onset to groin puncture with quality of reperfusion after mechanical thrombectomy: a meta-analysis of individual patient data from 7 randomized clinical trials. JAMA Neurol. 2019;76(4):405-411. doi: 10.1001/jamaneurol.2018.4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber R, Reimann G, Weimar C, et al. ; Neurovascular Net Ruhr . Outcome and periprocedural time management in referred versus directly admitted stroke patients treated with thrombectomy. Ther Adv Neurol Disord. 2016;9(2):79-84. doi: 10.1177/1756285615617081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayaraman MV, Hemendinger ML, Baird GL, et al. . Field triage for endovascular stroke therapy: a population-based comparison. J Neurointerv Surg. 2019;neurintsurg-2019-015033. doi: 10.1136/neurintsurg-2019-015033 [DOI] [PubMed] [Google Scholar]
- 25.Zaidi SF, Shawver J, Espinosa Morales A, et al. . Stroke care: initial data from a county-based bypass protocol for patients with acute stroke. J Neurointerv Surg. 2017;9(7):631-635. doi: 10.1136/neurintsurg-2016-012476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrera D, Gorchs M, Querol M, et al. ; Catalan Stroke Code and Reperfusion Consortium (Cat-SCR) . Revalidation of the RACE scale after its regional implementation in Catalonia: a triage tool for large vessel occlusion. J Neurointerv Surg. 2019;11(8):751-756. doi: 10.1136/neurintsurg-2018-014519 [DOI] [PubMed] [Google Scholar]
- 27.Helwig SA, Ragoschke-Schumm A, Schwindling L, et al. . Prehospital stroke management optimized by use of clinical scoring vs mobile stroke unit for triage of patients with stroke: a randomized clinical trial. JAMA Neurol. 2019. doi: 10.1001/jamaneurol.2019.2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fassbender K, Grotta JC, Walter S, Grunwald IQ, Ragoschke-Schumm A, Saver JL. Mobile stroke units for prehospital thrombolysis, triage, and beyond: benefits and challenges. Lancet Neurol. 2017;16(3):227-237. doi: 10.1016/S1474-4422(17)30008-X [DOI] [PubMed] [Google Scholar]
- 29.Yamal JM, Rajan SS, Parker SA, et al. . Benefits of stroke treatment delivered using a mobile stroke unit trial. Int J Stroke. 2018;13(3):321-327. doi: 10.1177/1747493017711950 [DOI] [PubMed] [Google Scholar]
- 30.Brekenfeld C, Goebell E, Schmidt H, et al. . ‘Drip-and-drive’: shipping the neurointerventionalist to provide mechanical thrombectomy in primary stroke centers. J Neurointerv Surg. 2018;10(10):932-936. doi: 10.1136/neurintsurg-2017-013634 [DOI] [PubMed] [Google Scholar]
- 31.Srinivas A, Bhagat N, Lynch J, et al. . Six months later: final helistroke pilot time analysis. J Vasc Interv Radiol. 2019;30(10):1714-1716. doi: 10.1016/j.jvir.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 32.Katz BS, Adeoye O, Sucharew H, et al. . Estimated impact of emergency medical service triage of stroke patients on comprehensive stroke centers: an urban population-based study. Stroke. 2017;48(8):2164-2170. doi: 10.1161/STROKEAHA.116.015971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H, Pesavento L, Coote S, et al. . Ambulance clinical triage for acute stroke treatment: paramedic triage algorithm for large vessel occlusion. Stroke. 2018;49(4):945-951. doi: 10.1161/STROKEAHA.117.019307 [DOI] [PubMed] [Google Scholar]
- 34.Xian Y, Xu H, Lytle B, et al. . Use of strategies to improve door-to-needle times with tissue-type plasminogen activator in acute ischemic stroke in clinical practice: findings from target: stroke. Circ Cardiovasc Qual Outcomes. 2017;10(1):e003227. doi: 10.1161/CIRCOUTCOMES.116.003227 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Quarterly evolution of medians of logistic time metrics from stroke onset
eFigure 2. Quarterly evolution of medians of logistic time metrics from hospital arrival