Abstract
SARS-CoV-2, the causal agent of COVID-19, first emerged in late 2019 in China. It has since infected more than 870,000 individuals and caused more than 43,000 deaths globally. Here, we discuss therapeutic and prophylactic interventions for SARS-CoV-2 with a focus on vaccine development and its challenges. Vaccines are being rapidly developed but will likely come too late to affect the first wave of a potential pandemic. Nevertheless, critical lessons can be learned for the development of vaccines against rapidly emerging viruses. Importantly, SARS-CoV-2 vaccines will be essential to reducing morbidity and mortality if the virus establishes itself in the population.
Amanat and Krammer discuss therapeutic and prophylactic interventions for SARS-CoV-2, the causal agent of COVID-19, with a focus on vaccine development and its challenges.
Main Text
On December 31, 2019, several cases of pneumonia of unknown etiology were reported in Wuhan, China. The outbreak had started in early December or November (Huang et al., 2020), and the number of cases rose quickly; more than 80,000 infections were reported in China as of March 15, 2020, including more than 3,000 deaths. At the time of this review (April 6, 2020), the disease, termed COVID-19 (coronavirus disease 2019), had become pandemic and spread to more than 203 countries and territories, including community transmission in countries like the United States, Germany, France, Spain, Japan, Singapore, South Korea, Iran, and Italy and a large-scale outbreak with more than 600 cases on the cruise ship Diamond Princess. As of April 1, more than 870,000 cases and 43,000 deaths had been reported globally, with rapid growth of numbers in many countries. The causative agent of the outbreak was swiftly identified as betacoronavirus with a genomic sequence closely related to that of the severe acute respiratory syndrome (SARS) coronavirus from 2003, earning the new virus the name SARS-CoV-2 (Gorbalenya et al., 2020, Wu et al., 2020, Zhou et al., 2020, Zhu et al., 2020). SARS-CoV-2 likely originated in bats but might have been amplified in an intermediate host. Initial work showed that it can use angiotensin-converting enzyme 2 (ACE2) from bats, civet cats, swine, cats, ferrets, non-human primates (NHPs), and humans as a receptor (Letko et al., 2020, Wan et al., 2020, Zhou et al., 2020). Transmission of the infection to a pet dog in Hong Kong suggests that canine ACE2 can also be recognized by SARS-CoV-2. Pangolins, protected animals that are traded illegally in Asia and elsewhere, have been proposed as a potential amplifying host by some studies (Lam et al., 2020, Zhang et al., 2020). The initial reports from China and elsewhere note that although most COVID-19 cases present mild to moderate pathology, approximately 20% percent of cases are severe (Chen et al., 2020, Guan et al., 2020, Huang et al., 2020, Novel Coronavirus Pneumonia Emergency Response Epidemiology Team and AuthorAnonymous, 2020, Wang et al., 2020). The case fatality rate (CFR) seems to be age dependent, with a higher percentage in the elderly, especially men, and an overall interim CFR of approximately 1%–3%. The number of individuals with undetected, mild cases could be much higher than the official case number, which would lead to a lower infection fatality rate (IRF). South Korea, a country that put a massive effort into testing and has already tested tens of thousands of samples, reports much lower CFRs than countries without extensive testing like Spain, Iran, or the United States. The CFR is disproportionally high in Italy (currently 7.3%), likely because of a large number of mild cases missed combined with a relatively older population and a healthcare system that is overwhelmed with cases. The reproductive number (R0) of the infection, that is, the number of cases directly generated by one case in a population in which all individuals are susceptible to infection, is estimated to be 2–3 (Li et al., 2020). Given the severity of the disease, which in most age groups is above that of seasonal influenza or pandemic H1N1 (2009) influenza, vaccines and therapeutics to tackle this novel virus are urgently needed.
Coronaviruses, in Brief
SARS-CoV-2 is part of the Coronaviridae family, whose members are named after their crown-like appearance under the electron microscope caused by the surface glycoproteins that decorate the virus. The family includes two subfamilies: Letovirinae and Orthocoronavirinae. Orthocoronavirinae includes the genera Alphacoronvirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. Alphacoronaviruses and betacoronaviruses typically infect only mammals, whereas gammacoronoviruses and deltacoronaviruses typically infect avian species and sometimes mammals (Cui et al., 2019). Coronaviruses are common human pathogens; two types of alphacoronaviruses (229E and NL63) and two types of betacoronaviruses (OC43 and HKU1) circulate in humans and cause common cold. More pathogenic coronaviruses for humans include SARS-CoV-1, the Middle Eastern respiratory syndrome coronavirus (MERS-CoV), and now SARS-CoV-2, which are all betacoronaviruses.
Coronaviruses have a large (30+ kb) single-stranded positive-sense RNA genome encoding for several open reading frames. One frame encodes the spike protein (S protein), a class I fusion protein that mediates attachment of the virus to cell surface receptors followed by uptake into endosomes (for most coronaviruses). Proteolytic cleavage of the S protein and fusion of viral and endosomal membranes trigger release of viral RNA into the cytosol (reviewed in Fehr and Perlman, 2015). The RNA contains a 5′ cap structure and a 3′ poly(A) tail that allows expression of the replicase, which is encoded by approximately two-thirds of the genome. The other third codes for the structural and accessory proteins. The replicase is expressed as two polyproteins: pp1a and pp1ab; these include up to 16 nonstructural proteins (nsps). The nsps are generated by processing of pp1a and pp1ab by 2–3 viral proteases encoded within the replicase. Many nsps then assemble into the replicase-transcriptase complex that—in the host cell cytosol—produces anti-sense genome, new viral genome, and subgenomic RNA that serves as mRNA. Structural proteins S, matrix (M) protein, and envelope (E) are then generated and inserted into the endoplasmatic reticulum and follow the secretory pathway to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC). A minority of coronaviruses also encode a hemagglutinin esterase (HE). In many coronaviruses, the S protein is cleaved into two subunits, S1 and S2, often by furin-like proteases. The RNA genome associates with nucleoprotein and then buds into the ERGIC, forming virus particles. After assembly, virions are transported to the cell surface in vesicles and are exocytosed. Several accessory proteins, which seem to be important for pathogenesis, are also expressed but not all are functionally characterized.
Therapeutics for SARS-CoV-2 Infections
Clinical trials with the nucleotide analog remdesivir (ClinicalTrials.gov: NCT04257656, NCT04252664, NCT04280705, etc.) and protease inhibitors (ClinicalTrials.gov: NCT04255017, NCT04276688, etc.), as well as other treatment options, are ongoing in China and the United States, and trial results are expected within weeks. Remdesivir works against coronaviruses closely related to SARS-CoV-2 in animal models, as well as against the related MERS-CoV, including in NHPs (Agostini et al., 2018, Brown et al., 2019, de Wit et al., 2020, Sheahan et al., 2017, Sheahan et al., 2020). Remdesivir was also tested for treatment of ebolavirus infections in humans (and found to be less successful than other treatments by Mulangu et al., 2019); therefore, safety data exist for this therapeutic agent, which should accelerate the process of clinical testing against SARS-CoV-2. Remdesivir’s mechanism of action as a nucleotide analog is not clear, but it likely terminates RNA synthesis, leads to incorporation mutagenesis, or both (Agostini et al., 2018). In addition, a combination of the two licensed HIV inhibitors, lopinavir and ritonavir, is also being tested in clinical trials (e.g., ClinicalTrials.gov: NCT04264858, etc.). Lopinavir is a bona fide protease inhibitor, whereas ritonavir was initially designed as protease inhibitor but was found to boost the half-life of lopinavir by inhibiting cytochrome P450 (Hull and Montaner, 2011). The combination was compassionately used as treatment for SARS-CoV-1 in 2003–2004 and showed some promise (Chu et al., 2004). Effectiveness of the combination was limited in mice but appreciable in NHP models of MERS-CoV (Chan et al., 2015, Sheahan et al., 2020). The mechanism of action of lopinavir is not clear, but it likely inhibits one or more coronavirus proteases. Other treatment options with ongoing or planned clinical trials include dosing recombinant human ACE2 to neutralize the virus and prevent lung damage (ClinicalTrials.gov: NCT04287686) and using the antiviral arbidol, a fusion inhibitor (Kadam and Wilson, 2017, Teissier et al., 2011). Another interesting option is the use of convalescent serum as treatment; clinical trials to test this are ongoing in China (ClinicalTrials.gov: NCT04264858, placebo control, not recruiting yet), and compassionate use of this strategy has recently started in the US (e.g. at Mount Sinai Medical Center, NY). Similarly, polyclonal human immunoglobulin G (IgG) derived from transgenic cows could be used, because this strategy has been successful for MERS-CoV in animal models (Luke et al., 2016) and has been tested for safety in clinical trials (ClinicalTrials.gov: NCT02788188). Many of these trials will have results within months, and if remdesivir (produced by Gilead) and/or lopinavir plus ritonavir (produced by AbbVie as Kaletra and Aluvia, respectively) show effectiveness, they could potentially be used widely within a short time frame. Compassionate use of these drugs has already been reported for SARS-CoV-2 infections (Holshue et al., 2020, Lim et al., 2020).
What Do We Know about Betacoronavirus Vaccine Design?
During the 2009 H1N1 influenza virus pandemic, vaccine producers switched their production pipelines quickly from producing trivalent seasonal influenza virus vaccines to monovalent pandemic vaccines. This was basically just a change of strains and established and approved processes, established release criteria, and existing correlates of protection could be used (Krammer and Palese, 2015). Still, it took six months until the vaccine was ready to be distributed and used, and it came too late to affect the second pandemic wave, which took place in the United States in fall 2009. This time, we are facing a new challenge in the form of a virus that has just emerged in humans, and the response will be more complex because there are no existing vaccines or production processes for coronavirus vaccines.
Vaccine technology has significantly evolved in the last decade, including the development of several RNA and DNA vaccine candidates, licensed vectored vaccines (e.g., Ervebo, a vesicular stomatitis virus [VSV]-vectored ebolavirus vaccine, licensed in the European Union), recombinant protein vaccines (e.g., Flublok, an influenza virus vaccine made in insect cells, licensed in the United States), and cell-culture-based vaccines (e.g., Flucelvax, an influenza virus vaccine made in mammalian cells). SARS-CoV-2 was identified in record time, and its genomic sequence was swiftly made widely available by Chinese researchers (Wu et al., 2020, Zhou et al., 2020, Zhu et al., 2020). In addition, we know from studies on SARS-CoV-1 and the related MERS-CoV vaccines that the S protein on the surface of the virus is an ideal target for a vaccine. In SARS-CoV-1 and SARS-CoV-2, this protein interacts with the receptor ACE2, and antibodies targeting the spike can interfere with this binding, thereby neutralizing the virus (Figure 1 ). The structure of the S protein of SARS-CoV-2 was solved in record time at high resolution, contributing to our understanding of this vaccine target (Lan et al., 2020a, Wrapp et al., 2020). Therefore, we have a target antigen that can be incorporated into advanced vaccine platforms.
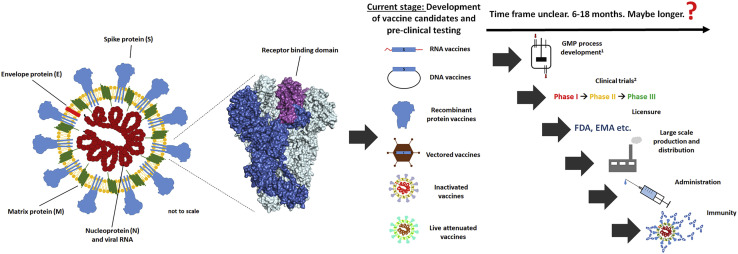
Figure 1.
Overview of Potential SARS-CoV-2 Vaccine Platforms
The structure of a coronavirus particle is depicted on the left, with the different viral proteins indicated. The S protein is the major target for vaccine development. The spike structure shown is based on the trimeric SARS-CoV-1 spike (PDB: 5XL3). One trimer is shown in dark blue, and the receptor binding domain, a main target of neutralizing antibodies, is highlighted in purple. The other two trimers are shown in light blue. SARS-CoV-2 vaccine candidates based on different vaccine platforms have been developed, and for some of them, pre-clinical experiments have been initiated. For one mRNA-based candidate, a clinical trial recently started to enroll volunteers shortly (ClinicalTrials.gov: NCT04283461). However, many additional steps are needed before these vaccines can be used in the population, and this process might take months, if not years. 1For some candidates, cGMP processes have already been established. 2Clinical trial design might be altered to move vaccines through clinical testing quicker.
Several vaccines for SARS-CoV-1 were developed and tested in animal models, including recombinant S-protein-based vaccines, attenuated and whole inactivated vaccines, and vectored vaccines (Roper and Rehm, 2009). Most of these vaccines protect animals from challenge with SARS-CoV-1, although many do not induce sterilizing immunity. In some cases, vaccination with the live virus results in complications, including lung damage and infiltration of eosinophils in a mouse model (e.g., Bolles et al., 2011, Tseng et al., 2012) and liver damage in ferrets (e.g., Weingartl et al., 2004). In another study, vaccination with inactivated SARS-CoV-1 led to enhancement of disease in one NHP, whereas it protected 3 animals from challenge (Wang et al., 2016). The same study identified certain epitopes on the S protein as protective, whereas immunity to others seemed to be enhancing disease. However, in almost all cases, vaccination is associated with greater survival, reduced virus titers, and/or less morbidity compared with that in unvaccinated animals. Similar findings have been reported for MERS-CoV vaccines (Agrawal et al., 2016, Houser et al., 2017). Therefore, whereas vaccines for related coronaviruses are efficacious in animal models, we need to ensure that the vaccines, which are developed for SARS-CoV-2, are sufficiently safe.
Another consideration for effective coronavirus vaccine development might be waning of the antibody response. Infection with human coronaviruses does not always induce long-lived antibody responses, and re-infection of an individual with the same virus is possible after an extended period of time (but only in a fraction of individuals and resulting in mild or no symtpoms), as shown in human challenge studies (Callow et al., 1990). Antibody titers in individuals that survived SARS-CoV-1 or MERS-CoV infections often waned after 2–3 years (Liu et al., 2006, Wu et al., 2007) or were weak initially (Choe et al., 2017). Despite that, re-infections are unlikely in the short term. Of note, re-infections after days of recovery have been reported recently but appear to be the consequences of false negative test results (Lan et al., 2020b). However, they could happen when humoral immunity wanes over months and years. An effective SARS-CoV-2 vaccine will need to overcome these issues to protect in a scenario in which the virus becomes endemic and causes recurrent seasonal epidemics.
SARS-CoV-2 infection causes the most severe pathology in individuals above 50 years of age. The reason for this is not clear, but many viral infections have milder manifestations in naive younger individuals than in naive older individuals. Because older individuals are more affected, it will be important to develop vaccines that protect this segment of the population. Unfortunately, older individuals typically respond less well to vaccination because of immune senescence (Sambhara and McElhaney, 2009). For influenza, which is problematic for older adults, specific formulations for this segment of the population include more antigen or an adjuvant (DiazGranados et al., 2013, Tsai, 2013). Protection in older individuals appears to require higher neutralization titers against influenza virus than in younger individuals (Benoit et al., 2015), and this issue might need to be addressed for SARS-CoV-2. If vaccination in older individuals is not effective, they could still benefit indirectly if vaccination is able to stop transmission of the virus in younger individuals.
Only a small number of SARS-CoV-1 vaccines made it to phase I clinical trials before funding dried up because of eradication of the virus from the human population through non-pharmaceutical interventions when case numbers were still small. Results from these trials, performed with an inactivated virus vaccine and a spike-based DNA vaccine, are encouraging because the vaccines were safe and induced neutralizing antibody titers (Lin et al., 2007, Martin et al., 2008). Some neutralizing monoclonal antibodies isolated against SARS-CoV-1, like CR3022 (ter Meulen et al., 2006, Tian et al., 2020), can cross-react to the receptor binding domain of SARS-CoV-2. This suggests that SARS-CoV-1 vaccines might cross-protect against SARS-CoV-2. However, because these vaccines have not been developed further than phase I, they are currently not available for use. Vaccines against MERS-CoV, also targeting the MERS-CoV S protein, are in pre-clinical and clinical development, including vaccines based on modified vaccinia Ankara vectors, adenovirus vectors, and DNA-based vaccines, and several of them are supported by the Coalition for Epidemic Preparedness Innovation (CEPI) (Yong et al., 2019). However, it is unlikely that MERS-CoV vaccines induce strong cross-neutralizing antibodies to SARS-CoV-2 because of the phylogenetic distance between the two viruses. Nevertheless, we can still learn a lot from these vaccines about how to move forward with SARS-CoV-2 vaccine design (Pallesen et al., 2017).
The Current Pipeline for SARS-CoV-2 Vaccines
The development of vaccines for human use can take years, especially when novel technologies are used that have not been extensively tested for safety or scaled up for mass production. Because no coronavirus vaccines are on the market and no large-scale manufacturing capacity for these vaccines exists as yet (Table 1 ), we will need to build these processes and capacities. Doing this for the first time can be tedious and time consuming (Figure 1). CEPI has awarded funds to several highly innovative players in the field, and many of them will likely succeed in eventually making a SARS-CoV-2 vaccine. However, none of these companies and institutions have an established pipeline to bring such a vaccine to late-stage clinical trials that allow licensure by regulatory agencies, and they do not currently have the capacity to produce the number of doses needed. An mRNA-based vaccine, which expresses target antigen in vivo in the vaccinee after injection of mRNA encapsulated in lipid nanoparticles, co-developed by Moderna and the Vaccine Research Center at the National Institutes of Health, is currently the furthest along, and a phase I clinical trial recently started (ClinicalTrials.gov: NCT04283461). Curevac is working on a similar vaccine but is still in the pre-clinical phase. Additional approaches in the pre-clinical stage include recombinant-protein-based vaccines (focused on the S protein, e.g., ExpresS2ion, iBio, Novavax, Baylor College of Medicine, University of Queensland, and Sichuan Clover Biopharmaceuticals), viral-vector-based vaccines (focused on the S protein, e.g., Vaxart, Geovax, University of Oxford, and Cansino Biologics), DNA vaccines (focused on the S protein, e.g., Inovio and Applied DNA Sciences), live attenuated vaccines (Codagenix with the Serum Institute of India, etc.), and inactivated virus vaccines (Figure 1; Table 1). All of these platforms have advantages and disadvantages (Table 1), and it is not possible to predict which strategy will be faster or more successful. Johnson & Johnson (J&J) (Johnson & Johnson, 2020) and Sanofi (2020) recently joined efforts to develop SARS-CoV-2 vaccines. However, J&J is using an experimental adenovirus vector platform that has not yet resulted in a licensed vaccine. Sanofi’s vaccine, to be made using a process similar to the process used for their approved Flublok recombinant influenza virus vaccine (Zhou et al., 2006), is also months, if not years, from being ready for use in the human population.
Table 1.
Overview of Vaccine Production Platforms and Technologies for SARS-CoV-2
| Platform | Target | Existing, Licensed Human Vaccines Using the Same Platform | Advantages | Disadvantages |
|---|---|---|---|---|
| RNA vaccines | S protein | No | No infectious virus needs to be handled, vaccines are typically immunogenic, rapid production possible. | Safety issues with reactogenicity have been reported. |
| DNA vaccines | S protein | No | No infectious virus needs to be handled, easy scale up, low production costs, high heat stability, tested in humans for SARS-CoV-1, rapid production possible. | Vaccine needs specific delivery devices to reach good immunogenicity. |
| Recombinant protein vaccines | S protein | Yes for baculovirus (influenza, HPV) and yeast expression (HBV, HPV) | No infectious virus needs to be handled, adjuvants can be used to increase immunogenicity. | Global production capacity might be limited. Antigen and/or epitope integrity needs to be confirmed. Yields need to be high enough. |
| Viral vector-based vaccines | S protein | Yes for VSV (Ervebo), but not for other viral vectored vaccines | No infectious virus needs to be handled, excellent preclinical and clinical data for many emerging viruses, including MERS-CoV. | Vector immunity might negatively affect vaccine effectiveness (depending on the vector chosen). |
| Live attenuated vaccines | Whole virion | Yes | Straightforward process used for several licensed human vaccines, existing infrastructure can be used. | Creating infectious clones for attenuated coronavirus vaccine seeds takes time because of large genome size. Safety testing will need to be extensive. |
| Inactivated vaccines | Whole virion | Yes | Straightforward process used for several licensed human vaccines, existing infrastructure can be used, has been tested in humans for SARS-CoV-1, adjuvants can be used to increase immunogenicity. | Large amounts of infectious virus need to be handled (could be mitigated by using an attenuated seed virus). Antigen and/or epitope integrity needs to be confirmed. |
Understanding the Time Frames
Why does this take so long? As mentioned earlier, there are currently no approved human coronavirus vaccines. In addition, many technologies used (production platforms, vectors, etc.) are new and need to be tested thoroughly for safety. The target for the vaccine, the S protein, has been identified, and vaccine candidates are being generated. This is usually followed by two important steps that are typically needed before bringing a vaccine into clinical trials. First, the vaccine is tested in appropriate animal models to see whether it is protective. However, animal models for SARS-CoV-2 might be difficult to develop. The virus does not grow in wild-type mice and only induced mild disease in transgenic animals expressing human ACE2 (Bao et al., 2020). Other potential animal models include ferrets and NHPs, for which pathogenicity studies are ongoing. Even in the absence of an animal model that replicates human disease, it is possible to evaluate the vaccine because serum from vaccinated animals can be tested in in vitro neutralization assays. Post-challenge safety data should also be collected in these cases to assess for complications such as the ones seen SARS-CoV-1 and MERS-CoV vaccines. Second, vaccines need to be tested for toxicity in animals, e.g., in rabbits. Usually, viral challenge is not part of this process, because only the safety of the vaccine will be evaluated. This testing, which has to be performed in a manner compliant with GLP (Good Laboratory Practice), typically takes 3–6 months to complete. For some vaccine platforms, parts of the safety testing might be skipped if there is already sufficient data available for similar vaccines made in the same production process.
Vaccines for human use are produced in processes that comply with current Good Manufacturing Practice (cGMP) to ensure constant quality and safety of vaccines. This requires dedicated facilities, trained personnel, proper documentation, and raw material that was produced to be of cGMP quality. These processes have to be designed or amended to fit SARS-CoV-2 vaccines. For many vaccine candidates in the preclinical phase, such processes do not yet exist and have to be developed from scratch.
Once sufficient pre-clinical data are available and initial batches of the vaccine have been produced that are of cGMP quality, clinical trials might be initiated. Typically, clinical development of vaccines starts with small phase I trials to evaluate the safety of vaccine candidates in humans. These are followed by phase II trials (formulation and doses are established to initially prove efficacy) and finally by phase III trials, in which the efficacy and safety of a vaccine need to be demonstrated in a larger cohort. However, in an extraordinary situation like the current one, this scheme might be compressed and an accelerated regulatory approval pathway might be developed. If efficacy is shown, a vaccine might be licensed by regulatory agencies.
Another important point is that production capacity to produce sufficient amounts of cGMP-quality vaccine needs to be available. For vaccines based on existing vaccine platforms, e.g., inactivated or live attenuated vaccines, this can be relatively easily achieved, because existing infrastructure can be used (Table 1). For vaccines based on novel technologies, e.g., mRNA, this capacity needs to be built, and this typically takes time. Although it would be beneficial if even a limited number of doses were available to protect health care workers and the most vulnerable segments of the population, the goal should be to make vaccines available to the global population. This will be challenging. Even for influenza virus vaccines, for which many production facilities exist in high-income countries, as well as low- and middle-income countries, the demand in the case of a pandemic would by far exceed the production capacity.
Finally, it takes time to distribute vaccines and administer them. To vaccinate a large proportion of the population would likely take weeks. Given that the population is currently naive to SARS-CoV-2, it is highly likely that more than one dose of the vaccine will be needed. Prime-boost vaccination regimens are typically used in such cases, and the two vaccinations are usually spaced 3–4 weeks apart. It is likely that protective immunity will be achieved only 1–2 weeks after the second vaccination. This therefore adds another 1–2 months to the timeline. Even if shortcuts for several of the steps mentioned earlier can be found, it is unlikely that a vaccine would be available earlier than 6 months after the initiation of clinical trials. Realistically, SARS-CoV-2 vaccines will not be available for another 12–18 months.
What are potential solutions for these long time frames in the future? One possibility is to build production capacity, to be globally distributed if possible, that can be activated in the event of new emerging viruses. From today’s perspective, only a few types of viruses are likely to cause respiratory disease that leads to rapid global spread. Surveillance in the animal reservoir paired with virus characterization studies can identify members of virus families that have the potential to cause pandemics. Vaccine candidates using these isolates could then be produced, tested in animals to determine the mechanisms of protection, and tested in humans to establish the safety of the vaccines. It is unlikely that the same viruses that are chosen as vaccine candidates will later cause outbreaks. However, if the vaccine candidate is sufficiently closely related, sequences for the vaccines could be quickly switched and the vaccines for the newly emerging viruses could be swiftly produced and moved to late-stage clinical trials right away (while large-scale production is ramped up globally). In addition, stockpiled vaccines based on the initial candidates could be deployed, even if slightly mismatched to the strain causing the outbreak (a strategy that is currently used for H5 and H7 avian influenza virus vaccines). This would allow a response within a few weeks and could potentially stop a virus locally before it becomes pandemic. An alternative but challenging solution would be the development of broadly protective vaccines that cover whole virus families or genera. This effort is ongoing for influenza viruses (Erbelding et al., 2018) and could potentially be applied to coronaviruses, or at least betacoronaviruses. Both of these options are costly and require global political will and vision.
Concluding Remarks
Considering the deep dive stock markets have taken in recent weeks and given the expected effect of a pandemic on the economy, funding for vaccine production infrastructure that would allow a swift response to emerging viruses looks like a great investment. However, without a pandemic looming, such investments have rarely been made in the past, except for H5 and H7 subtype influenza viruses. Now would be the right time to consider investing in vaccines against emerging viruses that can lead to loss of human lives and burden the global economy. An investment of a few billion dollars would allow us to have sufficient surveillance, appropriate vaccine candidates, and infrastructure ready that could churn out vaccines for use in the global population quickly and effectively, potentially stopping an emerging virus in its tracks. In addition, we need well-developed emergency plans that allow us to develop, test, produce, and distribute vaccines within weeks, not months or years. This would need tight coordination among pharmaceutical companies, governments, regulatory agencies, and the World Health Organization (WHO), as well as novel and out-of-the-box approaches to cGMP production, release processes, regulatory science, and clinical trial design.
For SARS-CoV-2, vaccines might come too late to affect the first wave of this pandemic. However, they might be useful if additional waves occur later or in a post-pandemic scenario in which SARS-CoV-2 continues to circulate as a seasonal virus. In addition, lessons learned from handling this outbreak will allow us to be better prepared in the future. The viruses will keep coming.
Acknowledgments
We thank Francesco Berlanda-Scorza for providing feedback on this manuscript. Work on influenza virus vaccines and immunity in the Krammer laboratory is supported by the National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00051, NIAID Centers of Excellence for Influenza Research and Surveillance (CEIRS) contract HHSN272201400008C, and NIAID grants AI117287 and AI128821, as well as funding from the U.S. Department of Defense and the Bill and Melinda Gates Foundation. Work on SARS-CoV-2 reagents is supported by CEIRS and institutional seed funding; reagents are being deposited into BEI Resources to support SARS-CoV-2 research and countermeasure development.
References
- Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. MBio. 2018;9 doi: 10.1128/mBio.00221-18. e00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A.S., Tao X., Algaissi A., Garron T., Narayanan K., Peng B.H., Couch R.B., Tseng C.T. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccin. Immunother. 2016;12:2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Deng W., Huang B., Gao H., Ren L., Wei Q., Yu P., Xu Y., Liu J., Qi F. The Pathogenicity of 2019 Novel Coronavirus in hACE2 Transgenic Mice. bioRxiv. 2020 doi: 10.1101/2020.02.07.939389. [DOI] [Google Scholar]
- Benoit A., Beran J., Devaster J.M., Esen M., Launay O., Leroux-Roels G., McElhaney J.E., Oostvogels L., van Essen G.A., Gaglani M. Hemagglutination Inhibition Antibody Titers as a Correlate of Protection Against Seasonal A/H3N2 Influenza Disease. Open Forum Infect. Dis. 2015;2:ofv067. doi: 10.1093/ofid/ofv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M., Baric R.S. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85:12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.J., Won J.J., Graham R.L., Dinnon K.H., 3rd, Sims A.C., Feng J.Y., Cihlar T., Denison M.R., Baric R.S., Sheahan T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow K.A., Parry H.F., Sergeant M., Tyrrell D.A. The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L., Li F., Xiao C., Gao H., Yu P. Treatment With Lopinavir/Ritonavir or Interferon-β1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. J. Infect. Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe P.G., Perera R.A.P.M., Park W.B., Song K.H., Bang J.H., Kim E.S., Kim H.B., Ko L.W.R., Park S.W., Kim N.J. MERS-CoV Antibody Responses 1 Year after Symptom Onset, South Korea, 2015. Emerg. Infect. Dis. 2017;23:1079–1084. doi: 10.3201/eid2307.170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., HKU/UCH SARS Study Group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA. 2020 doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados C.A., Dunning A.J., Jordanov E., Landolfi V., Denis M., Talbot H.K. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009–2010 season. Vaccine. 2013;31:861–866. doi: 10.1016/j.vaccine.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Erbelding E.J., Post D.J., Stemmy E.J., Roberts P.C., Augustine A.D., Ferguson S., Paules C.I., Graham B.S., Fauci A.S. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018;218:347–354. doi: 10.1093/infdis/jiy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C. Clinical characteristics of 2019 novel coronavirus infection in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Washington State 2019-nCoV Case Investigation Team First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser K.V., Broadbent A.J., Gretebeck L., Vogel L., Lamirande E.W., Sutton T., Bock K.W., Minai M., Orandle M., Moore I.N., Subbarao K. Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody. PLoS Pathog. 2017;13:e1006565. doi: 10.1371/journal.ppat.1006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull M.W., Montaner J.S. Ritonavir-boosted protease inhibitors in HIV therapy. Ann. Med. 2011;43:375–388. doi: 10.3109/07853890.2011.572905. [DOI] [PubMed] [Google Scholar]
- Johnson and Johnson . 2020. What You Need to Know About the Latest on the Coronavirus—and a Potential Preventive Vaccine.https://www.jnj.com/latest-news/what-you-need-to-know-about-coronavirus-and-a-potential-johnson-johnson-vaccine [Google Scholar]
- Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. USA. 2017;114:206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F., Palese P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 2015;14:167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- Lam T.T.-Y., Shum M.H.-H., Zhu H.-C., Tong Y.-G., Ni X.-B., Liao Y.-S., Wei W., Cheung W.Y.-M., Li W.-J., Li L.-F. Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. bioRxiv. 2020 doi: 10.1101/2020.02.13.945485. [DOI] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Crystal structure of the 2019-nCoV spike receptor-binding domain bound with the ACE2 receptor. bioRxiv. 2020 doi: 10.1101/2020.02.19.956235. [DOI] [PubMed] [Google Scholar]
- Lan L., Xu D., Ye G., Xia C., Wang S., Li Y., Xu H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage betacoronaviruses. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.W., Kang Y.M., Lee B., Park S.J. Case of the Index Patient Who Caused Tertiary Transmission of COVID-19 Infection in Korea: the Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Infected Pneumonia Monitored by Quantitative RT-PCR. J. Korean Med. Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.T., Zhang J.S., Su N., Xu J.G., Wang N., Chen J.T., Chen X., Liu Y.X., Gao H., Jia Y.P. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir. Ther. (Lond.) 2007;12:1107–1113. [PubMed] [Google Scholar]
- Liu W., Fontanet A., Zhang P.H., Zhan L., Xin Z.T., Baril L., Tang F., Lv H., Cao W.C. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J. Infect. Dis. 2006;193:792–795. doi: 10.1086/500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke T., Wu H., Zhao J., Channappanavar R., Coleman C.M., Jiao J.A., Matsushita H., Liu Y., Postnikova E.N., Ork B.L. Human polyclonal immunoglobulin G from transchromosomic bovines inhibits MERS-CoV in vivo. Sci. Transl. Med. 2016;8:326ra21. doi: 10.1126/scitranslmed.aaf1061. [DOI] [PubMed] [Google Scholar]
- Martin J.E., Louder M.K., Holman L.A., Gordon I.J., Enama M.E., Larkin B.D., Andrews C.A., Vogel L., Koup R.A., Roederer M., VRC 301 Study Team A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26:6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D., Lusakibanza Manzo M., Nzolo D., Tshomba Oloma A., Ibanda A., PALM Writing Group. PALM Consortium Study Team A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen J., Wang N., Corbett K.S., Wrapp D., Kirchdoerfer R.N., Turner H.L., Cottrell C.A., Becker M.M., Wang L., Shi W. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper R.L., Rehm K.E. SARS vaccines: where are we? Expert Rev. Vaccines. 2009;8:887–898. doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambhara S., McElhaney J.E. Immunosenescence and influenza vaccine efficacy. Curr. Top. Microbiol. Immunol. 2009;333:413–429. doi: 10.1007/978-3-540-92165-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanofi. 2020. Sanofi joins forces with U.S. Department of Health and Human Services to advance a novel coronavirus vaccine.http://www.news.sanofi.us/2020-02-18-Sanofi-joins-forces-with-U-S-Department-of-Health-and-Human-Services-to-advance-a-novel-coronavirus-vaccine [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. [Google Scholar]
- Teissier E., Zandomeneghi G., Loquet A., Lavillette D., Lavergne J.P., Montserret R., Cosset F.L., Böckmann A., Meier B.H., Penin F., Pécheur E.I. Mechanism of inhibition of enveloped virus membrane fusion by the antiviral drug arbidol. PLoS ONE. 2011;6:e15874. doi: 10.1371/journal.pone.0015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen J., van den Brink E.N., Poon L.L., Marissen W.E., Leung C.S., Cox F., Cheung C.Y., Bakker A.Q., Bogaards J.A., van Deventer E. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3:e237. doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T.F. Fluad®-MF59®-Adjuvanted Influenza Vaccine in Older Adults. Infect. Chemother. 2013;45:159–174. doi: 10.3947/ic.2013.45.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., Peters C.J., Couch R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE. 2012;7:e35421. doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS. J. Virol. 2020 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang L., Kuwahara K., Li L., Liu Z., Li T., Zhu H., Liu J., Xu Y., Xie J. Immunodominant SARS Coronavirus Epitopes in Humans Elicited both Enhancing and Neutralizing Effects on Infection in Non-human Primates. ACS Infect. Dis. 2016;2:361–376. doi: 10.1021/acsinfecdis.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J., Smith G., Jones S., Proulx R., Deschambault Y. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J. Virol. 2004;78:12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.P., Wang N.C., Chang Y.H., Tian X.Y., Na D.Y., Zhang L.Y., Zheng L., Lan T., Wang L.F., Liang G.D. Duration of antibody responses after severe acute respiratory syndrome. Emerg. Infect. Dis. 2007;13:1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong C.Y., Ong H.K., Yeap S.K., Ho K.L., Tan W.S. Recent Advances in the Vaccine Development Against Middle East Respiratory Syndrome-Coronavirus. Front. Microbiol. 2019;10:1781. doi: 10.3389/fmicb.2019.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Pangolin homology associated with 2019-nCoV. bioRxiv. 2020 doi: 10.1101/2020.02.19.950253. [DOI] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Post P., Chubet R., Holtz K., McPherson C., Petric M., Cox M. A recombinant baculovirus-expressed S glycoprotein vaccine elicits high titers of SARS-associated coronavirus (SARS-CoV) neutralizing antibodies in mice. Vaccine. 2006;24:3624–3631. doi: 10.1016/j.vaccine.2006.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., China Novel Coronavirus Investigating and Research Team A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]