Abstract
An animal laboratory in a teaching hospital is a possible cause of cross infection. We aimed to assess the infection control in our animal laboratory and evaluate the disinfectant effects of a portable pulsed xenon ultraviolet (PX-UV) machine. Samples were taken from the surface of research tables, other high touch places, such as doorknobs, weighing scales, and handles of trolleys, and from air in the barrier system pre- and post-manual cleaning and post-PX-UV disinfection. The bacteria types were identified. We found that routine manual cleaning significantly reduced bacterial colony form unit (CFU)/cm2 (P = .02), and the median of CFU/cm2 reduced from 0.5 pre-cleaning to zero post-cleaning. PX-UV disinfection also significantly reduced residual bacterial counts (P = .002), with the highest counts 10 pre-PX-UV disinfection and 1 afterwards. Without manual cleaning, PX-UV disinfected surfaces significantly (P < .001), median count 6 pre-PX-UV disinfection and zero afterwards. PX-UV significantly reduced bacterial colony counts in the air with the median count falling from 6 to zero (P < .001). Some of the 21 species of pathogens we identified in the current study are pathogenic, resistant to antibiotics, and able to cause nosocomial infections and zoonosis. PX-UV reduced counts of most of the pathogens. PX-UV is an effective agent against these pathogens.
Keywords: Pulsed xenon ultraviolet light, Disinfection, Animal laboratory
Highlights
-
•
An animal laboratory in a teaching hospital is a possible cause of cross infection.
-
•
The disinfectant effect of manual cleaning and portable pulsed xenon ultraviolet (PX-UV) was compared in our laboratory.
-
•
Manual cleaning significantly reduced bacterial counts on surfaces and PX-UV significantly reduced residual bacterial counts.
-
•
PX-UV, without manual cleaning, significantly reduced bacterial counts on surfaces.
-
•
PX-UV significantly reduced bacterial counts in the room air.
1. Introductions
Medical researchers and life scientists are often involved in animal studies. In China, staff in medical schools and teaching hospitals are the main resources for medical and life sciences research. Hence, hospitals may have one or more animal laboratory.
Our animal laboratory is on a hospital campus where the majority of investigators are Masters or PhD students from three affiliated Xiang Ya hospital, as well as from the medical school. As a result, there is a risk of cross infection by transmitting pathogens between a hospital clinic and the animal laboratory, and triggering a disaster like SARS, the severe acute respiratory syndrome that originated in China and spread to many countries in 2003 [1].
Pulsed xenon ultraviolet (PX-UV) is proven as an effective tool for disinfection of pathogens in various hospital settings, including wards [[2], [3], [4], [5], [6]], operating rooms [7], surgical sites [8,9], nursing rooms [10], human milk feeding rooms [11], and burns units [12]; and this disinfectant effect also applies to clinical laboratories and blood sampling rooms in China [13,14]. However, the effectiveness of PX-UV in disinfection of a hospital animal laboratory is unstudied. Hence, we aimed to evaluate the usefulness of PX-UV in this setting in China.
Our animal laboratory is specific pathogen free (SPF), which means that animals kept in the barrier system should be free of particular pathogens, such as those able to cause infections and zoonoses, and those that will interfere with research. Pathogens from outside the barrier system are not allowed to be transferred in according to the regulations. Thus, we did not introduce any bacteria from outside the system, but measured the pathogens that exist in the two rooms in the animal laboratory that are used for surgical operations and administering test agents.
2. Methods and Materials
2.1. Sampling
2.1.1. Sampling Sites
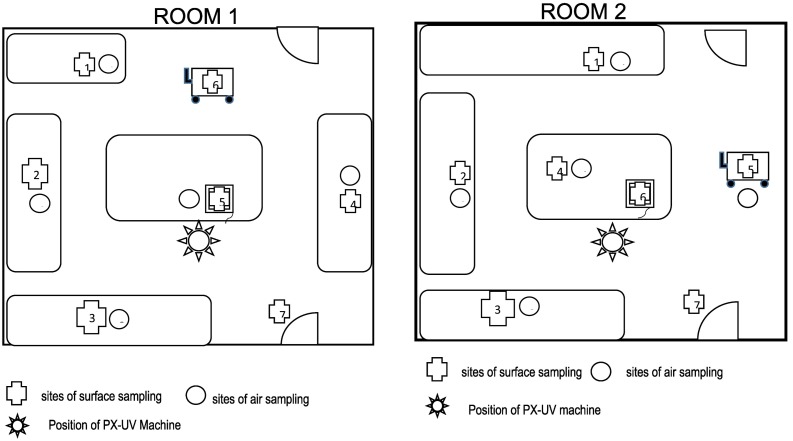
This study was conducted in the animal laboratory on the campus of the Third Xiang Ya Hospital, which has 2200 beds. The size of each room is 7 m × 7 m. Research tables are placed along the wall as shown in Fig. 1 . We measured pathogens in the air and on the surface of the research tables, and other high touch places such as doorknobs, weighing scales, and handles of trolleys.
Fig. 1.
The sampling sites for surfaces and air in the two rooms. Pathogens in the air and on the surface of the research tables, doorknobs, weighing scales, and handles of trolleys were measured.
The sampling sites for surfaces and air are indicated in Fig. 1. In each room we sampled 7 surface sites and 5 air sites for pathogens, with each site one meter away from the wall. The tables are 0.9 m high.
2.1.2. Routine Manual Cleaning
At the end of each day, after removing all unnecessary items from the tables and the room, the laboratory staff cleans the research tables using rags wetted with 0.5% peracetic acid and the air in the room is disinfected by spraying 1% peracetic acid.
2.1.3. Sampling Time
Samples were taken at the end of the working day, at about 7 pm, when the facility was about to close. The disinfectant effect of PX-UV was evaluated in two ways. First, after routine manual cleaning of the research tables, samples were taken at three time points: pre- and post-manual cleaning of the surfaces as well as post-PX-UV disinfection. Second, an evaluation was performed without manual cleaning, for which samples were taken pre- and post-PX-UV disinfection.
The comparison of colony counts pre- and post-manual cleaning and post-PX-UV on surfaces was repeated 4 times with 7 sampling sites; the comparison of colony counts pre- and post-PX-UV disinfection in the air was repeated 4 times with 10 sampling sites. The comparison of colony counts pre- and post-PX-UV without manual cleaning on surfaces was repeated twice with 7 sampling sites. We did not repeat the last comparison four times because the results were consistent with our other findings.
2.1.4. Sampling Methods
For air sampling, 64 cm2 tryptic soy agar plates were open for 30 mins to collect pathogens falling from air into the plates. The control plates, one in each room, were open and covered immediately. Other procedures were the same in both test and control plates.
For surface sampling, a sterilized specification board with a 5 cm × 5 cm window (Nanjing Bizheng Biological Technology Co., Ltd.) was used to define the sampling area. A total of 100 cm2 were sampled for each table. For the weighing scales and doorknobs, the whole surface of the plate containing the materials for measuring and the whole doorknob were sampled. Sterilized sponges moistened with saline were used to scrub the surface up and down five times in the specification window. The sponge was then placed in a 50 mL conical tube containing 10 mL sterile saline for testing. The tubes were then placed in a mixer for two minutes, from which 100 μL was cultured for 48 h at 36 ± 1 °C. Sterilized gloves were worn to prevent contamination during sampling.
2.2. Culture Count Results
The bacterial colonies in each plate were counted and the types of bacteria were classified with matrix-assisted laser desorption/ionization time of flight mass spectrometry (MicroflexLT/SH. BRUKER, Germany). For the surface study, the colony form unit (CFU)/cm2 is the number of colonies counted in each plate. For the air pathogen study, results were reported as total CFU per plate. When CFU was over 100 per plate, results were recorded as 100.
2.3. Device
The PX-UV machine MX-3600 (Xi'An Fukang Air Purification Equipment and Engineering Co. Ltd., Xi'an, China) was initially assessed for its efficacy and we found that it was set at a wavelength of 100 nm to 400 nm, a frequency of 3 Hz, and a duration of work of 6 mins. The disinfectant efficacy of the machine was 100% for Staphylococcus aureus and Escherichia coli. Thus, these parameters were used for this study. The machine was deployed on the floor close to the table in the center of the room. When working, the top of the machine's UV light lamp is 1.1 m high, well above the table surfaces (0.9 m). Hence, all the table surfaces and air was exposed to UV light.
2.4. Data Analysis
Data were analyzed by performing a Wilcoxon rank sum test, using a software program called MedCalc, Version 17 (MedCalc Software, Ostend, Belgium), to compare the difference between the data pre- and post-disinfection. Statistical significance was set at P < .05.
3. Results
3.1. Effect of Manual Cleaning and PX-UV Disinfection on Surfaces
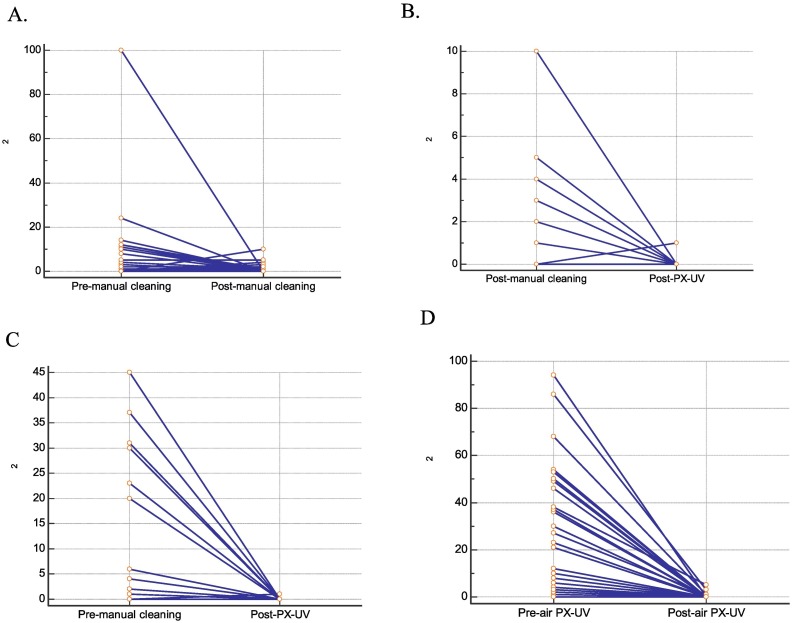
As demonstrated in Table 1A and Fig. 2A, routine manual cleaning significantly reduced bacterial colony counts (P = .02). The median of the colony count dropped from 0.5 before cleaning to zero after cleaning. PX-UV disinfection significantly reduced residual bacterial counts (P = .002), with the highest count being 10 before PX-UV disinfection and 1 afterwards (Table 1B and Fig. 2B). PX-UV disinfects surfaces without manual cleaning (P < .001), with a median count of 6 before PX-UV disinfection reducing to zero afterwards (Table 1C and Fig. 2 C).
Table 1.
Comparison of colony counts pre- and post-manual cleaning and post-PX-UV disinfection on surfaces and air.
| A | ||||||
|---|---|---|---|---|---|---|
| Pre- manual cleaning |
Post- manual cleaning |
|||||
| No. samples | Median (95% CV) | Lowest - highest values | No. samples | Median (95% CV) | Lowest - highest values | P |
| 56 | 0.5 (0.00–0.69) | 0.00–100.00 | 56 | 0 (0.00–1.00) | 0.00–10.00 | 0.02 |
| B | ||||||
|---|---|---|---|---|---|---|
| Post- manual cleaning |
Post- PX-UV |
|||||
| No. samples | Median (95% CV) | Lowest - highest values | No. samples | Median (95% CV) | Lowest - highest values | P |
| 56 | 0(0.00–1.00) | 0.00–10.00 | 56 | 0(0.00–0.00) | 0.00–1.00 | 0.002 |
| C | ||||||
|---|---|---|---|---|---|---|
| Pre- PX-UV without manual cleaning |
Post- PX-UV |
|||||
| No. samples | Median (95% CV) | Lowest - highest values | No. samples | Median (95% CV) | Lowest - highest values | P |
| 28 | 6(0.89–30.10) | 0.00–45.00 | 28 | 0 (0.00–0.00) | 0.00–1.00 | <0.001 |
| D | ||||||
|---|---|---|---|---|---|---|
| Before air disinfection |
After air PX-UV |
|||||
| No. samples | Median (95% CV) | Lowest - highest values | No. samples | Median (95% CV) | Lowest - highest values | P |
| 40 | 5(0.34–25.64) | 0.00–94.00 | 40 | 0 (0.00–0.00) | 0.00–5.00 | <0.001 |
Fig. 2.
Effect of manual cleaning and pulsed xenon ultraviolet light (PX-UV) on surfaces and air. Routine manual cleaning significantly reduces bacterial colony counts (CFU) (P = .02), with the median of the CFU reduced from 0.5 before cleaning to zero after cleaning (A). PX-UV disinfection significantly reduces residual bacterial counts (P = .002), with the highest count being 10 before PX-UV disinfection and 1 afterwards (B). PX-UV disinfects the surface without manual cleaning (P < .001), with the median count of 6 before PX-UV disinfection reducing to zero afterwards (C). PX-UV disinfection significantly reduces bacterial colony counts in the air, with a median count 6 reducing to zero (P < .001) (D).
3.2. Effect of PX-UV Disinfection on the Air
As shown in Table 1D and Fig. 2D, PX-UV disinfection significantly reduced bacterial colony counts in the air, with a median count 6 reducing to zero (P < .001).
All controls were bacteria free.
3.3. Pathogens Identified
As shown in Table 2 , 16 species of pathogens were identified from the surfaces of the research tables, weighing scales, handles of trolleys, and doorknobs. Ten species decreased, one remained unchanged, and five increased after manual cleaning. Due to technical reasons, pathogen identification was not performed after the first test when only one colony was found in each of the air and surface cultures after PX-UV disinfection. In the other tests, no pathogen was detected after PX-UV disinfection.
Table 2.
Comparison of the colony counts and bacteria kinds before and after annual cleaning and after PX-UV disinfection on surfaces.
| Name of the bacteria identified on surface of the table, balance, handle of trolley and doorknob | Staining | Counts identified before manual cleaning | Counts identified after manual cleaning | Counts identified after PX-UV | Characteristics |
|---|---|---|---|---|---|
| Aerococcus viridans | G+ | 3 | 0 | 0 | Generally acquired in hospital environment and pathogenic, can infect newborns[21,22] |
| Bacillus flexus | G+ | 2 | 0 | 0 | |
| Brevibacillus centrosporus | G+ | 4 | 0 | 0 | |
| Brevibacillus centrosporus | G+ | 0 | 2 | 0 | |
| Comamonas kerstersii | G− | 4 | 0 | 0 | |
| Corynebacterium glutamicum | G+ | 2 | 0 | 0 | |
| Corynebacterium stationis | G+ | 1 | 0 | 0 | |
| Jeotgalicoccus halotolerans | G+ | 0 | 1 | 0 | |
| Lactobacillus pantheris | G+ | 2 | 0 | 0 | |
| Lactobacillus paracasei ssp. paracasei | G+ | 1 | 1 | 0 | |
| Proteus mirabilis | G− | 2 | 0 | 0 | Causing urinary tract infection, nephrolith and cystic calculus, sepsis[16,17] |
| Proteus vulgaris | G− | 0 | 10 | 0 | |
| Staphylococcus nepalensis | G+ | 34 | 0 | 0 | Zoonotic potential[18] |
| Staphylococcus sciuri ssp. sciuri | G+ | 1 | 5 | 0 | pathogenic[19] |
| Staphylococcus succinus ssp. succinus | G+ | 0 | 17 | 0 | |
| Staphylococcus xylosus | G+ | 6 | 0 | 0 | Pathogenic and resistance to different kinds of antibiotics[20] |
Table 3 shows that 11 species of bacteria were identified in the air. Four decreased and six increased after manual cleaning. As was the case for the surface study, PX-UV disinfected most of the pathogens. Among these 11 species of pathogens, six were the same pathogens as found on the surfaces, and five were different.
Table 3.
Comparison of the colony counts and bacteria kinds before and after annual cleaning and after PX-UV disinfection in air.
| Name of the bacteria identified in the air | Staining | Counts identified before manual cleaning | Counts identified after manual cleaning | Counts identified after PX-UV | Characteristics |
|---|---|---|---|---|---|
| Brevibacillus centrosporus | G+ | 0 | 2 | 0 | |
| Flavobacterium gelidilacus | G− | 1 | 0 | 0 | |
| Hydrogenophaga pseudoflava | G− | 0 | 3 | 0 | |
| Lactobacillus brevis | G+ | 2 | 0 | 0 | |
| Lactobacillus paracasei ssp. paracasei | G+ | 3 | 10 | 0 | |
| Microbacterium lacticum | G+ | 1 | 0 | 0 | |
| Proteus mirabilis | G− | 0 | 6 | 0 | Causing urinary tract infection, nephrolith and cystic calculus, sepsis[16,17] |
| Staphylococcus nepalensis | G+ | 4 | 2 | 0 | Zoonotic potential[18] |
| Staphylococcus sciuri ssp. camaticus | G+ | 0 | 1 | 0 | |
| Staphylococcus sciuri ssp. sciuri | G+ | 0 | 11 | 0 | Pathogenic and resistance to different kinds of antibiotics[19] |
| Staphylococcus xylosus | G+ | 3 | 6 | 0 | Weak pathogenic but has strong resistance to antibiotics |
4. Discussion
In this study, we found that routine manual cleaning significantly reduces bacterial colony counts, but bacteria remain. PX-UV effectively disinfects the surfaces of research tables, weighing scales, doorknobs, handles of trolleys, and simultaneously the air in the room, with or without manual cleaning in a short time. Some of the bacteria identified in the barrier system are pathogenic and are able to cause nosocomial infection.
Careful cleaning and disinfection of environmental surfaces in hospital is essential for effective infection control. An animal laboratory should be cleaned and disinfected regularly. Traditionally, the surfaces and the air of an animal laboratory are cleaned and disinfected by spraying peracetic acid or hydrogen peroxide, or by ultraviolet radiation. These methods, however, have intrinsic shortcomings. Spraying peracetic acid or hydrogen peroxide may corrode the surfaces of the racks of the individual ventilation cages (IVC) system, and those of the tables, which are often made of stainless steel. The detergents may also affect animals if the IVC, the chamber for raising animals, draws air from inside the room.
The effectiveness of manual cleaning and disinfection is variable [5,7]. Inherent human error often results in incomplete cleaning, as demonstrated by our study. Continuous ultraviolet radiation takes about 30 min to disinfect target surfaces and air [15]. But this is too long when the surfaces and air must be disinfected quickly. In such cases, PX-UV disinfection has its advantages as shown in our study, with its fast (achieving satisfactory results in 6 min) and effective disinfection.
A number of pathogens can infect both animals and humans causing zoonosis, such as plague, Lyme disease, rabies, anthrax, tuberculosis, and epidemic hemorrhagic fever. In recent years, emerging zoonoses such as SARS, Ebola, and COVID-2019 have received worldwide attention because of major outbreaks [1,16,17]. Minor outbreaks of emerging and re-emerging zoonoses occur frequently in China [18]. Zoonotic outbreaks occurred in students and teachers because of using infected animals during teaching [19].
In our study, we found that several types of infectious bacteria exist on the surfaces and air inside the barrier system. Among them, proteus mirabilis can cause urinary tract infection, nephrolith and cystic calculus, sepsis [20,21]; staphylococcus nepalensis has zoonotic potential [22]; staphylococcus sciuri ssp sciuri [23], and staphylococcus xylosus [24] are pathogenic and resistant to different antibiotics; and aerococcus viridans can cause serious infection [25,26]. The existence of these bacteria in the barrier system suggests a risk of cross infection with the hospital environment. Strict control of the transmission of infectious pathogens in an animal laboratory is important, not only for the health of laboratory animals [27], which helps achieve reliable and reproducible research results, but also for the control of nosocomial infection. PX-UV disinfection is an optimal choice to achieve this with its advantages of fast disinfection and satisfactory efficacy without damaging equipment and apparatus.
In our study, the discrepancy between the bacterial species on the surfaces and in the air, as well as the increase in bacteria counts and species after manual cleaning, is possibly caused by contamination from the cloth used by the cleaner, or contamination from researchers as some students are still working while the cleaner is cleaning and sampling. As we only identified a few bacteria in each plate, we did not test their resistance to antibiotics.
In the barrier system of an animal laboratory, bacteria are being exchanged with the hospital environment. The bacteria are pathogenic, resistant to antibiotics, and have potential to cause cross-infection, resulting in nosocomial infections and zoonosis. PX-UV disinfection is an effective agent to disinfect the system.
Author Contributions
C.Q.G., Y.B.L., C.Y., and C.Z., conceptualized the study and wrote the manuscript. J.J.L, S.N. W., L.H.C., Y.L., Y.W., Y.X.D, M.M.W., J.J.Q. and Y.T. conducted the experiments. All authors reviewed the manuscript.
Ethics Approval
Not required.
Declaration of Competing Interest
None.
Acknowledgements
The study was supported by grants from the National Key Research and Development Program of China (2017YFA0105201 to Y.B.L) and the Science and Technology Department of Hunan Province (2016DK2004 to CQG) and a grant from the Municipal Science and Technology Bureau of Changsha (KQ1701086 to CQG). The sponsors have not played any role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- 1.Shi Z., Hu Z. A review of studies on animal reservoirs of the SARS coronavirus. Virus Res. 2008 Apr;133(1):74–87. doi: 10.1016/j.virusres.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagaraja A., Visintainer P., Haas J.P., Menz J., Wormser G.P., Montecalvo M.A. Clostridium difficile infections before and during use of ultraviolet disinfection. Am. J. Infect. Control. 2015 Sep 1;43(9):940–945. doi: 10.1016/j.ajic.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Hosein I., Madeloso R., Nagaratnam W., Villamaria F., Stock E., Jinadatha C. Evaluation of a pulsed xenon ultraviolet light device for isolation room disinfection in a United Kingdom hospital. Am. J. Infect. Control. 2016 Sep 1;44(9):e157–e161. doi: 10.1016/j.ajic.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 4.Jinadatha C., Villamaria F.C., Restrepo M.I., Ganachari-Mallappa N., Liao I.C., Stock E.M., Copeland L.A., Zeber J.E. Is the pulsed xenon ultraviolet light no-touch disinfection system effective on methicillin-resistant Staphylococcus aureus in the absence of manual cleaning? Am. J. Infect. Control. 2015 Aug;43(8):878–881. doi: 10.1016/j.ajic.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Zeber J.E., Pfeiffer C., Baddley J.W., Cadena-Zuluaga J., Stock E.M., Copeland L.A., Hendricks J., Mohammadi J., Restrepo M.I., Jinadatha C. Effect of pulsed xenon ultraviolet room disinfection devices on microbial counts for methicillin-resistant Staphylococcus aureus and aerobic bacterial colonies. Am. J. Infect. Control. 2018 Jun;46(6):668–673. doi: 10.1016/j.ajic.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Nerandzic M.M., Thota P., Sankar C.T., Jencson A., Cadnum J.L., Ray A.J., Salata R.A., Watkins R.R., Donskey C.J. Evaluation of a pulsed xenon ultraviolet disinfection system for reduction of healthcare-associated pathogens in hospital rooms. Infect. Control Hosp. Epidemiol. 2015 Feb;36(2):192–197. doi: 10.1017/ice.2014.36. [DOI] [PubMed] [Google Scholar]
- 7.Simmons S., Dale C., Jr., Holt J., Passey D.G., Stibich M. Environmental effectiveness of pulsed-xenon light in the operating room. Am. J. Infect. Control. 2018 Sep;46(9):1003–1008. doi: 10.1016/j.ajic.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Catalanotti A., Abbe D., Simmons S., Stibich M. Influence of pulsed-xenon ultraviolet light-based environmental disinfection on surgical site infections. Am. J. Infect. Control. 2016 Jun 1;44(6):e99–e101. doi: 10.1016/j.ajic.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Fornwalt L., Ennis D., Stibich M. Influence of a total joint infection control bundle on surgical site infection rates. Am. J. Infect. Control. 2016 Feb;44(2):239–241. doi: 10.1016/j.ajic.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Kovach C.R., Taneli Y., Neiman T., Dyer E.M., Arzaga A.J., Kelber S.T. Evaluation of an ultraviolet room disinfection protocol to decrease nursing home microbial burden, infection and hospitalization rates. BMC Infect. Dis. 2017 Mar 3;17(1):186. doi: 10.1186/s12879-017-2275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dippenaar R., Smith J. Impact of pulsed xenon ultraviolet disinfection on surface contamination in a hospital facility’s expressed human milk feed preparation area. BMC Infect. Dis. 2018 Feb 23;18(1):91. doi: 10.1186/s12879-018-2997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green C., Pamplin J.C., Chafin K.N., Murray C.K., Yun H.C. Pulsed-xenon ultraviolet light disinfection in a burn unit: impact on environmental bioburden, multidrug-resistant organism acquisition and healthcare associated infections. Burns. 2017 Mar;43(2):388–396. doi: 10.1016/j.burns.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Chen L.H., Li Y., Qi Y., Wang S.N., Gao C.Q., Wu Y. Evaluation of a pulsed xenon ultraviolet light device for reduction of pathogens with biofilm-forming ability and impact on environmental bioburden in clinical laboratories. Photodiagn. Photodyn. Ther. 2019 Aug 24;(19) doi: 10.1016/j.pdpdt.2019.08.026. pii: S1572–1000. 30356–4. [DOI] [PubMed] [Google Scholar]
- 14.Wang S.N., Li J.J., Liu Y.X., Lin Z., Qiao J.J., Chen L.H., Li Y., Wu Y., Wang M.M., Liu Y.B., Yan C., Chen Z.H., Gao C.Q. Pulsed xenon ultraviolet and non-thermal atmospheric plasma treatments are effective for the disinfection of air in hospital blood sampling rooms. Photodiagn. Photodyn. Ther. 2019 Sep;27:137–140. doi: 10.1016/j.pdpdt.2019.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber D.J., Rutala W.A., Anderson D.J., Chen L.F., Sickbert-Bennett E.E., Boyce J.M. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: focus on clinical trials. Am. J. Infect. Control. 2016 May 2;44(5 Suppl):e77–e84. doi: 10.1016/j.ajic.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baize S., Pannetier D., Oestereich L., Rieger T., Koivogui L., Magassouba N., Soropogui B., Sow M.S., Keïta S., De Clerck H., Tiffany A., Dominguez G., Loua M., Traoré A., Kolié M., Malano E.R., Heleze E., Bocquin A., Mély S., Raoul H., Caro V., Cadar D., Gabriel M., Pahlmann M., Tappe D., Schmidt-Chanasit J., Impouma B., Diallo A.K., Formenty P., Van Herp M., Günther S. Emergence of Zaire Ebola virus disease in Guinea. N. Engl. J. Med. 2014 Oct 9;371(15):1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 17.Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020 Feb 18;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q., Cao L., Zhu X.Q. Major emerging and re-emerging zoonoses in China: a matter of global health and socioeconomic development for 1.3 billion. Int. J. Infect. Dis. 2014 Aug;25:65–72. doi: 10.1016/j.ijid.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anon . China Animal Health. Vol. 10. 2011 Oct. Twenty-eight teachers and students in Northeast Agricultural University were infected with brucellosis. 90–90. [Google Scholar]
- 20.Schultz E., Haenni M., Mereghetti L., Siebor E., Neuwirth C., Madec J.Y., Cloeckaert A., Doublet B. Survey of multidrug resistance integrative mobilizable elements SGI1 and PGI1 in Proteus mirabilis in humans and dogs in France, 2010-13. J. Antimicrob. Chemother. 2015 Sep;70(9):2543–2546. doi: 10.1093/jac/dkv154. [DOI] [PubMed] [Google Scholar]
- 21.Torzewska A., Rozalski A. Inhibition of crystallization caused by Proteus mirabilis during the development of infectious urolithiasis by various phenolic substances. Microbiol. Res. 2014 Jul-Aug;169(7–8):579–584. doi: 10.1016/j.micres.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Rossi C.C., da Silva Dias I., Muniz I.M., Lilenbaum W., Giambiagi-deMarval M. The oral microbiota of domestic cats harbors a wide variety of Staphylococcus species with zoonotic potential. Vet. Microbiol. 2017 Mar;201:136–140. doi: 10.1016/j.vetmic.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 23.Hu X., Zheng B., Jiang H., Kang Y., Cao Q., Ning H., Shang J. Draft genome sequence of staphylococcus sciuri subsp. sciuri Strain Z8, isolated from human skin. Genome Announc. 2015 Jul 23;3(4) doi: 10.1128/genomeA.00714-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikulášová M., Valáriková J., Dušinský R., Chovanová R., Belicová A. Multiresistance of Staphylococcus xylosus and Staphylococcus equorum from Slovak Bryndza cheese. Folia Microbiol. (Praha). 2014 May;59(3):223–227. doi: 10.1007/s12223-013-0286-y. [DOI] [PubMed] [Google Scholar]
- 25.Yadav K., Sharma M., Agarwal S., Bhatia N., Yadav N. Aortic pseudoaneurysm & endocarditis caused by Aerococcus viridans: a case report and literature review. Cardiovasc. Revasc. Med. 2018 Mar;19(2):201–203. doi: 10.1016/j.carrev.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Pan Z., Ma Y., Ma J., Dong W., Yao H. Acute meningitis of piglets and mice caused by co-infected with Streptococcus suis and Aerococcus viridans. Microb. Pathog. 2017 May;106:60–64. doi: 10.1016/j.micpath.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Franklin C.L., Ericsson A.C. Microbiota and reproducibility of rodent models. Lab. Anim. (NY). 2017 Mar 22;46(4):114–122. doi: 10.1038/laban.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]