The pathogenesis of coronavirus disease 2019 (COVID-19) remains unclear and there is presently no evidence for efficient therapeutics. The pathogenesis of severe acute respiratory syndrome (SARS) related to coronavirus involves a cytokine storm with high serum levels of proinflammatory cytokines and chemokines interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), interferon-γ, IL-1 and IL-12, and IL-8.1, 2, 3, 4 Similarly, in COVID-19, higher plasma levels of cytokines IL-6, IL-2, IL-7, IL-10, interferon-γ inducible protein (IP10), monocyte chemoattractant protein (MCP1), macrophage inflammatory protein (MIP1A), and TNF-α have been found in patients admitted to intensive care units, and the cytokine storm syndrome was proportional to the severity of disease.5 The proinflammatory IL-6 appears as one of the key cytokines leading to the inflammatory storm, which may result in increased alveolar–capillary blood–gas exchange dysfunction.4 , 6 The proinflammatory cytokine IL-6 seems to have a prominent role in this inflammatory cascade.4
These data suggest that IL-6 may be a potential actionable target cytokine to treat COVID-19-related acute respiratory distress syndrome (ARDS). We report here the case of a patient with respiratory failure linked to COVID-19 who had a rapid favorable outcome after two infusions of the anti-IL-6 receptor inhibitor tocilizumab. This suggests that anti-IL-6 receptor inhibitor treatment could decrease the risk of progression toward SARS by mitigating the cytokine storm in lungs with COVID-19.
Case description
A 42-year-old male recently diagnosed with metastatic sarcomatoid clear cell renal cell carcinoma, had been hospitalized for fever, symptomatic bone metastases pain management, and first-line systemic treatment decisions. The patient had no other significant medical history. He presented initially with isolated fever of 39.0°C on 12 March 2020 for which he received ceftriaxone outside our center. On day 6, he developed mild cough and fever (38.3°C) prompting a SARS-Cov-2 real time polymerase chain reaction test, which was positive. The patient was admitted to the COVID-19 ward of our hospital and closely monitored. Chest computerized tomography (CT) scan revealed bilateral patchy ground glass opacities related to COVID-19 (Figure 1 ). Antiviral therapy lopinavir-ritonavir use (400–100 mg orally) was begun on day 7 and maintained for 5 days, according to local guidelines. On day 8, sudden dyspnea and saturation drop required oxygen supplementation increase to 6 l/min, without the need for artificial ventilation. He received two doses of intravenous tocilizumab, 8 mg/kg for each dose, 8 h apart, with a good tolerability. Thereafter, he experienced clinical improvement, became rapidly afebrile, and required gradually decreased oxygen consumption. This was fully discontinued on day 12 (Figure 1). Chest CT on day 19 confirmed improvement by showing partial regression of the pulmonary infiltrates and ground glass appearance (Figure 1). C-reactive protein in blood, a surrogate marker of cytokine storm, decreased from 225 mg/l to 33 mg/l in 4 days (Figure 1). No major change was observed in circulating lymphocytic subpopulations after tocilizumab, and the percentage of CD4+ CD25+ lymphocytes was found to be high, before and after tocilizumab (Figure 2 ). The patient ultimately clinically fully recovered from COVID-19 symptoms.
Figure 1.
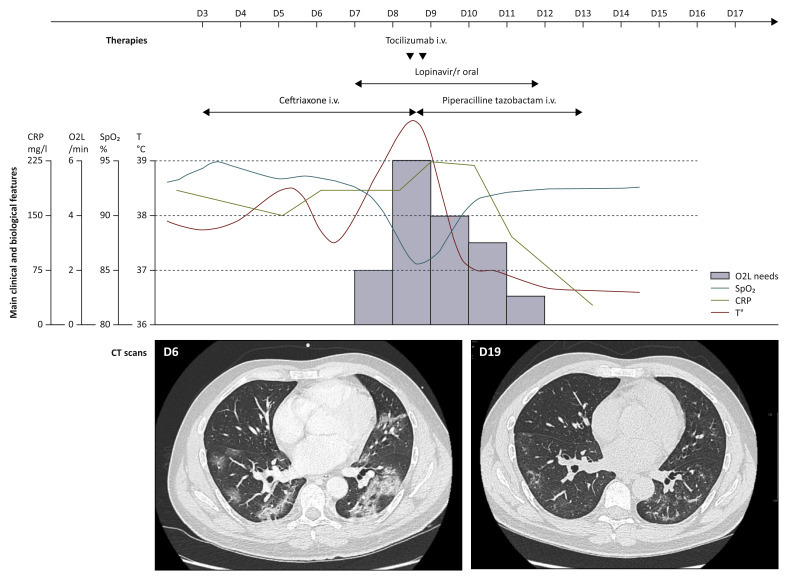
Outcome of patient with COVID-19 before and after tocilizumab.
The top of the figure shows evolution of clinical vital signs, requirement for supplemental O2 in l/min, C-reactive protein in blood. The bottom of the figure shows a pulmonary CT scan of the patient before (day 6) and after (day 12) tocilizumab. The patient received two doses of i.v. tocilizumab at 8 mg/kg for each dose (small black arrows). All therapies given for COVID-19 are summarized at the top of the figure.
COVID-19, coronavirus disease 2019; CRP, C-reactive protein in blood; CT, computerized tomography; i.v., intravenously; Lopinavir/r, lopinavir plus ritonavir; O2L, oxygen supplementation in l/min; SpO2, saturation pulsed in oxygen; T°, temperature in degrees Celsius.
Figure 2.
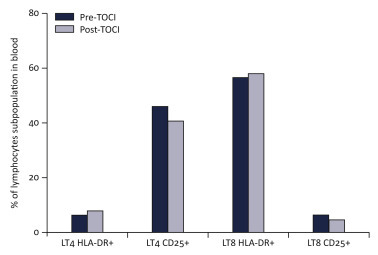
CD25 and HLA-DR expression among CD4+ and CD8+ T cells in blood of a patient with COVID-19 before and after tocilizumab.
Percentage of HLA-DR (LT4 HLA-DR+) and CD25 (LT4 CD25+) among CD3+CD4+ (LT4) and of HLA-DR (LT8 HLA-DR+) and CD25 (LT8 CD25+) among CD3+CD8+ (LT8) before introduction of tocilizumab (Pre_TOCI) and 4 days after initiation of treatment (Post_TOCI).
COVID-19, coronavirus disease 2019.
Discussion and conclusion
As a recombinant humanized anti-human IL-6 receptor monoclonal antibody, tocilizumab, can specifically bind soluble IL-6 receptor and inhibit signal transduction.7 Tocilizumab is currently mainly used to treat patients with rheumatoid arthritis. It is given every 4 weeks, for up to 24 weeks, with a favorable safety profile.8 Other indications for tocilizumab are juvenile arthritis, giant cell arthritis, and—more recently—cytokine release syndromes associated with chimeric antigen receptor T-cell therapies.7 , 9 In these mainly rheumatologic indications, the tolerance of tocilizumab is generally good; the main adverse events are a transient decrease in leukocytes, increase in liver enzymes, and a slight increase of bacterial infection.7 , 8 In patients with COVID-19, the safety profile, and especially the potential drug interactions with antivirals, remain to be investigated in clinical trials. Clinical trials are also required to explore whether tocilizumab can be used effectively in patients with respiratory failure due to COVID-19 and to investigate at what stage of the disease this treatment could be the most appropriate.
Here we report the first successful treatment of a patient with respiratory failure related to COVID-19 and treated with tocilizumab. The present report has several limitations. First, the patient was immunosuppressed because of his cancer, and this case is therefore not generalizable to the non-cancer population. Interestingly, his lymphocytic subpopulation presented a high level of senescence, as frequently observed in cancer patients (data not shown). To what extent this could explain the sensitivity to tocilizumab should be further explored. Second, the patient received concomitant therapies, especially lopinavir-ritonavir, but it seems unlikely that this changed the disease trajectory, since lopinavir-ritonavir has recently been shown not to be effective in patients with severe COVID-19 in a randomized, controlled trial.10 It thus seems likely that the rapid control of the pulmonary hyperinflammation resulted from tocilizumab treatment.
In summary, COVID-19 with hyperinflammatory pulmonary symptoms is associated with a cytokine storm involving interleukins and chemokine dysregulation. Among these, the actionable proinflammatory IL-6 axis seems to play a major role. We report the first observation of a patient with severe COVID-19-related lung disease successfully treated with anti-IL-6 receptor treatment. Blocking the cytokine IL-6 axis appears to us to be a promising therapy to be studied urgently in patients developing SARS related to coronavirus.
Acknowledgments
Funding
None declared.
Disclosure
JMM reports sponsored research at Gustave Roussy Cancer Center with Abbvie, Agios, Amgen, Astex, AstraZeneca, Bayer, Beigene, Blueprint, Bristol-Myers Squibb, Boeringer Ingelheim, Celgene, Chugai, Forma, Genentech, GSK, H3 Biomedecine, Incyte, Innate Pharma, Janssen, Lilly, Loxo, Medimmune, MSD, Novartis, Oncopeptides AB, Roche, Sanofi, Taiho, and Xencor, outside the submitted work, and personal fees or travel grants or ad-board: Astex, Iqone, Mundipharma, and Bristol-Myers Squibb, outside the submitted work.
BB reports sponsored research at Gustave Roussy Cancer Center with Abbvie, Amgen, AstraZeneca, BeiGene, Blueprint Medicines, BMS, Boehringer Ingelheim, Celgene, Cristal Therapeutics, Daiichi-Sankyo, Eli Lilly, GSK, Ignyta, IPSEN, Inivata, Janssen, Merck KGaA, MSD, Nektar, Onxeo, OSE immunotherapeutics, Pfizer, Pharma Mar, Roche-Genentech, Sanofi, Servier, Spectrum Pharmaceuticals, Takeda, Tiziana Pharma, and Tolero Pharmaceuticals, outside the submitted work.
CR reports personal fees for advisory boards from Bristol-Myers Squibb (BMS), Pierre Fabre, Novartis, Amgen, Merck, and Roche, outside the submitted work.
FB reports personal fees from AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Eli Lilly Oncology, F. Hoffmann-La Roche Ltd, Novartis, Merck, MSD, Pierre Fabre, Pfizer, and Takeda, outside the submitted work.
AM reports sponsored research at Gustave Roussy Cancer Center with: Abbvie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveo pharmaceuticals, Bayer, Beigene, Blueprint, BMS, Boeringer Ingelheim, Celgene, Chugai, Clovis, Daiichi Sankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, Gamamabs, Genentech, Gortec, GSK, H3 Biomedecine, Incyte, Innate Pharma, Janssen, Kura Oncology, Kyowa, Lilly, Loxo, Lysarc, Lytix Biopharma, Medimmune, Menarini, Merus, MSD, Nanobiotix, Nektar Therapeutics, Novartis, Octimet, Oncoethix, Oncopeptides AB, Orion, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Sierra Oncology, Taiho, Takeda, Tesaro, and Xencor, outside the submitted work.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong C.K., Lam C.W.K., Wu A.K.L. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conti P., Ronconi G., Caraffa A. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (CoV-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(1):1. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka T., Narazaki M., Ogata A., Kishimoto T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin Immunol. 2014;26(1):88–96. doi: 10.1016/j.smim.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Gabay C., Emery P., van Vollenhoven R. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–1550. doi: 10.1016/S0140-6736(13)60250-0. [DOI] [PubMed] [Google Scholar]
- 9.Lee D.W., Gardner R., Porter D.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao B., Wang Y., Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]


