Abstract
The organization of estrogenic signaling in the CNS is exceedingly complex. It is comprised of peripherally and centrally synthesized estrogens, and a plethora of types of estrogen receptor that can localize to both the nucleus and the plasma membrane. Moreover, CNS estrogen receptors can exist independent of aromatase (aka estrogen synthase) as well as oligomerize with it, along with a host of other membrane signaling proteins. This ability of CNS estrogen receptors to either to physically pair or exist separately enables locally produced estrogens to act on multiple spatial levels, with a high degree of gradated regulation and plasticity, signaling either in-phase or out-of phase with circulating estrogens. This complexity explains the numerous contradictory findings regarding sex-dependent pain processing and sexually dimorphic opioid antinociception. This review highlights the increasing awareness that estrogens are major endogenous arbiters of both opioid analgesic actions and the mechanisms used to achieve them. This behooves us to understand, and possibly intercede at, the points of intersection of estrogenic signaling and opioid functionality. Factors that integrate estrogenic actions at subcellular, synaptic, and CNS regional levels are likely to be prime drug targets for novel pharmacotherapies designed to modulate CNS estrogen-dependent opioid functionalities and possibly circumvent the current opioid epidemic.
1. Introduction
Most hormones and neurotransmitters that are present in males and females utilize similar molecular mechanisms in both sexes. Interestingly, this is not the case with opioids, which can act via substantially different mechanisms in males vs. females to achieve the same end result. Assuming that sex-dependent responsiveness to exogenous opioids reflects analogous divergence in endogenous opioid systems, the lack of parallelism in mechanisms underlying opioid actions in males and females not only reveals an extraordinary degree of plasticity among systems mediating opioid actions, but also provides insight into the ways in which endogenous opioid systems might be manipulated for medicinal purposes. Although sexual dimorphism in pain and opioid antinociception has long been recognized (Craft, 2003; Fillingim & Gear, 2004; Fillingim, King, Ribeiro-Dasilva, Rahim-Williams, & Riley, 2009; Ibironke & Aji, 2011; Liu & Gintzler, 2013; Loyd, Morgan, & Murphy, 2007; Mogil, Chesler, Wilson, Juraska, & Sternberg, 2000; Teepker, Peters, Vedder, Schepelmann, & Lautenbacher, 2010), we are only now beginning to understand its biochemical and molecular underpinnings, and the ability of endogenous estrogens to act as key arbiters of mechanisms utilized by opioids and sexual dimorphism thereof.
Estrogens (predominantly comprised of estradiol and estriol) belong to the steroidal class of hormones, which are derivatives of cholesterol. Enzymes largely control the synthesis and release of steroidal hormones since they are exceedingly lipid soluble and cannot be stored in vesicles. The ovaries are the predominant source of estrogens. However, we now know that throughout the central nervous system (CNS) there is expression of aromatase (Balthazart, Baillien, & Ball, 2001, 2006; Balthazart, Cornil, et al., 2006; Cornil, Ball, & Balthazart, 2006; Evrard, 2006; Evrard et al., 2000; Evrard & Balthazart, 2003; Evrard, Willems, Harada, & Balthazart, 2003; Jakab, Harada, & Naftolin, 1994; Liu, Chakrabarti, Schnell, Wessendorf, & Gintzler, 2011; Liu, Murugaiyan, Storman, Schnell, Wessendorf, et al., 2017b) (a key enzyme in the synthesis of estrogens), along with multiple types of estrogen receptor (ER) (Hazell et al., 2009; Merchenthaler, Lane, Numan, & Dellovade, 2004; Mitra et al., 2003; Perez, Chen, & Mufson, 2003; Shughrue & Merchenthaler, 2001). This resulted in a profound change in the functional categories used to describe estrogenic actions, i.e., not exclusively as hormones, but also as neuroactive agents that are intrinsic to the CNS. Additionally, the realization that the CNS is an estrogen-producing organ, and that some CNS-derived estrogens are poised to be secreted into the periphery (Storman, Liu, Wessendorf, & Gintzler, 2018), illustrates that the brain should be considered as an neuroendocrine organ.
Estrogens are known to influence a myriad of CNS functions. These include mood, memory, cognition, neuroprotection and, more recently, nociception (pain processing) and opioid antinociception (pain relief) (reviewed in Boulware & Mermelstein, 2005). This review will focus exclusively on the effect of estrogens on opioid functionality (exogenous as well as endogenous), the importance of estrogens to sex-dependent analgesic responsiveness, and related underlying mechanisms.
Estrogens and their receptors are now established to influence the physiological consequences and underlying analgesic mechanism(s) used by morphine (Liu, von Gizycki, & Gintzler, 2007), endomorphin 2 (EM2) (Liu & Gintzler, 2013) (a highly mu-opioid receptor (MOR)-selective opioid (Zadina, Hackler, Ge, & Kastin, 1997)), and dynorphin (kappa opioid receptor (KOR) agonist) (Gear, Gordon, et al., 1996; Gear, Miaskowski, et al., 1996; Miaskowski & Levine, 1999), as well as the heterodimerization of MOR with KOR (Chakrabarti, Liu, & Gintzler, 2010; Liu et al., 2011), the endogenous release of spinal opioids (e.g., EM2, dynorphin) (Kumar, Storman, Liu, & Gintzler, 2015; Liu, Murugaiyan, Storman, Schnell, Kumar, et al., 2017a), and the physiological collaboration among them (e.g., EM2 and dynorphin) (Liu, Murugaiyan, Storman, Schnell, Kumar, et al., 2017a). Complicating the influence of estrogens on opioid sequelae is the fact that at least two physiological distinguishable pools of estrogens exist: peripheral (ovarian) and estrogens produced within CNS. Furthermore, CNS-produced estrogens are not necessarily synchronized with the synthesis of ovarian estrogens, i.e., the production of CNS-derived estrogens can be either in-phase or out-of-phase with the ebb and flow of circulating estrogens (Storman et al., 2018). This complicates associating estrogen-dependent CNS functionalities with a particular stage of reproductive cycle. Additionally, nuclear ERs also traffic to the plasma membrane (Bjornstrom & Sjoberg, 2005; Boulware & Mermelstein, 2005; Dewing et al., 2007; Levin, 2005, 2008, 2009a, 2009b, 2009c; Razandi et al., 2003; Vasudevan & Pfaff, 2007), where they can either oligomerize with aromatase (estrogen synthase) or exist independent (or “free”) of it (Storman et al., 2018). This effectively creates multiple subpopulations of ERs that differ in function and regulation.
2. Sexually dimorphic opioid mechanisms mediate comparable opioid analgesia
Estrogens can profoundly influence the mechanisms that underlie analgesic responsiveness to the spinal application of morphine and their sexually dimorphic presentation. The spinal application of morphine produces thermal antinociception that is similar in magnitude and time course in rats of both sexes. However, despite the similarity in analgesic responsiveness in males and females, the antinociception results from strikingly sex-specific differential recruitment of spinal opioid analgesic components (Liu et al., 2007). Activation of spinal MOR, the predominant opioid receptor that is targeted for medicinal pain management, is critical for spinal morphine antinociception in both sexes. However, in females, but not males, activation of spinal KOR is also a prerequisite for spinal morphine antinociception, as is the recruitment of spinal dynorphin (an endogenous KOR-selective agonist) (Liu et al., 2007).
Gonadal hormonal action can be either “activational” or “organizational” (Phoenix, Goy, Gerall, & Young, 1959). The sexually dimorphic dynorphin/KOR component of spinal morphine analgesia is strikingly dependent on organizational actions of ovarian steroids. Elimination of acute activational effects of gonadal steroids via ovariectomy or orchiectomy fails to eliminate in females or unmask in males the dynorphin/KOR component of spinal morphine analgesia (Liu et al., 2007). This indicates that activational actions of gonadal hormones are not a determinant of phenotypic responsiveness to intrathecal morphine. In striking contrast, the spinal KOR component of spinal morphine antinociception is not manifest in sexually mature female rats that had been androgenized during the neonatal period (Liu et al., 2007). Thus, organizational effects of ovarian sex steroids are critical to the ability of intrathecal morphine to recruit KOR antinociceptive mechanisms. Notably, neonatal castration does not unveil a KOR component of spinal antinociception in sexually mature males. Thus, it is the presence of ovarian sex steroids during development, not the absence of testicular steroids, that is the critical ingredient for the ability of spinal morphine to harness KOR antinociception in females.
3. Influence of estrogens on the physical relationship of MOR and KOR
The female-specific dependence of spinal morphine antinociception on the concomitant utilization of spinal MOR and KOR/dynorphin bespeaks of an organization between spinal MOR and KOR in females that is not manifest in males. Indeed, MOR/KOR heterodimers are vastly more prevalent in the spinal cord of females than males, and in the spinal cord of proestrous than diestrous females (Chakrabarti et al., 2010).
Female-specific and estrous cycle stage-dependent formation of spinal MOR/KOR heterodimers suggests estrogens to be critical determinants of MOR/KOR oligomerization. Furthermore, the parallel occurrence of MOR/KOR heterodimerization and dynorphin/KOR mediation of spinal morphine antinociception during proestrus (when circulating levels of estrogens are high), but not during diestrus (when circulating estrogens are low) (Chakrabarti et al., 2010), is consistent with the ability of spinal dynorphin to activate the KOR protomer within heterodimeric MOR/KOR (Chakrabarti et al., 2010), providing the required KOR component of spinal morphine antinociception during proestrus. These findings strongly indicate MOR/KOR to be an estrogen-dependent female-specific molecular transducer for spinal morphine antinociception.
Abundant formation of spinal MOR/KOR during proestrus but not diestrus (or in males) suggests, but does not definitively establish, that estrogens are the driving force. This was validated by demonstrating the effect on MOR/KOR of spinally administered ER antagonists and ovariectomy. These studies indicated that CNS-derived as well as ovarian-derived estrogens are essential determinants of the ebb and flow of the heterodimerization of MOR and KOR (Chakrabarti et al., 2010) and thus the spinal antinociceptive mechanisms that are available, i.e., KOR-dependent and KOR-independent. Additionally, this oligomerization also requires concomitant, but non-additive, activation of multiple spinal mERs (ERα,ERβ and ER1 {aka GPR30, a G protein coupled ER}) (Liu et al., 2011).
Importantly, spinal estrogenic signaling, or lack thereof, interconverts the mechanistic underpinnings of spinal morphine analgesia (without altering its magnitude or temporal characteristics), shifting it between KOR-dependent and KOR-independent. There are substantial physiological and medicinal implications of the parallel existence of readily exchangeable morphine-activated spinal analgesic systems, which oscillate in accordance with stage of cycle. For example, in proestrous females (or the corresponding menstrual cycle stage in women), when spinal morphine analgesia requires dynorphin activation of the KOR protomer of MOR/KOR, impaired spinal dynorphin release could result in lack of analgesic responsiveness to spinal morphine. However, spinal morphine analgesic responsiveness can be rescued by interfering with spinal estrogenic signaling (via either spinal aromatase inhibition, or blockade of spinal mERs). This realization suggests that the spinal application of fadrozole (an aromatase inhibitor) or selective antagonists of mERα, mERβ, or GPR30 are likely to be effective adjuvants with intrathecal morphine in these situations.
4. Estrogens can influence the balance between antinociception and pronociception induced by dynorphin-KOR signaling
Dynorphin and KOR have been proposed to be pronociceptive (pain promoting) (Dubner & Ruda, 1992; Hollt, Haarmann, Millan, & Herz, 1987; Lai, Ossipov, Vanderah, Malan, & Porreca, 2001; Ruda, Iadarola, Cohen, & Young III, 1988; Stiller, Grubb, & Schaible, 1993; Wang et al., 2001) as well as antinociceptive (pain reducing) (Guirimand, Strimbu-Gozariu, Willer, & Le Bars, 1994; Han & Xie, 1982; Herman & Goldstein, 1984; Menendez, Andres-Trelles, Hidalgo, & Baamonde, 1993; Pelissier, Paeile, Soto-Moyano, Saavedra, & Hernandez, 1990; Przewlocki et al., 1983; Schmauss, 1987; Schmauss & Yaksh, 1984; Watkins, Wiertelak, & Maier, 1992). Formation of spinal cord MOR/KOR heterodimers provides a way to adjust the balance between antinociceptive vs. pronociceptive functions of the spinal dynorphin/KOR opioid system. The estrogen-driven chemical partnering of KOR with MOR facilitates spinal KOR-mediated antinociception to be manifest without being compromised by the pain-promoting functions that have also been associated with spinal KOR. Thus, dimeric MOR/KOR represents an estrogen-dependent female-specific signaling molecule that could underlie reports of much greater KOR-mediated antinociception in women than men (Gear et al., 2003; Gear, Gordon, et al., 1996; Gear et al., 1999; Gear, Miaskowski, et al., 1996; Holtman & Wala, 2006). The more robust, estrogen-driven heterodimerization of MOR and KOR in spinal cord of females than males suggests that in females dynorphin is capable of sub-serving antinociception, whereas in males dynorphin is much more likely to act as a pronociceptive agent.
5. Spinal ER-mGluR1 signaling suppresses EM2 analgesia
In addition to modulating the mechanistic underpinnings of analgesic responsiveness to spinal morphine (without altering the analgesia itself), estrogens are also endogenous modulators of the analgesia elicited by EM2 (the predominant endogenous MOR ligand in the spinal cord (Martin-Schild, Gerall, Kastin, & Zadina, 1999)). Antinociception elicited by spinal EM2 manifests not only a striking sexual dimorphism, but also dependence on stage of cycle. In females, the spinal EM2 antinociceptive system oscillates between analgesically active and inactive states. During diestrus, when circulating estrogens are low, spinal EM2 antinociception is minimal, whereas during proestrus spinal EM2 antinociception is robust and comparable in magnitude to that observed in males. Additionally, in proestrous females, spinal EM2 antinociception also requires spinal dynorphin and KOR activation, concomitant with MOR activation (Liu & Gintzler, 2013), as was observed for morphine.
However, instead of altering the mechanistic underpinnings of comparable analgesic responsiveness (as occurs for spinal morphine), estrogens actively suppress the spinal EM2 analgesia that is elicited by intrathecal EM2. This effect is mediated via mERα activation of mGluR1 (Liu & Gintzler, 2013). Furthermore, illustrating the complexity of estrogenic modulation, estrogenic suppression of spinal EM2 analgesia occurs, paradoxically, during diestrus (when circulating estrogens are low).
This lack synchronicity with circulating estrogens of spinal estrogenic signaling that suppresses spinal EM2 analgesia results from the actions of mERα within a spinal membrane-bound oligomer that contains aromatase (aka estrogen synthase), metabotropic glutamate receptors (mGluR1,mGluR2/3), MOR and KOR (Liu, Murugaiyan, Storman, Schnell, Kumar, et al., 2017a; Liu, Murugaiyan, Storman, Schnell, Wessendorf, et al., 2017b). In the case of EM2, during diestrus, suppressive spinal estrogenic signaling is mediated by mERα that signals via mGluR1 (Boulware et al., 2005; Liu, Murugaiyan, Storman, Schnell, Wessendorf, et al., 2017b). Estrogen-activated mERα-mGluR1 signaling reduces spinal dynorphin release, lessening KOR activation (Liu, Murugaiyan, Storman,Schnell, Kumar, et al., 2017a). This impedes spinal EM2 analgesic responsiveness since it requires both dynorphin and KOR activity, as is the case during proestrus (Liu & Gintzler, 2013).
6. Estrogenic signaling suppresses endogenous spinal EM2 release
The ability of estrogens to inhibit release of spinal dynorphin is not restricted to this opioid. Spinal estrogenic signaling is also a critical regulator of the utilization of endogenous spinal EM2. However, whereas mERα signaling (when coupled to mGluR1) is sufficient to suppress spinal dynorphin release, suppression of spinal EM2 release requires the concomitant activity of both mERα and GPR30 (Kumar et al., 2015). The need for cooperative signaling by mERα and GPR30 is consistent with earlier reports of their co-expression by neurons of the spinal dorsal horn (Liu et al., 2011), and their cooperative effects on gene transcription (Albanito et al., 2007) and formation of spinal MOR/KOR heterodimers (Liu et al., 2011).
Notably, the ability of mERα/GPR30 activity to inhibit EM2 release is sexually dimorphic; mERα/GPR30 blockade does not have any effect on spinal EM2 release in spinal cord of males (Kumar et al., 2015). Furthermore, the magnitude of estrogenic inhibition of spinal EM2 release is dependent on stage of estrous cycle (Kumar et al., 2015); blockade of spinal mERα/GPR30 during proestrus results in the largest increase in spinal EM2 release, an intermediate increase during diestrus, and no detectable increment during estrus (when circulating estrogens are at their lowest).
The negative correlation between the magnitude of basal EM2 release and circulating estrogens levels (i.e., basal EM2 release is highest during estrus, when circulating estrogens are lowest), suggests that spinal estrogenic activity acts as a physiological clamp to suppress EM2 release. Strikingly, the enhancement of spinal EM2 release by concomitant blockade of ERα and GPR30 is eliminated by either inhibition of spinal aromatase (via intrathecal fadrozole) or ovariectomy (Kumar et al., 2015). This indicates that both ovarian and spinally synthesized estrogens act collaboratively to regulate release of spinal EM2.
7. Estrogenic signaling enables coordination of spinal EM2 utilization with physiological demand
It is interesting to note that suppression of spinal EM2 release by estrogens directly parallels estrogen-dependent heterodimerization of MOR and KOR, which is also greater in proestrus than diestrus (Chakrabarti et al., 2010; Liu et al., 2011). Many studies have concluded that monomeric spinal KOR subserves pronociception (see Lai et al., 2001 for review) but not antinociception (Piercey & Einspahr, 1989; Przewlocka, Dziedzicka, Lason, & Przewlocki, 1992; Schmauss, 1987; Stevens & Yaksh, 1986). However, activation of KOR with MOR within MOR/KOR reveals antinociceptive attributes of KOR. Thus, during diestrus, when levels of MOR/KOR are low, the pronociceptive functions of spinal KOR prevail, while during proestrus, the pronociceptive functions of monomeric KOR are counterbalanced by the increased formation of MOR/KOR. So, during proestrus, when there is maximum estrogenic suppression of the release of EM2, this opioid would be less essential for counterbalancing endogenous dynorphin/KOR pronociception than during diestrus, when levels of pronociceptive monomeric KOR are high.
8. Collaboration between spinally- and ovarian-produced estrogens
Several studies have reported the relevance of either peripheral or central estrogens in nociception. As examples, ovariectomized rats demonstrate diminished sensitivity to formalin-induced pain (Gaumond, Arsenault, & Marchand, 2002); inhibition of spinal aromatase decreases pain sensitivity in both male and female quail (Evrard & Balthazart, 2004); in proestrous female rats, inhibition of spinal aromatase reduces MOR/KOR dimerization and eliminates the dynorphin/KOR dependency of morphine antinociception (Liu et al., 2011). There are much fewer examples of collaboration between peripheral and central estrogens in nociception and opioid antinociception. Nevertheless, their functional interrelationship remains subject of much speculation (Schlinger, Remage-Healey, & Rensel, 2014).
CNS-derived estrogens are synthesized by CNS aromatase, which is located near synaptic structures. CNS-derived estrogens are thereby poised to activate nearby mERs relevant to pain modulation (Evrard, 2006;Evrard & Balthazart, 2004; Hojo et al., 2004; Naftolin et al., 1996; Peterson, Yarram, Schlinger, & Saldanha, 2005). Such estrogenic signaling would be expected to be highly spatially restricted. In contrast, peripherally synthesized estrogens reach the CNS by penetrating the blood-brain barrier and diffusing from cerebrospinal fluid into extracellular fluid (Guyton & Hall, 2010) to act on CNS ERs. Such signaling, at first blush, would be expected to produce uniform activation of all CNS ERs, which is inconsistent with segregated, partitioned CNS functionality and is incongruous with the graded (not all or none) suppression of spinal EM2 release over the estrous cycle (Kumar et al., 2015). Thus, it is more likely that peripheral and central estrogens cooperate to modulate CNS functionality (the relative contributions of each and specific points of intersection remaining largely unknown). The mechanism(s) underpinning hypothesized synergistic interactions between centrally and peripherally synthesized estrogens remain obscure. Given reports that diffusion of centrally synthesized estrogens, and, by extension, diffusion of peripherally synthesized estrogens into the CNS, is highly spatially restricted (Charlier et al., 2011; Fokidis, Prior, & Soma, 2013; Remage-Healey, Maidment, & Schlinger, 2008; Schlinger et al., 2014), it is possible that CNS-and ovarian-derived estrogens activate different populations of spinal ERs that are functionally convergent and act in a cooperative, possibly synergistic fashion.
9. Multiple subpopulations of CNS aromatase and mERα
The plethora and complexity of the actions of estrogens on the CNS is undergirded by a multiplicity of types of ER (e.g., ERα, ERβ, GPR30,Gq-mER, ER-X). The multi-faceted nature of estrogenic modulation of neuronal function is supported by the ability of ERs to localize to the nucleus to regulate transcription, as well as to the plasma membrane (Filardo, Quinn, Bland, & Frackelton, 2000; Razandi, Pedram, Greene, & Levin, 1999; Revankar, Cimino, Sklar, Arterburn, & Prossnitz, 2005), where they activate (within sec to min) signaling cascades identical to those initiated by G protein-coupled receptors (Levin, 2009c; Mermelstein, 2009; Micevych & Dominguez, 2009; Vasudevan & Pfaff, 2008).
Actions of estrogens fall into five main categories: endocrine, paracrine, autocrine, synaptocrine and oligocrine. The first three differ only with respect to the distance between the point of synthesis and the site of action (reviewed in Saldanha, Remage-Healey, & Schlinger, 2011). A fourth category, synaptocrine (Saldanha et al., 2011), involves specific modulation of estrogen concentrations at the synapse. Synaptocrine estrogenic signaling is enabled by the presence of aromatase and ERs on dendritic/somatic membranes and presynaptic terminals (Beyer, Pawlak, & Karolczak, 2003; Blaustein, 1992; Blaustein, Lehman, Turcotte, & Greene, 1992; McEwen et al., 2001; McEwen & Alves, 1999; Schlinger & Callard, 1989; Wu et al., 2009). The fifth category, oligocrine, was defined by our recent finding that aromatase and mERs are present in the same oligomer along with other plasma membrane-associated signaling molecules. Oligocrine signaling, the ability of estrogens to function as intracellular messengers whose synthesis and actions occur within the same macromolecular signaling complex, confers exquisite discrete spatial and temporal specificity to estrogenic signaling. Furthermore, the presence of aromatase activity within a signaling complex containing mERs permits the differential activation/deactivation of discrete subcellular ER-coupled signaling, independent of variations in circulating levels of estrogens—either out of phase (as occurs for estrogenic suppression of spinal EM2 antinociception) or in-phase with ovarian steroid production (as occurs for estrogenic regulation of MOR/KOR heterodimerization).
Oligocrine estrogenic signaling in the CNS complements endocrine (ovarian estrogens acting on CNS ERs) and neurotransmitter-like synaptocrine estrogenic signaling. Maximum physiological utilization of the multiple modalities of CNS estrogenic signaling suggests the importance of coordinating the actions of peripheral and central estrogens. Additionally, the ability of the CNS as well as the ovary to make estrogens means that there are at least two functional pools of aromatase. This duality is further complicated by the presence of aromatase in a CNS plasma membrane oligomer that contains mERs, as well as other signaling proteins. This organization creates two additional populations of mERs and aromatase, those that are oligomerized with each other and those that exist separately, i.e., “free.” Thus, the multi-dimensional nature of estrogenic actions in the CNS, particularly as pertains to modulating nociception and opioid antinociception, likely requires not only coordination between the actions of central and peripheral estrogens, but also the activity of spatially and functionally segregated pools of aromatase and mERs in the CNS. Given this complexity and multidimensional nature of estrogenic signaling, it is not surprising that estrogens have been reported to be pronociceptive (Bradshaw, Miller, Ling, Malsnee, & Ruda, 2000; Ji, Murphy, & Traub, 2003; Ji, Tang, & Traub, 2011; Li et al., 2009; Lu, Chen, Wang, & Wu, 2009; Sanoja & Cervero, 2005), as well as antinociceptive (Aloisi et al., 2010; Cao, Ji, Tang, & Traub, 2012; Fischer et al., 2008; Giamberardino, Affaitati, Valente, Iezzi, & Vecchiet, 1997; Kramer & Bellinger, 2009; Lawson, Nag, Thompson, & Mokha, 2010; Mannino, South, Quinones-Jenab, & Inturrisi, 2007; Riley, Robinson, Wise, & Price, 1999; Sarajari & Oblinger, 2010).
The existence of mERα/aromatase oligomerized and free populations holds out the promise that their various subpopulations can be independently targeted for medicinal purposes. The putative utility of doing so is underscored by our recent finding that although aromatase and mERα physically associate with each other in both spinal cord and hypothalamus, the distribution between mERα-associated and free is essentially opposite in these areas—in the spinal cord, essentially all of the aromatase is oligomerized with mERα, whereas in the hypothalamus, essentially the opposite pertains (Storman et al., 2018). Thus, a drug that is selective for the mERα/aromatase oligomer would likely affect estrogenic signaling in the spinal cord, but markedly less so in hypothalamus whereas targeting free mERα or aromatase would likely alter estrogenic signaling in hypothalamus but not spinal cord.
Perhaps the best example of the potency of sex steroids in influencing opioid analgesia is the increase in nociceptive thresholds that occurs during physiological gestation. During physiological pregnancy, there is a naturally occurring elevation of pain tolerance, peaking just prior to parturition (Gintzler, 1980). Systemic administration of the general opioid receptor blocker, naloxone, abolishes pregnancy-induced analgesia, indicating participation of the endogenous opioid system. Strikingly, the opioid antinociception associated with pregnancy is also seen when the pregnancy blood profile of estrogen and progesterone are simulated (in the absence of physiological pregnancy). Moreover, both the antinociception induced by pregnancy and its hormonal simulation are mediated not only by the same types of opioid receptor but also the same CNS compartment, spinal cord (Dawson-Basoa & Gintzler, 1993, 1996, 1997, 1998; Medina, Dawson-Basoa, & Gintzler, 1993). This underscores that the profile of change in activity of sex steroids is not only necessary, but also sufficient for pregnancy-induced analgesia to be manifest, highlighting the potential of estrogens, acting in combination with progesterone, to unlock the powerful ability of endogenous opioids to mitigate pain.
10. Conclusions
Estrogens impact multiple parameters of opioid functionality (see Scheme 1). Given the multi-faceted influence of estrogens on opioid systems, it is imperative to understand, and possibly intercede at, the points of intersection of estrogenic signaling and opioid functionality, particularly in the face of the current opioid epidemic. The propensity of aromatase to either to physically pair with or exist separately from mERα in combination with a synaptic relationship between aromatase and mERα, enables locally produced estrogens to act on multiple spatial levels, spanning the subcellular to synaptic, with a high degree of gradated regulation and plasticity. Factors that integrate estrogenic actions at subcellular, synaptic, and CNS regional levels are likely to be prime drug targets for novel pharmacotherapies designed to modulate CNS estrogen-dependent functionalities and possibly circumvent the current opioid epidemic.
Scheme 1.
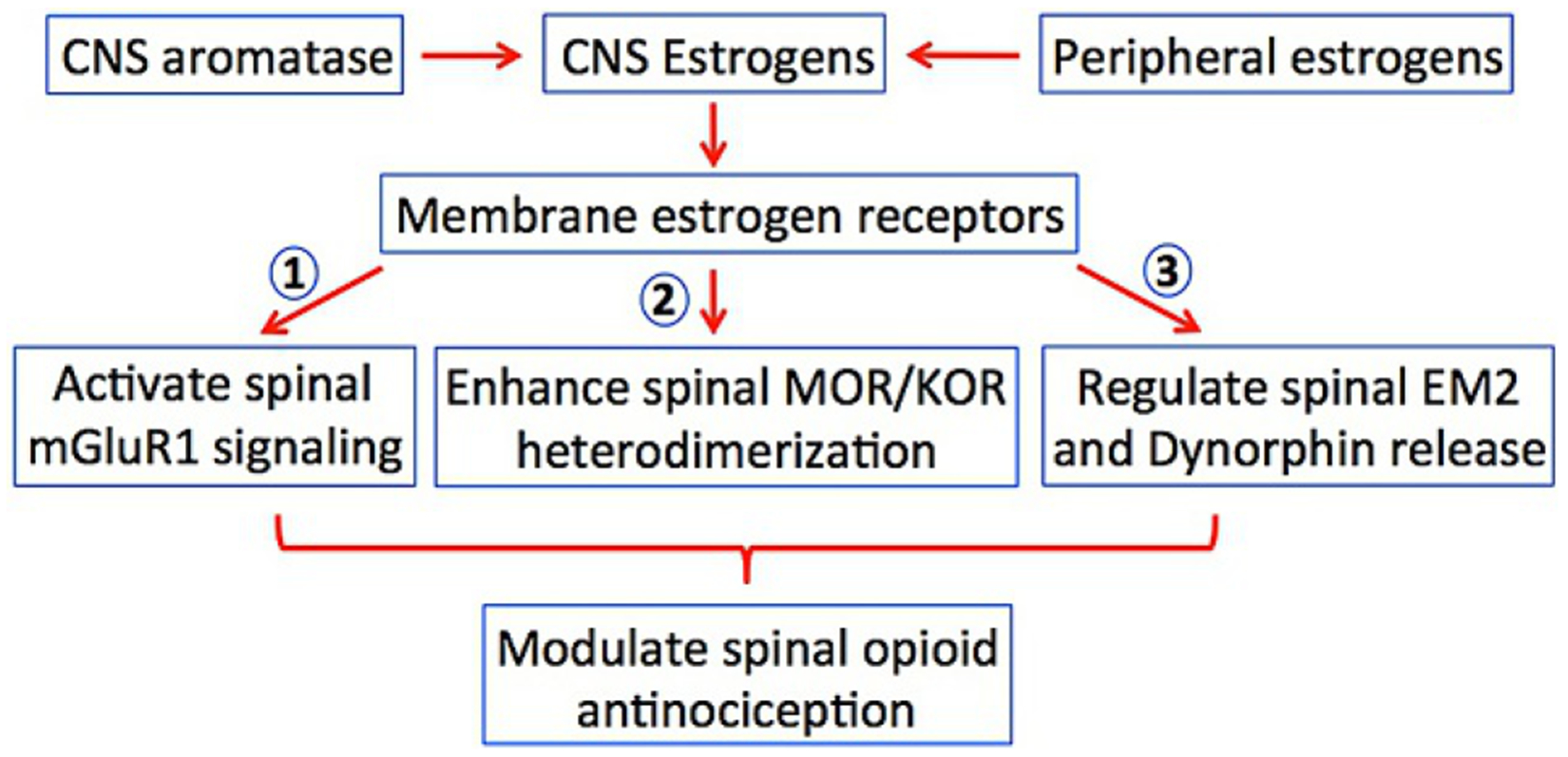
CNS membrane estrogen receptors are activated by ovarian-produced and CNS-produced estrogens. Opioid parameters modulated by CNS estrogenic signaling include (1) membrane estrogen receptor alpha activation of spinal mGluR1 (to inhibit spinal EM2 analgesia during diestrus), (2) enhancement of the heterodimerization of spinal MOR and KOR (as occurs during proestrus), (3) graded inhibition of spinal release of EM2 (as occurs over the reproductive cycle) and dynorphin release (as occurs during diestrus). All impact exogenous as well as endogenous opioid antinociception.
Footnotes
Disclosures
None of the authors hold any conflicts of interest.
References
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, et al. (2007). G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Research, 67(4), 1859–1866. 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Affaitati G, Ceccarelli I, Fiorenzani P, Lerza R, Rossi C, et al. (2010). Estradiol and testosterone differently affect visceral pain-related behavioural responses in male and female rats. European Journal of Pain, 14(6), 602–607. 10.1016/j.ejpain.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, & Ball GF (2001). Rapid and reversible inhibition of brain aromatase activity. Journal of Neuroendocrinology, 13(1), 63–73. jne598 [pii]. 10.1111/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, & Ball GF (2006). Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology, 147(1), 359–366. 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Cornil CA, Taziaux M, Charlier TD, Baillien M, & Ball GF (2006). Rapid changes in production and behavioral action of estrogens. Neuroscience, 138(3), 783–791. 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Beyer C, Pawlak J, & Karolczak M (2003). Membrane receptors for oestrogen in the brain. Journal of Neurochemistry, 87(3), 545–550. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, & Sjoberg M (2005). Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Molecular Endocrinology, 19(4), 833–842. 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Blaustein JD (1992). Cytoplasmic estrogen receptors in rat brain: Immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology, 131(3), 1336–1342. 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Lehman MN, Turcotte JC, & Greene G (1992). Estrogen receptors in dendrites and axon terminals in the Guinea pig hypothalamus. Endocrinology, 131(1), 281–290. 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- Boulware MI, & Mermelstein PG (2005). The influence of estradiol on nervous system function. Drug News & Perspectives, 18(10), 631–637. 10.1358/dnp.2005.18.10.959578. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, & Mermelstein PG (2005). Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. The Journal of Neuroscience, 25(20), 5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw H, Miller J, Ling Q, Malsnee K, & Ruda MA (2000). Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain, 85(1–2), 93–99. [DOI] [PubMed] [Google Scholar]
- Cao DY, Ji Y, Tang B, & Traub RJ (2012). Estrogen receptor beta activation is anti-nociceptive in a model of visceral pain in the rat. The Journal of Pain, 13(7), 685–694. 10.1016/j.jpain.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Liu NJ, & Gintzler AR (2010). Formation of mu-/kappa-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proceedings of the National Academy of Sciences of the United States of America, 107(46), 20115–20119. 10.1073/pnas.1009923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Newman AE, Heimovics SA, Po KW, Saldanha CJ, & Soma KK (2011). Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. Journal of Neuroendocrinology, 23(8), 742–753. 10.1111/j.1365-2826.2011.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, & Balthazart J (2006). Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Research, 1126(1), 2–26. 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM (2003). Sex differences in opioid analgesia: “From mouse to man” The ClinicalJournal of Pain, 19(3), 175–186. [DOI] [PubMed] [Google Scholar]
- Dawson-Basoa ME, & Gintzler AR (1993). 17-b-Estradiol and progesterone modulate an intrinsic opioid analgesic system. Brain Research, 601, 241–245. [DOI] [PubMed] [Google Scholar]
- Dawson-Basoa ME, & Gintzler AR (1996). Estrogen and progesterone activate spinal kappa-opiate receptor analgesic mechanisms. Pain, 64, 607–615. [DOI] [PubMed] [Google Scholar]
- Dawson-Basoa ME, & Gintzler AR (1997). Involvement of spinal cord delta opiate receptors in the antinociception of gestation and its hormal simulation. Brain Research, 757, 37–42. [DOI] [PubMed] [Google Scholar]
- Dawson-Basoa ME, & Gintzler AR (1998). Gestational and ovarian sex steroid antinociception: Synergy between spinal k and d opioid systems. Brain Research, 794, 61–67. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, & Micevych P (2007). Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(35), 9294–9300. 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R, & Ruda M (1992). Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends in Neurosciences, 15, 96–103. [DOI] [PubMed] [Google Scholar]
- Evrard HC (2006). Estrogen synthesis in the spinal dorsal horn: A new central mechanism for the hormonal regulation of pain. American Journal of Physiology. Regulatory. Integrative and Comparative Physiology, 291(2), R291–R299. 10.1152/ajpregu.00930.2005. [DOI] [PubMed] [Google Scholar]
- Evrard H, Baillien M, Foidart A, Absil P, Harada N, & Balthazart J (2000). Localization and controls of aromatase in the quail spinal cord. The Journal of Comparative Neurology, 423(4), 552–564. . [DOI] [PubMed] [Google Scholar]
- Evrard HC, & Balthazart J (2003). Aromatase (estrogen synthase) activity in the dorsal horn of the spinal cord: Functional implications. Annals of the New York Academy of Sciences, 1007, 263–271. [DOI] [PubMed] [Google Scholar]
- Evrard HC, & Balthazart J (2004). Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24(33), 7225–7229. 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard HC, Willems E, Harada N, & Balthazart J (2003). Specific innervation of aromatase neurons by substance P fibers in the dorsal horn of the spinal cord in quail. The Journal of Comparative Neurology, 465(2), 309–318. 10.1002/cne.10854. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, & Frackelton AR Jr. (2000). Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Molecular Endocrinology, 14(10), 1649–1660. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, & Gear RW (2004). Sex differences in opioid analgesia: Clinical and experimental findings. European Journal of Pain, 8(5), 413–425. 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, & Riley JL 3rd. (2009). Sex, gender, and pain: A review of recent clinical and experimental findings. The Journal of Pain, 10(5), 447–485. 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L, Torres-Chavez KE, Clemente-Napimoga JT, Jorge D, Arsati F, deArruda Veiga MC, et al. (2008). The influence of sex and ovarian hormones on temporomandibular joint nociception in rats. The Journal of Pain, 9(7), 630–638. 10.1016/j.jpain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Fokidis HB, Prior NH, & Soma KK (2013). Fasting increases aggression and differentially modulates local and systemic steroid levels in male zebra finches. Endocrinology, 154(11), 4328–4339. 10.1210/en.2013-1171. [DOI] [PubMed] [Google Scholar]
- Gaumond I, Arsenault P, & Marchand S (2002). The role of sex hormones on formalin-induced nociceptive responses. Brain Research, 958(1), 139–145. S0006899302036612 [pii]. 10.1016/S0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- Gear RW, Gordon NC, Heller PH, Paul S, Miaskowski C, & Levine JD (1996). Gender difference in analgesic response to the kappa-opioid pentazocine. Neuroscience Letters, 205(3), 207–209. [DOI] [PubMed] [Google Scholar]
- Gear RW, Gordon NC, Miaskowski C, Paul SM, Heller PH, & Levine JD (2003). Sexual dimorphism in very low dose nalbuphine postoperative analgesia. Neuroscience Letters, 339(1), 1–4. [DOI] [PubMed] [Google Scholar]
- Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, & Levine JD (1996). Kappa-opioids produce significantly greater analgesia in women than in men. Nature Medicine, 2(11), 1248–1250. [DOI] [PubMed] [Google Scholar]
- Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, & Levine JD(1999). The kappa opioid nalbuphine produces gender-and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain, 83(2), 339–345. [DOI] [PubMed] [Google Scholar]
- Giamberardino MA, Affaitati G, Valente R, Iezzi S, & Vecchiet L (1997). Changes in visceral pain reactivity as a function of estrous cycle in female rats with artificial ureteral calculosis. Brain Research, 774(1–2), 234–238. doi: S0006–8993(97)81711–8 [pii]. 10.1016/s0006-8993(97)81711-8. [DOI] [PubMed] [Google Scholar]
- Gintzler AR (1980). Endorphin-mediated increases in pain threshold during pregnancy.Science, 210, 193–195. [DOI] [PubMed] [Google Scholar]
- Guirimand F, Strimbu-Gozariu M, Willer J-C, & Le Bars D (1994). Effects of mu, delta and kappa opioid antagonists on the depression of a C-fiber reflex by intrathecal morphine and DAGO in the rat. The Journal of Pharmacology and Experimental Therapeutics, 269, 1007–1020. [PubMed] [Google Scholar]
- Guyton A, & Hall JE (2010). Guyton and Hall textbook of medical physiology (12th ed).Philadelphia, PA: Saunders. [Google Scholar]
- Han JS, & Xie CW (1982). Dynorphin: Potent analgesic effect in spinal cord of the rat.Life Sciences, 31, 1781–1784. [DOI] [PubMed] [Google Scholar]
- Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, & Lolait SJ (2009). Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. The Journal of Endocrinology, 202(2), 223–236. 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman BH, & Goldstein A (1984). Antinociception and paralysis induced by intrathecal dynorphin a. The Journal of Pharmacology and Experimental Therapeutics, 232, 27–32. [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, et al. (2004). Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proceedings of the National Academy of Sciences of the United States of America, 101(3), 865–870. 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollt V, Haarmann I, Millan MJ, & Herz A (1987). Prodynorphin gene expression is enhanced in the spinal cord of chronic arthritic rats. Neuroscience Letters, 73, 90–94. [DOI] [PubMed] [Google Scholar]
- Holtman JR Jr., & Wala EP (2006). Characterization of the antinociceptive effect of oxycodone in male and female rats. Pharmacology, Biochemistry, and Behavior, 83(1), 100–108. 10.1016/j.pbb.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Ibironke GF, & Aji KE (2011). Pain threshold variations in female rats as a function of the estrus cycle. Nigerian Journal of Physiological Sciences, 26(1), 67–70. [PubMed] [Google Scholar]
- Jakab RL, Harada N, & Naftolin F (1994). Aromatase- (estrogen synthetase) immunoreactive neurons in the rat septal area. A light and electron microscopic study. Brain Research, 664(1–2), 85–93. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, & Traub RJ (2003). Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(9), 3908–3915. 23/9/3908 [pii]. 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Tang B, & Traub RJ (2011). Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain, 152(5), 1182–1191. 10.1016/j.pain.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PR, & Bellinger LL (2009). The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology, 150(8), 3680–3689. 10.1210/en.2008-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Storman EM, Liu NJ, & Gintzler AR (2015). Estrogens suppress spinal Endomorphin 2 release in female rats in phase with the estrous cycle. Neuroendocrinology, 102(1–2), 33–43. 10.1159/000430817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Ossipov MH, Vanderah TW, Malan TP Jr., & Porreca F (2001). Neuropathic pain: The paradox of dynorphin. Molecular Interventions, 1(3), 160–167. [PubMed] [Google Scholar]
- Lawson KP, Nag S, Thompson AD, & Mokha SS (2010). Sex-specificity and estrogen-dependence of kappa opioid receptor-mediated antinociception and anti-hyperalgesia. Pain, 151(3), 806–815. 10.1016/j.pain.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER (2005). Integration of the extranuclear and nuclear actions of estrogen. MolecularEndocrinology, 19(8), 1951–1959. 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER (2008). Rapid signaling by steroid receptors. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 295(5), R1425–R1430. 10.1152/ajpregu.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER (2009a). G protein-coupled receptor 30: Estrogen receptor or collaborator? Endocrinology, 150(4), 1563–1565. 10.1210/en.2008-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER (2009b). Membrane oestrogen receptor alpha signalling to cell functions. The Journal of Physiology, 587(Pt. 21), 5019–5023. 10.1113/jphysiol.2009.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER (2009c). Plasma membrane estrogen receptors. Trends in Endocrinology and Metabolism, 20(10), 477–482. 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Fan X, Warner M, Xu XJ, Gustafsson JA, & Wiesenfeld-Hallin Z (2009). Ablation of estrogen receptor alpha or beta eliminates sex differences in mechanical pain threshold in normal and inflamed mice. Pain, 143(1–2), 37–40. 10.1016/j.pain.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Liu NJ, Chakrabarti S, Schnell S, Wessendorf M, & Gintzler AR (2011). Spinal synthesis of estrogen and concomitant signaling by membrane estrogen receptors regulate spinal {kappa}- and {mu}-opioid receptor heterodimerization and female-specific spinal morphine antinociception. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(33), 11836–11845. 10.1523/JNEUROSCI.1901-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NJ, & Gintzler AR (2013). Spinal endomorphin 2 antinociception and the mechanisms that produce it are both sex- and stage of estrus cycle-dependent in rats. The Journal of Pain, 14(11), 1522–1530. 10.1016/j.jpain.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NJ, Murugaiyan V, Storman EM, Schnell SA, Kumar A, Wessendorf MW, et al. (2017a). Plasticity of signaling by spinal estrogen receptor alpha, kappa-opioid receptor and mGluRs over the rat reproductive cycle regulates spinal endomorphin 2 antinociception: Relevance of endogenous biased agonism. The Journal of Neuroscience, 37, 11181–11191. 10.1523/JNEUROSCI.1927-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NJ, Murugaiyan V, Storman EM, Schnell SA, Wessendorf MW, & Gintzler AR (2017b). Estrogens synthesized and acting within a spinal oligomer suppress spinal endomorphin 2 antinociception: Ebb and flow over the rat reproductive cycle. Pain, 158(10), 1903–1914. 10.1097/j.pain.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NJ, von Gizycki H, & Gintzler AR (2007). Sexually dimorphic recruitment of spinal opioid analgesic pathways by the spinal application of morphine. The Journal of Pharmacology and Experimental Therapeutics, 322(2), 654–660. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Morgan MM, & Murphy AZ (2007). Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: A potential mechanism for sex differences in antinociception. Neuroscience, 147(2), 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Chen CW, Wang SY, & Wu FS (2009). 17Beta-estradiol mediates the sex difference in capsaicin-induced nociception in rats. The Journal of Pharmacology and Experimental Therapeutics, 331(3), 1104–1110. 10.1124/jpet.109.158402. [DOI] [PubMed] [Google Scholar]
- Mannino CA, South SM, Quinones-Jenab V, & Inturrisi CE (2007). Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. The Journal of Pain, 8(4), 334–342. 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, & Zadina JE (1999). Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. The Journal of Comparative Neurology, 405, 450–471. [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, et al. (2001). Tracking the estrogen receptor in neurons: Implications for estrogen-induced synapse formation. Proceedings of the National Academy of Sciences of the United States of America, 98(13), 7093–7100. 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Alves SE (1999). Estrogen actions in the central nervous system. Endocrine Reviews, 20(3), 279–307. [DOI] [PubMed] [Google Scholar]
- Medina VM, Dawson-Basoa ME, & Gintzler AR (1993). 17-b-estradiol and progesterone positively modulate spinal cord dynorphin: Relevance to the analgesia of pregnancy. Neuroendocrinology, 58, 310–315. [DOI] [PubMed] [Google Scholar]
- Menendez L, Andres-Trelles F, Hidalgo A, & Baamonde A (1993). Involvement of spinal k opioid receptors in a type of footshock induced analgesia in mice. Brain Research, 611, 264–271. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, & Dellovade TL (2004). Distribution of estrogen receptor alpha and beta in the mouse central nervous system: In vivo autoradiographic and immunocytochemical analyses. The Journal of Comparative Neurology, 473(2), 270–291. 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG (2009). Membrane-localised oestrogen receptor alpha and beta influence neuronal activity through activation of metabotropic glutamate receptors. Journal of Neuroendocrinology, 21(4), 257–262. 10.1111/j.1365-2826.2009.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, & Levine JD (1999). Does opioid analgesia show a gender preference for females? Pain Forum, 8, 34–44. [Google Scholar]
- Micevych P, & Dominguez R (2009). Membrane estradiol signaling in the brain. Frontiers in Neuroendocrinology, 30(3), 315–327. 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, et al. (2003). Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology, 144(5), 2055–2067. 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, & Sternberg WF (2000). Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neuroscience and Biobehavioral Reviews, 24(3), 375–389. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, & Balthazart J (1996). Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology, 63(2), 149–155. [DOI] [PubMed] [Google Scholar]
- Pelissier T, Paeile C, Soto-Moyano R, Saavedra H, & Hernandez A (1990). Analgesia is produced by intrathecal administration of the k opioid agonist, U50,488H, on formalin-evoked cutaneous pain in the rat. European Journal of Pharmacology, 190, 287–293. [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, & Mufson EJ (2003). Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Research. Developmental Brain Research, 145(1), 117–139. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, & Saldanha CJ (2005). Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings of the Biological Sciences, 272(1576), 2089–2096. 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, & Young WC (1959). Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female Guinea pig. Endocrinology, 65, 369–382. 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Piercey MF, & Einspahr FJ (1989). Spinal analgesic actions of kappa receptor agonists, U-50488H and spiradoline (U-62066). The Journal of Pharmacology and Experimental Therapeutics, 251(1), 267–271. [PubMed] [Google Scholar]
- Przewlocka B, Dziedzicka M, Lason W, & Przewlocki R (1992). Differential effects of opioid receptor agonists on nociception and cAMP level in the spinal cord of monoarthritic rats. Life Sciences, 50(1), 45–54. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Stala L, Greczek M, Shearmen GT, Przewlocka B, & Herz A (1983). Analgesic effects of mu, delta, kappa-opiate agonists and in particular, dynorphin at the spinal level. Life Sciences, 33(1), 649–652. [DOI] [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, & Levin ER (2003). Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Molecular and Cellular Biology, 23(5), 1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, & Levin ER (1999). Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Molecular Endocrinology, 13(2), 307–319. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, & Schlinger BA (2008). Forebrain steroid levels fluctuate rapidly during social interactions. Nature Neuroscience, 11(11), 1327–1334. 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, & Prossnitz ER (2005). A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science, 307(5715), 1625–1630. 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Riley JL 3rd, Robinson ME, Wise EA, & Price DD (1999). A meta-analytic review of pain perception across the menstrual cycle. Pain, 81(3), 225–235. S0304395998002589 [pii]. 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Iadarola MJ, Cohen LV, & Young WS III. (1988). In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America, 85, 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, & Schlinger BA (2011). Synaptocrine signaling: Steroid synthesis and action at the synapse. Endocrine Reviews, 32(4), 532–549. 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanoja R, & Cervero F (2005). Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: A model of functional abdominal pain. Pain, 118(1–2), 243–253. 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Sarajari S, & Oblinger MM (2010). Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Experimental Neurology, 224(1), 163–169. 10.1016/j.expneurol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, & Callard GV (1989). Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology, 49(4), 434–441. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Remage-Healey L, & Rensel M (2014). Establishing regional specificity of neuroestrogen action. General and Comparative Endocrinology, 205, 235–241. 10.1016/j.ygcen.2014.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmauss C (1987). Spinal k-opioid receptor-mediated antinociception is stimulus specific.European Journal of Pharmacology, 137, 197–205. [DOI] [PubMed] [Google Scholar]
- Schmauss C, & Yaksh TL (1984). In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. The Journal of Pharmacology and Experimental Therapeutics, 228(1), 1–12. [PubMed] [Google Scholar]
- Shughrue PJ, & Merchenthaler I (2001). Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. The Journal of Comparative Neurology, 436(1), 64–81. [PubMed] [Google Scholar]
- Stevens CW, & Yaksh TL (1986). Dynorphin A and related peptides administered intrathecally in the rat: A search for putative kappa opiate receptor activity. The Journal of Pharmacology and Experimental Therapeutics, 238(3), 833–838. [PubMed] [Google Scholar]
- Stiller RU, Grubb BD, & Schaible H-G (1993). Neurophysiological evidence for increased kappa opioidergic control of spinal cord neurons in rats with unilateral inflammation at the ankle. The European Journal of Neuroscience, 5, 1520–1527. [DOI] [PubMed] [Google Scholar]
- Storman EM, Liu NJ, Wessendorf MW, & Gintzler AR (2018). Physical linkage of estrogen receptor alpha and aromatase in rat: Oligocrine and endocrine actions of CNS-produced estrogens. Endocrinology, 159(7), 2683–2697. 10.1210/en.2018-00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teepker M, Peters M, Vedder H, Schepelmann K, & Lautenbacher S (2010). Menstrual variation in experimental pain: Correlation with gonadal hormones. Neuropsychobiology, 61(3), 131–140. 10.1159/000279303. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, & Pfaff DW (2007). Membrane-initiated actions of estrogens in neuroendocrinology: Emerging principles. Endocrine Reviews, 28(1), 1–19. 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, & Pfaff DW (2008). Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Frontiers in Neuroendocrinology, 29(2), 238–257. 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, et al. (2001). Pronociceptive actions of dynorphin maintain chronic neuropathic pain. The Journal of Neuroscience, 21(5), 1779–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, & Maier SF (1992). Kappa opiate receptors mediate tail-shock induced antinociception at spinal levels. Brain Research, 582, 1–9. [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, et al. (2009). Estrogen masculinizes neural pathways and sex-specific behaviors. Cell, 139(1), 61–72. 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, & Kastin AJ (1997). A potent and selective endogenous agonist for the mu-opiate receptor. Nature, 386(6624), 499–502. 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]


