Abstract
The associations between different combinations of metabolic abnormalities and the risk of all and site-specific cancers remain unclear. We aimed to estimate the association and interplay between serum cholesterol, glycemic status and risk of cancer in the China Cardiometabolic Disease and Cancer Cohort (4C)-Study, a nationwide, multicenter, prospective, population-based study. The investigation was performed in 137,884 participants during 2014-2016. Incident cancer was defined as the first occurrence of any type cancer of all sites during follow-up. After 510,164 person-years of follow-up, 1,710 were detected as incident cancer after exclusion of participants diagnosed as cancer within 6 months from baseline. A relatively low level of LDL cholesterol (<100 mg/dl) was related to a significant higher risk of incident cancer [1.20 (1.08-1.34); P=0.0007]. Diabetic individuals have a significantly higher risk of incident cancer, especially those with poorly glycemic control. Diabetic participants with both lower levels of LDL cholesterol and poorly glycemic control were at a higher risk of incident cancer [1.42 (1.10-1.81); P=0.006]. Our study showed a positive association of cancer risk with low-level LDL cholesterol and diabetes and found that participants with both lower levels of LDL cholesterol and poorly controlled diabetes had the higher risk of incident cancer, which indicates the compelling need of achieving glycemic control goal and maintaining appropriate LDL cholesterol levels.
Keywords: Cancer, cholesterol, diabetes, HbA1c, LDL
Introduction
Cancer is considered as a major threat to public health, and action needs to be taken on all fields of prevention. Findings from epidemiological study and experimental investigations have linked factors that may alter metabolic status, such as diet, smoking, and obesity, with an increased cancer risk [1-3]. Diabetes and higher levels of serum LDL cholesterol are believed to be major metabolic risk factors of several non-communicable chronic diseases, including stroke and coronary heart diseases [4-6]. However, the association between metabolic elements and cancer is complex and incongruous. Diabetes is a well-established risk factor for all cancer and several site-specific cancers [7-12]. On the other hand, hypercholesterolemia has been found of an incongruous association with cancer risk [13-16]. A few prospective epidemiological studies have detected that serum LDL cholesterol level is inversely associated with the risk of cancer, but these studies have been limited by some residual confounding factors, such as the use of lipid-lowering medication and findings from previous studies are inconsistent [13-18]. Moreover, the combination of glycemic status and serum cholesterol in the association of cancer is not well understood and nor do current recommendations on optimal management of these two factors with regard to cancer. To our knowledge, the associations between different combinations of metabolic abnormalities and the risk of all and site-specific cancers remain unclear.
Thus, the present study aimed to investigate the association and joint effect between serum cholesterol, glycemic status and the risk of incident cancer in middle-aged and elderly Chinese population.
Materials and methods
Study design
The China Cardiometabolic Disease and Cancer Cohort (4C)-Study is a nationwide, multicenter, prospective, population-based study that was designed to explore the associations of metabolic factors with clinical outcomes, including incident diabetes, cardiovascular events and cancer in middle-aged and elderly Chinese individuals [19]. The 4C study included 20 community sites, covering 16 provinces, autonomous regions, or municipalities of mainland China. We used data from this study of 193,846 individuals for this investigation. The study protocol and informed consent were approved by the Committee on Human Research at Rui-Jin Hospital Affiliated to the Jiao-Tong University School of Medicine. All participants signed the written informed consent.
Baseline data collection
At each study site, baseline data was collected according to a standardized protocol in examination centers at local health stations or community clinics in 2010-2011.
Using a standard questionnaire, trained staff collected information face-to-face about sociodemographic characteristics, history of diseases and medication, family history, and lifestyle factors (including current smoking status, current drinking status, physical activity, sedentary behavior and diet habits) [20] Height, weight and blood pressure were measured by the trained personnel using a standardized protocol.
Fasting blood samples were collected to evaluate the levels of fasting glucose and cholesterol. All participants underwent a 75-g load OGTT, and OGTT postload plasma glucose level was obtained at 2 hours. Plasma glucose concentrations were evaluated at local hospitals using the glucose oxidase or hexokinase method and serum total cholesterol, LDL cholesterol, and HDL cholesterol were measured at the central laboratory using an auto-analyzer (ARCHITECT ci16200 analyzer, Abbott Laboratories, Illinois, USA). A Hemoglobin Capillary Collection System (HCCS, Bio-Rad Laboratories, CA, USA) was used to collect finger capillary whole blood and determined by high-performance liquid chromatography (VARIANT™ II Systems, BIO-RAD, Hercules, CA, USA).
According to the 2010 American Diabetes Association (ADA) criteria, diabetes was diagnosed at baseline if meeting at least one of the following criteria (1) Fasting plasma glucose (FPG) level of 126 mg/dL or higher, (2) OGTT 2-hour postload plasma glucose (PPG) level of 200 mg/dL or higher, or (3) HbA1c level of 6.5% or higher, or (4) A self-reported diagnosis by professionals. Prediabetes was defined as (1) FPG level between 100 mg/dL and 125 mg/dL, (2) OGTT 2-hour PPG level between 140 mg/dL and 199 mg/dL, or (3) HbA1c level between 5.7% and 6.4%. Participants who had an FPG level <100 mg/dL and OGTT 2-hour PPG level <140 mg/dL and HbA1c level <5.7% were defined as normal glucose regulation (NGR).
Follow-up investigation and outcome assessment
The follow-up investigation was conducted during 2014-2016. If patients were hospitalized or visited an emergency department, their medical records were abstracted using a standard form that including inpatient record, pathology reports and their photocopies. Two members of the outcome adjudication committee who are masked to the baseline characteristics of participants verified each clinical event independently, and discrepancies were adjudicated by discussion involving other members of the committee. Incident cancer was defined as the first occurrence of any type of cancer at all sites during follow-up.
Statistical analyses
Baseline characteristics were summarized as the means (± standard deviation), medians (interquartile ranges) or proportions. One-way ANOVA was used to compare continuous variables and chi-square tests for categorical variables. The incidence rate of cancer with 95% CI was calculated per 1000 person-years with the number of persons with new-onset cancer during follow-up as the numerator and the total person-years as denominator. Participants with a diagnosis of cancer within 6 months from baseline were withdrawn to avoid the prediagnostic effect.
Cox proportional hazards models were used to investigate the associations of baseline cholesterol measures and glycemic status with subsequent incident cancer. Multivariate analyses were adjusted for potential confounders: age, sex, BMI, family history of cancer, smoking status, drinking status, education level, physical activity, consumption of vegetables and fruit, insulin therapy, lipid-lowering medication, diabetes status and systolic blood pressure. Potential nonlinear associations between the levels of LDL cholesterol and the incident cancer were examined with restricted cubic splines.
All analyses were conducted by SAS version 9.2 (SAS Institute, Cary, NC), and a two-tailed test with P<0.05 was considered as statistically significant.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request; some restrictions will apply.
Results
General flowchart
The 4C study was conducted among 193,846 participants recruited from 20 community sites of 16 provinces in mainland China. The baseline survey was performed in 2010-2011. Of those participants, 23,606 individuals were lost to follow-up (12.18%) and 170,240 remained in the study. Among these 170,240, 2,798 with cancer at baseline were excluded and 24,572 participants without follow-up data on cancer status were also excluded. Participants with cholesterol or glucose measures missing at baseline were further excluded (n=4,639), and 347 were withdrawn because of a diagnosis of cancer within 6 months from baseline. Finally, 137,884 participants were included for the current analysis and the mean follow-up duration was 3.8 years (Figure 1).
Figure 1.
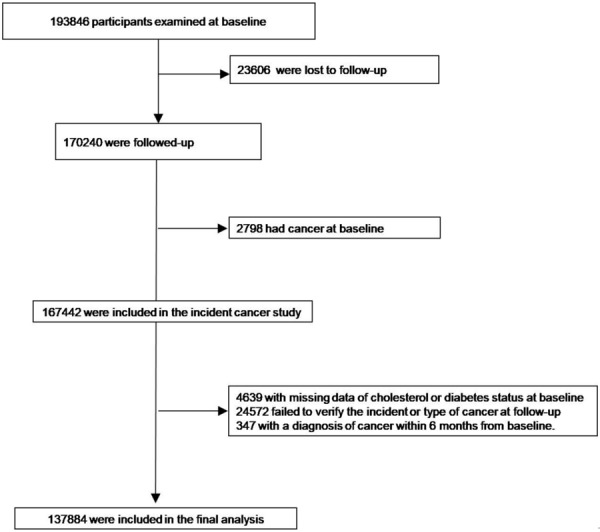
Participant Flow Diagram of the China Cardiometabolic Disease and Cancer Cohort (4C) Study.
Baseline characteristics
After 510,164 person-years of follow-up, 1,710 (1.24%) were detected as incident cancer based on their medical records (1.60% in men and 1.06% in women). The new-onset cancer mainly consisted of lung cancer (n=357), breast cancer (n=188), colorectal cancer (n=187), liver cancer (n=156) and stomach cancer (n=156). The baseline characteristics stratified by incident cancer status are shown in Table 1. Generally, participants with incident cancer were older, were more likely to be men, were current smokers and drinkers, and had significantly higher levels of systolic blood pressure, FPG, OGTT 2-hour PPG, and HbA1c and lower levels of LDL-cholesterol, HDL-cholesterol, total cholesterol (P<0.05). Additionally, higher levels of education, less consumption of vegetables and fruit and insulin therapy were associated with incident cancer (P<0.05). There was no significant difference for the use of lipid-lowering drugs between the two groups.
Table 1.
Characteristics of the study population according to the incident cancer status
| All Cancer | |||
|---|---|---|---|
|
|
|||
| Incident cancer (-) | Incident cancer (+) | P value | |
| N, % | 136,174 (98.76) | 1,710 (1.24) | - |
| Age at recruitment, years | 56.80 ± 9.10 | 61.84 ± 9.56 | <0.0001 |
| Male sex, n (%) | 47,346 (34.77) | 769 (44.97) | <0.0001 |
| Education attainment (high school or above), n (%) | 48,806 (36.78) | 547 (32.81) | 0.0008 |
| Family history of cancer | 14,772 (12.10) | 179 (12.82) | 0.41 |
| BMI, kg/m2 | 24.68 ± 3.61 | 24.61 ± 3.69 | 0.48 |
| Systolic blood pressure, mmHg | 133.59 ± 20.94 | 137.59 ± 21.77 | <0.0001 |
| Diastolic blood pressure, mmHg | 78.57 ± 11.20 | 78.38 ± 11.40 | 0.49 |
| Smoking status | |||
| Never | 104,301 (75.64) | 1,201 (70.23) | <0.0001 |
| Former | 6,488 (4.76) | 118 (6.90) | |
| Current | 19,950 (14.65) | 326 (19.06) | |
| Alcohol consumption | |||
| Never | 112,192 (82.39) | 1,353 (79.12) | 0.0003 |
| Former | 2,944 (2.16) | 58 (3.39) | |
| Current | 13,858 (10.18) | 200 (11.70) | |
| Vegetables and fruit intake <4.5 cups/day, n (%) | 74,256 (57.19) | 1,020 (62.69) | <0.0001 |
| Red meat intake, g/day | 28.57 (7.14-58.81) | 21.43 (0.00-57.14) | 0.67 |
| Fasting plasma glucose, mmol/L | 5.97 ± 1.65 | 6.09 ± 1.84 | 0.003 |
| 2 h Postprandial plasma glucose, mmol/L | 8.27 ± 3.86 | 9.04 ± 4.49 | <0.0001 |
| HbA1c, % | 6.02 ± 1.03 | 6.12 ± 1.13 | 0.0001 |
| LDL cholesterol, mg/dl | 111.93 ± 33.95 | 108.67 ± 35.00 | <0.0001 |
| HDL cholesterol, mg/dl | 51.91 ± 14.05 | 50.71 ± 14.42 | 0.0005 |
| Total cholesterol, mg/dl | 176.92 (83.26-230.95) | 173.37 (82.37-262.98) | <0.0001 |
| insulin therapy, n (%) | 1,795 (1.32) | 35 (2.05) | 0.009 |
| Lipid-lowering medications, n (%) | 1,261 (0.93) | 21 (1.23) | 0.20 |
Association of serum cholesterol level with incident cancer
The HRs and 95% CIs for the association between serum cholesterol level with incident cancer are shown in Table 2. We stratified the participants according to their fasting total cholesterol level, LDL cholesterol and HDL cholesterol level at baseline into quartile groups. Multiple-adjusted hazard ratios of all incident cancer associated with serum cholesterol level based on their quartiles interval were 1.36 (1.18-1.55), 1.11 (0.97-1.28), and 1.03 (0.89-1.18) for LDL cholesterol; 1.07 (0.93-1.23), 0.98 (0.85-1.12), and 0.95 (0.82-1.10) for HDL cholesterol; and 1.41 (1.22-1.62), 1.28 (1.11-1.47), and 1.11 (0.96-1.28) for total cholesterol, respectively (Table 2). Furthermore, according to the guideline-recommended cut-off values, compared with higher levels of LDL cholesterol (≥130 mg/dl), lower LDL cholesterol (<100 mg/dl) was associated with an increased risk of incident cancer [<70 mg/dl: 1.48 (1.25-1.75); P<0.0001; 70-100 mg/dl: 1.21 (1.06-1.38); P=0.005], whereas no significantly elevated risk was observed for a moderate level of LDL cholesterol (100-130 mg/dl: 1.06 (0.93-1.20; P=0.39). A similar association was also detected for total cholesterol, but not for HDL-cholesterol.
Table 2.
Incidence rate and adjusted hazard ratios for incident cancer in participants according to serum lipids level
| No. of person-years | No. of events | Incidence, per 1000 person-year | Unadjusted | Adjusted* | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||||
| LDL cholesterol, mg/dl | |||||||
| Groups according to quartile range | |||||||
| Q1 | 125,709 | 501 | 4.0 | 1.24 (1.09-1.42) | 0.001 | 1.36 (1.18-1.55) | <0.0001 |
| Q2 | 125,408 | 415 | 3.3 | 1.04 (0.91-1.20) | 0.54 | 1.11 (0.97-1.28) | 0.14 |
| Q3 | 124,192 | 394 | 3.2 | 0.998 (0.87-1.15) | 0.97 | 1.03 (0.89-1.18) | 0.53 |
| Q4 | 125,818 | 400 | 3.2 | 1.00 (ref.) | - | 1.00 (ref.) | - |
| Groups according to guideline-recommended cut-off values | |||||||
| <70 | 49,510 | 212 | 4.3 | 1.34 (1.13-1.57) | <0.0001 | 1.48 (1.25-1.75) | <0.0001 |
| 70-100 | 140,767 | 504 | 3.6 | 1.13 (0.99-1.28) | 0.06 | 1.21 (1.06-1.38) | 0.005 |
| 100-130 | 173,255 | 559 | 3.2 | 1.02 (0.90-1.16) | 0.77 | 1.06 (0.93-1.20) | 0.39 |
| ≥130 | 137,596 | 435 | 3.2 | 1.00 (ref.) | - | 1.00 (ref.) | - |
| HDL cholesterol, mg/dl | |||||||
| Groups according to quartile range | |||||||
| Q1 | 128,941 | 497 | 3.9 | 1.12 (0.99-1.28) | 0.08 | 1.07 (0.93-1.23) | 0.34 |
| Q2 | 124,383 | 416 | 3.3 | 0.997 (0.87-1.14) | 0.97 | 0.98 (0.85-1.12) | 0.73 |
| Q3 | 125,296 | 396 | 3.2 | 0.95 (0.83-1.09) | 0.49 | 0.95 (0.82-1.10) | 0.49 |
| Q4 | 122,508 | 401 | 3.3 | 1.00 (ref.) | - | 1.00 (ref.) | - |
| Groups according to guideline-recommended cut-off values | |||||||
| <40 | 97,621 | 371 | 3.8 | 1.11 (0.98-1.24) | 0.09 | 1.07 (0.95-1.21) | 0.25 |
| ≥40 | 403,507 | 1,339 | 3.3 | 1.00 (ref.) | - | 1.00 (ref.) | - |
| Total cholesterol, mg/dl | |||||||
| Groups according to quartile range | |||||||
| Q1 | 127,116 | 493 | 3.9 | 1.28 (1.12-1.47) | 0.0003 | 1.41 (1.22-1.62) | <0.0001 |
| Q2 | 125,136 | 446 | 3.6 | 1.19 (1.04-1.37) | 0.01 | 1.28 (1.11-1.47) | 0.0007 |
| Q3 | 123,868 | 399 | 3.2 | 1.08 (0.94-1.25) | 0.27 | 1.11 (0.96-1.28) | 0.16 |
| Q4 | 125,008 | 372 | 3.0 | 1.00 (ref.) | - | 1.00 (ref.) | - |
| Groups according to guideline-recommended cut-off values | |||||||
| <150 | 77,273 | 310 | 4.0 | 1.27 (1.11-1.45) | 0.0006 | 1.39 (1.20-1.59) | <0.0001 |
| 150-<200 | 214,085 | 750 | 3.5 | 1.13 (1.01-1.25) | 0.03 | 1.18 (1.06-1.31) | 0.003 |
| ≥200 | 209,769 | 650 | 3.1 | 1.00 (ref.) | - | 1.00 (ref.) | - |
Adjusted for age, sex, BMI, family history of cancer, smoking, drinking, education status, physical activity, consumption of vegetables and fruit, insulin therapy, lipid-lowering medication, diabetes status and systolic blood pressure.
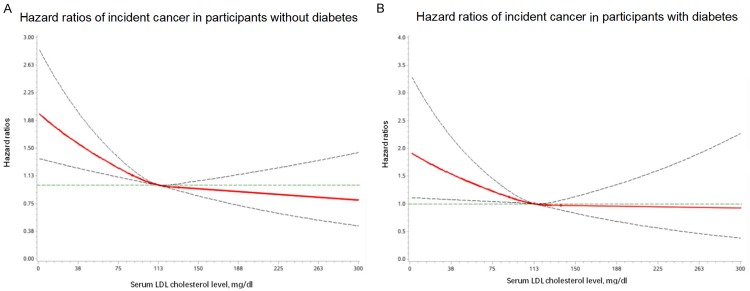
Multivariable adjusted restricted cubic spline analyses suggested an association between incident cancer and LDL cholesterol as a continuous variable, regardless of the glycemic status. The findings suggested that risk of incident cancer may be lower with higher LDL cholesterol and the risk reduction may level off after 100-130 mg/dL (Figure 2).
Figure 2.
Hazard ratios and 95% CIs for combination of LDL cholesterol and diabetic status in relation to incident cancer. A. Multivariable-adjusted hazard ratios of incident cancer in participants without diabetes. B. Multivariable-adjusted hazard ratios of incident cancer in participants with diabetes. The solid lines are multivariate-adjusted hazard ratios and the dashed lines indicate 95% confidence intervals derived from restricted cubic spline regression. Four knots were located at the 25%, 50%, and 75% percentiles for serum LDL cholesterol. The Cox regression was adjusted for age, sex, BMI, family history of cancer, smoking, drinking, education status, physical activity, consumption of vegetables and fruit, insulin therapy, lipid-lowering medication, and systolic blood pressure at baseline.
Association of glycemic status with incident cancer
Table 3 shows the hazard ratios and 95% CIs for the association of glycemic status with incident cancer, which detected diabetes was an independent risk factor; but no statistically significant association was observed for prediabetes. In addition, diabetic participants with poorly glycemic control had a significantly higher risk of incident cancer [diabetes with HbA1c <7.0% vs. normal glucose regulation: 1.24 (1.04-1.50); diabetes with HbA1c ≥7.0% vs. normal glucose regulation: 1.34 (1.08-1.66)].
Table 3.
Incidence rate and adjusted hazard ratios for incident cancer in participants according to glycemic status and LDL cholesterol
| No. of person-years | No. of events | Incidence, per 1000 person-year | Unadjusted | Adjusted* | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Groups according to glycemic status | |||||||
| NGR | 109,627 | 315 | 2.9 | 1.00 (ref.) | - | 1.00 (ref.) | - |
| Prediabetes | 268,649 | 845 | 3.1 | 1.12 (0.98-1.27) | 0.09 | 0.99 (0.86-1.16) | 0.94 |
| Diabetes with HbA1c <7.0% | 74,010 | 322 | 4.4 | 1.54 (1.32-1.80) | <0.0001 | 1.24 (1.04-1.50) | 0.02 |
| Diabetes with HbA1c ≥7.0% | 48,842 | 228 | 4.7 | 1.65 (1.39-1.95) | <0.0001 | 1.34 (1.08-1.66) | 0.007 |
| Groups according to combination of glycemic status and LDL cholesterol | |||||||
| Group 1: LDL cholesterol <100 mg/dl | |||||||
| Group 1 vs. others | 190,277 | 716 | 3.8 | 1.17 (1.06-1.29) | 0.002 | 1.20 (1.08-1.34) | 0.0007 |
| Group 2: Diabetes | |||||||
| Group 2 vs. others | 105,017 | 482 | 4.6 | 1.48 (1.33-1.65) | <0.0001 | 1.31 (1.15-1.49) | <0.0001 |
| Group 3: Diabetes with LDL cholesterol <100 mg/dl | |||||||
| Group 3 vs. others | 37,392 | 200 | 5.3 | 1.64 (1.41-1.90) | <0.0001 | 1.40 (1.18-1.65) | <0.0001 |
| Group 4: Diabetes with HbA1c ≥7.0% and LDL cholesterol <100 mg/dl | |||||||
| Group 4 vs. others | 14,983 | 85 | 5.7 | 1.69 (1.36-2.10) | <0.0001 | 1.42 (1.10-1.81) | 0.006 |
NGR, normal glucose regulation.
Adjusted for age, sex, BMI, family history of cancer, smoking, drinking, education status, physical activity, consumption of vegetables and fruit, insulin therapy, lipid-lowering medication, and systolic blood pressure.
Joint effect of LDL cholesterol and glycemic status with incident cancer
We then examined whether combinations of baseline glycemic status and LDL cholesterol could predict the incidence of cancer. We found that the risk of incident cancer may be lower with higher LDL cholesterol and the risk reduction may level off after 100-130 mg/dl, especially in diabetic participants. Table 3 shows the hazard ratios and 95% CI of the incident cancer according to several combinations of LDL cholesterol and glycemic status. Individuals with both lower levels of LDL cholesterol (<100 mg/dl) and poorly controlled diabetes (Diabetes with HbA1c ≥7.0%) were at higher risk of incident cancer than others [1.42 (1.10-1.81); P=0.006].
Association and joint effect between LDL cholesterol, glycemic status and site-specific cancers
When all site-specific cancers were evaluated, only digestive cancer was significantly associated with LDL cholesterol [LDL cholesterol <100 mg/dl vs. ≥100 mg/dl: 1.70 (1.41-2.05); P<0.0001]. Individuals with both lower levels of LDL cholesterol (<100 mg/dl) and poorly controlled diabetes (diabetes with HbA1c ≥7.0%) were at higher risks of digestive cancer than others [2.03 (1.39-2.96); P=0.0002]. The multivariable Cox regression revealed a similar relationship for incident colorectal cancer and liver risk. Those with both lower levels of LDL cholesterol and poorly controlled diabetes had an increased risk of pancreatic cancer than others [2.97 (1.08-8.17); P=0.03], whereas diabetic individuals had a higher risk of esophageal cancer [2.10 (1.09-4.07); P=0.03] (Table 4).
Table 4.
Incidence rate and adjusted hazard ratios for site-specific cancer according to glycemic status and LDL cholesterol
| No. of events | LDL cholesterol <100 mg/dl vs. others | Diabetes vs. others | Diabetes with LDL cholesterol <100 mg/dl vs. others | Diabetes with HbA1c ≥7.0% and LDL cholesterol <100 mg/dl vs. others | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Digestive organs | |||||||||
| Total | 614 | 1.70 (1.41-2.05) | <0.0001 | 1.62 (1.31-2.01) | <0.0001 | 2.03 (1.57-2.62) | <0.0001 | 2.03 (1.39-2.96) | 0.0002 |
| Colorectal Cancer | 187 | 1.47 (1.06-2.04) | 0.02 | 1.68 (1.16-2.43) | 0.006 | 2.42 (1.58-3.73) | <0.0001 | 1.82 (0.89-3.70) | 0.10 |
| Liver Cancer | 156 | 2.13 (1.50-3.03) | <0.0001 | 2.15 (1.47-3.16) | <0.0001 | 2.97 (1.97-4.50) | <0.0001 | 3.59 (2.07-6.21) | <0.0001 |
| Stomach Cancer | 156 | 1.02 (0.71-1.46) | 0.92 | 0.95 (0.60-1.50) | 0.82 | 0.54 (0.26-1.14) | 0.11 | 0.73 (0.26-2.05) | 0.54 |
| Pancreatic Cancer | 68 | 1.58 (0.89-2.80) | 0.12 | 1.06 (0.52-2.16) | 0.88 | 1.69 (0.75-3.78) | 0.20 | 2.97 (1.08-8.17) | 0.03 |
| Esophageal cancer | 47 | 1.69 (0.91-3.15) | 0.10 | 2.10 (1.09-4.07) | 0.03 | 1.96 (0.87-4.41) | 0.10 | 0.52 (0.07-3.98) | 0.52 |
| Other sites other than digestive organs | |||||||||
| Total | 1096 | 1.03 (0.90-1.18) | 0.65 | 1.19 (1.01-1.40) | 0.03 | 1.13 (0.91-1.41) | 0.27 | 1.16 (0.84-1.61) | 0.37 |
| Lung Cancer | 357 | 1.03 (0.81-1.30) | 0.84 | 1.12 (0.84-1.49) | 0.44 | 1.18 (0.81-1.72) | 0.38 | 1.36 (0.78-2.34) | 0.28 |
| Female breast Cancer | 188 | 0.79 (0.56-1.11) | 0.17 | 1.26 (0.82-1.96) | 0.29 | 1.17 (0.64-2.14) | 0.62 | 1.57 (0.69-3.56) | 0.28 |
| Thyroid Cancer | 108 | 0.83 (0.54-1.28) | 0.40 | 0.99 (0.55-1.78) | 0.98 | 0.78 (0.31-1.99) | 0.61 | 0.81 (0.19-3.49) | 0.78 |
| Endometrial Cancer | 77 | 0.88 (0.53-1.45) | 0.61 | 1.26 (0.67-2.37) | 0.48 | 0.89 (0.34-2.35) | 0.81 | 0.74 (0.17-3.30) | 0.69 |
| Haematological Malignancies | 59 | 1.15 (0.64-2.06) | 0.64 | 1.38 (0.69-2.74) | 0.36 | 1.69 (0.75-3.82) | 0.21 | 0.38 (0.05-2.94) | 0.38 |
| Cervical Cancer | 52 | 0.98 (0.54-1.76) | 0.93 | 0.47 (0.14-1.51) | 0.20 | 1.05 (0.30-3.65) | 0.94 | 1.92 (0.40-9.17) | 0.41 |
HRs were adjusted for age, sex, BMI, family history of cancer, smoking, drinking, education status, physical activity, consumption of vegetables and fruit, insulin therapy, lipid-lowering medication, and systolic blood pressure.
Discussion
The present study showed that relatively low LDL cholesterol was associated with the risk of incident cancer, whereas diabetes was significantly associated with the incident cancer in Chinese adults aged 40 years or older. Moreover, participants with both lower levels of LDL cholesterol and poorly controlled diabetes had the higher risk of incident cancer and this association seemed to be driven mainly by digestive cancer.
Despite the controversial results, there is a growing of evidence linking cholesterol to cancer risk [13-18]. A study from Korea showed total cholesterol levels were positively associated with breast cancer in women and prostate and colon cancer in men, and inversely associated with liver and stomach cancer [14]. Another study of European populations indicated that HDL cholesterol is inversely associated with colorectal cancer risk and no relationship was detected for LDL cholesterol [15]. In addition, the association is often confounded and the role of lipid-lowering medication and baseline health status in causation is questionable [17,18]. In our study, an inverse association for LDL cholesterol with all cancer incidence is observed, even after adjusting for lipid-lowering medication. There was a strong association of incident cancer with colorectal cancer and liver cancer; whereas a borderline significant relationship was detected for pancreatic cancer.
The mechanisms through which low LDL cholesterol levels could lead to an increased risk of cancer are still unclear. Actually, cholesterol is believed to play a role in several biochemical pathways of cancer initiation or progression, such as steroid hormone synthesis and vitamin D [21,22]. Previous experimental studies showed that cancer cells have higher levels of cholesterol-rich lipid rafts in the plasma membrane, which may be relevant to cancer cell survival [23,24]. Moreover, evidence is emerging that cholesterol related to the regulation of immune cell function, by improving their antitumor activity and activating immune signalling, which may provide novel insights into the role of cholesterol in the development of cancer [25-27].
Previous population studies have suggested that people with diabetes are at an increased risk for incident cancer and related mortality. Current evidence from the China Kadoorie Biobank (CKB) has also demonstrated a positive relationship between diabetes and an increased risk of site-specific cancers such as colorectal, liver and pancreatic [28-31]. Actually, our study is the first study that evaluates diabetes in China using the latest diabetes diagnosis criteria in a nationwide, multicenter, population-based, prospective cohort study. All glycemic indicators for the diagnosis of diabetes (FPG, OGTT-2 hour PPG, and HbA1c) were employed, which provided a comprehensive evaluation of diabetes in Chinese population and reflected a precise relationship of diabetes with cancer risk. We found that diabetes was significantly associated with risk of total cancer and several site-specific cancers such as colorectal, liver, and esophageal cancer.
As important manifestations of the metabolic syndrome, hypercholesterolemia and diabetes are often co-occurring. Given the proposed potentiation of the development of atherosclerotic cardiovascular disease, LDL cholesterol has been considered a principal target of lipid-lowering therapy, especially in diabetic participants. The ACC/AHA 2013 guideline lowered the threshold level of LDL cholesterol for initiating statin therapy from 100 mg/dl to 70 mg/dl in population with a very high risk of cardiovascular disease [32-34]. Apparently, the association of LDL cholesterol level with cancer is inconsistent with that for cardiovascular diseases. Our population study showed that the risk of incident cancer was inversely related to LDL cholesterol level, especially in the lower range (<100 mg/dl). These findings suggest an awareness of striking a balance to avoid too low LDL cholesterol levels as for cancer, yet to still provide benefit for cardiovascular diseases.
As a high-risk group for cardiovascular disease, diabetic patients have a more stringent goal for LDL cholesterol in clinical guidelines. According to the ACC/AHA 2018 guideline, adults of 40 to 75 years of age with diabetes mellitus should initiate a moderate-intensity statin regimen, and a high-intensity statin therapy may be recommended if multiple risk factors exist [35]. In our study, the diabetic subjects who had lower levels of LDL cholesterol (<100 mg/dl) had an increased risk of incident cancer and those with poorly glycemic control conferred higher cancer risk. The synergistic effects of these two conditions highlight the need for clinicians to evaluate lipid-lowering targets and achieve glycemic control goals in the clinical routine among diabetic patients.
Strengths and limitations
The major strength of this analysis lies in the large number of participants with comprehensive measurements of cholesterol and glycemic biomarkers, all measured at the same clinical laboratory. To our best knowledge, this is the first large-sample prospective study on cancer risk with detailed information on cholesterol and glucose biomarkers in a Chinese population.
Our study has several limitations. First, the study participants were only followed-up for an average of 3.8 years. This relatively short follow-up duration limited the number of clinical events and influenced the study’s statistical power for several site-specific cancers. However, we have observed 1,710 incident cancer cases. Second, 12.18% of study participants were lost to follow-up in this study. Rural-urban migration and urban redevelopment in China contributed to this loss-to-follow-up. Finally, the potential existence of precancerous conditions or prediagnostic cancer may influence the cholesterol levels and lead to bias in the study. Nevertheless, this study was conducted in the general population after excluding participants with cancer diagnosed within the first 6 months after the baseline investigation.
Conclusions
In summary, our study showed a positive association of cancer risk with low-level LDL cholesterol and diabetes, which lends support to the postulation that a lower level of LDL cholesterol may confer a higher cancer risk. These findings indicate the compelling need of achieving glycemic control goal among diabetic patients and maintaining appropriate LDL cholesterol levels to provide benefit for cancer, as well as cardiovascular diseases in clinical practice.
Acknowledgements
We thank the field workers for their contribution and the participants for their cooperation. Research reported in this publication was supported by the Ministry of Science and Technology of China [grant number 2016YFC1305601, 2016YFC1305202, 2016YFC1304904, 2017YFC1310700, 2018YFC1311800] and the National Natural Science Foundation of China [grant number 81700764, 81670795, and 81561128019]; National Major Scientific and Technological Special Project for “Significant New Drugs Development” [grant number 2017ZX09304007], Shanghai Pujiang Program [grant number 18PJ1409600], Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support from Shanghai Jiao Tong University School of Medicine [grant number 20171901], and Shanghai Sailing Program [grant number 17YF1416800].
Disclosure of conflict of interest
None.
References
- 1.Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Martin-Hirsch P, Tsilidis KK. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477. doi: 10.1136/bmj.j477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massetti GM, Dietz WH, Richardson LC. Excessive weight gain, obesity, and cancer: opportunities for clinical intervention. JAMA. 2017;318:1975–1976. doi: 10.1001/jama.2017.15519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tu H, Wen CP, Tsai SP, Chow WH, Wen C, Ye Y, Zhao H, Tsai MK, Huang M, Dinney CP, Tsao CK, Wu X. Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ. 2018;360:k134. doi: 10.1136/bmj.k134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katzke VA, Sookthai D, Johnson T, Kühn T, Kaaks R. Blood lipids and lipoproteins in relation to incidence and mortality risks for CVD and cancer in the prospective EPIC-Heidelberg cohort. BMC Med. 2017;15:218. doi: 10.1186/s12916-017-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103–S123. doi: 10.2337/dc19-S010. [DOI] [PubMed] [Google Scholar]
- 6.Bloomgarden Z, Handelsman Y. World congress on insulin resistance, diabetes and cardiovascular disease. J Diabetes. 2018;10:776–777. doi: 10.1111/1753-0407.12787. [DOI] [PubMed] [Google Scholar]
- 7.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 8.Liao WC, Tu YK, Wu MS, Lin JT, Wang HP, Chien KL. Blood glucose concentration and risk of pancreatic cancer: systematic review and dose-response meta-analysis. BMJ. 2015;350:g7371. doi: 10.1136/bmj.g7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu F, Rabidou K, Fang R, Tan L, Xu S, Liu H, Argueta C, Zhang L, Mao F, Yan G, Chen J, Dong Z, Lv R, Xu Y, Wang M, Ye Y, Zhang S, Duquette D, Geng S, Yin C, Lian CG, Murphy GF, Adler GK, Garg R, Lynch L, Yang P, Li Y, Lan F, Fan J, Shi Y, Shi YG. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559:637–641. doi: 10.1038/s41586-018-0350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum MJ, Shaw RJ. Genomics: drugs, diabetes and cancer. Nature. 2011;470:338–9. doi: 10.1038/470338a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Lee HM, Chan JC. Drug-subphenotype interactions for cancer in type 2 diabetes mellitus. Nat Rev Endocrinol. 2015;11:372–9. doi: 10.1038/nrendo.2015.37. [DOI] [PubMed] [Google Scholar]
- 13.Radišauskas R, Kuzmickienė I, Milinavičienė E, Everatt R. Hypertension, serum lipids and cancer risk: a review of epidemiological evidence. Medicina (Kaunas) 2016;52:89–98. doi: 10.1016/j.medici.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Kitahara CM, Berrington de González A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011;29:1592–8. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, Frohlich J, Ayyobi A, Overvad K, Toft-Petersen AP, Tjønneland A, Hansen L, Boutron-Ruault MC, Clavel-Chapelon F, Cottet V, Palli D, Tagliabue G, Panico S, Tumino R, Vineis P, Kaaks R, Teucher B, Boeing H, Drogan D, Trichopoulou A, Lagiou P, Dilis V, Peeters PH, Siersema PD, Rodríguez L, González CA, Molina-Montes E, Dorronsoro M, Tormo MJ, Barricarte A, Palmqvist R, Hallmans G, Khaw KT, Tsilidis KK, Crowe FL, Chajes V, Fedirko V, Rinaldi S, Norat T, Riboli E. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut. 2011;60:1094–102. doi: 10.1136/gut.2010.225011. [DOI] [PubMed] [Google Scholar]
- 16.Iso H, Ikeda A, Inoue M, Sato S, Tsugane S JPHC Study Group. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer. 2009;125:2679–86. doi: 10.1002/ijc.24668. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, So WY, Ma RC, Ko GT, Kong AP, Zhao H, Luk AO, Lam CW, Ho CS, Tong PC, Chan JC. Low LDL cholesterol, albuminuria, and statins for the risk of cancer in type 2 diabetes: the Hong Kong diabetes registry. Diabetes Care. 2009;32:1826–32. doi: 10.2337/dc09-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, He J, Li M, Tang X, Hu R, Shi L, Su Q, Peng K, Xu M, Xu Y, Chen Y, Yu X, Yan L, Wang T, Zhao Z, Qin G, Wan Q, Chen G, Dai M, Zhang D, Gao Z, Wang G, Shen F, Luo Z, Qin Y, Chen L, Huo Y, Li Q, Ye Z, Zhang Y, Du R, Cheng D, Liu C, Wang Y, Wu S, Yang T, Deng H, Li D, Lai S, Bloomgarden ZT, Chen L, Zhao J, Mu Y, Ning G, Wang W, Bi Y 4C Study Group. Predictive value of fasting glucose, postload glucose, and hemoglobin a1c on risk of diabetes and complications in Chinese adults. Diabetes Care. 2019;42:1539–1548. doi: 10.2337/dc18-1390. [DOI] [PubMed] [Google Scholar]
- 20.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 21.Chimento A, Casaburi I, Avena P, Trotta F, De Luca A, Rago V, Pezzi V, Sirianni R. Cholesterol and its metabolites in tumor growth: therapeutic potential of statins in cancer treatment. Front Endocrinol (Lausanne) 2019;9:807. doi: 10.3389/fendo.2018.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. 2018;50:20. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107–18. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–31. [PubMed] [Google Scholar]
- 25.Miguez MJ, Rosenberg R, Burbano X, Malow R. Cholesterol as a mediator of alcohol-induced risks for respiratory disease hospitalizations among people living with HIV. J AIDS Clin Res. 2011;(Suppl 1) doi: 10.4172/2155-6113.S1-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C, Yan C, Wang L, Chang CC, Chang TY, Zhang T, Zhou P, Song BL, Liu W, Sun SC, Liu X, Li BL, Xu C. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651–5. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasumasu T, Takahara K, Sadayasu T, Date H, Isozumi K, Kouzuma R, Nakashima Y. Effect of plasma lipoproteins on natural killer cell activity in the elderly population. J Gerontol A Biol Sci Med Sci. 2003;58:561–5. doi: 10.1093/gerona/58.6.m561. [DOI] [PubMed] [Google Scholar]
- 28.Pang Y, Kartsonaki C, Guo Y, Chen Y, Yang L, Bian Z, Bragg F, Millwood IY, Shen L, Zhou S, Liu J, Chen J, Li L, Holmes MV, Chen Z. Diabetes, plasma glucose and incidence of colorectal cancer in Chinese adults: a prospective study of 0.5 million people. J Epidemiol Community Health. 2018;72:919–925. doi: 10.1136/jech-2018-210651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang Y, Kartsonaki C, Turnbull I, Guo Y, Clarke R, Chen Y, Bragg F, Yang L, Bian Z, Millwood IY, Hao J, Han X, Zang Y, Chen J, Li L, Holmes MV, Chen Z. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology. 2018;68:1308–1318. doi: 10.1002/hep.30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan XF, He M, Yu C, Lv J, Guo Y, Bian Z, Yang L, Chen Y, Wu T, Chen Z, Pan A, Li L China Kadoorie Biobank Collaborative Group. Type 2 diabetes and risk of incident cancer in china: a prospective study among 0.5 million chinese adults. Am J Epidemiol. 2018;187:1380–1391. doi: 10.1093/aje/kwx376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang Y, Kartsonaki C, Guo Y, Bragg F, Yang L, Bian Z, Chen Y, Iona A, Millwood IY, Lv J, Yu C, Chen J, Li L, Holmes MV, Chen Z. Diabetes, plasma glucose and incidence of pancreatic cancer: a prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int J Cancer. 2017;140:1781–1788. doi: 10.1002/ijc.30599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 33.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Penson PE, Long DL, Howard G, Toth PP, Muntner P, Howard VJ, Safford MM, Jones SR, Martin SS, Mazidi M, Catapano AL, Banach M. Associations between very low concentrations of low density lipoprotein cholesterol, high sensitivity C-reactive protein, and health outcomes in the Reasons for Geographical and Racial Differences in Stroke (REGARDS) study. Eur Heart J. 2018;39:3641–3653. doi: 10.1093/eurheartj/ehy533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2018 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request; some restrictions will apply.