Abstract
Cullin-RING E3 ligases are involved in the ubiquitination of substrates that regulate important biological processes and are a potential therapeutic target in many types of cancer. MLN4924, a small molecule of NEDD8-activating enzyme inhibitor, inactivates CRL by blocking cullin neddylation and has been reported to elicit anti-tumor effect. In this study, In this study, we aimed to investigate the effects of MLN4924 on angiogenesis in human umbilical vascular endothelial cells (HUVECs) and four types of cancer cells. Our results showed that MLN4924 inhibits cell viability and induced apoptosis in HUVECs in a dose-dependent manner. MLN4924 inhibits proliferation and interferes with the cell cycle checkpoint regulators, p21, p27, and phospho-histone H3. Vascular endothelial growth factor (VEGF) treatment increased the level of UBC12 in HUVECs, indicating that neddylation pathway is involved in VEGF-activated angiogenesis. MLN4924 decreased VEGF-activated cell proliferation via neddylation inhibition. MLN4924 inhibited VEGF-activated cell migration, capillary tube formation and VEGF-mediated Erk1/2 activation in HUVECs. We also examined antitumor effect of MLN4924 using xenograft SCID mouse models of four different types of cancer cells. The in vivo results showed MLN4924 inhibited tumor growth in all four types of cancers with decreasing CD31 expression in xenograft tumor. In conclusion, MLN4924 inhibited viability, migration, and VEGF-promoted angiogenic activity in HUVECs; consistently, MLN4924 inhibited tumor growth in four types of cancers with suppression of angiogenesis. These findings provide evidence to develop therapeutic strategy for cancer treatment through anti-angiogenesis through neddylation inhibition.
Keywords: MLN4924, anti-angiogenesis, neddylation
Introduction
Emerging evidence has shown that the growth, invasion, and metastasis of solid tumors are closely associated with angiogenesis [1,2]. The hypoxic conditions that result from dysregulated tumor growth induce the expression of transcription factor HIF-1α. The expression of vascular endothelial growth factor A (VEGFA) and vascular endothelial growth factor receptor 2 (VEGFR2) are subsequently increased in endothelial cells [3]. VEGFR2 is the major mediator of the mitogenic, angiogenic, and permeability-enhancing effects of VEGF [4]. Accordingly, anti-angiogenesis is increasingly regarded as a promising target for cancer therapy.
The balance between protein synthesis and degradation is critical for maintaining cellular homeostasis and normal cellular functions. Ubiquitin, a small regulatory protein, can be covalently linked to target proteins and direct them to the 26S proteasome for degradation [5]. The ubiquitin conjugation pathway consists of three-steps: activation, conjugation, and ligation. First, ubiquitin is activated by ubiquitin-activating enzyme (E1) with energy acquired from ATP hydrolysis. Then, the ubiquitin molecule is transferred to the second enzyme of the complex, E2 (ubiquitin-conjugating enzyme). The final reaction requires E3 (ubiquitin ligase), which binds and recognizes the target substrate and labels them with ubiquitin [6]. E3 ligases are crucial factors and play the most important role in this pathway because they modulate substrate specificity.
Culling-RING ligases (CRLs) are the largest family of E3 ubiquitin ligases [7]. CRLs regulate several important biological processes, such as cell-cycle progression, cell-proliferation, DNA repair, and apoptosis via degradation of various key substrates [8]. Among the eight cullin family members, cullin-1 is known to bind adaptor protein SKP1 and an F-box protein at the N-terminus, and a RING protein, RBX1 or RBX2 (also known as ROC2 or SAG) at the C-terminus, thus forming the Skp1, Cullin and F-box protein (SCF) E3 complex [9].
NEDD8 (neural precursor cell-expressed developmentally downregulated protein 8) is a ubiquitin-like protein. Neddylation refers to the process of conjugating NEDD8 to target proteins. Like ubiquitination, neddylation requires E1 NEDD8-activating enzyme (NAE), E2 NEDD8-conjugating enzyme (UBC12), and E3 NEDD8 ligase, which catalyzes the transfer of NEDD8 to a target molecule [7,10]. NEDD8 is a ubiquitin-like molecule that can be attached to proteins of the cullin family [11], and is required for the activity of one subclass of ubiquitin E3 ligases. Cullin-based E3 ligases comprise a large family of ubiquitin ligases that mediate the ubiquitination of numerous cellular proteins with diverse functions, including proteins involved in cell signaling, transcriptional regulation, and cell cycle control [11-15]. Disruption of neddylation can lead to accumulation of numerous intracellular proteins and can lead to DNA damage responses, autophagy, apoptosis, and abnormal cellular responses [16].
Previous studies have indicated that E1, E2, and E3 enzymes are expressed in human umbilical vein endothelial cells [17]. Proteasome inhibitors suppress angiogenesis via inhibition of VEGFR 2 expression in endothelial cells [18]. Moreover, various ubiquitin E3 ligase complexes have been reported to be involved in the regulation of angiogenesis and VEGFR 2 expression [19].
MLN4924, a selective NAE inhibitor, has been reported as a promising anti-cancer drug [10,20]. MLN4924 treatment significantly decreases levels of cullin neddylation and total NEDD8-conjugated proteins. The anti-angiogenic effect of MLN4924 has been reported in endothelial cells [21]. Mechanistic studies indicated that MLN4924 triggers the anti-angiogenic effects by inducing RhoA accumulation in HUVECs [21].
In this study, we aimed to investigate the antiangiogenetic effects of MLN4924 on human umbilical vein endothelial cells (HUVECs). Additionally, we tried to validate the role of neddylation pathway in VEGF-promoted angiogenic activity in HUVECs and examine the involvement of anti-angiogenesis in MLN4924-induced anti-tumor effect in xenograft tumors of four different types of cancers.
Materials and methods
Reagents and antibodies
MLN4924 was purchased from Millennium Pharmaceuticals (Cambridge, MA, USA). Antibodies for western blotting of various proteins such as CHOP, cleaved PARP, cleaved caspase-3, cleaved caspase-7, p21, p27, UBC12, phospho-Erk1/2, Erk1/2 and CD31 were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-phospho-histone H3 (Ser10) antibody was obtained from Merck Millipore (Billerica, MA, USA). The antibody against Erk1/2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and the antibodies against GAPDH, β-actin and α-tubulin were purchased from GeneTex (Irvine, CA, USA). All the other chemicals and reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA) or Merck Millipore (Billerica, MA, USA).
Cell lines
We used five distinct cell lines to perform our experiments: human urothelial cell carcinoma (BFTC-905), cervical cancer (HeLa), renal cell carcinoma (Caki-2), and pharyngeal squamous cell carcinoma (FaDu) cells. All the cell lines were obtained from the Bioresource Collection and Research Center, Hsinchu, Taiwan. The BFTC-905, HeLa, Caki-2 and Fadu cell lines were cultured in high-glucose Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The HUVECs were cultured with M199 medium containing 20% FBS, endothelial cell growth supplement (Millipore, Billerica, MA, USA) in the gelatin-coated dish. These five types of cells were maintained at 37°C in humidified air containing 5% CO2. All the other culture media and supplements were obtained from Invitrogen. Furthermore, all other culture media and supplements were purchased from Invitrogen (Carlsbad, CA, USA).
Apoptosis assay
Cells were stained with the annexin V-FITC apoptosis detection kit (Invitrogen), and apoptotic cells were identified and quantified by flow cytometry according to the manufacturer’s instructions. In brief, after treatment with varying conditions, cells were washed with PBS and collected via trypsin-EDTA solution (Invitrogen). The cell suspensions were then centrifuged at 1000 rpm for 5 minutes to remove the trypsin-EDTA solution. Then, the cells were re-suspended and incubated with propidium iodide (PI), annexin V-FITC, and annexin V binding buffer for 15 minutes at room temperature. The stained cells were analyzed via flow cytometry with a Becton Dickinson LSR II (BD Bioscience, San Jose, CA, USA).
Cell viability assay
Cell viability was determined using WST-1 (BioTools, Taipei, Taiwan). In brief, each of the five cell lines were seeded with culture medium in 96-well microplates (4500 cells/well) and incubated at 37°C for 24 h before drug treatments. After treatment for the indicated times, cells were incubated with complete medium containing WST-1 reagent at 37°C for 4 h. Then the absorbance was detected using a Thermo Scientific Multiskan GO plate reader (Thermo Scientific, Rockford, IL, USA) at a wavelength of 450 nm.
Cell proliferation assay
HUVECs (2000 cells/well) were treated with various concentrations of MLN4924 for 24 h. The BrdU cell proliferation assay was performed according to the protocol provided by the manufacturer (Roche Applied Science, Indianapolis IN, USA) [20,22].
Western blotting
After various treatments, the cells from each cell line were washed with ice-cold PBS and exposed to cell lysis buffer (Cell Signaling Technology) on ice for 15 minutes, followed by centrifugation at 14000 rpm for 15 minutes at 4°C. The supernatants were harvested and total protein concentration was determined via the BCA protein assay (Thermo Scientific). An equal quantity of each sample was subjected to SDS-PAGE and then transferred to a PVDF membrane (GE Healthcare, Menlo Park, California, USA). The membranes were blocked with 5% BSA in TBST for at least 1 h followed by incubation with their respective primary antibodies at 4°C overnight. The membranes were then washed by TBST with a 10-minute time interval three times and incubated at room temperature for 1 h with applicable horseradish peroxidase (HRP)-conjugated secondary antibodies (Genetex, Irvine, CA, USA).
Antibody-conjugated membranes were visualized by enhanced chemiluminescence (ECL) substrates (Merck Millipore and Biotools) under QmageQuant LAS 4000 (GE Healthcare) system [20].
Transwell migration assay
Transwell migration assay was performed using TranswellTM chambers (Corning, New York, NY, USA) with a polycarbonate membrane (6.5 mm diameter, 8 μm pore size). HUVECs (2 × 104 cells) in 100 μL M199 medium containing 5% FBS were loaded into the upper chambers of the Transwell apparatus. VEGF (50 ng/mL), a potent chemotactic agent of endothelial cells, with or without various concentrations of THZ1 (250, and 500 nM) in 5% FBS-containing M199 were loaded in the lower chambers for HUVEC migration. After 4 h, the cells on the top surface of the membrane were scraped with a cotton swab. The migrated cells on the lower surface of the membrane were fixed with methanol for 30 min. Next, cells remaining in the upper chamber were wiped out with a cotton swab, and cells migrated to the lower chamber were fixed with 4% paraformaldehyde, followed by 0.1% crystal violet staining. The numbers of migrated cells were determined by Image J (version 1.52q, NIH, Bethesda, MD, USA).
In vivo Matrigel plug assay
The MatrigelTM plug assay was performed as described previously [23,24]. Briefly, 0.5 mL MatrigelTM containing MLN4924 (0.5 or 5 mM) or 125 ng VEGF (Invitrogen) and 20 units of heparin was injected subcutaneously to the ventral area of 6-week-old FVB mice (n = 8 for each dose of MLN4924). The mice were sacrificed after 5 days and the MatrigelTM plugs were excised and photographed [24]. Each well was then photographed for quantification of angiogenic efficacy of HUVECs by gauging the formation of tubes on MatrigelTM Matrigel with the Image J software.
Capillary tube formation assay
We coated 48-well culture plates with MatrigelTM (150 μL/well) at 4°C and incubated them for 1 hour at 37°C. HUVECs were seeded on the surface of the MatrigelTM and treated with various concentrations of MLN4924 (10-500 nM) for 24 h. Capillary tube formation was photographed using inverted light microscopy. The length of tube formation was quantified based on counts from 10 low-power fields randomly chosen from each well [24].
In vivo xenograft SCID mice model
A total of 1 × 106 cells from human urothelial cell carcinoma (BFTC-905), cervical cancer (HeLa), renal cell carcinoma (Caki-2), and pharyngeal squamous cell carcinoma (FaDu) cells were suspended in 200 μL serum-free medium and mixed with an equal volume of Matrigel (BD Biosciences, Bedford, MA), followed by subcutaneous injection to the dorsal flank of 8-week-old Nude mice (obtained from the Taiwan National Laboratory Animal Center, Taipei, Taiwan). After tumor growth had reached roughly 200 mm3, the mice were randomly assigned to a MLN4924-treated group or saline-treated sham control group. The MLN4924-treated group received intraperitoneal injections of MLN4924 10 mg/kg in DMSO twice a day for over four weeks, while the control group received DMSO in PBS only. Tumor volume was measured with calipers twice per week. Tumor volume was calculated using the formula V = LD × (SD)2/2, where V is the tumor volume, LD is the longest tumor diameter, and SD is the shortest tumor diameter. Then themice were sacrificed by CO2 asphyxiation, and the tumors were surgically removed and photographed. All studies involving animal experiments, animal care and experimental procedures were approved by the National Taiwan University College of Medicine and College of Public Health Institutional Animal Care and Use Committee (IACUC, No. 20160280, 20170268, 20150236 and 20180483). All studies involving animals complied with the ARRIVE guidelines for the reporting of experiments involving animals.
Statistical analysis
GraphPad Prism® 5 software was used to perform all data analysis. All data are expressed as mean ± SD. Data with two groups were analyzed using a two-tailed Student’s t-test; data with multiple groups were analyzed via one-way ANOVA followed by Bonferroni’s post hoc test. Comparisons were considered statistically different when P < 0.05.
Results
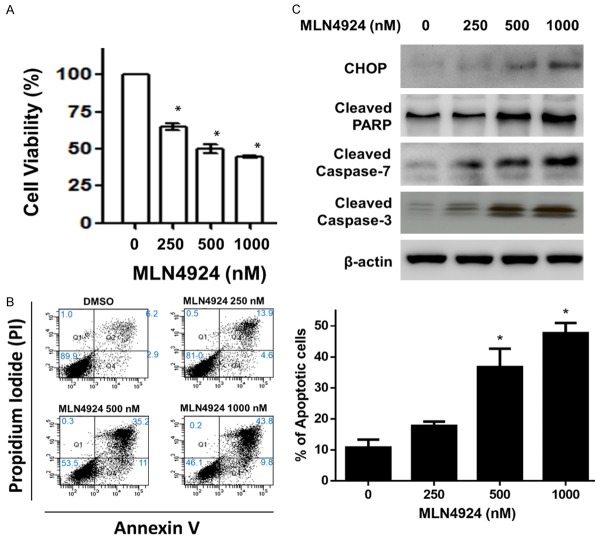
MLN4924 inhibits viability and induces apoptosis in HUVECs in a dose-dependent manner
To investigate the role of neddylation in the regulation of angiogenesis, we first examined the effects of MLN4924 on the viability of HUVECs by MTT assay. As shown in Figure 1A, MLN4924 inhibited viability of HUVECs in a dose-dependent manner at 24 h. In addition, MLN4924 induced dose-dependent apoptotic effects in HUVECs at 24 h (Figure 1B). Consistently, western blotting analysis showed a dose-dependent increase in CHOP, PARP cleavage, and caspases-3 and -7 activation at 24 h after exposure to 100-1000 nM MLN4924.
Figure 1.
MLN4924 inhibits cell viability and induces apoptosis in HUVECs in a dose-dependent manner. A. HUVECs were treated with various concentrations of MLN4924 (10-1000 nM) and DMSO (as non-treated control) for 24 h. Cell viability was analyzed via MTT assay. Data are presented as mean ± SD. Asterisks indicate the degree of statistical difference; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001, as compared with non-treated control. B. HUVECs were treated with various concentrations of MLN4924 (250-1000 nM) and DMSO for 24 h. Apoptotic cells were measured by flow cytometry with propidium iodide (PI) and annexin V staining. Quantitative analysis of apoptosis was calculated. Data are presented as mean ± SD. Asterisks indicate the degree of statistical difference; *P ≤ 0.05 as compared with non-treated control. C. Cell lysates were harvested and subjected to western blot using specific antibodies against CHOP, cleaved PARP, and caspases-3 and -7. Results are representative of at least three independent experiments.
MLN4924 inhibits proliferation and interferes with cell cycle checkpoint regulators p21, p27 and phospho-histone H3
We further investigated the effects of MLN4924 on cell proliferation and cell cycle transition in HUVECs. A BrdU proliferation assay was used to analyze the proliferation of HUVECs. As shown in Figure 2A, MLN4924 inhibited cell proliferation in a dose-dependent manner at 24 h. BrdU is commonly used in the detection of proliferating cells in living tissue because BrdU is incorporated into the newly synthesized DNA of replicating cells during S phase. Previous studies have shown that MLN4924 induced cell cycle arrest in cancer cells [20,25]. An impact of MLN4924 on cell cycle transition has been hypothesized due to the accumulation of several critical CRL substrates involved in the regulation the of cell cycle [20,26]. The impact of MLN4924 on HUVECs has been previously reported in another study [21]. Our results showed that MLN4924 induced the accumulation of cell cycle regulators (p21 and p27) and substrates of CRLs (Figure 2B) [27]. MLN4924, a potent and selective NAE inhibitor, suppressed NEDD8 conjunction and cullin neddylation, subsequently inducing the accumulation of substrates (p21 and p27) [27]. Additionally, histone H3 is a nuclear core histone protein regulating chromatin and functions in chromosome condensation and cell-cycle progression during the mitotic phase, after phosphorylation of the serine-10 residue [28]. Consistently, we found that MLN4924 suppressed the expression of phospho-histone H3 (Ser10) in HUVECs in a dose-dependent manner (Figure 2B). Extracellular signal-regulated protein kinases 1 and 2 (Erk1/2) are members of the mitogen-activated protein kinase super family that plays a critical role in proliferation, cell cycle progression, and apoptosis [29]. As shown in Figure 2C, MLN4924 decreased phospho-ERK1/2 expression in HUVECs. In summary, MLN4924 diminished cell proliferation and phospho-Erk1/2 expression; moreover, it interfered with cell cycle regulators in HUVECs.
Figure 2.
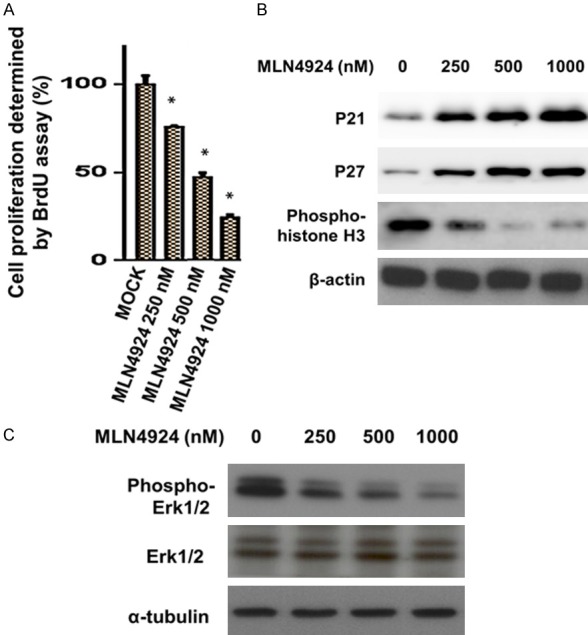
MLN4924 inhibits proliferation and interferes with cell cycle checkpoint regulators p21, p27 and phospho-histone H3. HUVECs were treated with DMSO as a control, or with various concentrations of MLN4924, for 24 h. A. Percentage of BrdU positive HUVECs after 24-h treatment with MLN4924 is shown. Data are presented as mean ± SD. Asterisks indicate the degree of statistical difference; *P ≤ 0.05 as compared with non-treated control. B. Cell lysates were analyzed for levels of p21, p27, and phospho-Histone-H3 (Ser10) via western blotting with specific antibodies. C. Thr-202/Tyr-204 phosphorylation of ERK1/2 and Erk in HUVECs at 24 h after exposure to MLN4924.
The neddylation pathway is involved in VEGF-activated angiogenesis; MLN4924 diminished the cell proliferation induced by VEGF in HUVECs via neddylation blockade
To investigate the role of neddylation in the regulation of VEGF-dependent angiogenesis in HUVECs, we measured the expression of related proteins in the neddylation pathway. Neddylation is a three-step enzymatic cascade mediated by NEDD8-activating enzyme E1 (NAE), which initiates the NEDD8 transfer cascade by activating NEDD8, NEDD8-conjugating enzyme E2 (UBC12), and NEDD8 ligase E3 [7,10]. We examined the expression levels of UBC12 in VEGF-treated HUVECs. Western blotting showed that VEGF treatment increased the levels of and UBC12 in HUVECs at different timepoints (Figure 3A). MLN4924, a potent NAE inhibitor, specifically inhibits the neddylation pathway. Correspondingly, MLN4924 (500 nM) suppressed the expression of UBC12 in VEGF-activated HUVECs (Figure 3C). Moreover, MLN4924 inhibited VEGF-activated cell viability and induced apoptosis in HUVECs (Figure 3B and 3C). These findings indicate that MLN4924 inhibits protein neddylation and suppresses VEGF-activated HUVEC proliferation. Erk1/2 signaling, a pathway downstream of EGFR-activated angiogenesis, plays a key role in angiogenic activity and maintaining vascular integrity in HUVECs. We therefore analyzed the expression of phospho-Erk1/2 in HUVECs at 24 h after MLN4924 treatment. MLN4924 suppressed VEGF-mediated Erk1/2 activation in a dose-dependent manner (Figure 3D).
Figure 3.
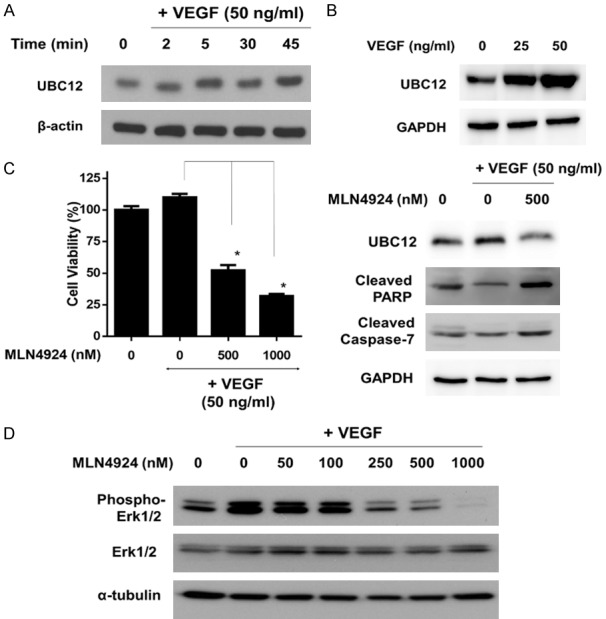
Neddylation pathway is involved in VEGF-activated angiogenesis; MLN4924 diminished cell proliferation induced by VEGF in HUVECs via Neddylation Blockade. A. HUVECs were treated with VEGF (50 ng/mL) at various time points (2, 5, 30, 45 minutes) and various concentrations of VEGF (0, 25 and 50 ng/ml) for 24 h. Cell lysates were analyzed by western blot for UBC12. B. HUVECs were treated with VEGF (50 ng/mL) without or with MLN4924 (50-500 nM). Percentage of cell viability in HUVECs after 24-h treatment with MLN4924 is shown. Data are presented as mean ± SD. Asterisks and pounds indicate the degree of statistical difference; *P ≤ 0.05 as compared with VEGF-treated control. C. HUVECs were treated with VEGF (50 ng/mL) without or with MLN4924 (500 nM). Cell lysates were analyzed by western blot for UBC12, cleaved PARP, cleaved caspase-3 and cleaved caspase-7. D. HUVECs were treated with VEGF (50 ng/mL) with or without MLN4924 (50-1000 nM). Cell lysates were harvested and subjected to western blot using specific antibodies against phosphor-Erk1/2 and Erk1/2. Results are representative of at least three independent experiments.
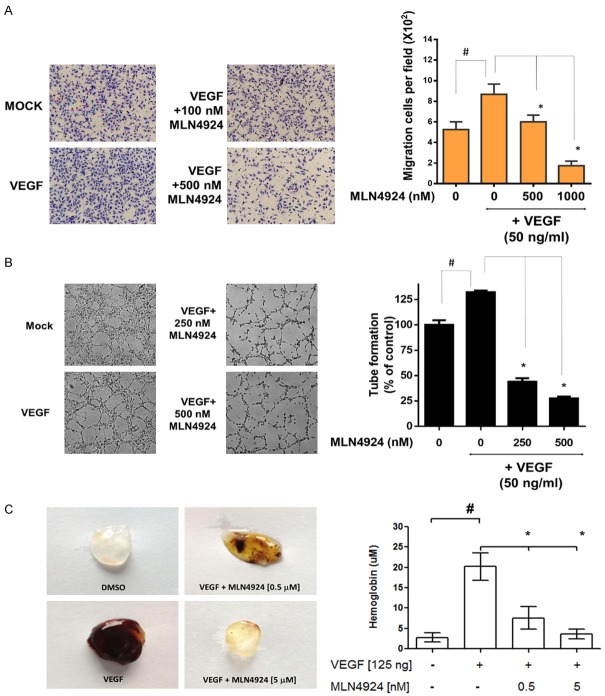
MLN4924 inhibits VEGF-activated cell migration, capillary tube formation, new vessel formation, and VEGF-mediated downstream Erk1/2 activation in HUVECs
To further investigate the anti-angiogenic effects of MLN4924, we first examined the effect of MLN4924 on the VEGF-activated cell migration of HUVECs by TranswellTM migration assay. As shown in Figure 4A, MLN4924 (100 and 500 nM) inhibited VEGF-activated cell migration in HUVECs. Quantitative analyses showed that VEGF significantly enhanced cell migration in HUVEC (P < 0.01). MLN4924 significantly inhibited the VEGF-activated cell migration of HUVECs in a concentration-dependent manner. Consistently, VEGF increased and MLN4924 decreased VEGF-activated capillary vessel formation in HUVECs (Figure 4B) at 24 h. Moreover, the MatrigelTM plug assay has been widely adopted for detection of neo-vascular formation in vivo. We further evaluated the anti-angiogenic effects of MLN4924 using this assay. As shown in Figure 4C, MLN4924 significantly inhibited VEGF-induced MatrigelTM plug angiogenesis and decreased hemoglobin levels in a concentration-dependent manner.
Figure 4.
MLN4924 inhibits VEGF-activated cell migration, capillary tube formation, and the downstream angiogenetic pathway in HUVECs. A. Representative image of TranswellTM migration of HUVECs recorded via time-lapse microscope (100×). Data are presented as mean ± SD. Asteris ks indicate the degree of statistical difference: *P ≤ 0.05 as compared with VEGF-treated control. #P ≤ 0.05 as compared with non-treated control. B. HUVECs were seeded into a MatrigelTM-coated 48-well plate and treated with 100 or 500 nM MLN4924 for 24 h. Photographs of tube formation are shown (100×). C. In vivo MatrigelTM plug assay. MatrigelTM containing the indicated amount of DMSO, VEGF (125 ng), or VEGF plus MLN4924 (0.5 mM and 5 mM) was injected into 6-week-old FVB mice subcutaneously (n = 8/dose). After 5 days, MatrigelTM plugs were excised and photographed. The hemoglobin concentration in each plug was analyzed. Asterisks and pound symbols indicate the degree of statistical difference: *P ≤ 0.05 as compared to VEGF-treated group. #P < 0.05 as compared to DMSO-treated control group.
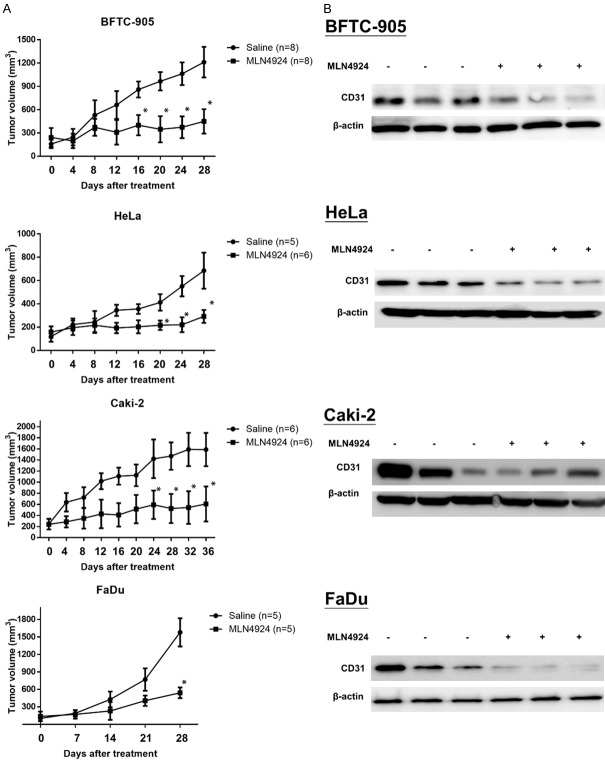
MLN4924 elicits anti-tumor effects via the suppression of angiogenesis in human urothelial cell carcinoma (BFTC-905), cervical cancer (HeLa), renal cell carcinoma (Caki-2), and pharyngeal squamous cell carcinoma (FaDu) cells
MLN4924 suppresses tumor growth and elicits anti-angiogenesis and decreased CD31 expression in four human malignant tumor xenografts. We then examined the anti-tumor effects of MLN4924 in Nude mice carrying four types of solid malignant tumors, including urothelial cell carcinoma (BFTC-905), cervical cancer (HeLa), renal cell carcinoma (Caki-2), and pharyngeal squamous cell carcinoma (FaDu). Mice bearing tumor xenografts were treated with saline as a sham control or MLN4924 at 10 mg/kg twice a day for over 4 weeks. At the end of treatment, tumor volume in the MLN4924-treated group shrunk significantly compared to the control group in all four xenografts (Figure 5A). Consistent with a previous study, MLN4924 had no effect on the weight of the mice compared to the control group (data not shown), indicating minimal toxicity for MLN4924 [20,30]. These observations suggest that MLN4924 effectively inhibited the growth of xenografted tumors in SCID mice with minimal general toxicity. To clarify the correlation between anti-angiogenesis and the anti-tumor effects of MLN4924, we examined the expression of CD31, a marker for the vascular endothelium, in tumor xenografts in both the MLN4924-treated and saline-treated groups. We found that MLN4924 suppressed the expression of CD31 in tumor xenografts compared to the saline-treated controls (Figure 5B).
Figure 5.
MLN4924 suppresses tumor growth and elicits anti-angiogenesis and decreased CD31 expression in four human malignant tumor xenografts. A. SCID mice bearing xenograft tumors from human urothelial cell carcinoma (BFTC-905), cervical cancer (HeLa), renal cell carcinoma (Caki-2), or pharyngeal squamous cell carcinoma (FaDu) cells were treated with saline as a sham control or MLN4924 (10 mg/kg i.p.) twice a day for over 4 weeks. Tumor volume was measured at sacrifice. Data are presented as mean ± SD. Asterisks indicate the degree of statistical difference; *P ≤ 0.05 as compared with saline-treated sham control. B. Western blot was used to analyzed CD 31 protein levels in the tumor xenograft.
Discussion
Tumor angiogenesis plays an important role in the processes of tumor progression and metastasis [1]. Neddylation regulates several important biological processes [8], and dysregulated neddylation leads to accumulation of numerous intracellular proteins and subsequently induces cell stress and even cell death, but research on the regulatory effects of neddylation with regards to angiogenesis is scarce [21,31].
In the present study, we found that inactivation of neddylation by MLN4924 suppressed the proliferation, survival, and cell cycle transition of HUVECs. In addition, we observed activation of the neddylation pathway, with increased expression of UBC12 noted in VEGF-treated HUVECs. MLN4924 effectively suppressed VEGF-activated cell proliferation, migration, and capillary vessel formation and the downstream phospho-Erk1/2 signaling pathway in HUVECs. Our results indicated the regulatory role for neddylation in angiogenic activity of vascular endothelial cells. Most importantly, our in vivo xenograft data indicates that neddylation inactivation by MLN4924 elicits significant anti-tumor effects in four human malignant solid tumors, with concomitant suppression of CD 31, the marker of vascular endothelial cells. MLN4924 is currently in several phase I clinical trials for cancer therapy with well-tolerated side effects. These findings provide evidence for developing novel anti-angiogenic agents that target the regulation of neddylation.
Selective protein degradation is an efficient way of terminating protein activity. NEDD8 conjugated to Cullin is necessary for the activation of CRLs, the largest multiunit ubiquitin ligase family, which are responsible for the ubiquitination and degradation of about 20% of ubiquitinated cellular proteins, including transcription factors, tumor suppressors, and onco-proteins [32].
Neddylation inhibition with MLN4924 or knockdown of ROC1/RBX1, an essential cullin-associated subunit of CRL, induced anti-angiogenesis [21]. The anti-angiogenic effects via are largely mediated by inactivating CRLE3 ligase [21], induce the accumulation of several CRL substrates such as RhoA, cell cycle inhibitors p21, p27, and wee1, and DNA replication licensing proteins CDT1 and ORC1 [21]. Our unpublished data also indicated that the specific inhibition of Cullin-1 via dominant-negative construct effectively suppressed VEGF-promoted angiogenic activity (phospho-Erk1/2) and capillary formation in HUVECs (data not shown here). The cullin-1 mediated neddylation process may play an essential role in regulating the angiogenic processes of HUVECs. Enzymatic inactivation by an NAE inhibitor, MLN4924 induced anti-angiogenesis effect in HUVECs.
In summary, neddylation inhibition decreased HUVEC survival, migration and VEGF-promoted angiogenic. Moreover, neddylation inhibition reduced tumor growth with diminished expression of vascular endothelial marker, CD31. These findings provide novel insights toward treating cancer via anti-angiogenetic effect of neddylation inhibition.
Acknowledgements
This work was supported by grants from National Taiwan University Hospital (108-4104, 108-M4137, and 107-S3784), the Taiwan Ministry of Science and Technology (106-2320-B-182-034, 108-2314-B-002-054, and 108-2320-B-182-010), Chiayi Chang Gung Memorial Hospital (CMRPD6F0031-3 and CMRPG6G0291).
Disclosure of conflict of interest
None.
References
- 1.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 2.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 3.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 5.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 7.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 8.Sarikas A, Hartmann T, Pan ZQ. The cullin protein family. Genome Biol. 2011;12:220. doi: 10.1186/gb-2011-12-4-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, Conaway RC, Conaway JW, Harper JW, Pavletich NP. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 10.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 11.Podust VN, Brownell JE, Gladysheva TB, Luo RS, Wang C, Coggins MB, Pierce JW, Lightcap ES, Chau V. A Nedd8 conjugation pathway is essential for proteolytic targeting of p27Kip1 by ubiquitination. Proc Natl Acad Sci U S A. 2000;97:4579–4584. doi: 10.1073/pnas.090465597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Furukawa M, Matsumoto T, Xiong Y. NEDD8 modification of CUL1 dissociates p120 (CAND1), an inhibitor of CUL1-SKP1 binding and SCF ligases. Mol Cell. 2002;10:1511–1518. doi: 10.1016/s1097-2765(02)00783-9. [DOI] [PubMed] [Google Scholar]
- 13.Read MA, Brownell JE, Gladysheva TB, Hottelet M, Parent LA, Coggins MB, Pierce JW, Podust VN, Luo RS, Chau V, Palombella VJ. Nedd8 modification of cul-1 activates SCF(beta(TrCP))-dependent ubiquitination of IkappaBalpha. Mol Cell Biol. 2000;20:2326–2333. doi: 10.1128/mcb.20.7.2326-2333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amir RE, Iwai K, Ciechanover A. The NEDD8 pathway is essential for SCF (bet-TrCP)-mediated ubiquitination and processing of the NF-kappa B precursor p105. J Biol Chem. 2002;277:23253–23259. doi: 10.1074/jbc.M200967200. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto M, Nishida T, Nagayama Y, Yasuda H. Nedd8-modification of Cul1 is promoted by Roc1 as a Nedd8-E3 ligase and regulates its stability. Biochem Biophys Res Commun. 2003;301:392–398. doi: 10.1016/s0006-291x(02)03051-6. [DOI] [PubMed] [Google Scholar]
- 16.Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D, Pan Y, Ding C, Qian J, Wu L, Chu Y, Yi J, Wang X, Sun Y, Jeong LS, Liu J, Jia L. The Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and apoptosis to suppress liver cancer cell growth. Cancer Res. 2012;72:3360–3371. doi: 10.1158/0008-5472.CAN-12-0388. [DOI] [PubMed] [Google Scholar]
- 17.Drexler HC, Risau W, Konerding MA. Inhibition of proteasome function induces programmed cell death in proliferating endothelial cells. FASEB J. 2000;14:65–77. doi: 10.1096/fasebj.14.1.65. [DOI] [PubMed] [Google Scholar]
- 18.Meissner M, Reichenbach G, Stein M, Hrgovic I, Kaufmann R, Gille J. Down-regulation of vascular endothelial growth factor receptor 2 is a major molecular determinant of proteasome inhibitor-mediated antiangiogenic action in endothelial cells. Cancer Res. 2009;69:1976–1984. doi: 10.1158/0008-5472.CAN-08-3150. [DOI] [PubMed] [Google Scholar]
- 19.Murdaca J, Treins C, Monthouel-Kartmann MN, Pontier-Bres R, Kumar S, Van Obberghen E, Giorgetti-Peraldi S. Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J Biol Chem. 2004;279:26754–26761. doi: 10.1074/jbc.M311802200. [DOI] [PubMed] [Google Scholar]
- 20.Kuo KL, Ho IL, Shi CS, Wu JT, Lin WC, Tsai YC, Chang HC, Chou CT, Hsu CH, Hsieh JT, Chang SC, Pu YS, Huang KH. MLN4924, a novel protein neddylation inhibitor, suppresses proliferation and migration of human urothelial carcinoma: in vitro and in vivo studies. Cancer Lett. 2015;363:127–136. doi: 10.1016/j.canlet.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Yao WT, Wu JF, Yu GY, Wang R, Wang K, Li LH, Chen P, Jiang YN, Cheng H, Lee HW, Yu J, Qi H, Yu XJ, Wang P, Chu YW, Yang M, Hua ZC, Ying HQ, Hoffman RM, Jeong LS, Jia LJ. Suppression of tumor angiogenesis by targeting the protein neddylation pathway. Cell Death Dis. 2014;5:e1059. doi: 10.1038/cddis.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu FS, Wu JT, Lin JY, Yang SP, Kuo KL, Lin WC, Shi CS, Chow PM, Liao SM, Pan CI, Hong JY, Chang HC, Huang KH. Histone deacetylase inhibitor, trichostatin A, synergistically enhances paclitaxel-induced cytotoxicity in urothelial carcinoma cells by suppressing the ERK pathway. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20051162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinda KM. In vivo matrigel migration and angiogenesis assay. Methods Mol Biol. 2009;467:287–294. doi: 10.1007/978-1-59745-241-0_17. [DOI] [PubMed] [Google Scholar]
- 24.Shi CS, Kuo KL, Chen MS, Chow PM, Liu SH, Chang YW, Lin WC, Liao SM, Hsu CH, Hsu FS, Chang HC, Huang KH. Suppression of angiogenesis by targeting cyclin-dependent kinase 7 in human umbilical vein endothelial cells and renal cell carcinoma: an in vitro and in vivo study. Cells. 2019;8 doi: 10.3390/cells8111469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei D, Li H, Yu J, Sebolt JT, Zhao L, Lawrence TS, Smith PG, Morgan MA, Sun Y. Radiosensitization of human pancreatic cancer cells by MLN4924, an investigational NEDD8-activating enzyme inhibitor. Cancer Res. 2012;72:282–293. doi: 10.1158/0008-5472.CAN-11-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia L, Li H, Sun Y. Induction of p21-dependent senescence by an NAE inhibitor, MLN4924, as a mechanism of growth suppression. Neoplasia. 2011;13:561–569. doi: 10.1593/neo.11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 28.Hans F, Dimitrov S. Histone H3 phosphorylation and cell division. Oncogene. 2001;20:3021–3027. doi: 10.1038/sj.onc.1204326. [DOI] [PubMed] [Google Scholar]
- 29.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho IL, Kuo KL, Liu SH, Chang HC, Hsieh JT, Wu JT, Chiang CK, Lin WC, Tsai YC, Chou CT, Hsu CH, Pu YS, Shi CS, Huang KH. MLN4924 synergistically enhances cisplatin-induced cytotoxicity via JNK and Bcl-xL pathways in human urothelial carcinoma. Sci Rep. 2015;5:16948. doi: 10.1038/srep16948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin Y, Zhang P, Wang Y, Jin B, Zhou J, Zhang J, Pan J. Neddylation blockade diminishes hepatic metastasis by dampening cancer st-em-like cells and angiogenesis in uveal melanoma. Clin Cancer Res. 2018;24:3741–3754. doi: 10.1158/1078-0432.CCR-17-1703. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Kang J, Zhang W, Cai L, Wang S, Liang Y, Jiang Y, Liu X, Zhang Y, Ruan H, Chen G, Wang M, Jia L. Validation of NEDD8-conjugating enzyme UBC12 as a new therapeutic target in lung cancer. EBioMedicine. 2019;45:81–91. doi: 10.1016/j.ebiom.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]