Abstract
5-Fluorouracil (5-FU) is an effective anticancer drug. However, high drug resistance limits its chemotherapeutic efficacy. Cancer cell resistance in colon cancer to 5-FU has been attributed to endoplasmic reticulum (ER) stress. But little is known about any similar role in resistance of breast cancer (BC). Here, we aim to investigate the role of ER stress played in BC cell resistance to 5-FU and to describe relevant molecular mechanisms. The expression patterns of 78-kDa glucose-regulated protein (GRP78), octamer 4 (OCT4), long non-coding RNA (lncRNA) myocardial infarction associated transcript (MIAT), and Protein kinase B (AKT) in BC MCF-7 cells resistant to 5-FU were determined by Western blot assay. Next, gain- and loss of-function experiments were conducted to verify effects of GRP78, OCT4, MIAT, and AKT on the to 5-FU sensitivity of MCF-7 cells and 5-FU resistant MCF cells (MCF-7/5-FU). Besides, the in vivo roles of the GRP78/OCT4/lncRNA MIAT/AKT pathway were assessed in tumor-bearing nude mice. 5-FU induced ER stress increased the expression of GRP78 in MCF-7 cells. GRP78 could positively regulate the expression of MIAT and AKT through upregulating OCT4, thereby contributing to 5-FU resistance in BC cells. Additionally, the function of GRP78 silencing in promoting tumor cell sensitivity was confirmed in vivo. These data supported an important role of the ER stress-mediated GRP78/OCT4/lncRNA MIAT/AKT pathway in BC cell resistance to 5-FU, highlighting potential molecular targets for combating 5-FU resistance in BC.
Keywords: Breast cancer, 5-fluorouracil, 78-kDa glucose-regulated protein, octamer 4, protein kinase B, long non-coding RNA myocardial infarction associated transcript
Introduction
Breast cancer (BC) is the most prevalent malignancy and the leading cause of cancer-related death in women [1], characterized by the presence of cancer cells with stem cell-like characteristics and tumor initiation potential [2]. 5-fluorouracil (5-FU) is an antimetabolite designed to achieve chemotherapeutic effects by inhibiting thymidine synthase [3], and is currently one of the most common chemotherapeutic agents applied in treatment of several cancers including gastric cancer [4], colon cancer [5] and BC [6]. It is well established that drug resistance is the major barrier to the efficacy of chemotherapy in cancer patients [7]. Despite recent progress, biological mechanisms underlying drug resistance remain incompletely understood [8]. An understanding of the mechanism of 5-FU resistance is necessary for the advancement of BC treatment.
It has been reported that stress-induced cell defense mechanisms play a crucial role in mediating tumor resistance, and targeting stress-related signals comprise a novel strategy to improve chemosensitivity [9]. In particular, endoplasmic reticulum (ER) stress is increasingly linked to drug resistance in human cancers, especially in BC [10-12]. Thus, the investigations into specific mechanisms of ER stress might help in developing treatments that mitigate 5-FU resistance in BC. Glucose regulatory protein (GRP78), is known to regulate ER stress [13], and its high expression is correlated to chemoresistance [14]. Findings from a recent study suggest that GRP78 mediates the sensitivity of human colon cancer cells to 5-FU via ER stress induction [15]. Therefore, GRP78 may also be involved in the control sensitivity of BC cells to 5-FU. Besides, GRP78 is reported to affect chemo-radioresistance via regulating octamer 4 (OCT4) in head-neck cancer [16]. OCT4, a transcription factor of embryonic stem cells, has been associated with tamoxifen resistance in BC [17]. OCT4 can potentiate the activation of Protein kinase B (AKT) and exert functions over chromatin plasticity and pluripotency [18]. Recently, the AKT signaling pathway has been revealed as a critical pathway mediating drug resistance in many cancers such as hepatocellular carcinoma [19], lung adenocarcinoma [20] and BC [21]. It has been shown that OCT4 can regulate the expression of long non-coding RNA (lncRNA) ‘myocardial infarction associated transcript’ (MIAT) [22], which was found to regulate AKT and thereby mediate drug resistance in lung cancer [23].
Based on such existing evidence, we hypothesized that 5-FU could potentially induce ER stress and activate the GRP78/OCT4/lncRNA MIAT/AKT pathway, by which the chemoresistance of BC cells to 5-FU is affected. Our study thus aims to testify this hypothesis in an attempt to improve the understanding of chemoresistance-associated molecular mechanisms and suggest new therapeutic targets for reversing drug resistance in BC.
Materials and methods
Ethics statement
The study was conducted after approval by the Ethics Committee of Renmin Hospital of Wuhan University. All participants or their guardians provided signed informed consent. The experiments involving animals were performed in compliance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Study subjects
BC tissues and adjacent normal tissues were collected from 56 BC patients who received surgical treatment at Renmin Hospital of Wuhan University from February 2017 to May 2018. All patients had received 5-FU for a long period prior to the surgery, with evidence of drug resistance. All of these patients were diagnosed with BC based on the American Joint Committee on Cancer criteria for BC. The harvested tissues were immediately stored in liquid nitrogen.
Immunohistochemistry
Tissue sections were dewaxed and dehydrated in ascending series of alcohol. The sections were heat-pretreated in citrate buffer for 1.5 min, and then cooled down at room temperature. After phosphate buffered saline (PBS) washes, each section was incubated with 50 μL 3% H2O2 for 20 min at room temperature to eliminate endogenous peroxidase activity. The sections were incubated with the primary rabbit antibodies against GRP78 (ab108615), OCT4 (ab109183, 1:1000) and AKT (ab179463, 1:1000) all from Abcam (Cambridge, UK) overnight at 4°C. After PBS washes, the sections were incubated at 37°C for 20 min with addition of 50 μL polymer enhancer and 50 μL enzyme-labeled rabbit anti-polymer for 30 min at 37°C. Thereafter, the sections were developed with 2 drops or 100 μL diaminobenzidine and observed under a microscope for 3-10 min. Brown cells were considered as positive cells. After being rinsed with distilled water, the sections were counterstained with hematoxylin, dehydrated with gradient alcohol (75%, 95% and 100% ethanol), and sealed with neutral gum, and observed under the microscope.
Cells positive for GRP78, OCT4, and AKT were defined as the presence of brown-yellow fine particles in the tumor cells. The percentage of positive cells was scored as follows: less than 10% was scored as 0 point (negative), 11% to 51% was scored as 2 points, 51% to 81% was scored as 3 points; and more than 81% was scored as 4 points. The staining intensity was scored as follows: 1 point referred to weak staining, 2 points referred to medium intensity and 3 points indicated high intensity staining. The proportion of positive cells less than 10% was regarded as negative (-) regardless of their staining intensity. Based on these two scores, 3 points was weak positive (+), 4-5 points was positive (+), and 6-7 points was strong positive (+++).
Cell culture
The 293T cell line was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). MDA-MB-468 and MCF-7 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). 293T, MDA-MB-468, and MCF-7 cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM; 3100022, Gibco, Grand Island, NY, USA). A total of 5 mL of the culture medium was pipetted into a 15 mL sterile centrifuge tube, and placed in a water-bath at 37°C. Cells were removed from the liquid nitrogen and thawed in a 37°C water bath, placed in a 15 mL centrifuge tube, and centrifuged at 800 r/min for 5 min. The supernatant was removed and cells were resuspended in 1 mL of culture medium. The cell suspension was transferred into a 6 cm culture dish containing 2 mL culture medium, followed by incubation at 37°C with 5% CO2. Cell growth was observed the next day. The culture medium was renewed every 1-2 days.
Culture of 5-FU-resistant BC cell line
After normal passage of cells, 5-FU was added to the culture medium at incremental concentrations of 10 to 150 ng/mL, each 10 ng/mL. If the cells did not require passage, the medium was replaced with fresh medium containing the same concentration of 5-FU every 2 days. The cells were passaged for at least 3 times with each concentration of 5-FU. The cells were cultured continuously for 8 months and passaged a total of 50 times.
Cell counting kit-8 (CCK8) assay
CCK8 assay was utilized to quantify cell viability. 5-FU-resistant cells and parental cells in logarithmic growth phase were seeded at 1×104 cells per well in a 96-well plate and cultured with 100 μL medium per well. After 24 h, the cells were cultured with medium containing different concentrations of 5-FU (100 μL/well). Three parallel wells were set up for each concentration. A total of 100 μL medium was used as control. After 48 h, 10 μL CCK8 solution (C0038, Beyotime, Shanghai, China) was added to each well. After 2 h of further incubation, the supernatant was removed. The optical density (OD) at 450 nm was quantified using an enzyme-linked immunosorbent assay (ELISA) plate reader.
Cell transfection
Puromycin-resistant Amp+ pLKO.1 vector expressing siRNA against GRP78, lncRNA MIAT, or OCT4 (si-GRP78, si-MIAT or si-OCT4), and G418-resistant pBABE vector overexpressing GRP78, lncRNA MIAT, or OCT4 (oe-GRP78, oe-MIAT or oe-OCT4) as well as packaging plasmids pVSVG, pREV, and pMDL were purchased from Cyagen Biosciences Inc. (Sunnyvale, CA). After the above-mentioned plasmids were transfected into DH5α competent cells, the corresponding plasmids were extracted using a plasmid extraction kit (DP103-03; TIANGEN Co., Ltd, Beijing, China). The extracted plasmids were transfected into 293T cells with Turbofect transfection reagent (R0531, Thermo Fisher Scientific, Waltham, MA, USA). After 12 h, the culture medium was renewed. The culture medium was collected at 24th h and 48th h separately and the virus was filtered and collected using a 0.45 μm filter. The BC cell line was infected with lentiviruses. Stable knockdown or overexpression cell lines were screened with puromycin (2 μg/mL) and G418 (500 μg/mL).
Colony formation assay
Cells in the logarithmic growth phase were seeded at 2×103 cells per well in a 6-well plate for a 10-d incubation. All samples were assayed in triplicates. The formation of cell clones was observed under an inverted microscope. Cells were washed, fixed with 1 mL formaldehyde for 10 min, and stained with 1 mL 0.1% crystal violet for 10 min. The remaining crystal violet was washed out with double distilled water. The cells were then air-dried and photographed. Stained cells were counted and a curve was plotted.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA from tissues and cells was extracted using TRIzol reagent (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China). The synthesis of primers was conducted by Takara (Dalian, Liaoning, China) (Table 1). Reverse transcription was performed according to the instructions of reverse transcription kit (K1622, Beijing Yaanda Biotechnology Co., Ltd) to generate cDNA. Real-Time fluorescence-based quantitative PCR assay was developed using a PCR instrument (ViiA 7, Daan Gene Co., Ltd., of Sun Yat-sen University, Guangzhou, China). The relative expression of target genes was measured by the 2-ΔΔCt method and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels.
Table 1.
Primer sequences for RT-qPCR
| Gene | Primer sequence (5’-3’) |
|---|---|
| LncRNA MIAT | Forward: 5’-AGAGAGGACATGAGGACCCC-3’ |
| Reverse: 5’-CCTACCTCACAGGGCTGTTG-3’ | |
| GAPDH | Forward: 5’-CGACTTCAACAGCAACTCCCACTCTTCC-3’ |
| Reverse: 5’-TGGGTGGTCCAGGGTTTCTTACTCCTT-3’ |
Note: RT-qPCR, reverse transcription quantitative polymerase chain reaction; lncRNA, long non-coding RNA; MIAT, myocardial infarction associated transcript; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blot analysis
Total protein was extracted from cells using lysis buffer containing phenylmethylsulfonyl fluoride and phosphatase inhibitors. The protein concentration was detected using a bicinchoninic acid protein assay kit. Totally 50 µg isolated protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane (Merck Millipore, Billerica, MA, USA), and then blocked with 5% skimmed milk for 2 h. The PVDF membrane was probed with diluted primary rabbit antibodies to GRP78 (ab108615, 1:1000), OCT4 (ab109183, 1:1000), AKT (ab179463), phosphorylated (p)-PERK (ab192591), p-IRE-1 (ab48187), ATF6 (ab203119) and mouse antibody to β-actin (ab8226) (Abcam, Cambridge, UK), overnight at 4°C. After incubation of the membranes with the secondary antibody, horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobulin G (IgG) (TransGen Biotech, Beijing, China), for 1 h at room temperature, bands were detected using enhanced chemiluminescence (BB-3501, Amersham-Pharmacia Biotech, Freiburg, Germany). Images were captured using an image analysis system (Bio-Rad, Hercules, CA, USA), and then subjected to gray scale analysis using Quantity One v4.6.2 Software. The relative gray scale ratio of the target proteins to β-actin was calculated.
Tumor xenografts in nude mice
Thirty male BALB/c mice (aged 6 weeks, weighting about 18-22 g) were purchased from Shanghai Lingchang Company (SLAC, Shanghai, China) and maintained for 7 days in a specific pathogen free (SPF) environment with free access to aseptic feed and water under a 12 h light/dark cycle. The cells were stably transfected with si-NC, si-GRP78, or co-transfected with si-GRP78 and oe-NC or co-transfected with si-GRP78 and oe-MIAT. Cell suspensions were prepared at a density of 5×106 cells/mL. Mice were inoculated subcutaneously with 0.2 mL of the cell suspension via left armpit. After inoculation, all nude mice were kept in a laminar flow hood in the SPF animal room.
Tumor growth was observed every 3 days after inoculation, and the data were recorded. Six days later, a total of 100 μL 5-FU (30 mg/kg) dissolved in PBS or an equal volume of PBS was intraperitoneally injected into the nude mice every 3 days. The short diameter (a) and long diameter (b) of the tumor were recorded using a Vernier caliper. The tumor volume was calculated based on the formula: tumor volume =π(a2b)/6.
Statistical analysis
The data were processed using SPSS 21.0 statistical software (IBM SPSS Statistics, Chicago, IL, USA). Measurement data were expressed as mean ± standard deviation. Unpaired data with normal distribution and homogeneity between two groups were compared using unpaired t-test. Comparisons among multiple groups were conducted by one-way analysis of variance (ANOVA) with Tukey’s post hoc test. Temporal measurements within each group were compared using repeated measures ANOVA, followed by a Bonferroni’s post-hoc test for multiple comparisons. Pearson’s correlation coefficient was used to assess the relationship between two indices. A value of P<0.05 indicated significant difference.
Results
5-FU induces ER stress in BC cells and enhances drug resistance
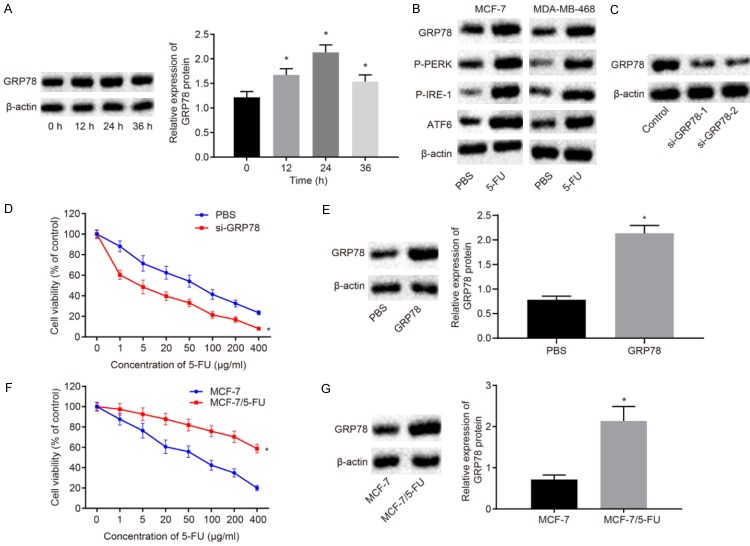
The efficacy of 5-FU, a chemotherapy drug for BC, is limited by drug resistance that may develop after long-term treatment and 5-FU treatment may induce ER stress in tumor cells, thereby reducing drug sensitivity [15]. In order to explore the mechanisms of 5-FU resistance in BC, the effect of 5-FU on ER stress in the BC cell line MCF-7 was investigated. The expression of ER stress marker protein GRP78 after treatment with 10 μg/mL 5-FU was measured by Western blot analysis. The results showed that GRP7 was increased after 5-FU treatment and peaked at the 24th h (P<0.05; Figure 1A), indicating increased ER stress. At the same time, the expression of ER stress protein in BC cells MDA-MB-468 and MCF-7 was determined by Western blot analysis. The results showed that the expression of GRP78 and ATF6 as well as the extents of PERK and IRE-1 phosphorylation was significantly increased in both MDA-MB-468 and MCF-7 cells after 5-FU treatment (Figure 1B). To investigate the effects of upregulation of GRP78, the sensitivity of MCF-7 to 5-FU after knock-down or overexpression of GRP78 was examined by CCK8 assay. The results showed that the knockdown of GRP78 elevated the sensitivity of MCF-7 to 5-FU (P<0.05), while the overexpression of GRP78 decreased the sensitivity of MCF-7 to 5-FU (P<0.05; Figure 1C-E). The 5-FU-resistant cell line MCF-7/5-FU was constructed, in order to further explore the relationship between GRP78 and 5-FU resistance in BC. CCK8 assay demonstrated that the sensitivity of MCF-7/5-FU cells to 5-FU was significantly lower than that of MCF-7 cells to 5-FU (Figure 1F). The expression of GRP78 between MCF-7/5-FU and MCF-7 cells was determined by Western blot analysis. The expression of GRP78 in MCF-7/5-FU cells was found to be higher than that in MCF-7 cells (P<0.05; Figure 1G). Together these results demonstrated that 5-FU induced ER stress in BC cells and increased the expression of GRP78.
Figure 1.
5-FU contributes to induced ER stress and enhanced drug resistance in BC cells. A. The GRP78 expression induced by 5-FU treatment in MCF-7 cells measured by Western blot analysis. B. The expression of ER stress-related proteins in MCF-7 and MDA-MB-468 cells after treatment with 5-FU for 24 h determined by Western blot analysis. C. The transfection efficiency of si-GRP78-1 and si-GRP78-2 in 5-FU-treated MCF-7 cells measured by Western blot assay. D. Viability of MCF-7 cells exposed to different concentration of 5-FU after knockdown of GRP78 by CCK8 assay. E. The transfection efficiency of oe-GRP78 in 5-FU-treated MCF-7 cells measured by Western blot assay. F. Viability of MCF-7/5-FU and MCF-7 cells exposed to different concentration of 5-FU measured by CCK8 assay. G. The protein expression of GRP78 in the MCF-7/5-FU and MCF-7 cells measured by Western blot analysis. *P<0.05 vs. the PBS group or MCF-7 cells. Measurement data were expressed as mean ± standard deviation. Data in compliance with normal distribution and homogeneity between two groups were compared using t-test. Comparisons among multiple groups were conducted by one-way ANOVA with Tukey’s post-hoc test. Statistical analysis in relation to time-based measurements within each group was realized using repeated measures ANOVA, followed by a Bonferroni’s post-hoc test for multiple comparisons. The experiment was repeated three times.
GRP78 increases resistance of BC cells to 5-FU by increasing the expression of OCT4
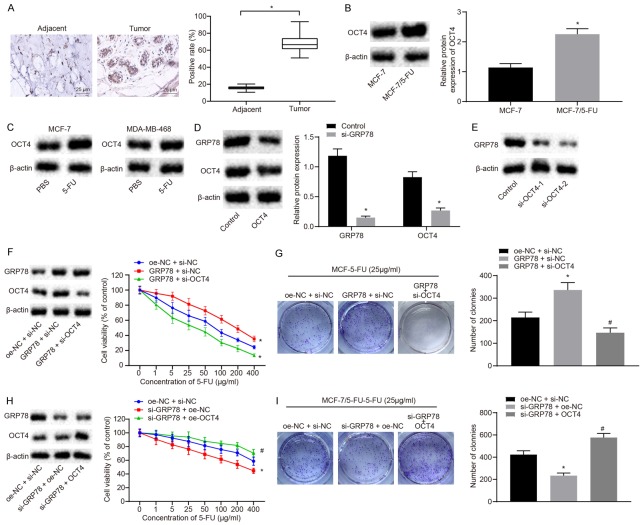
As one of the stem cell markers, OCT4 often induces an increase in cell stemness and can lead to cell resistance [17]. It has been reported that GRP78 increases the expression of OCT4 [16]. Thus, we speculated that GRP78 might promote the resistance of BC cells to 5-FU via regulating OCT4. The expression of OCT4 in BC tissues and adjacent normal tissues (n=56) was detected by immunohistochemistry. The results suggested that OCT4 was expressed at a higher level in BC tissues than in the adjacent normal tissues (P<0.05; Figure 2A). We further compared the expression levels of OCT4 between MCF-7/5-FU and MCF-7 cells. As measured by Western blot analysis, OCT4 expression levels were higher in MCF-7/5-FU cells than in MCF-7 cells (P<0.05; Figure 2B). Once BC cells were treated with 5-FU for 24 h, the expression levels of OCT4 were measured and Western blot analysis showed OCT4 levels were elevated after 5-FU treatment (Figure 2C). After the knockdown of GRP78 in MCF-7/5-FU cells, Western blot analysis showed OCT4 protein expression was down-regulated (P<0.05; Figure 2D), suggesting OCT4 was positively regulated by GRP78. To investigate whether GRP78 promoted 5-FU resistance in BC through OCT4, MCF-7 cells were co-transfected with si-OCT4 and oe-GRP78. CCK8 and colony formation assays were carried out to examine the sensitivity of the transfected cells to 5-FU. The results showed that overexpression of GRP78 increased resistance of cells to 5-FU (Figure 2E, 2F) and colony formation ability (P<0.05; Figure 2G), which were reduced upon knockdown of OCT4. These results suggested OCT4 knockdown inhibited the GRP78-induced resistance to 5-FU. At the same time, MCF-7/5-FU cells were co-transfected with si-GRP78 and oe-OCT4. It was demonstrated that knockdown of GRP78 suppressed the resistance of cells to 5-FU (Figure 2H) and reduced colony formation rate (P<0.05; Figure 2I), but those were rescued by overexpression of OCT4. Thus, the results suggested overexpression of OCT4 restrained the increase in sensitivity of MCF-7/5-FU cells induced by GRP78 knockdown.
Figure 2.
GRP78 upregulates OCT4 to enhance resistance of BC cells to 5-FU. A. The expression of OCT4 in BC tissues and adjacent normal tissues detected by immunohistochemistry (×400). B. The protein expression of OCT4 in MCF-7/5-FU and MCF-7 cells measured by Western blot analysis. C. The protein expression of OCT4 after 5-FU treatment measured by Western blot analysis. D. The GRP78 and OCT4 protein expression in MCF-7 cells transfected with si-GRP78 measured by Western blot analysis. E. The OCT4 protein expression in MCF-7 cells transfected with si-OCT4-1 or si-OCT4-2 measured by Western blot analysis. F. The protein expression of GRP78 and OCT4 in MCF-7 cells measured by Western blot analysis and the sensitivity of MCF-7 cells to 5-FU assessed by CCK8 assay after GRP78 overexpression with or without the presence of OCT4 knockdown. G. The colony formation rate of 5-FU-treated MCF-7 cells after overexpression of GRP78 with or without the presence of silencing of OCT4 evaluated by colony formation assay. H. The protein expression of GRP78 and OCT4 in MCF-7/5-FU cells measured by Western blot analysis and sensitivity to 5-FU examined by CCK8 assay after GRP78 knockdown with or without the presence of OCT4 overexpression. I. The colony formation rate of MCF-7/5-FU cells after knockdown of GRP78 with or without the presence of overexpression of OCT4 assessed by colony formation assay. *P<0.05 vs. adjacent normal tissues, MCF-7 cells, the control group or MCF-7/5-FU cells treated with oe-NC + si-NC. Measurement data were expressed as mean ± standard deviation. Data in compliance with normal distribution and homogeneity between two groups were compared using t-test. Comparisons among multiple groups were conducted by ANOVA with Tukey’s post-hoc test. Statistical analysis in relation to time-based measures within each group was realized using repeated measurement ANOVA, followed by a Bonferroni’s post-hoc test for multiple comparisons. The experiment was repeated three times.
GRP78 upregulates AKT to enhance the drug resistance of BC cells through the recruitment of OCT4
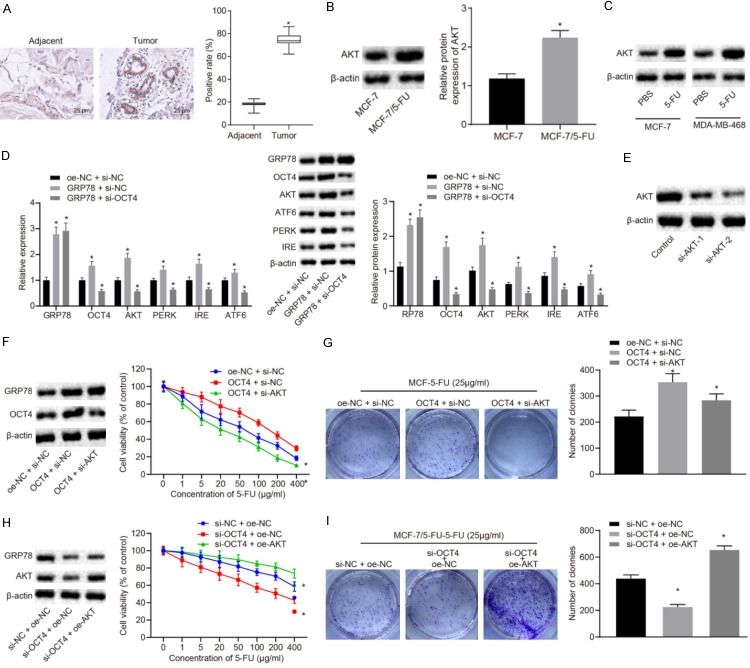
AKT has been found to elevate the resistance of tumor cells to chemotherapy. Moreover, OCT4 has been reported to increase the expression of AKT [18]. Next, whether GRP78 could regulate AKT and OCT4 to affect the resistance of BC cells to 5-FU was explored. The expression levels of AKT in BC tissues and adjacent normal tissues were detected by immunohistochemistry. The results demonstrated that AKT was highly expressed in BC tissues (P<0.05; Figure 3A). As measured by Western blot analysis, the expression levels of AKT in MCF-7/5-FU cells were higher than those in MCF-7 cells (P<0.05; Figure 3B). The results of Western blot analysis indicated that AKT was upregulated when MCF-7 and MDA-MB-468 cells were treated with 5-FU for 24 h (Figure 3C). The role played by GRP78 in drug resistance to 5-FU through OCT4-mediated AKT was further demonstrated by RT-qPCR and western blot analysis. As shown in Figure 3D, GRP78 overexpression upregulated GRP78, OCT4, AKT, PERK, IRE1, and ATF6, while silencing of OCT4 in the presence of GRP78 overexpression reduced GRP78, OCT4, AKT, PERK, IRE1, and ATF6 (P<0.05). Hence, GRP78 may elevate the expression of AKT through the upregulation of OCT4.
Figure 3.
GRP78 upregulates AKT through increasing OCT4, thereby enhancing resistance of BC cells to 5-FU. A. The expression of AKT in BC tissues and adjacent normal tissues detected by immunohistochemistry (×400). B. The expression of AKT in MCF-7/5-FU and MCF-7 cells measured by Western blot analysis. C. The expression of AKT in MCF-7 and MDA-MB-468 cells after 5-FU treatment. D. The mRNA and protein expression of GRP78, OCT4, AKT, PERK, IRE1 and ATF6 in MCF-7/5-FU cells after GRP78 overexpression with or without the presence of OCT4 silencing. E. Western blot analysis for expression of AKT protein in MCF-7/5-FU cells transfected with si-AKT-1 or si-AKT-2. F. The expression of OCT4 and AKT protein in MCF-7 cells measured by Western blot analysis and sensitivity of MCF-7 cells to 5-FU evaluated by CCK8 assay after transfection with oe-OCT4 with or without the presence of si-AKT. G. The colony formation rate in 5-FU-treated MCF-7 cells after transfection with oe-OCT4 with or without the presence of si-AKT. H. The expression of OCT4 and AKT protein in MCF-7/5-FU cells and sensitivity of MCF-7/5-FU cells to 5-FU after transfection with si-OCT4 with or without the presence of oe-AKT. I. The colony formation rate in MCF-7/5-FU cells after transfection with si-OCT4 with or without the presence of oe-AKT. *P<0.05 vs. adjacent normal tissues, MCF-7 cells, the control group or MCF-7/5-Fu cells treated with oe-NC + si-NC. Measurement data were expressed as mean ± standard deviation. Data in compliance with normal distribution and homogeneity between two groups were compared using t-test. Comparisons among multiple groups were conducted by ANOVA with Tukey’s post-hoc test. Statistical analysis in relation to time-based measurements within each group was realized using repeated measurement ANOVA, followed by a Bonferroni’s post-hoc test for multiple comparisons. The experiment was repeated three times.
MCF-7 cells were transfected with si-AKT or oe-OCT4 to further investigate the effect of AKT and OCT4 on the sensitivity of BC cells to 5-FU. It was demonstrated that overexpressing OCT4 inhibited the sensitivity of BC cells to 5-FU, which was rescued by si-AKT-mediated inhibition of AKT (Figure 3E, 3F). Furthermore, the enhanced colony formation ability conferred by OCT4 overexpression was repressed by AKT knockdown (P<0.05; Figure 3G). MCF-7/5-FU cells were then transfected with oe-AKT and si-OCT4. The results revealed that overexpression of AKT inhibited the induced sensitivity of MCF-7/5-FU cells to 5-FU (Figure 3H) and colony formation ability (Figure 3I) by knockdown of OCT4. These results together demonstrated that GRP78 was able to upregulate AKT through OCT4, thereby promoting resistance of BC cells to 5-FU.
GRP78 regulates OCT4/MIAT/AKT to mediate resistance of BC cells to 5-FU
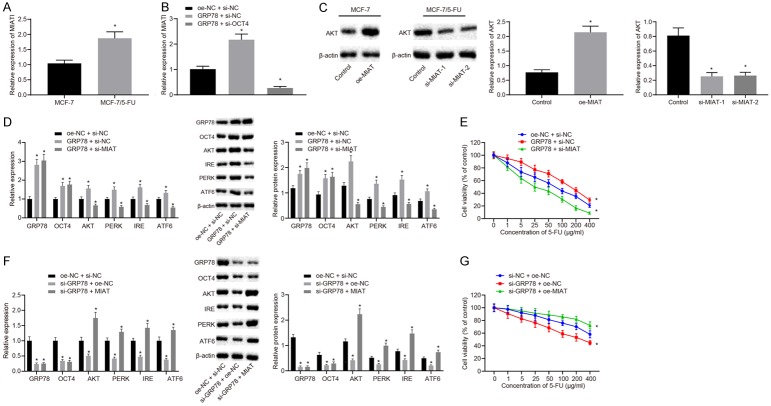
It has been reported that there exists a regulatory loop between lncRNA MIAT and OCT4 [24], and lncRNA MIAT elevates the expression of AKT [23]. We investigated whether OCT4 regulated AKT through lncRNA MIAT to promote the resistance of BC to 5-FU. As determined by RT-qPCR assay, the expression of lncRNA MIAT in MCF-7/5-FU cells was higher than that in MCF-7 cells (P<0.05; Figure 4A). Besides, the expression of lncRNA MIAT was increased in MCF-7 cells co-transfected with oe-GRP78 and si-NC relative to those co-transfected with oe-NC and si-NC, but decreased in MCF-7 cells co-transfected with oe-GRP78 and si-OCT4 as compared to the cells co-transfected with oe-GRP78 and si-NC (P<0.05; Figure 4B). Subsequently, the results of Western blot analysis demonstrated that protein expression of AKT was elevated in the MCF-7/5-FU cells transfected with oe-MIAT but reduced in cells transfected with si-MIAT (P<0.05; Figure 4C). The expression levels of GRP78, OCT4, AKT, PERK, IRE1, and ATF6 were increased in the MCF-7/5-FU cells by oe-GRP78 transfection. The increase in AKT, PERK, IRE1 and ATF6 expression mediated by GRP78 overexpression was diminished by silencing of MIAT (P<0.05), while the expression of GRP78 and OCT4 was not altered (Figure 4D). These results together demonstrated that GRP78 up-regulated OCT4 to upregulate lncRNA MIAT, thereby increasing the expression of AKT.
Figure 4.
GRP78-mediated upregulation of OCT4 increases the expression of AKT through regulating MIAT, thereby enhancing the resistance of BC cells to 5-FU. A. The expression of lncRNA MIAT in MCF-7/5-FU and MCF-7 cells determined by RT-qPCR assay. B. The expression of lncRNA MIAT after MCF-7/5-FU and MCF-7 cells transfected with oe-GRP78 with or without the presence of si-OCT4 examined by RT-qPCR assay. C. The expression of AKT protein after MCF-7/5-FU and MCF-7 cells transfected with oe-MIAT or si-MIAT measured by Western blot analysis. D. The mRNA and protein expression of GRP78, OCT4, AKT, PERK, IRE1 and ATF6 in the MCF-7/5-FU cells transfected with oe-GRP78 with or without the presence of si-MIAT measured by RT-qPCR and Western blot analysis. E. The sensitivity of MCF-7 cells to 5-FU after transfected with oe-GRP78 with or without the presence of si-MIAT assessed by CCK8 assay. F. The mRNA and protein expression of GRP78, OCT4, AKT, PERK, IRE1 and ATF6 in MCF-7/5-FU cells transfected with si-GRP78 with or without the presence of oe-MIAT measured by RT-qPCR and Western blot analysis. G. The sensitivity of MCF-7 cells to 5-FU in response to si-GRP78 with or without the presence of oe-MIAT transfection assessed by CCK8 assay. *P<0.05 vs. adjacent normal tissues, MCF-7 cells, the control group or MCF-7/5-FU cells treated with oe-NC + si-NC. Measurement data were expressed as mean ± standard deviation. Data in compliance with normal distribution and homogeneity between two groups were compared using t-test. Comparisons among multiple groups were conducted by one-way ANOVA with Tukey’s post-hoc test. Statistical analysis in relation to time-based measurements within each group was realized using repeated measures ANOVA, followed by a Bonferroni’s post-hoc test for multiple comparisons. The experiment was repeated three times.
Next, to investigate whether GRP78 upregulated lncRNA MIAT and AKT to enhance BC cell resistance to 5-FU, MCF-7 cells were transfected with oe-GRP78 and si-MIAT, and sensitivity to 5-FU was assessed by CCK8 assay. The data showed that enhanced resistance of BC cells to 5-FU induced by GRP78 overexpression was diminished by si-MIAT (Figure 4E). Meanwhile, MCF-7/5-FU cells were transfected with si-GRP78 and oe-MIAT, and 5-FU sensitivity was detected by CCK8 assay. It was shown that overexpression of lncRNA MIAT inhibited the increase in sensitivity of MCF-7/5-FU cells to 5-FU which was induced by inhibition of GRP78 (P<0.05; Figure 4F, 4G). In summary, the expression of GRP78 was increased through ER stress in BC cells, which then elevated the expression of OCT4. Upregulation of OCT4 increased the expression of AKT through lncRNA MIAT, thereby enhancing the resistance of BC cells to 5-FU.
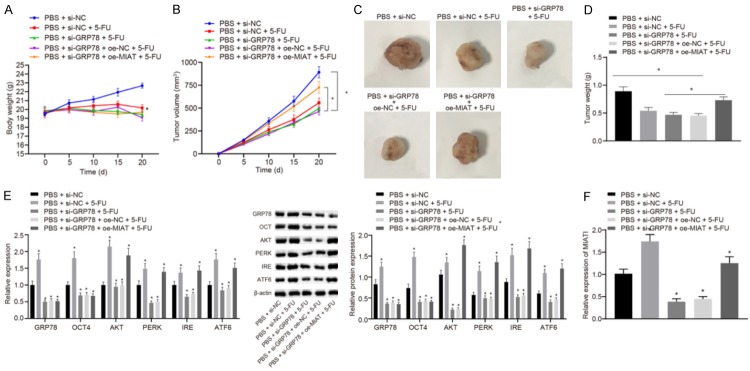
Knockdown of GRP78 in nude mice enhances the BC cell sensitivity to 5-FU
In order to investigate the effect of ER stress-mediated high expression of GRP78 on BC cell resistance to 5-FU in vivo, mice were subcutaneously injected with the MCF-7 cells stably transfected with si-NC, si-GRP78 alone, or co-transfected with si-GRP78 and oe-NC or si-GRP78 and oe-MIAT, and intraperitoneal injection of 5-FU was applied for treatment during the experiment. It was demonstrated that mice lost weight after 5-FU treatment (Figure 5A). The treatment with 5-FU inhibited tumor growth, and knockdown of GRP78 enhanced the inhibitory effect of 5-FU on tumor growth. However, this enhancement was inhibited by overexpression of lncRNA MIAT (P<0.05; Figure 5B). After mice were euthanized, the tumors were excised. The nude mice injected with si-GRP78-transfected MCF-7 cells and treated with 5-FU had tumors of a minimum volume. However, the tumor volume in nude mice injected with MCF-7 cells co-transfected with si-GRP78 and oe-NC was larger than that in mice injected with MCF-7 cells co-transfected with si-GRP78 and si-MIAT (P<0.05; Figure 5C, 5D). The protein and mRNA were extracted from the tumors for RT-qPCR and western blot analysis. The mRNA and protein expression of GRP78, OCT4, AKT, PERK, IRE1 and ATF6 was decreased in tumors of mice upon GRP78 knockdown. The mRNA and protein expression of GRP78 and OCT4 was decreased but that of AKT, PERK, IRE1, and ATF6 was increased in tumors of mice by additional overexpression of MIAT in the presence of si-GRP78 (P<0.05; Figure 5E). At the same time, the results of RT-qPCR assay showed that the expression of lncRNA MIAT increased upon treatment of 5-FU but reduced in the nude mice injected with si-MIAT-transfected MCF-7 cells (P<0.05; Figure 5F). These results demonstrated that knockdown of GRP78 promoted BC cell sensitivity to 5-FU by downregulating lncRNA MIAT.
Figure 5.
Knockdown of GRP78 promotes BC cell sensitivity to 5-FU in nude mice. A. Body weight of nude mice. B. Tumor volume in nude mice. C. The representative tumors formed in nude mice. D. The tumor weight in nude mice. E. The expression of GRP78, OCT4, AKT, PERK, IRE1 and ATF6 in tumors formed in nude mice measured by Western blot analysis. F. The expression of MIAT in tumors formed in nude mice determined by RT-qPCR assay. *P<0.05 vs. nude mice injected with MCF-7/5-FU cells stably transfected with si-NC and PBS. Measurement data were expressed as mean ± standard deviation. Comparisons among multiple groups were conducted by one-way ANOVA with Tukey’s post-hoc test. Statistical analysis in relation to time-based measurements within each group was realized using repeated measures ANOVA, followed by a Bonferroni’s post-hoc test for multiple comparisons.
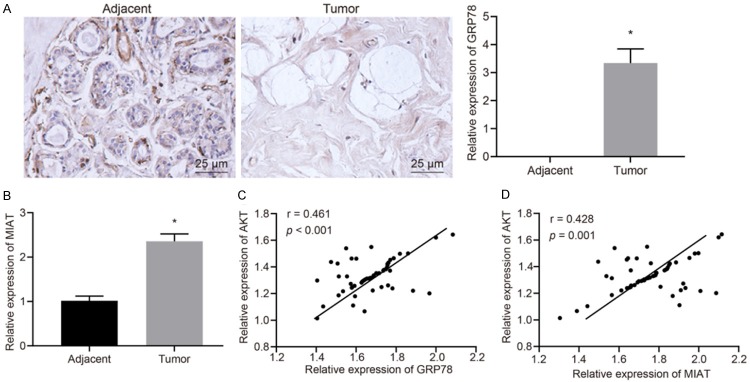
GRP78 and lncRNA MIAT are highly expressed in BC tissues
BC tissues and adjacent normal tissues from BC patients were collected to investigate the expression levels of GRP78 in BC. The results of immunohistochemistry displayed that GRP78 was highly expressed in BC tissues as compared to adjacent normal tissues (P<0.05; Figure 6A). At the same time, the results of RT-qPCR assay showed that the expression of lncRNA MIAT in BC tissues was significantly higher than that in adjacent normal tissues (P<0.05; Figure 6B). Furthermore, we analyzed the correlation between GRP78 and AKT expression in BC tissues. The results showed that the expression of AKT in BC tissues was positively correlated with the expression levels of GRP78 (P<0.05; Figure 6C). Additionally, the expression of lncRNA MIAT and AKT in BC tissues was examined by RT-qPCR assay and Western blot analysis, and their relationship was analyzed. The results indicated lncRNA MIAT was positively correlated with AKT (P<0.05; Figure 6D). Overall the findings showed that the high expression of GRP78 may affect the development of BC cells and the resistance to chemotherapeutic drugs by increasing the expression levels of lncRNA MIAT and AKT.
Figure 6.
GRP78 and lncRNA MIAT are overexpressed in BC tissues. A. The expression of GRP78 in BC tissues and adjacent normal tissues detected by immunohistochemistry (×400). B. The expression of lncRNA MIAT in BC tissues and adjacent normal tissues determined by RT-qPCR assay. C. The correlation analysis between GRP78 and AKT expression in BC tissues. D. The relationship between lncRNA MIAT and AKT in BC tissues. *P<0.05 vs. adjacent normal tissues. Measurement data were expressed as mean ± standard deviation. Unpaired data in compliance with normal distribution and homogeneity between two groups were compared using unpaired t-test. Comparisons for data in scatter plots were conducted by analysis of covariance. The experiment was repeated three times.
Discussion
5-FU is a commonly used chemotherapy drug for BC. However, its use often leads to drug resistance [25]. Recently, an association of the molecular mechanism of ER stress with chemoresistance has drawn increasing research attention [26,27]. Besides, it has been shown that ER stress and unfolded protein response activation can modulate the levels and activities of cell survival- and autophagy-related regulators, and thereby crucially function in controlling the drug resistance of BC cells [28]. More importantly, 5-FU has been linked to the stimulation of ER stress in colon cancer [29] and hepatocellular carcinoma [30]. The current study mainly investigated the involvement of ER stress in resistance of BC to 5-FU and the underlying molecular mechanisms. The results revealed that 5-FU induced BC cell resistance through the induction of ER stress, as a result of which the GRP78/OCT4/lncRNA MIAT/AKT pathway was activated.
First of all, our finding demonstrated that 5-FU was able to stimulate ER stress, which in turn induced the resistance of BC cells to 5-FU. The expression levels of ER stress marker proteins GRP78 and ATF6 and the extent of PERK and IRE-1 phosphorylation levels were significantly upregulated when BC cells were treated with 5-FU. These results are consistent with a previous study that found 5-FU treatment elevates the expression of ER stress marker proteins such as PERK, IRE-1 and ATF4 in colon cancer [15]. ATF6 is a membrane precursor, located in the endoplasmic reticulum and its transport can be induced under ER stress [31]. Moreover, a high expression of GRP78 was observed in the 5-FU-resistant BC cell line MCF-7/5-FU. GRP78, an ER stress survival mediator, is involved in the regulation of chemoresistance triggered by ER stress in pancreatic adenocarcinoma cells [32]. Similar to these results, we also found that a high expression level of GRP78 induced by ER stress could enhance the resistance of BC cells to 5-FU. Based on these findings, we established that 5-FU induced ER stress in BC cells and increased the expression of GRP78, which enhanced cell resistance to 5-FU.
In addition, OCT4 was highly expressed in MCF-7/5-FU cells. The stem cell factor OCT4 has been found to play a promotive role in drug resistance in a variety of cancers. For example, overexpression of OCT4 increases bladder cancer cell resistance to cisplatin [33]. Additionally, OCT4 contributes to tamoxifen resistance in BC [34]. Also, OCT4 enhances the resistance of non-small cell lung cancer cells to gefitinib by potentiating gefitinib-induced apoptosis [35]. Notably, a previous study has reported that OCT4 is involved in drug-resistance in lung cancer through downstream PTEN and TNC genes [36]. Similar results have been found in our study, where OCT4 was shown to promote BC cell resistance to 5-FU via enhancing 5-FU-induced proliferation. The knockdown of GRP78 has shown tumor-suppressing properties in head-neck cancer and was found to be accompanied by downregulation of OCT4 [16], which partially concurred with our finding that GRP78 promoted 5-FU resistance by increasing the expression of OCT4 in BC cells. Furthermore, our study demonstrated that AKT was overexpressed in MCF-7/5-FU cells. Multiple lines of evidence have revealed that the AKT pathway plays a key role in drug resistance of many cancer cells. For instance, AKT was noted to enhance the resistance of lung cancer cells to antineoplastic drugs [37]. Besides, AKT, upregulated by miR-222, was found capable of increasing the drug resistance of BC cells to adriamycin [38]. Aligned with these findings, the present study demonstrated that AKT mediated by OCT4 enhanced 5-FU resistance in BC cells. A high expression of lncRNA MIAT has been found in MCF-7/5-FU cells [39], supporting the current paradigm that lncRNA MIAT is highly expressed in BC cells and functions as an oncogene in BC. Interestingly, there exists a regulatory loop between lncRNA MIAT and OCT4 in malignant mature B cells [24]. More importantly, the inhibition of lncRNA MIAT is shown to elevate the sensitivity of BC cells by enhancing apoptotic response to drugs, accompanied with a decrease in OCT4 expression [40]. Besides, the oncogenic role of lncRNA MIAT has been attributed to its induction of AKT phosphorylation [41]. These previous findings are partially consistent with our result showing OCT4 upregulated AKT via lncRNA MIAT to enhance 5-FU resistance in BC cells. Additionally, we further confirmed that knockdown of GRP78 promoted BC cell sensitivity to 5-FU by downregulating lncRNA MIAT in nude mice.
In summary, our study demonstrated that induction of ER stress conferred BC cell resistance to 5-FU by activating the GRP78/OCT4/lncRNA MIAT/AKT pathway (Figure 7). The present study elucidated that the mechanism of 5-FU-induced chemoresistance was mediated by GRP78 upregulated MIAT and AKT via increasing OCT4, laying the groundwork for the development of new therapeutic targets for BC. However, the specific mechanisms underpinning the role of AKT in 5-FU resistance remain unclear and future investigations are warranted for a greater understanding of detailed mechanisms in this context.
Figure 7.
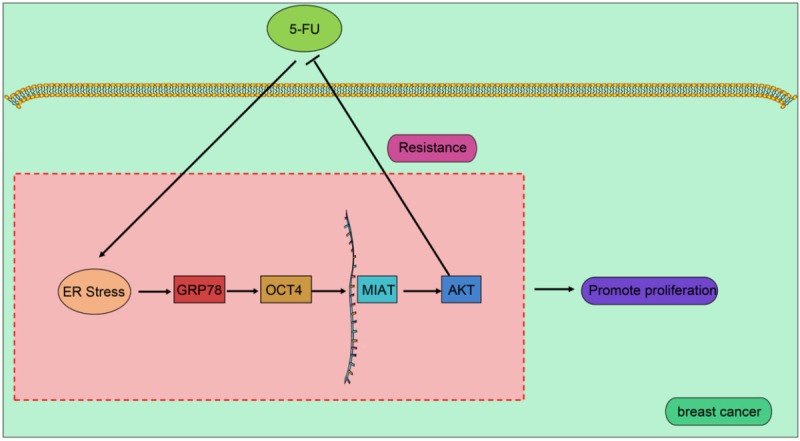
5-FU enhances resistance of BC cells to 5-FU by regulating GRP78/lncRNA MIAT/AKT pathway through inducing ER stress. After BC cells were treated with 5-FU, the expression of GRP78 was elevated through ER stress, thereby increasing the expression of OCT4. Upregulation of OCT4 increased the expression of AKT through increasing lncRNA MIAT, thereby inducing resistance in BC cells to 5-FU.
Acknowledgements
The authors acknowledge and appreciate their colleagues for their valuable efforts and comments regarding this paper. This study was supported by Hubei Provincial Natural Science Foundation (No. 2019CFB303).
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Dittmer J. Breast cancer stem cells: features, key drivers and treatment options. Semin Cancer Biol. 2018;53:59–74. doi: 10.1016/j.semcancer.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Vinod BS, Antony J, Nair HH, Puliyappadamba VT, Saikia M, Narayanan SS, Bevin A, Anto RJ. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013;4:e505. doi: 10.1038/cddis.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Xing Y, Gao Q, Sun X, Zhang D, Cao G. Combination of nrp1-mediated irgd with 5-fluorouracil suppresses proliferation, migration and invasion of gastric cancer cells. Biomed Pharmacother. 2017;93:1136–1143. doi: 10.1016/j.biopha.2017.06.103. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z, Li Z, Feng X, Hao J, Zhang K, Xiao B, Chen M, Huang W, Xiong S, Wu X, Deng W. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing pi3k/akt and nf-kappab/inos signaling pathways. J Pineal Res. 2017;62 doi: 10.1111/jpi.12380. [DOI] [PubMed] [Google Scholar]
- 6.Jahani M, Azadbakht M, Norooznezhad F, Mansouri K. L-arginine alters the effect of 5-fluorouracil on breast cancer cells in favor of apoptosis. Biomed Pharmacother. 2017;88:114–123. doi: 10.1016/j.biopha.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 7.Wijdeven RH, Pang B, Assaraf YG, Neefjes J. Old drugs, novel ways out: drug resistance toward cytotoxic chemotherapeutics. Drug Resist Updat. 2016;28:65–81. doi: 10.1016/j.drup.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Konieczkowski DJ, Johannessen CM, Garraway LA. A convergence-based framework for cancer drug resistance. Cancer Cell. 2018;33:801–815. doi: 10.1016/j.ccell.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Y, Zheng Y, Gu J, Wang S, Wang N, Yang B, Zhang F, Wang D, Fu W, Wang Z. Betulinic acid chemosensitizes breast cancer by triggering er stress-mediated apoptosis by directly targeting grp78. Cell Death Dis. 2018;9:636. doi: 10.1038/s41419-018-0669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikesitch N, Lee JM, Ling S, Roberts TL. Endoplasmic reticulum stress in the development of multiple myeloma and drug resistance. Clin Transl Immunology. 2018;7:e1007. doi: 10.1002/cti2.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopisetty MK, Kovacs D, Igaz N, Ronavari A, Belteky P, Razga Z, Venglovecz V, Csoboz B, Boros IM, Konya Z, Kiricsi M. Endoplasmic reticulum stress: major player in size-dependent inhibition of P-glycoprotein by silver nanoparticles in multidrug-resistant breast cancer cells. J Nanobiotechnology. 2019;17:9. doi: 10.1186/s12951-019-0448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong JT, Yu J, Wang HJ, Shi Y, Zhao TS, He BX, Qiao B, Feng ZW. Effects of endoplasmic reticulum stress on the autophagy, apoptosis, and chemotherapy resistance of human breast cancer cells by regulating the pi3k/akt/mtor signaling pathway. Tumour Biol. 2017;39:1010428317697562. doi: 10.1177/1010428317697562. [DOI] [PubMed] [Google Scholar]
- 13.Shi-Chen Ou D, Lee SB, Chu CS, Chang LH, Chung BC, Juan LJ. Transcriptional activation of endoplasmic reticulum chaperone grp78 by hcmv ie1-72 protein. Cell Res. 2011;21:642–653. doi: 10.1038/cr.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailly C, Waring MJ. Pharmacological effectors of grp78 chaperone in cancers. Biochem Pharmacol. 2019;163:269–278. doi: 10.1016/j.bcp.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Yun S, Han YS, Lee JH, Kim S, Lee SH. Enhanced susceptibility to 5-fluorouracil in human colon cancer cells by silencing of grp78. Anticancer Res. 2017;37:2975–2984. doi: 10.21873/anticanres.11651. [DOI] [PubMed] [Google Scholar]
- 16.Chiu CC, Lee LY, Li YC, Chen YJ, Lu YC, Li YL, Wang HM, Chang JT, Cheng AJ. Grp78 as a therapeutic target for refractory head-neck cancer with cd24(-)cd44(+) stemness phenotype. Cancer Gene Ther. 2013;20:606–615. doi: 10.1038/cgt.2013.64. [DOI] [PubMed] [Google Scholar]
- 17.Gwak JM, Kim M, Kim HJ, Jang MH, Park SY. Expression of embryonal stem cell transcription factors in breast cancer: Oct4 as an indicator for poor clinical outcome and tamoxifen resistance. Oncotarget. 2017;8:36305–36318. doi: 10.18632/oncotarget.16750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell PA, Rudnicki MA. Oct4 interaction with hmgb2 regulates akt signaling and pluripotency. Stem Cells. 2013;31:1107–1120. doi: 10.1002/stem.1365. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Dong X, He C, Tan G, Li Z, Zhai B, Feng J, Jiang X, Liu C, Jiang H, Sun X. Lncrna SNHG1 contributes to sorafenib resistance by activating the Akt pathway and is positively regulated by mir-21 in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2019;38:183. doi: 10.1186/s13046-019-1177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu GS, Li M, Xu CX, Wang D. APE1 stimulates EGFR-TKI resistance by activating Akt signaling through a redox-dependent mechanism in lung adenocarcinoma. Cell Death Dis. 2018;9:1111. doi: 10.1038/s41419-018-1162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J, Yao JF, Deng XF, Zheng XD, Jia M, Wang YQ, Huang Y, Zhu JH. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin alphavbeta3 and activating FAK/PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37:23. doi: 10.1186/s13046-018-0694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheik Mohamed J, Gaughwin PM, Lim B, Robson P, Lipovich L. Conserved long noncoding RNAs transcriptionally regulated by Oct4 and nanog modulate pluripotency in mouse embryonic stem cells. RNA. 2010;16:324–337. doi: 10.1261/rna.1441510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Y, Li C, Luo Y, Li L, Liu J, Gui R. Silencing of long non-coding RNA MIAT sensitizes lung cancer cells to gefitinib by epigenetically regulating mir-34a. Front Pharmacol. 2018;9:82. doi: 10.3389/fphar.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sattari A, Siddiqui H, Moshiri F, Ngankeu A, Nakamura T, Kipps TJ, Croce CM. Upregulation of long noncoding RNA MIAT in aggressive form of chronic lymphocytic leukemias. Oncotarget. 2016;7:54174–54182. doi: 10.18632/oncotarget.11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen XX, Lam KH, Chen QX, Leung GP, Tang SCW, Sze SC, Xiao JB, Feng F, Wang Y, Zhang KY, Zhang ZJ. Ficus virens proanthocyanidins induced apoptosis in breast cancer cells concomitantly ameliorated 5-fluorouracil induced intestinal mucositis in rats. Food Chem Toxicol. 2017;110:49–61. doi: 10.1016/j.fct.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Bahar E, Kim JY, Yoon H. Chemotherapy resistance explained through endoplasmic reticulum stress-dependent signaling. Cancers (Basel) 2019;11 doi: 10.3390/cancers11030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jing D, Zhou W, Shen L, Zhang Q, Xie WT, Shen E, Li Z, Shen LF, Sun LQ. RIG-I promotes IFN/JAK2 expression and the endoplasmic reticulum stress response to inhibit chemoradiation resistance in nasopharyngeal carcinoma. Cancer Med. 2019;8:6344–6357. doi: 10.1002/cam4.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sisinni L, Pietrafesa M, Lepore S, Maddalena F, Condelli V, Esposito F, Landriscina M. Endoplasmic reticulum stress and unfolded protein response in breast cancer: the balance between apoptosis and autophagy and its role in drug resistance. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JK, Kang KA, Piao MJ, Ryu YS, Han X, Fernando PM, Oh MC, Park JE, Shilnikova K, Boo SJ, Na SY, Jeong YJ, Jeong SU, Hyun JW. Endoplasmic reticulum stress induces 5-fluorouracil resistance in human colon cancer cells. Environ Toxicol Pharmacol. 2016;44:128–133. doi: 10.1016/j.etap.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Yadunandam AK, Yoon JS, Seong YA, Oh CW, Kim GD. Prospective impact of 5-Fu in the induction of endoplasmic reticulum stress, modulation of GRP78 expression and autophagy in SK-HEP1 cells. Int J Oncol. 2012;41:1036–1042. doi: 10.3892/ijo.2012.1506. [DOI] [PubMed] [Google Scholar]
- 31.Schindler AJ, Schekman R. In vitro reconstitution of ER-stress induced ATF6 transport in COPII vesicles. Proc Natl Acad Sci U S A. 2009;106:17775–17780. doi: 10.1073/pnas.0910342106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke WR, Amundadottir L, James MA. CLPTM1L/CRR9 ectodomain interaction with GRP78 at the cell surface signals for survival and chemoresistance upon ER stress in pancreatic adenocarcinoma cells. Int J Cancer. 2019;144:1367–1378. doi: 10.1002/ijc.32012. [DOI] [PubMed] [Google Scholar]
- 33.Lu CS, Shieh GS, Wang CT, Su BH, Su YC, Chen YC, Su WC, Wu P, Yang WH, Shiau AL, Wu CL. Chemotherapeutics-induced Oct4 expression contributes to drug resistance and tumor recurrence in bladder cancer. Oncotarget. 2017;8:30844–30858. doi: 10.18632/oncotarget.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatt S, Stender JD, Joshi S, Wu G, Katzenellenbogen BS. Oct-4: a novel estrogen receptor-alpha collaborator that promotes tamoxifen resistance in breast cancer cells. Oncogene. 2016;35:5722–5734. doi: 10.1038/onc.2016.105. [DOI] [PubMed] [Google Scholar]
- 35.Li B, Yao Z, Wan Y, Lin D. Overexpression of OCT4 is associated with gefitinib resistance in non-small cell lung cancer. Oncotarget. 2016;7:77342–77347. doi: 10.18632/oncotarget.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang YA, Chen CH, Sun HS, Cheng CP, Tseng VS, Hsu HS, Su WC, Lai WW, Wang YC. Global Oct4 target gene analysis reveals novel downstream PTEN and TNC genes required for drug-resistance and metastasis in lung cancer. Nucleic Acids Res. 2015;43:1593–1608. doi: 10.1093/nar/gkv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu H, Wu C, Wu T, Xia W, Ci S, He W, Zhang Y, Li L, Zhou S, Edick AM, Zhang A, Zhang J, Pan F, Hu Z, He L, Guo Z. Inhibition of Akt sensitizes cancer cells to antineoplastic drugs by down-regulating flap endonuclease 1. Mol Cancer Ther. 2019;18:2407–2420. doi: 10.1158/1535-7163.MCT-18-1215. [DOI] [PubMed] [Google Scholar]
- 38.Shen H, Wang D, Li L, Yang S, Chen X, Zhou S, Zhong S, Zhao J, Tang J. Mir-222 promotes drug-resistance of breast cancer cells to adriamycin via modulation of PTEN/Akt/FOXO1 pathway. Gene. 2017;596:110–118. doi: 10.1016/j.gene.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 39.Alipoor FJ, Asadi MH, Torkzadeh-Mahani M. MIAT lncRNA is overexpressed in breast cancer and its inhibition triggers senescence and G1 arrest in MCF7 cell line. J Cell Biochem. 2018;119:6470–6481. doi: 10.1002/jcb.26678. [DOI] [PubMed] [Google Scholar]
- 40.Almnaseer ZA, Mourtada-Maarabouni M. Long noncoding RNA MIAT regulates apoptosis and the apoptotic response to chemotherapeutic agents in breast cancer cell lines. Biosci Rep. 2018;38 doi: 10.1042/BSR20180704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Zhang Z, Wu Z, Lin W, Yu M. Downregulation of the expression of the lncRNA MIAT inhibits melanoma migration and invasion through the PI3K/Akt signaling pathway. Cancer Biomark. 2019;24:203–211. doi: 10.3233/CBM-181869. [DOI] [PubMed] [Google Scholar]