Abstract
Cancer immunotherapy has been accompanied by promising results over the past few years. Programmed Cell Death Protein 1 (PD-1) plays a vital role in inhibiting immune responses and promoting self-tolerance through modulating the activity of T-cells, activating apoptosis of antigen-specific T cells and inhibiting apoptosis of regulatory T cells. Programmed Cell Death Ligand 1 (PD-L1) is a trans-membrane protein that is considered to be a co-inhibitory factor of the immune response, it can combine with PD-1 to reduce the proliferation of PD-1 positive cells, inhibit their cytokine secretion and induce apoptosis. PD-L1 also plays an important role in various malignancies where it can attenuate the host immune response to tumor cells. Based on these perspectives, PD-1/PD-L1 axis is responsible for cancer immune escape and makes a huge effect on cancer therapy. This review is aimed to summarize the role of PD-1 and PD-L1 in cancer, looking forward to improve the therapy of cancer.
Keywords: PD-1, PD-L1, cancer
Introduction
Cancer is a serious health problem and one of the primary diseases leading to morbidity and mortality in the world [1]. Chemoradiotherapy is currently the primary treatment option for patients with advanced cancer but can be limited due to severe side effects and drug resistance [2]. Therefore, developing new therapies to overcome the disadvantages is extremely required.
Cancer immunotherapy has been recently developed with the aim of designing effective treatments to improve the specificity and strength of the immune system against cancer [3]. James P. Allison and Tasuku Honjo won the 2018 Nobel Prize of Physiology or Medicine for discovering a cancer treatment by suppressing negative immunomodulation. Their research on the immune checkpoints programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), demonstrated that they acted as a “brake” role in immune function, and suggested that immune checkpoint inhibition may reactivate T cells and eliminate cancer cells more effectively [4]. An increasing number of studies report that immune checkpoint inhibitors may be of significant therapeutic value.
Accumulating evidence indicates that the inhibition of PD-1 promotes an effective immune response against cancer cells [5]. PD-1 signaling pathway suppression has shown that the clinical response of patients with different solid tumors and hematological malignancies, mainly relies on T-cells effectively to penetrating the tumor [6]. In addition, targeting PD-L1 has been associated with a significant clinical response in a wide range of cancer patients [7]. The purpose of the present review was to elucidate the role of PD-1/PD-L1 signaling in cancer, aiming to provide novel anticancer therapeutic strategies in the future.
Overview of PD-1/PD-L1 pathway
PD-1/PD-L1 pathway controls the induction and maintenance of immune tolerance within the tumor microenvironment. The activity of PD-1 and its ligands PD-L1 or PD-L2 are responsible for T cell activation, proliferation, and cytotoxic secretion in cancer to degenerating anti-tumor immune responses (Figure 1).
Figure 1.
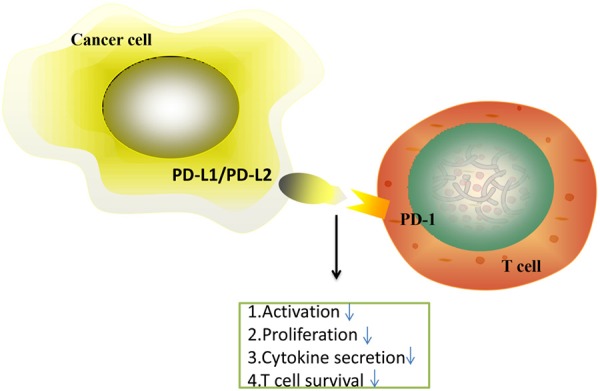
The PD-1/PD-L1 axis inhibits T cell activation, proliferation, and survival and cytotoxic secretion within cancer cell.
PD-1
PD-1, also referred to as CD279, was first discovered in interleukin-3 (IL-3)-deprived LyD9 (murine hematopoietic progenitor) and 2B4-11 (murine T-cell hybridoma) cell lines in 1992 [8]. PD-1 is 15% similar to the amino acid sequence of CD28, 20% similar to CTLA4, and 13% similar to induced T-cell co-stimulator [9]. PD-1 is a 55-kDa transmembrane protein containing 288 amino acids with an extracellular N-terminal domain (IgV-Like), a membrane-permeating domain and a cytoplasmic tail located at the N and C ends, respectively, with two tyrosine base [10].
PD-1 is an inhibitor of both adaptive and innate immune responses, and is expressed on activated T, natural killer (NK) and B lymphocytes, macrophages, dendritic cells (DCs) and monocytes [11]. Of note, PD-1 is highly expressed on tumor-specific T cells [11]. Transcription factors such as nuclear factor of activated T cells (NFAT), NOTCH, Forkhead box protein (FOX) O1 and interferon (IFN) regulatory factor 9 (IRF9) may trigger the transcription of PD-1 [12]. The conserved upstream regulatory regions B and C (CR-B and COR-C) are important for the expression of the PD-1 gene. There is a binding site in the CR-C region that is connected to NFATc1 (NFAT2) in TCD4 and TCD8 units [13]. Instead, c-FOS connects to sites in the CR-B region and enhances PD-1 expression when it stimulates T-cell receptors upon Ag detection in naive T cells [13]. NFATc is activated and binds to the promoter region of the pdcd1 gene [13]. In addition, IFN-α combined with IRF9 may result in PD-1 expression via binding to the promoter of the pdcd1 gene in exhausted T cells [14]. During chronic infections, PD-1 is expressed in exhausted TCD8 cells due to its demethylated promoter, and the FOXO1 transcription factor binds to the PD-1 promoter to increase its expression [14]. Cancer cell leakage increases the expression of the c-FOS subunit of AP1, thereby increasing the expression of PD-1 [15].
PD-1 plays two opposing roles, as it can be both beneficial and harmful. As regards its beneficial effects, it plays a key role in reducing the regulation of ineffective or harmful immune responses and maintaining immune tolerance. However, PD-1 causes the dilation of malignant cells by interfering with the protective immune response [16].
PD-L1
PD-1 ligand (PD-L1; also referred to as CD279 and B7-H1), belongs to the B7 series and is a 33-kDa type 1 transmembrane glycoprotein that contains 290 amino acids with Ig- and IgC domains in its extracellular region [17].
PD-L1 is usually expressed by macrophages, some activated T cells and B cells, DCs and some epithelial cells, particularly under inflammatory conditions [18]. In addition, PD-L1 is expressed by tumor cells as an “adaptive immune mechanism” to escape anti-tumor responses [19]. PD-L1 is associated with an immune environment rich in CD8 T cells, production of Th1 cytokines and chemical factors, as well as interferons and specific gene expression characteristics [20]. It has been demonstrated that IFN-γ causes PD-L1 upregulation in ovarian cancer cells, which is responsible for disease progression, whereas IFN-γ receptor 1 inhibition can reduce PD-L1 expression in acute myeloid leukemia mouse models through the MEK/extracellular signal-regulated kinase (ERK) and MYD88/TRAF6 pathways [21]. IFN-γ induces protein kinase D isoform 2 (PKD2), which is important for the regulation of PD-L1. Inhibition of PKD2 activity inhibits the expression of PD-L1 and promotes a strong antitumor immune response. NK cells secrete IFN-γ through the Janus kinase (JAK)1, JAK2 and signal transducer and activator of transcription (STAT)1 pathways, increasing the expression of PD-L1 on the surface of the tumor cells [22]. Studies on melanoma cells have shown that IFN-γ secreted by T cells through the JAK1/JAK2-STAT1/STAT2/STAT3-IRF1 pathway may regulate the expression of PD-L1. T and NK cells appear to secrete IFN-γ, which induces PD-L1 expression on the surface of the target cells, including tumor cells [23].
PD-L1 acts as a pro-tumorigenic factor in cancer cells via binding to its receptors and activating proliferative and survival signaling pathways [24]. This finding further indicated that PD-L1 is implicated in subsequent tumor progression. In addition, PD-L1 has been shown to exert non-immune proliferative effects on a variety of tumor cell types. For example, PD-L1 induced epithelial-to-mesenchymal transition (EMT) and stem cell-like phenotypes in renal cancer cells, indicating that the presence of the intrinsic pathway of PD-L1 promotes kidney cancer progression [25].
Effects of signaling pathways on PD-1/PD-L1 in cancer
PD-1/PD-L1 axis can be modulated by various signals in cancer cells (Figure 2), which exert a critical role in tumorigenesis. Therefore, it is imperative to observe this signaling network in order to improve the presently available understanding.
Figure 2.
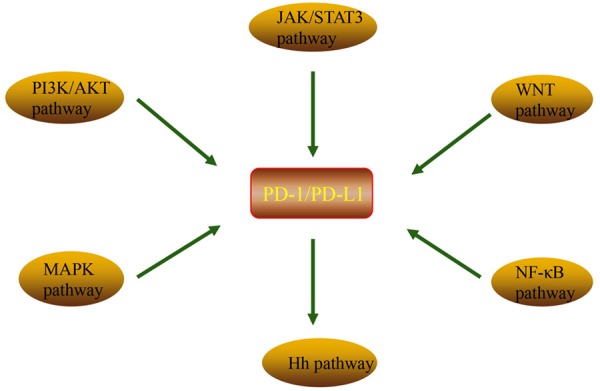
Various pathways regulation of PD-1/PD-L1 expression. PI3K/AKT pathway, MAPK pathway, JAK/STAT pathway, WNT pathway, NF-κB pathway and Hedgehog (Hh) pathway promote the expression of PD-1/PD-L1 axis.
PI3K/AKT signaling pathway
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway is associated with cell proliferation and can regulate a variety of cell proliferation and apoptosis-related processes [26]. Increased mammalian target of rapamycin (mTOR) activity has also been reported in human cancers. In addition, the mTOR pathway is involved in the regulation of the innate and adaptive immune systems [27]. Previous data have reported that the activation of PI3K/AKT enhances the nutritional intake and energy production of CD8- T cells, and mTOR is responsible for regulating the biological effects of immune cell stimulation [28]. Activation of PI3K/AKT can promote PD-L1 expression through increased extrinsic signaling or decreased expression of negative regulators, such as phosphatase and tensin homolog (PTEN). Downregulation of PTEN may lead to the activation of PI3K/AKT and facilitate the expression of PD-L1 [29]. Similarly, Zhao et al demonstrated that PD-1/PD-L1 blockade may attenuate apoptosis of CD8+ T cells through regulating the PI3K/AKT/mTOR pathway in gastrointestinal stromal tumors (GIST), indicating that PD-1/PD-L1 plays an important role in the immune system in combination with the PI3K/AKT/mTOR pathway. It has also been reported that PD-L1 knockdown in GIST cells can decrease the expression of PI3K, p-AKT and p-PI3K [30]. In addition, Wei et al demonstrated that the overexpression of PD-L1 activated PI3K/AKT in the nucleus in colorectal cancer cells [31].
MAPK signaling pathway
The mitogen-activated protein kinase (MAPK) signaling pathway is an important signal transduction system that is associated with the conversion of extracellular signals to intracellular responses, and it can also regulate cell proliferation, differentiation, invasion, metastasis and death through phosphorylation activation [32]. c-Jun amino-terminal kinase (c-Jun), p38MAPK and ERK are three parallel pathways involved in the MAPK pathway [33]. Recent research has gradually become focused on the association between the PD-1/PD-L1 axis and the MAPK pathway. For example, Stutvoet et al demonstrated that inhibition of the MAPK pathway prevented epidermal growth factor and IFN-γ-induced CD274 mRNA and PD-L1 protein and membrane upregulation in lung adenocarcinoma cells [34]. In addition, Jalali et al reported that PD-L1 antibody is linked to the MAPK signaling molecules in Hodgkin’s lymphoma (HL) cells; they also found that p-P38 and p-ERK were decreased in all HL lines after using an anti-PD-L1 antibody. Similarly, inhibition of MEK1/2, an important factor of the MAPK pathway, can markedly prevent PD-L1 expression in renal cell carcinoma [35].
JAK-STAT signaling pathway
JAK signaling activation of the STAT pathway is an evolutionarily conserved signaling pathway used by a variety of cytokines, IFNs, growth factors and related molecules [36]. This approach provides a crucial mechanism for extracellular factors to control gene expression. Therefore, it may be used as a basic example of how cells respond to environmental conditions and interpret these signals to regulate cell growth and differentiation [37]. Recently, the JAK/STAT pathway was reported to induce PD-L1 expression in cancers, which may be of value in cancer therapy. Toshifumi et al reported that AG490, a JAK2 inhibitor, suppressed the upregulation of PD-L1 at both the mRNA and protein levels [38]. These results confirmed that the JAK/STAT pathway regulates the expression of PD-L1. In addition, fibroblast growth factor receptor (FGFR)2 signaling effectively activated the JAK/STAT3 signaling pathway, which was accompanied by increased PD-L1 expression in vitro. In colorectal cancer xenograft models, overexpression of FGFR2 promoted PD-L1 expression and increased the size of the tumors. This effect may be blocked via downregulation of the JAK/STAT3 pathway by JAK inhibitors [39].
WNT signaling pathway
Deregulated WNT signaling has been shown to facilitate malignant transformation, tumor progression and resistance to conventional cancer therapy [40]. A large body of evidence indicates that abnormal WNT signals can also disrupt cancer immunomonitoring, thereby promoting immune escape and resistance to a variety of immunotherapies, including immune checkpoint blockers [41]. For example, in mouse models of breast cancer, the proteasomal degradation of non-glycosylated PD-L1 was facilitated by active signaling from the GSK3B-β-TrCP axis (e.g., without WNT ligands). By contrast, in mouse melanoma models, GSK3 blockers improve tumor eradication by repressing the gene encoding PDCD1 [42]. Furthermore, the use of selective WNT inhibitors or activators to reduce or upregulate PD-L1 expression, respectively, for the treatment of triple-negative breast cancer (TNBC), is based on functional crosstalk between WNT activity and PD-L1 expression [43].
NF-κB signaling pathway
It was recently demonstrated that the expression of the PD-L1 gene can be induced by Toll-like receptor (TLR) or IFN-γ-driven nuclear factor (NF)-κB. Lim et al observed that the NF-κB inhibitor curcumin, in combination with anti-CTLA-4 checkpoint inhibition therapy, reduced the growth of breast cancer, colon carcinoma, and melanoma cell lines, suggesting that NF-κB inhibition may play a dual role: targeting tumor cell proliferation and survival, as well as tumor immune checkpoints [44]. NF-κB is likely involved in LMP1-induced PD-L1 expression, as the NF-κB inhibitor caffeic acid phenethyl ester decreased PD-L1 induction. NF-κB is also a major mediator of INF-γ-induced PD-L1 expression. The NF-κB inhibitor, but not MAPK, PI3K or STAT3 inhibitors, abolished IFN-induced PD-L1 expression [45]. Furthermore, Peng et al reported that chemotherapy induces local immune suppression through NF-κB-mediated PD-L1 upregulation in ovarian cancer [46].
Hedgehog signaling pathway
The Hedgehog (Hh) signaling pathway is currently known to be crucial for the proliferation of substrate cells, and mutations in this pathway may lead to tumor formation; as a result, small molecular inhibitors that alter the composition of this pathway, including SMO and GLI, have been the focus of recent therapeutic developments [47]. Importantly, it was suggested that Hh signaling can regulate PD-L1 expression, and inhibition of Hh signaling may induce lymphocyte antitumor activity [48]. Jayati et al reported that Hh signals help induce PD-L1 expression in gastric cancer, PD-1/PD-L1 inhibition reverses Gli2-induced tolerance, and it is recommended that combined drug therapy inhibition of Hh signaling and immune checkpoints may be suitable for selected patients [49].
Effects of microRNAs on PD-1/PD-L1 in cancer
Overview of microRNAs
Recently, small nucleotide molecules called microRNAs (miRNAs) have become a focus of attention during tumor development by regulating cell cycle, metastasis, angiogenesis, metabolism and apoptosis of cancer cells [50]. miRNAs regulate gene expression through transcriptional regulation of mRNA. However, only few studies have acknowledged the role of miRNAs in activating gene expression [51]. Extensive data from a large number of studies suggest the role of miRNAs as tumor suppressors, carcinogenic, diagnostic and prognostic biomarkers in lung cancer [52]. They are also involved in regulating cancer cell metabolism and resistance or sensitivity to chemotherapy and radiotherapy [53]. Furthermore, the role of miRNAs in the regulation of PD-1 and PD-L1 was investigated, as described below and in Table 1. These molecules play an important role in tumor immune escape, leading to the development of microenvironments conducive to tumor growth and progression.
Table 1.
The effects of microRNAs on PD-1/PD-L1 in different cancers
| microRNAs | function | cancer | mechanism | reference |
|---|---|---|---|---|
| miR-155 | activation of the immune system | lymphoma | promoting PD-L1 | 45 |
| miR-34 | inducing a tumor immune response | Non-small cell lung cancer | inhibiting PD-L1 | 47 |
| miR-33a | regulating the expression of immune markers | lung adenocarcinoma | suppressing PD-1/PD-L1 | 50 |
| miR-21 | regulating the immune response | gastric cancer | suppressing PD-1 | 53-54 |
| miR-146a | regulating immune cells | melanoma | promoting PD-L1 | 68 |
| miR-873 | regulating the expression of immune markers | breast cancer | inhibiting PD-L1 | 55 |
microRNAs and PD-1/PD-L1
miR155 miR-155 plays a role in malignant hematological diseases, and is overexpressed in several hematopoietic and certain solid cancers. Some of the targets of miR-155 regulate cell growth, invasion, migration and stemness, and inhibit apoptosis [54]. Recent data also indicate that miR-155 plays a key role in the activation of the immune system, as miR-155 inhibition of tumor-related immune cells may promote tumor immune escape and promote tumor growth [55]. Zheng et al found that miR-155 can induce Fas-mediated apoptosis in CD8- T cells in an immune cell/B lymphoma cell co-culture system, and can target anti-PD-1 and anti-PD-L1 antibodies [56]. In addition, miR-155 enhances PD-L1 expression of lymphoma cells, recruits CD8- T cells through PD-1/PD-L1 interaction, and inhibits CD8- T-cell function by dephosphorylating AKT and ERK [57].
miR-34 The members of the miR-34 family have been reported to possess tumor-suppressive properties, such as inhibition of cell migration, invasion, proliferation, survival, EMT and stemness, and drug resistance [58]. A recent study demonstrated the synergy between miR-34a delivery and radiation therapy, which induces an immune response to the tumor through targeting PD-L1, leading to an increase in tumor infiltration by CD8+ cells and a significant reduction in tumor volume compared with either treatment alone [59]. In addition, Cortez et al reported that overexpression of miR-34b or miR34c inhibits the expression of the PD-L1 protein, while MRX34, a liposome nanoparticle possessing miR-34a simulation properties, increased miR34a levels in tumors and reduced PD-L1 mRNA and PD-L1 protein levels in non-small-cell lung cancer (NSCLC) [59].
miR-33a Recent studies demonstrated that miR-33a can regulate the expression of immune markers [60]. Laura et al analyzed the association between the level of expression of PD-1/PD-L1 and miR-33a, and found that miR-33a was negatively correlated with the expression of PD-1/PD-L1. miR-33a levels were found to be lower in patients with higher expression of PD-1, PD-L1 and CTLA4. To assess the association among patient survival, miR-33a and PD-1, patients with lung adenocarcinoma were grouped based on the joint expression of miR-33a and PD-1, and then the differences in survival of the two groups (high miR-33a/low PD-1 and low miR-33a/high PD-1 expression) were compared. The former group (patients with high miR-33a and low PD-1 levels) had a better prognosis, suggesting that miR-33a is a good prognostic marker as a result of PD-1 regulation [61].
miR-21 miR-21 is one of the most abundant miRNAs in mammalian tissues, comprising~10% of total miRNAs in several types of tumor cells, and is a critical factor involved in immune responses [62]. Iliopoulos et al reported that the level of miR-21 was upregulated in PD-1-/- T cells compared with wild-type controls by miRNA analysis of antigen-specific CD4+ T cells [63]. Zheng et al observed that PD-1 inhibition increased miR-21 expression, reducing the percentage of Treg cells, but increasing the percentage of Th17 cells. It was demonstrated that PD-1 is a negative regulator of miR-21, which may affect the Th17/Treg balance in patients following gastric cancer resection [64]. Thus, PD-1 may affect the differentiation of Th17/Treg cells, partially through regulating miR-21 expression in CD4+ T cells.
miR-873 Previous studies have demonstrated that miR-873 acts as a tumor suppressor by inhibiting IGF2BP1 expression in glioblastoma and by targeting the differentiated embryonic chondrocyte expressed gene 2 (DEC2) in esophageal cancer [65]. Recently, Gao et al reported an increase in the expression of several pluripotent transcription factors (OCT4, ALDH1A1, Nanog and SOX2) in MCF-7 cells with inhibitor-873 or plvx-PD-L1 transfection, while co-transfection with inhibitor-873 and si-PD-L1-2 attenuated or even reversed the promoting effects induced by inhibitor-873. By contrast, in MDA-MB-231 cells with miR-873 overexpression or PD-L1 knockdown, the expression of stemness markers was inhibited, while the inhibitory effect of miR-873 was reduced by PD-L1 overexpression. In addition, the transfection of inhibitor-873 or plvx-PD-L1 increases the formation of CD44-/CD24- cell spheres in MCF-7 cells, which can identify stem cells among breast cancer cells, whereas inhibitor-873-mediated promotion through PD-L1 knockdown shows that miR-873 reduces the number of stem cells among breast cancer cells via PD-L1 [66].
miR-146a miR-146a is a major regulator of multiple proinflammatory events, including effects on the TRAF6/TNF axis in T cells and the JAK/STAT/MHC axis in antigen-presenting cells; therefore, it appears to play a key role in the immune cells residing in the melanoma microenvironment [67]. Justin et al demonstrated that miR-146a plays an important role within the STAT1/IFNg axis in the melanoma microenvironment, affecting melanoma cell migration, proliferation and mitochondrial fitness, as well as PD-L1 levels. Additionally, they compared the combination therapy of miR-146a antagomiR with anti-PD-1 vs. anti-PD-1 treatment alone, and isotype with scrambled oligonucleotide controls [68]. Their observations in melanoma-bearing mice indicated improved survival when treated with the miR-146a antagomiR/anti-PD1 combination, suggesting that combined inhibition of PD-1 and miR-146a may be a novel strategy for enhancing antitumor immune response elicited by checkpoint therapy in cancer.
Effects of lncRNAs on PD-1/PD-L1 in cancer
Overview of lncRNAs
Long non-coding RNAs (lncRNAs) are generally defined as endogenous cellular RNA molecules of >200 nucleotides in length that lack an open reading frame of significant length (<100 amino acids) and contain 2 or more exons [69]. They were originally discovered in mice during large-scale sequencing of full-length cDNA libraries as part of the FANTOM project [70]. Currently, there are 16,066 lncRNA genes and 29,566 lncRNA transcripts documented in the human genome database (version 29) of GENCODE, compared with 19,940 protein-coding genes [71]. LncRNAs have also been shown to be subjected to transcriptional editing, such as splicing, polyadenylation and 5’ capping. Subsequently, each lncRNA develops a final stable structure that determines its unique cellular function, enabling it to interact with other molecules [72]. Recent data also reported that lncRNAs play important roles in multiple biological pathways, including modulation of the innate immune response (Table 2).
Table 2.
The effects of LncRNAs on PD-1/PD-L1 in different cancers
| LncRNAs | function | cancer | mechanism | reference |
|---|---|---|---|---|
| AFAP1-AS1 | regulating the immune response | nasopharyngeal carcinoma | promoting PD-L1 | 74 |
| NKX2-1-AS1 | regulating the expression of immune markers | lung cancer | inhibiting PD-L1 | 76 |
| UCA1 | regulating the expression of immune markers | bladder cancer | promoting PD-L1 | 77 |
| SNHG20 | regulating the expression of immune markers | esophageal squamous cell carcinoma | promoting PD-L1 | 79 |
| MALAT1 | regulating the expression of immune markers | diffuse large B cell lymphoma | inhibiting PD-L1 | 82 |
lncRNAs
AFAP1-AS1 The expression of the lncRNA actin filament-associated protein 1 antisense RNA1 (AFAP1-AS1) was significantly enhanced in nasopharyngeal carcinoma (NPC), and was found to promote invasion and metastasis of cancer cells by regulating the expression of several small GTPase family members and molecules in the actin cytokeratin signaling pathway [73]. In addition, Tang et al found that there were 4,196 differentially expressed genes in the GSE12452 database. Among these differentially expressed genes, AFAP1-AS1 was highly expressed in NPC cells and was positively correlated with PD-1 expression. However, it is unclear whether AFAP1-AS1 promotes pD-1 expression, and further studies are required [74].
NKX2-1-AS1 NKX2-1-AS1 is located in the 14q13.3 chromosome region and is amplified in 15% of lung cancer cases [75]. The upregulation of CD274 mRNA and PD-L1 protein was validated in H661 cells by qPCR and cilantro via RT-qPCR in H441 cells, respectively, after NKX2-1-AS1 knockout. In the specimens tested herein and in the publicly available GEO dataset (GDS3627), the reverse trends in tumor sympathising NKX2-1-AS1 and PD-L1 regulation and non-tumor control were observed [76]. These findings suggested that NKX2-1-AS1 inhibits the expression of CD274 (PD-L1) mRNA and protein in lung cancer and high levels of NKX2-1-AS1 are crucial for maintaining low levels of PD-L1 expression, in order to limit the ability of tumor cells to evade the immune system.
UCA1 Recent data demonstrated that gRNA/cas9-targeted UCA1 induces apoptosis of bladder cancer cells, and the downregulation of the PD-1 gene may be achieved by electroporation of gRNA/cas9 targeting PD-1, which was detected by PCR. The combination of anti-PD-1 and anti-UCA1 treatment suppressed tumor growth and effectively improved the survival of xenografted mice. Additionally, this treatment increases the production of T-cell IFN-γ, which is further enhanced by the expression of Th1-related immune stimulation genes to reduce the transcription of regulatory/inhibitory immune genes and to reshape the immunosuppression of tumor micro-environmental stimulation states. Finally, anti-UCA1 treatment was shown to induce IFN-γ-dependent PD-L1 expression in xenograft tumors in vivo [77]. These results demonstrate the efficacy of combination therapy using lncRNA UCA1-targeted therapy and PD-1 immune checkpoint inhibition, thereby supporting this combination for the clinical treatment of bladder cancer.
SNHG20 The small nuclear RNA host gene 20 (SNHG20) at 17q25.2 was first identified in hepatocellular carcinoma (HCC) and has been shown to play an important role in several cancers [78]. Zhang et al demonstrated that SNHG20 modulates the expression of ataxia telangiectasia-mutated kinase (p-ATM), p-JAK1/2, and PD-L1 in esophageal squamous cell carcinoma (ESCC) cells, and observed that the levels of p-ATM, p-JAK1/2 and PD-L1 are downregulated in KYSE450 cells with SNHG20 inhibition but upregulated in Het-1A cells following pcDNA3.1/SNHG20 transfection [79].
lncRNA MALAT1 lncRNA MALAT1 has long been known as a drug target in cancer treatment [80]. MALAT1 promotes EMT and angiogenesis by sponging miR-126-5p in colorectal cancer [81]. In addition, MALAT1, PD-L1 and CD8 were more highly expressed in diffuse large B-cell lymphoma (DLBCL) tissues, while the miR-195 expression levels were lower. The expression of miR-195 is negatively correlated with MALAT1 and PD-L1. MALAT1 can sponge miR-195 to regulate the expression of PD-L1. MALAT1 increases miR-195 levels and lowers PD-L1 levels, indicating that MALAT1 can regulate tumorigenesis and immune escape of DLBCL remained unclear [82].
Function of PD-1/PD-L1 in cancer
Breast cancer
Recent research demonstrated that PD-L1 expression is associated with EMT in human breast cancer stem cells (BCSCs). Compared with the cell line, PD-L1 was found to be upregulated in MCF-7 and BT-549 tumorspheres, which depends in part on PD-L1 promoter demethylation. In addition, the distribution of slow histones in the PD-L1 promoter region is less prominent compared with that in the cell line, and the overexpression of histone acetylase also contributes to the PD-L1 upregulation in the tumor [83]. These findings suggest that PD-L1 plays an important role in BCSCs. Recent pathological studies have revealed that the level of PD-L1 was higher in several subtypes of estrogen receptor (ER) α-negative breast cancer. Lu et al reported that the average PD-L1 mRNA level in ERα-positive breast cancer cell lines was markedly lower compared with that of ERα-negative breast cancer cell lines. Therefore, they investigated PD-L1 expression in the ER-positive breast cancer cell lines MCF7 and T47D and the ER-negative breast cancer cell lines BT549 and MDA-MB-231. RT-qPCR and western blot analysis revealed that the mRNA and protein levels of PD-L1 in MCF7 and T47D cells were markedly lower compared with those in BT549 and MDA-MB-231 cells [84]. Interestingly, recent reports have demonstrated that PD-L1 inhibits the growth of cancer cells, and PD-L1 silencing leads to an increase in spontaneous apoptosis and doxorubicin-induced apoptosis in breast cancer cells [85].
Lung cancer
Daniel et al hypothesized that anti-C5a therapy can enhance the ability of PD-1 to control lung cancer growth. In the subcutaneous 393P model, anti-C5a l-aptamer AON-D21 combined with the anti-PD-1 monoclonal antibody RMP1-14 resulted in more significant suppression of tumor growth compared with the effect of each treatment alone [86]. Kazuki et al demonstrated that PD-L1 was positively associated with male sex, smoking, advanced cancer stage, vascular invasion, squamous cell carcinoma histology, and wild-type epidermal growth factor receptor gene mutations in lung cancer. Univariate and multivariate survival analyses revealed that PD-L1-positive patients had a worse prognosis than PD-L1-negative patients [87]. In addition, recent data reported that the PD-1 molecules on the surface of Lewis lung cancer cells, C57BL/6 mouse spleen T lymphocytes and peripheral blood T lymphocytes were positively expressed. Compared with the control group, the volume of the transplanted tumor from Lewis lung cancer cells in C57BL/6 mice was larger with a 10-μg soluble form of PD-L1 (sPD-L1) injection, whereas no significant difference in tumor volume was observed with a 2.5- and 5-μg injection. Therefore, a certain dose of sPD-L1 was able to promote the growth of Lewis lung cancer cell xenograft tumors in C57BL/6 mice [88].
Colorectal cancer
PD-L1 expression has been associated with poor clinical outcomes. Low levels of differentiation and right-colon CRCs indicated a poor prognosis. The expression of PD-L1 was not associated with sex, age, tumor size, T stage, lymph node metastasis, or TNM stage. A number of inflammatory cytokines regulate the expression of PD-L1, and PD1/PD-L1 binding can induce active T-cell apoptosis and interleukin-10 (IL-10) expression as a negative feedback system [89]. PD-L1 expression can help tumor cells escape the surveillance of the immune system and enhance Treg function in CRC. PD-L1 expression is more common in metastatic CRC, and the expression of PD-L1 in primary CRC may not represent tumors that have spread to distant organs. PD-L1 expression in metastatic CRC should be considered as an independent factor when assessing the patient eligibility for immunotherapy [90].
Gastric cancer
Wu et al observed that PD-1, PD-L1 and PD-L2 mRNA levels were upregulated in gastric cancer tissues. PD-L1 is on the rise in gastric cancer patients infected with EBV in TC (P-0.009) and TIIC. The Hp state is not associated with PD-1 or PD-L1/PD-L2 expression. In TIIC, PD-L1 expression was found to be independently associated with better gastric cancer prognosis. The expression of PD-1 and PD-L1 is a favorable prognostic marker indicating the dose effect on gastric cancer-related mortality. A comprehensive evaluation of PD-1 and PD-L1 in TC and TIICs may help predict the prognosis of gastric cancer and identify patients who may benefit from targeted treatment [91]. Recently, PD-L1 has been reported to be expressed in TAFs. Mu et al found that the mRNA expression of PD-L1 increased significantly in a gastric cancer cell line cultured with TAFs and this effect was time-dependent. By increasing the expression of PD-L1, TAFs promote the growth of gastric cancer cells [92]. Wang et al found that pharmacological inhibitors or small interfering RNA inhibit edphatoe, which can increase PD-L1 levels in cultured gastric cancer cells and xenografts. Autophagy inhibition leads to accumulation of p62/SQSTM1 and activation of NF-κB, whereas NF-κB inhibition or p62/SQSTM1 knockdown attenuates PD-L1 induction by autophagy inhibition [93].
Bladder cancer
PD-L1 expression levels have been shown to be associated with the severity of bladder cancer. It was previously found that tumors with a higher PD-L1 content were more likely to be more malignant, with higher recurrence frequency and lower survival rate [94]. In addition, PD-L1 tumor cell expression is responsible for enhanced resistance to BCG therapy, which is considered to be associated with immune system suppression, as a fully functioning immune system is required for BCG efficacy [95]. Zhu et al reported that the PD-L1 protein is regulated by the ATG7 autophagy protein in bladder cancer (BC), and demonstrated that ATG7 overexpression increased PD-L1 protein levels, mainly by promoting autophagy-mediated degradation of FOXO3a. These results revealed that combination therapy with autophagy inhibitors and PD-1/PD-L1 immune checkpoint inhibitors can improve the efficacy of BCG in humans [96].
Pancreatic cancer
PD-L1 is considered to be a new predictor of prognosis in pancreatic cancer patients. It has been suggested that blocking PD-L1 can effectively suppress pre-established pancreatic cancer in mouse models by increasing IFN-r and reducing IL-10 [97]. In addition, the level of tumor-infiltrating Tregs in PD-L1-positive tumors is higher compared with that in PD-L1-negative tumors. Such outcomes provided the rationale for therapy targeting the PD-1/PD-L1 pathway against pancreatic cancer [98]. Another study demonstrated that the expression of PD-1 on peripheral CD8+ T cells was markedly higher in pancreatic ductal adenocarcinoma (PDAC) patients compared with that in intraductal papillary mucinous neoplasm (IPMN) patients or healthy donors. ROC analysis further confirmed the diagnostic significance of PD-1 expression in PDAC. There is a significant correlation between PD-1 expression on peripheral CD8- T cells and the clinical stage, N classification and M classification of PDAC [99].
Prostate cancer
Currently available studies report that pD-1, PD-L1, and PD-L2 exhibit higher levels of expression than Il-17rc in the prostates of Il-17rc wild-type mice. Il-17rc wild-type mice were found to have more invasive prostate cancer in a PTEN-null background compared with il-17rc knockout mice. This finding indicates that increasing PD-1/PD-L1/2 expression can enhance immune inhibition in the tumor microenvironment, thereby promoting the formation of prostate cancer. PD-L1 is differentially expressed among primary prostate cancer patients. Furthermore, high PD-L1 expression has been associated with a poor prognosis [101]. Accordingly, PD-1/PD-L1-targeted therapy may be a promising novel treatment option for hormone-naïve prostate cancer patients.
DLBCL
PD-L1 is also expressed in DLBCL tumor cells and tumor-infiltrating non-malignant cells. By contrast, PD-1 expression on tumor-infiltrating lymphocytes (TILs) in patients with DLBCL has been associated with favorable overall survival (OS) [102]. Furthermore, high levels of plasma PD-L1 is associated with poor OS in DLBCL. The PD-1/PD-L1 pathway contributes to tumor cell survival, and inhibiting this pathway may be an effective approach to DLBCL treatment. Andorsky et al described a PD-L1+ DLBCL cell line that exhibited properties of non-GCB phenotype (e.g., inhibition of T-cell proliferation and IFN-γ secretion by tumor-associated T cells), suggesting that PD-L1 plays a pivotal role in the DLBCL tumor microenvironment and results in an aggressive clinical phenotype and a worse outcome [103]. PD-L1+ DLBCL is also associated with EBV infection, suggesting that EBV-related DLBCL patients may be suitable candidates for anti-PD-1/PD-L1 immunotherapy.
Inhibitors of PD-1/PD-L1 in cancer
PD-1/PD-L1-targeted inhibitors have been reported to play a critical role in cancers. Here, we summarized several regiments which are essential to improve cancer treatment (Figure 3).
Figure 3.
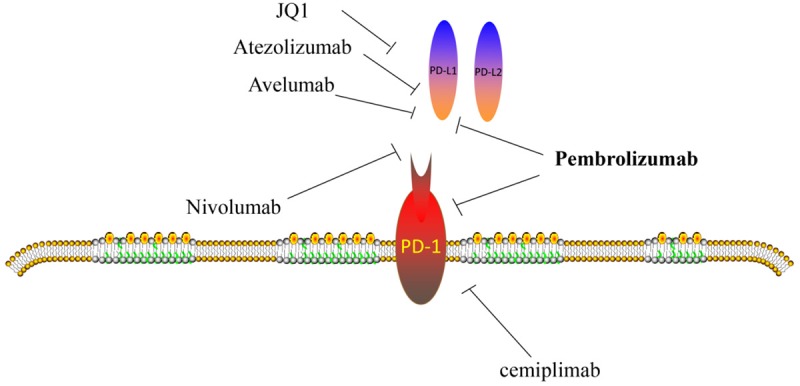
Inhibitors of PD-1/PD-L1 in cancers.
Nivolumab
Nivolumab is a fully human monoclonal antibody that inhibits the interaction between PD-1 and PD-L1. Zhao et al reported the case of a patient with advanced liver carcinosarcoma treated with nivolumab plus apatinib, which resulted in partial remission [104]. However, grade 3 increase of aminotransferase levels occurred during treatment, suggesting that the combination therapy should be recommended only after risk assessment. Nivolumab has been approved for patients with advanced UC who failed to respond to previous platinum-based chemotherapy on the basis of results from the phase 2 CheckMate 275 trial [105]. A key advantage of immunotherapy over the limited chemotherapy options available after platinum-based chemotherapy is the greater durability of treatment benefit, as illustrated by the long-term survival data from multiple studies, including the phase 3 CheckMate 017 (n=272) and CheckMate 057 (n=582) studies of nivolumab vs. docetaxel in patients with previously treated squamous and non-squamous advanced NSCLC, respectively [106]. The estimated 2-year OS rates with nivolumab vs. docetaxel were 23 vs. 8%, respectively, for patients with squamous NSCLC, and 29 vs. 16%, respectively, for those with non-squamous NSCLC; the estimated 3-year OS rate for the combined CheckMate 017 and 057 study populations was 17% with nivolumab vs. 8% with docetaxel.
Pembrolizumab
Joseph et al demonstrated that patients with lung metastases respond to pembrolizumab better compared with those with liver metastases (62 vs. 22%, respectively) [107]. Similarly, patients with lung metastases have a 1-year OS of 89%, in contrast to those with liver metastases who have a 1-year OS rate of 53%. Patients with head and neck recurrence and/or metastatic squamous cell carcinoma (SCCHN) had limited overall survival rates of 6 months or less. Immunotherapy using anti-PD-1 checkpoint inhibitors has led to improved tumor response and survival. For example, pembrolizumab has been shown in the phase Ib KEYNOTE-012 and single-arm phase II KEYNOTE-055 studies to have a response rate of 18% and a median overall survival of 6 to 8 months in treated, recurrent, and metastatic patients [108]. In addition, pembrolizumab plus trastuzumab has been used to treat patients with PD-L1-positive, trastuzumab-resistant, and HER2-positive breast cancer [109].
JQ1
Liu et al studied the effects of JQ1 on PD-L1 mRNA and protein levels in renal cell carcinoma primary culture cells, as well as in prostate, liver and lung cancer cell lines [110]. They found that JQ1 inhibits cell growth in a dose-dependent manner. The expression of PD-L1 decreased in the primary culture of JQ1-treated renal, prostate, liver, and lung cancer cell lines. In addition, they also observed that PD-L2 levels decreased in the JQ1-treated cells. Thereby, JQ1 appears to regulate the PD-1/PD-L1 axis.
Atezolizumab
Atezolizumab is a humanized engineered anti-PD-L1 monoclonal antibody that blocks interactions between PD-L1 and its receptors PD-1 and B7.1, thereby enhancing T-cell mediated anticancer immunity [111]. Atezolizumab monotherapy has been approved for metastatic UC and NSCLC. Recently, atezolizumab was combined with bevacizumab (anti-VEGF) and compared with sunitinib for first-line treatment of ccRCC in the phase 3 IMmotion 151 trial. Studies indicate that atezolizumab/bevacizumab met one of its co-primary endpoints, with improved investigator-assessed PFS versus sunitinib in patients with advanced ccRCC and expression of PD-L1 on ≥1% of tumor-infiltrating immune cells via immunohistochemistry [112].
Avelumab
Avelumab is a human IgG1 monoclonal antibody PD-L1 inhibitor. In the phase 1 b trial (JAVELIN Solid Tumor) investigating avelumab in previously treated patients with metastatic or recurrent NSCLC, PD-L1 expression on tumor and immune cells was attenuated. Preliminary data from a single-group, nonrandomized, phase 1 b trial involving 55 patients with advanced renal-cell carcinoma showed that avelumab in combination with axitinib can lead to effective responses in 58% of patients and a rate of disease control of 78% [113].
Cemiplimab
Inhibitors of the PD-1 have generated promising outcomes in advanced Cutaneous Squamous Cell Carcinoma (cSCC). Cemiplimab binds to PD-1 and inhibits its interaction with PD-L1 and PD-L2 by acting as a human programmed death receptor-1 monoclonal antibody. In September 2018, the FDA approved cemiplimab to treat patients with metastatic or locally advanced cSCC who are not candidates for surgery or radiotherapy [114].
Conclusion
Taken together, PD-1/PD-L1 plays a vital role in most cancers, making it a key area for further research. From the data described, PD-1/PD-L1 is an opportunity and challenge for cancer treatment. A large number of solid cancers exhibit higher expression of PD-1/PD-L1, PD-1/PD-L1-targeted inhibitors will be responsible for cancer treatment. Immunotherapy is a promising treatment that may lead to further survival benefits for some patients. The advantages of immunotherapy based on PD-1/PD-L1 blockades for various tumors appear to be the logical next step. Although, there are a great number of unknowns, including dose, safety, durability, and indeed efficacy.
Acknowledgements
We apologize to the many authors whose studies are important but could not be cited due to space limitation. We are grateful for members of pathology department for their discussion and suggestion during the course of this study.
Disclosure of conflict of interest
None.
References
- 1.Sameiyan E, Hayes AW, Karimi G. The effect of medicinal plants on multiple drug resistance through autophagy: a review of in vitrostudies. Eur J Pharmacol. 2019;852:244–253. doi: 10.1016/j.ejphar.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Seebacher N, Shi H, Kan Q, Duan Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget. 2017;8:84559–84571. doi: 10.18632/oncotarget.19187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, Sahebkar A. PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234:16824–16837. doi: 10.1002/jcp.28358. [DOI] [PubMed] [Google Scholar]
- 4.Ljunggren HG, Jonsson R, Hoglund P. Seminal immunologic discoveries with direct clinical implications: the 2018 nobel prize in physiology or medicine honours discoveries in cancer immunotherapy. Scand J Immunol. 2018;88:e12731. doi: 10.1111/sji.12731. [DOI] [PubMed] [Google Scholar]
- 5.Messenheimer DJ, Jensen SM, Afentoulis ME, Wegmann KW, Feng Z, Friedman DJ, Gough MJ, Urba WJ, Fox BA. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin Cancer Res. 2017;23:6165–6177. doi: 10.1158/1078-0432.CCR-16-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24:26. doi: 10.1186/s12929-017-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol. 2016;2:1217–1222. doi: 10.1001/jamaoncol.2016.0639. [DOI] [PubMed] [Google Scholar]
- 8.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 10.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ, Cui G, Li MO, Kaech SM. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8+ T cells during chronic infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Li W, Xiao J, Jiao S, Teng F, Xue S, Zhang C, Sheng C, Leng Q, Rudd CE, Wei B, Wang H. ADAP and SKAP55 deficiency suppresses PD-1 expression in CD8+ cytotoxic T lymphocytes for enhanced anti-tumor immunotherapy. EMBO Mol Med. 2015;7:754–769. doi: 10.15252/emmm.201404578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youngblood B, Oestreich KJ, Ha SJ, Duraiswamy J, Akondy RS, West EE, Wei Z, Lu P, Austin JW, Riley JL, Boss JM, Ahmed R. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity. 2011;35:400–412. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao G, Deng A, Liu H, Ge G, Liu X. Activator protein 1 suppresses antitumor T-cell function via the induction of programmed death 1. Proc Natl Acad Sci U S A. 2012;109:15419–15424. doi: 10.1073/pnas.1206370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmaninejad A, Khoramshahi V, Azani A, Soltaninejad E, Aslani S, Zamani MR, Zal M, Nesaei A, Hosseini SM. PD-1 and cancer: molecular mechanisms and polymorphisms. Immunogenetics. 2018;70:73–86. doi: 10.1007/s00251-017-1015-5. [DOI] [PubMed] [Google Scholar]
- 17.Sanmamed MF, Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 2014;20:256–261. doi: 10.1097/PPO.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 19.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji M, Liu Y, Li Q, Li XD, Zhao WQ, Zhang H, Zhang X, Jiang JT, Wu CP. PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J Transl Med. 2015;13:5. doi: 10.1186/s12967-014-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501–1509. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellucci R, Martin A, Bommarito D, Wang K, Hansen SH, Freeman GJ, Ritz J. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology. 2015;4:e1008824. doi: 10.1080/2162402X.2015.1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19:1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong P, Xiong Y, Yue J, Hanley SJB, Watari H. Tumor-intrinsic PD-L1 signaling in cancer initiation, development and treatment: beyond immune evasion. Front Oncol. 2018;8:386. doi: 10.3389/fonc.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunes-Xavier CE, Angulo JC, Pulido R, López JI. A critical insight into the clinical translation of PD-1/PD-L1 blockade therapy in clear cell renalcell carcinoma. Curr Urol Rep. 2019;20:1. doi: 10.1007/s11934-019-0866-8. [DOI] [PubMed] [Google Scholar]
- 26.Sharma VR, Gupta GK, Sharma AK, Batra N, Sharma DK, Joshi A, Sharma AK. PI3K/Akt/mTOR intracellular pathway and breast cancer: factors, mechanism and regulation. Curr Pharm Des. 2017;23:1633–1638. doi: 10.2174/1381612823666161116125218. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell JS, Massi D, Teng MWL, Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91–103. doi: 10.1016/j.semcancer.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Gowrishankar K, Gunatilake D, Gallagher SJ, Tiffen J, Rizos H, Hersey P. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-κB. PLoS One. 2015;10:1–19. doi: 10.1371/journal.pone.0123410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409–416. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 30.Zhao R, Song Y, Wang Y, Huang Y, Li Z, Cui Y, Yi M, Xia L, Zhuang W, Wu X, Zhou Y. PD-1/PD-L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif. 2019;52:e12571. doi: 10.1111/cpr.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei F, Zhang T, Deng SC, Wei JC, Yang P, Wang Q, Chen ZP, Li WL, Chen HC, Hu H, Cao J. PD-L1 promotes colorectal cancer stem cell expansion by activating HMGA1-dependent signaling pathways. Cancer Lett. 2019;450:1–13. doi: 10.1016/j.canlet.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Peng Q, Deng Z, Pan H, Gu L, Liu O, Tang Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol Lett. 2018;15:1379–1388. doi: 10.3892/ol.2017.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pradhan R, Singhvi G, Dubey SK, Gupta G, Dua K. MAPK pathway: a potential target for the treatment of non-small-cell lung carcinoma. Future Med Chem. 2019;11:793–795. doi: 10.4155/fmc-2018-0468. [DOI] [PubMed] [Google Scholar]
- 34.Stutvoet TS, Kol A, de Vries EG, de Bruyn M, Fehrmann RS, Terwisscha van Scheltinga AG, de Jong S. MAPK pathway activity plays a key role in PD-L1 expression of lung adenocarcinoma cells. J Pathol. 2019;249:52–64. doi: 10.1002/path.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jalali S, Price-Troska T, Bothun C, Villasboas J, Kim HJ, Yang ZZ, Novak AJ, Dong H, Ansell SM. Reverse signaling via PD-L1 supports malignant cell growth and survival in classical Hodgkin lymphoma. Blood Cancer J. 2019;9:22. doi: 10.1038/s41408-019-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groner B, von Manstein V. Jak Stat signaling and cancer: opportunities, benefits and side effects of targeted inhibition. Mol Cell Endocrinol. 2017;451:1–14. doi: 10.1016/j.mce.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Doi T, Ishikawa T, Okayama T, Oka K, Mizushima K, Yasuda T, Sakamoto N, Katada K, Kamada K, Uchiyama K, Handa O, Takagi T, Naito Y, Itoh Y. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancercell lines. Oncol Rep. 2017;37:1545–1554. doi: 10.3892/or.2017.5399. [DOI] [PubMed] [Google Scholar]
- 39.Li P, Huang T, Zou Q, Liu D, Wang Y, Tan X, Wei Y, Qiu H. FGFR2 promotes expression of PD-L1 in colorectal cancer via the JAK/STAT3 signaling pathway. J Immunol. 2019;202:3065–3075. doi: 10.4049/jimmunol.1801199. [DOI] [PubMed] [Google Scholar]
- 40.Harb J, Lin PJ, Hao J. Recent development of WNT signaling pathway inhibitors for cancer therapeutics. Curr Oncol Rep. 2019;21:12. doi: 10.1007/s11912-019-0763-9. [DOI] [PubMed] [Google Scholar]
- 41.Galluzzi L, Spranger S, Fuchs E, López-Soto A. WNT signaling in cancer immunosurveillance. Trends Cell Biol. 2019;29:44–65. doi: 10.1016/j.tcb.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor A, Rothstein D, Rudd CE. Small-molecule inhibition of PD-1 transcription is an effective alternative to antibody blockade in cancer therapy. Cancer Res. 2018;78:706–717. doi: 10.1158/0008-5472.CAN-17-0491. [DOI] [PubMed] [Google Scholar]
- 43.Castagnoli L, Cancila V, Cordoba-Romero SL, Faraci S, Talarico G, Belmonte B, Iorio MV, Milani M, Volpari T, Chiodoni C, Hidalgo-Miranda A, Tagliabue E, Tripodo C, Sangaletti S, Di Nicola M, Pupa SM. WNT signaling modulates PD-L1 expression in the stem cell compartment of triple-negative breast cancer. Oncogene. 2019;38:4047–4060. doi: 10.1038/s41388-019-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim W, Jeong M, Bazer FW, Song G. Curcumin Suppresses proliferation and migration and induces apoptosis on human placental choriocarcinoma cells via ERK1/2 and SAPK/JNK MAPK signaling pathways. Biol Reprod. 2016;95:83. doi: 10.1095/biolreprod.116.141630. [DOI] [PubMed] [Google Scholar]
- 45.Bi XW, Wang H, Zhang WW, Wang JH, Liu WJ, Xia ZJ, Huang HQ, Jiang WQ, Zhang YJ, Wang L. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9:109. doi: 10.1186/s13045-016-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Murphy SK, Konishi I, Mandai M. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–5045. doi: 10.1158/0008-5472.CAN-14-3098. [DOI] [PubMed] [Google Scholar]
- 47.Wu F, Zhang Y, Sun B, McMahon AP, Wang Y. Hedgehog signaling: from basic biology to cancer therapy. Cell Chem Biol. 2017;24:252–280. doi: 10.1016/j.chembiol.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin AM, Nirschl CJ, Polanczyk MJ, Bell WR, Nirschl TR, Harris-Bookman S, Phallen J, Hicks J, Martinez D, Ogurtsova A, Xu H, Sullivan LM, Meeker AK, Raabe EH, Cohen KJ, Eberhart CG, Burger PC, Santi M, Taube JM, Pardoll DM, Drake CG, Lim M. PD-L1 expression in medulloblastoma: an evaluation by subgroup. Oncotarget. 2018;9:19177–19191. doi: 10.18632/oncotarget.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakrabarti J, Holokai L, Syu L, Steele NG, Chang J, Wang J, Ahmed S, Dlugosz A, Zavros Y. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. Oncotarget. 2018;9:37439–37457. doi: 10.18632/oncotarget.26473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Catela Ivkovic T, Voss G, Cornella H, Ceder Y. microRNAs as cancer therapeutics: a step closer to clinical application. Cancer Lett. 2017;407:113–122. doi: 10.1016/j.canlet.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braicu C, Zimta AA, Harangus A, Iurca I, Irimie A, Coza O, Berindan-Neagoe I. The function of non-coding RNAs in lung cancer tumorigenesis. Cancers (Basel) 2019;11 doi: 10.3390/cancers11050605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iqbal MA, Arora S, Prakasam G, Calin GA, Syed MA. MicroRNA in lung cancer: role, mechanisms, pathways and therapeutic relevance. Mol Aspects Med. 2019;70:3–20. doi: 10.1016/j.mam.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Bayraktar R, Van Roosbroeck K. miR-155 in cancer drug resistance and as target for miRNA-based therapeutics. Cancer Metastasis Rev. 2018;37:33–44. doi: 10.1007/s10555-017-9724-7. [DOI] [PubMed] [Google Scholar]
- 55.Mashima R. Physiological roles of miR-155. Immunology. 2015;145:323–333. doi: 10.1111/imm.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng Z, Sun R, Zhao HJ, Fu D, Zhong HJ, Weng XQ, Qu B, Zhao Y, Wang L, Zhao WL. MiR155 sensitized B-lymphoma cells to anti-PD-L1 antibody via PD-1/PD-L1-mediated lymphoma cell interaction with CD8+ T cells. Mol Cancer. 2019;18:54. doi: 10.1186/s12943-019-0977-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767–1778. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Zhao H, Zhou X, Song L. Inhibition of lactate dehydrogenase A by microRNA-34a resensitizes colon cancer cells to 5-fluorouracil. Mol Med Rep. 2015;11:577–582. doi: 10.3892/mmr.2014.2726. [DOI] [PubMed] [Google Scholar]
- 59.Cortez MA, Ivan C, Valdecanas D, Wang X, Peltier HJ, Ye Y, Araujo L, Carbone DP, Shilo K, Giri DK, Kelnar K, Martin D, Komaki R, Gomez DR, Krishnan S, Calin GA, Bader AG, Welsh JW. PDL1 regulation by p53 via miR-34. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang M, Li Y, Zhao Y, He S, Shi J. miR-33a inhibits cell proliferation and invasion by targeting CAND1 in lung cancer. Clin Transl Oncol. 2018;20:457–466. doi: 10.1007/s12094-017-1730-2. [DOI] [PubMed] [Google Scholar]
- 61.Boldrini L, Giordano M, Niccoli C, Melfi F, Lucchi M, Mussi A, Fontanini G. Role of microRNA-33a in regulating the expression of PD-1 in lung adenocarcinoma. Cancer Cell Int. 2017;17:105. doi: 10.1186/s12935-017-0474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfeffer SR, Yang CH, Pfeffer LM. The role of miR-21 in cancer. Drug Dev Res. 2015;76:270–277. doi: 10.1002/ddr.21257. [DOI] [PubMed] [Google Scholar]
- 63.Iliopoulos D, Kavousanaki M, Ioannou M, Boumpas D, Verginis P. The negative costimulatory molecule PD-1 modulates the balance between immunity and tolerance via miR-21. Eur J Immunol. 2011;41:1754–1763. doi: 10.1002/eji.201040646. [DOI] [PubMed] [Google Scholar]
- 64.Zheng X, Dong L, Wang K, Zou H, Zhao S, Wang Y, Wang G. MiR-21 participates in the PD-1/PD-L1 pathway-mediated imbalance of Th17/Treg cells in patients after gastric cancer resection. Ann Surg Oncol. 2019;26:884–893. doi: 10.1245/s10434-018-07117-6. [DOI] [PubMed] [Google Scholar]
- 65.Wang RJ, Li JW, Bao BH, Wu HC, Du ZH, Su JL, Zhang MH, Liang HQ. MicroRNA-873 (miRNA-873) inhibits glioblastoma tumorigenesis and metastasis by suppressing the expression of IGF2BP1. J Biol Chem. 2015;290:8938–8948. doi: 10.1074/jbc.M114.624700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao L, Guo Q, Li X, Yang X, Ni H, Wang T, Zhao Q, Liu H, Xing Y, Xi T, Zheng L. MiR-873/PD-L1 axis regulates the stemness of breast cancer cells. EBioMedicine. 2019;41:395–407. doi: 10.1016/j.ebiom.2019.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stickel N, Hanke K, Marschner D, Prinz G, Kohler M, Melchinger W, Pfeifer D, Schmitt-Graeff A, Brummer T, Heine A, Brossart P, Wolf D, von Bubnoff N, Finke J, Duyster J, Ferrara J, Salzer U, Zeiser R. MicroRNA-146a reduces MHC-II expression via targeting JAK/STAT signaling in dendritic cells after stem cell transplantation. Leukemia. 2017;31:2732–2741. doi: 10.1038/leu.2017.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mastroianni J, Stickel N, Andrlova H, Hanke K, Melchinger W, Duquesne S, Schmidt D, Falk M, Andrieux G, Pfeifer D, Dierbach H, Schmitt-Graeff A, Meiss F, Boerries M, Zeiser R. miR-146a controls immune response in the melanoma microenvironment. Cancer Res. 2019;79:183–195. doi: 10.1158/0008-5472.CAN-18-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, Nikaido I, Osato N, Saito R, Suzuki H, Yamanaka I, Kiyosawa H, Yagi K, Tomaru Y, Hasegawa Y, Nogami A, Schonbach C, Gojobori T, Baldarelli R, Hill DP, Bult C, Hume DA, Quackenbush J, Schriml LM, Kanapin A, Matsuda H, Batalov S, Beisel KW, Blake JA, Bradt D, Brusic V, Chothia C, Corbani LE, Cousins S, Dalla E, Dragani TA, Fletcher CF, Forrest A, Frazer KS, Gaasterland T, Gariboldi M, Gissi C, Godzik A, Gough J, Grimmond S, Gustincich S, Hirokawa N, Jackson IJ, Jarvis ED, Kanai A, Kawaji H, Kawasawa Y, Kedzierski RM, King BL, Konagaya A, Kurochkin IV, Lee Y, Lenhard B, Lyons PA, Maglott DR, Maltais L, Marchionni L, McKenzie L, Miki H, Nagashima T, Numata K, Okido T, Pavan WJ, Pertea G, Pesole G, Petrovsky N, Pillai R, Pontius JU, Qi D, Ramachandran S, Ravasi T, Reed JC, Reed DJ, Reid J, Ring BZ, Ringwald M, Sandelin A, Schneider C, Semple CA, Setou M, Shimada K, Sultana R, Takenaka Y, Taylor MS, Teasdale RD, Tomita M, Verardo R, Wagner L, Wahlestedt C, Wang Y, Watanabe Y, Wells C, Wilming LG, Wynshaw-Boris A, Yanagisawa M, Yang I, Yang L, Yuan Z, Zavolan M, Zhu Y, Zimmer A, Carninci P, Hayatsu N, Hirozane-Kishikawa T, Konno H, Nakamura M, Sakazume N, Sato K, Shiraki T, Waki K, Kawai J, Aizawa K, Arakawa T, Fukuda S, Hara A, Hashizume W, Imotani K, Ishii Y, Itoh M, Kagawa I, Miyazaki A, Sakai K, Sasaki D, Shibata K, Shinagawa A, Yasunishi A, Yoshino M, Waterston R, Lander ES, Rogers J, Birney E, Hayashizaki Y FANTOM Consortium; RIKEN Genome Exploration Research Group Phase I & II Team. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–573. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- 71.Gencode. GENCODE. Statistics about the Current GENCODE Release (version 29) Available online: https://www.gencodegenes.org/human/stats.html. 2019.
- 72.Blythe AJ, Fox AH, Bond CS. The ins and outs of lncRNA structure: how, why and what comes next? Biochim Biophys Acta. 2016;1859:46–58. doi: 10.1016/j.bbagrm.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Bo H, Gong Z, Zhang W, Li X, Zeng Y, Liao Q, Chen P, Shi L, Lian Y, Jing Y, Tang K, Li Z, Zhou Y, Zhou M, Xiang B, Li X, Yang J, Xiong W, Li G, Zeng Z. Upregulated long non-coding RNA AFAP1-AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget. 2015;6:20404–20418. doi: 10.18632/oncotarget.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang Y, He Y, Shi L, Yang L, Wang J, Lian Y, Fan C, Zhang P, Guo C, Zhang S, Gong Z, Li X, Xiong F, Li X, Li Y, Li G, Xiong W, Zeng Z. Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma. Oncotarget. 2017;8:39001–39011. doi: 10.18632/oncotarget.16545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braicu C, Zimta AA, Harangus A, Iurca I, Irimie A, Coza O, Berindan-Neagoe I. The function of non-coding rnas in lung cancer tumorigenesis. Cancers (Basel) 2019;11 doi: 10.3390/cancers11050605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kathuria H, Millien G, McNally L, Gower AC, Tagne JB, Cao Y, Ramirez MI. NKX2-1-AS1 negatively regulates CD274/PD-L1, cell-cell interaction genes, and limits human lung carcinoma cell migration. Sci Rep. 2018;8:14418. doi: 10.1038/s41598-018-32793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhen S, Lu J, Chen W, Zhao L, Li X. Synergistic antitumor effect on bladder cancer by rational combination of programmed cell death 1 blockade and crispr-cas9-mediated long non-coding RNA urothelial carcinoma associated 1 knockout. Hum Gene Ther. 2018;29:1352–1363. doi: 10.1089/hum.2018.048. [DOI] [PubMed] [Google Scholar]
- 78.Zhang D, Cao C, Liu L, Wu D. Up-regulation of LncRNA SNHG20 predicts poor prognosis in hepatocellular carcinoma. J Cancer. 2016;7:608–617. doi: 10.7150/jca.13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang C, Jiang F, Su C, Xie P, Xu L. Upregulation of long noncoding RNA SNHG20 promotes cell growth and metastasis in esophageal squamous cell carcinoma via modulating ATM-JAK-PD-L1 pathway. J Cell Biochem. 2019 doi: 10.1002/jcb.28444. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 80.Arun G, Spector DL. MALAT1 long non-coding RNA and breast cancer. RNA Biol. 2019;16:860–863. doi: 10.1080/15476286.2019.1592072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Z, Ou C, Liu J, Chen C, Zhou Q, Yang S, Li G, Wang G, Song J, Li Z, Zhang Z, Yuan W, Li X. YAP1-induced MALAT1 promotes epithelial-mesenchymal transition and angiogenesis by sponging miR-126-5p in colorectal cancer. Oncogene. 2019;38:2627–2644. doi: 10.1038/s41388-018-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Wang QM, Lian GY, Song Y, Huang YF, Gong Y. LncRNA MALAT1 promotes tumorigenesis and immune escape of diffuse large B cell lymphoma by sponging miR-195. Life Sci. 2019;231:116335. doi: 10.1016/j.lfs.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 83.Darvin P, Sasidharan Nair V, Elkord E. PD-L1 expression in human breast cancer stem cells is epigenetically regulated through posttranslational histone modifications. J Oncol. 2019;2019:3958908. doi: 10.1155/2019/3958908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu L, Shen Y, Zhu X, Lv R, Li S, Zhang Z, Shi YG, Tan L. ERalpha is a negative regulator of PD-L1 gene transcription in breast cancer. Biochem Biophys Res Commun. 2018;505:157–161. doi: 10.1016/j.bbrc.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Liu S, Chen S, Yuan W, Wang H, Chen K, Li D, Li D. PD-1/PD-L1 interaction up-regulates MDR1/P-gp expression in breast cancer cells via PI3K/AKT and MAPK/ERK pathways. Oncotarget. 2017;8:99901–99912. doi: 10.18632/oncotarget.21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ajona D, Ortiz-Espinosa S, Moreno H, Lozano T, Pajares MJ, Agorreta J, Bertolo C, Lasarte JJ, Vicent S, Hoehlig K, Vater A, Lecanda F, Montuenga LM, Pio R. A combined PD-1/C5a blockade synergistically protects against lung cancer growth and metastasis. Cancer Discov. 2017;7:694–703. doi: 10.1158/2159-8290.CD-16-1184. [DOI] [PubMed] [Google Scholar]
- 87.Takada K, Toyokawa G, Okamoto T, Shimokawa M, Kozuma Y, Matsubara T, Haratake N, Akamine T, Takamori S, Katsura M, Shoji F, Oda Y, Maehara Y. A comprehensive analysis of programmed cell death Ligand-1 expression with the clone SP142 antibody in non-small-cell lung cancer patients. Clin Lung Cancer. 2017;18:572–582. e571. doi: 10.1016/j.cllc.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Yang KN, Han W, Qin YJ, Chen LN. Effects of different levels of soluble PD-L1 protein on the growth of Lewis lung cancer transplanted tumor. J Biol Regul Homeost Agents. 2019;33:537–542. [PubMed] [Google Scholar]
- 89.Li Y, He M, Zhou Y, Yang C, Wei S, Bian X, Christopher O, Xie L. The prognostic and clinicopathological roles of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis. Front Pharmacol. 2019;10:139. doi: 10.3389/fphar.2019.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang HB, Yao H, Li CS, Liang LX, Zhang Y, Chen YX, Fang JY, Xu J. Rise of PD-L1 expression during metastasis of colorectal cancer: implications for immunotherapy. J Dig Dis. 2017;18:574–581. doi: 10.1111/1751-2980.12538. [DOI] [PubMed] [Google Scholar]
- 91.Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, Liu X. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:661–674. doi: 10.1016/j.csbj.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mu L, Yu W, Su H, Lin Y, Sui W, Yu X, Qin C. Relationship between the expressions of PD-L1 and tumour-associated fibroblasts in gastric cancer. Artif Cells Nanomed Biotechnol. 2019;47:1036–1042. doi: 10.1080/21691401.2019.1573741. [DOI] [PubMed] [Google Scholar]
- 93.Wang X, Wu WKK, Gao J, Li Z, Dong B, Lin X, Li Y, Li Y, Gong J, Qi C, Peng Z, Yu J, Shen L. Autophagy inhibition enhances PD-L1 expression in gastric cancer. J Exp Clin Cancer Res. 2019;38:140. doi: 10.1186/s13046-019-1148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 2017;54:58–67. doi: 10.1016/j.ctrv.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 96.Zhu J, Li Y, Luo Y, Xu J, Liufu H, Tian Z, Huang C, Li J, Huang C. A feedback loop formed by ATG7/Autophagy, FOXO3a/miR-145 and PD-L1 regulates stem-like properties and invasion in human bladder cancer. Cancers (Basel) 2019;11 doi: 10.3390/cancers11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okudaira K, Hokari R, Tsuzuki Y, Okada Y, Komoto S, Watanabe C, Kurihara C, Kawaguchi A, Nagao S, Azuma M, Yagita H, Miura S. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35:741–749. doi: 10.3892/ijo_00000387. [DOI] [PubMed] [Google Scholar]
- 98.Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, Laschinger M, W Büchler M, Friess H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 99.Masugi Y, Abe T, Ueno A, Fujii-Nishimura Y, Ojima H, Endo Y, Fujita Y, Kitago M, Shinoda M, Kitagawa Y, Sakamoto M. Characterization of spatial distribution of tumor-infiltrating CD8(+) T cells refines their prognostic utility for pancreatic cancer survival. Mod Pathol. 2019;32:1495–1507. doi: 10.1038/s41379-019-0291-z. [DOI] [PubMed] [Google Scholar]
- 100.Yang S, Zhang Q, Liu S, Wang AR, You Z. PD-1, PD-L1 and PD-L2 expression in mouse prostate cancer. Am J Clin Exp Urol. 2016;4:1–8. [PMC free article] [PubMed] [Google Scholar]
- 101.Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, Majores M, Stein J, Uhl B, Muller S, Ellinger J, Stephan C, Jung K, Brossart P, Kristiansen G. The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin Cancer Res. 2016;22:1969–1977. doi: 10.1158/1078-0432.CCR-15-2042. [DOI] [PubMed] [Google Scholar]
- 102.Chen BJ, Dashnamoorthy R, Galera P, Makarenko V, Chang H, Ghosh S, Evens AM. The immune checkpoint molecules PD-1, PD-L1, TIM-3 and LAG-3 in diffuse large B-cell lymphoma. Oncotarget. 2019;10:2030–2040. doi: 10.18632/oncotarget.26771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17:4232–4244. doi: 10.1158/1078-0432.CCR-10-2660. [DOI] [PubMed] [Google Scholar]
- 104.Zhao L, Yang Y, Gao Q. Efficacy and safety of nivolumab plus apatinib in advanced liver carcinosarcoma: a case report. Immunotherapy. 2019;11:651–656. doi: 10.2217/imt-2018-0214. [DOI] [PubMed] [Google Scholar]
- 105.Hussain SA, Birtle A, Crabb S, Huddart R, Small D, Summerhayes M, Jones R, Protheroe A. From clinical trials to real-life clinical practice: the role of immunotherapy with PD-1/PD-L1 inhibitors in advanced urothelial carcinoma. Eur Urol Oncol. 2018;1:486–500. doi: 10.1016/j.euo.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 106.Schiwitza A, Schildhaus HU, Zwerger B, Ruschoff J, Reinhardt C, Leha A, Andreas S, Rittmeyer A. Monitoring efficacy of checkpoint inhibitor therapy in patients with non-small-cell lung cancer. Immunotherapy. 2019;11:769–782. doi: 10.2217/imt-2019-0039. [DOI] [PubMed] [Google Scholar]
- 107.Joseph RW, Elassaiss-Schaap J, Kefford R, Hwu WJ, Wolchok JD, Joshua AM, Ribas A, Hodi FS, Hamid O, Robert C, Daud A, Dronca R, Hersey P, Weber JS, Patnaik A, de Alwis DP, Perrone A, Zhang J, Kang SP, Ebbinghaus S, Anderson KM, Gangadhar TC. Correction: baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res. 2018;24:6098. doi: 10.1158/1078-0432.CCR-18-3340. [DOI] [PubMed] [Google Scholar]
- 108.Pelster MS, Mott F, Lewin J. Pembrolizumab-induced mucositis in a patient with recurrent hypopharynx squamous cell cancer. Laryngoscope. 2019 doi: 10.1002/lary.28038. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 109.Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, Bartsch R, Rabaglio-Poretti M, Kammler R, Maibach R, Smyth MJ, Di Leo A, Colleoni M, Viale G, Regan MM, André F International Breast Cancer Study Group and the Breast International Group. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019;20:371–382. doi: 10.1016/S1470-2045(18)30812-X. [DOI] [PubMed] [Google Scholar]
- 110.Liu K, Zhou Z, Gao H, Yang F, Qian Y, Jin H, Guo Y, Liu Y, Li H, Zhang C, Guo J, Wan Y, Chen R. JQ1, a BET-bromodomain inhibitor, inhibits human cancer growth and suppresses PD-L1 expression. Cell Biol Int. 2019;43:642–650. doi: 10.1002/cbin.11139. [DOI] [PubMed] [Google Scholar]
- 111.Crist M, Balar A. Atezolizumab in invasive and metastatic urothelial carcinoma. Expert Rev Clin Pharmacol. 2017;10:1295–1301. doi: 10.1080/17512433.2017.1389275. [DOI] [PubMed] [Google Scholar]
- 112.Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev. 2018;70:127–137. doi: 10.1016/j.ctrv.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 113.Lantuejoul S, Damotte D, Hofman V, Adam J. Programmed death ligand 1 immunohistochemistry in non-small cell lung carcinoma. J Thorac Dis. 2019;11:S89–S101. doi: 10.21037/jtd.2018.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ogata D, Tsuchida T. Systemic immunotherapy for advanced cutaneous squamous cell carcinoma. Curr Treat Options Oncol. 2019;20:30. doi: 10.1007/s11864-019-0629-2. [DOI] [PubMed] [Google Scholar]


