Short abstract
Objective
Acute hematogenous osteomyelitis (AHO) has declining incidence in developed countries. AHO can cause rapid destruction of bone that can lead to functional impairment or even death if misdiagnosed and not treated urgently. In this study, we aimed to identify the main factors that may lead to a poor outcome and to establish a profile of patients with AHO who might have a negative outcome.
Methods
We conducted a retrospective single-center study including 94 patients with AHO, over a 10-year interval. Complete medical history including age at diagnosis, sex, socioeconomic status, symptoms, entry portal, pathogenic agent, location of infection, radiological features, treatment, and outcome were recorded.
Results
Male predominance was observed, with boys from rural areas more prone to a poor outcome. This is likely because they are more exposed to trauma and do not have proper access to emergency medical care. Staphylococcus aureus was the most common etiologic agent, with 84 patients testing positive. Disease evolution was toward chronicity in patients diagnosed late. The most frequent complications were sepsis and pathological fractures.
Conclusions
In our study, patients with negative outcomes were characterized by young age, repeated negative cultures, delayed surgery, rural residence, and male sex.
Keywords: Acute hematogenous osteomyelitis, pediatric orthopedics, bone infection, outcome, patient profile, Staphylococcus aureus
Introduction
Acute osteomyelitis is an infection of the bone and marrow caused by bacteria. Worldwide incidence ranges between 1/1000 to 1/20,000 population, with 50% of cases occurring in children younger than 5 years of age.1–3 A study conducted in Glasgow, Scotland showed an incidence decline of 44% from 1970 to 1990 and a 50% decline between 1990 and 1997; that study was carried out in a population of children younger than 13 years of age.4,5 The incidence of pediatric acute hematogenous osteomyelitis (AHO) has been continuously decreasing in developed countries. As shown by Street et al. (2015)6 in a 10-year retrospective study performed in two hospitals of New Zealand, the incidence was 1 in 4000 children, with a predominance of male patients.
The most common pathogen involved in AHO is Staphylococcus aureus, present in 70% to 90% of AHO infections in children. Other pathogens responsible for AHO are streptococci, Staphylococcus epidermidis, enterococci, Escherichia coli, group A β-hemolytic Streptococcus (GABHS), Haemophilus influenzae, anaerobic organisms, and fungi.7–9
Bacterial dissemination in AHO can be hematogenous, contiguous (infection originating from soft tissues that spreads to the bone), or via direct inoculation (open fractures, puncture wounds, prostheses).10 Risk factors include trauma, sepsis, bacteremia, chronic catheterization or indwelling vascular lines, and immunodeficiency.11
AHO most frequently affects the metaphyseal region of long bones (27% in the femur, 22% the tibia, 5% fibula, 12% humerus, 4% radius, and 3% the ulna).12,13 The rich metaphyseal blood supply, with vascular loops and turbulent flow, facilitates bacterial colonization.14,15 Bacterial exponential growth leads to bone resorption. A purulent exudate forms, which may exit the metaphyseal cortex, creating a subperiosteal abscess.9,16
Acute osteomyelitis must be considered whenever a child has a fever and localized bone pain. The localization of pain can lead to a difficult diagnosis, as this might be reported as abdominal or back pain. Local examination reveals erythema, swelling, warmth, or other skin changes. These signs of inflammation may appear when the infection has progressed through the metaphyseal cortex into the subperiosteal space.17,18
The treatment of AHO is long-lasting and complex. It consists of appropriate antimicrobial therapy and may require surgical incision and drainage, and debridement for removal of all infective and necrotic material.17 Cast immobilization can be used to reduce muscular activity, dissemination, inflammation, decrease pain, and prevent complications.
The objective of the present study was to identify the main factors that may lead to a poor outcome and to establish a profile of patients with AHO who might have a negative outcome.
Methods
We conducted a retrospective single-center study, evaluating data from the medical records of 94 patients with AHO over a 10-year interval. The study was approved by the Ethics Board of our institution. Patient consent was obtained verbally when reevaluation was performed.
The patients included in our study were children with AHO; exclusion criteria were open fractures or bone surgery at the site of infection, nosocomial infections, and presence of prosthetic materials. A complete medical history was obtained from the clinical notes, including age at diagnosis, sex, socioeconomic status, symptoms, entry portal, pathogenic agent, location of infection, radiological features, treatment, and outcome.
An AHO diagnosis was established based on the findings of clinical examination, laboratory tests, and radiological investigation. Clinical examination revealed general and local symptoms such as the extent of swelling, erythema, antalgic posture, gait changes, the site of maximum pain, and functional impairment. Laboratory tests comprised red blood cell (RBC) and white blood cell counts (WBC > 12,000 cells/mL), erythrocyte sedimentation rate (ESR > 40 mm/h), C-reactive protein (CRP > 10.5 mg/dL), urinalysis, blood cultures, and pus cultures. When possible, the latter two were obtained before the administration of antibiotics. Radiological investigations were based on plain X-rays that were closely inspected for lytic or sclerotic lesions of the bone, osteopenia, periosteal elevation or calcification, cortical disruptions, joint effusions, and the aspect of the surrounding soft tissues. Ultrasonography of the affected area was performed to reveal potential subperiosteal abscess.
Medical treatment consisted of antibiotics, supportive care, casting, and surgical intervention by means of incision, evacuation and debridement of any subperiosteal abscess, drilling holes in the affected bone, and antiseptic lavage.
Resolution of osteomyelitis with a positive outcome was based on improvement in clinical signs, decreased inflammatory markers, and no relapse. Chronicization, death, and chronic septic arthritis were considered negative outcomes.
Statistical analysis
The statistical data were processed using Microsoft Excel and MedCalc (www.medcalc.org). A non-parametric chi-square test was used to compare variables.
In the univariate analyses, we included the following variables: age, sex, socioeconomic status, site of infection, infectious pathogen, entrance site of the pathogen, and signs and symptoms at admission.
Results
The patient distribution by age (0–18 years with a median age of 11 years) showed a high incidence of AHO among 11- to 13-year olds, and 11 AHO cases were encountered among 0- to 2-year olds. A male predominance was observed, with 52 (55%) boys versus 42 (45%) girls and sex ratio 1.23. The rural/urban patient distribution was 56/38, and 53.5% of rural patients had negative outcomes. The rural/urban distribution was significantly different in patients with negative outcomes (P = 0.002),
Concerning the site of infection, the femur (35 patients, 37.2%) and tibia (30 patients, 32%) were the most affected bones, followed by the humerus, forearm bones, and fibula. Ten patients (10.6%) had multifocal osteomyelitis.
The entry portal was suspected to be cutaneous in most patients (74.4%). In 16 patients, the site was pulmonary or otorhinolaryngology-related, and in 8 patients the entrance site could not be established (Table 1).
Table 1.
Epidemiological and clinical data versus outcome.
| Epidemiological features | Positive outcome: n = 58 (61.7%) | Negative outcome (criticization, chronic septic arthritis, and death): n = 36 (38.3%) | P* |
|---|---|---|---|
| Age, y | |||
| Median (standard deviation), 11 (4.7) | 11.5 (4.019) | 9 (5.177) | 0.002 |
| Sex | |||
| F (N = 42), n (%) | 31 (25.91%) [1.00] | 11 (16.09%) [1.61] | 0.03 |
| M (N = 52), n (%) | 27 (32.09%) [0.81] | 25 (19.91%) [1.30] | |
| Geographic residence | |||
| Rural (N = 56), n (%) | 26 (34.55%) [2.12] | 30 (21.45%) [3.41] | 0.0002 |
| Urban (N = 38), n (%) | 32 (23.45%) [3.12] | 6 (14.55%) [5.03] | |
| Entry site | |||
| Cutaneous (N = 70), n (%) | 47 (43.19%) [0.34] | 23 (26.81%) [0.54] | 0.088 |
| Pulmonary or oral (N = 16), n (%) | 6 (9.87%) [1.52] | 10 (6.13%) [2.45] | |
| Not established (N = 8), n (%) | 5 (4.94%) [1.52] | 3 (3.06%) [0.00] | |
| Pathological agent | |||
| Staphylococcus aureus (N = 84, 89%), n (%) | 55 (52.96%) [0.08] | 29 (31.04%) [0.13] | 0.11 |
| Group A β-hemolytic Streptococcus (N = 8), n (%) | 3 (5.04%) [0.08] | 5 (2.96%) [1.41] | |
| Other pathogens (N = 2), n (%) | 0 | 2 | |
| Location of the infectious process | |||
| Lower limb long bones | 46 (44.88%) [0.03] | 19 (20.12%) [0.06] | 0.132 |
| Upper limb long bones | 10 (8.98%) [0.12] | 3 (4.02%) [0.26] | |
| Other** | 2 (4.14%) [1.11] | 4 (1.86%) [2.47] | |
| Multifocal arthritis | 0 | 10 | |
*The chi-square test was used for a contingency table. A p-value of <0.05 was considered to be statistically significant.
**Rare sites like the calcaneus, pelvis, ribs or scapula.
The main symptoms included pain (94 patients), functional impairment (42 patients), swelling (60 patients) and general signs. General symptoms were those of severe infection (fever, shivering, pallor, increased heart rate), as well as gastrointestinal (nausea, vomiting), and urinary tract-related (oliguria) symptoms (Table 2).
Table 2.
Diagnostic approach.
| Diagnostic approach | Number of patients | Sensitivity | Comment |
|---|---|---|---|
| Clinical manifestation | |||
| Local pain | 94 | 100% | |
| Position of relief | 90 | Most frequently encountered in the lower limbs, especially in osteomyelitis of the femur, the thigh being in abduction and internal rotation. | |
| Erythema | 84 | 95% | |
| Local warmth | 82 | 87% | |
| Swelling | 60 | 63% | |
| Complete functional impairment | 42 | 44% | In patients with pathological fractures. |
| General symptoms | 48 | 51% | General signs of severe infection (fever, shivering, pallor, increased heart rate), gastrointestinal (nausea, vomiting), and urinary tract-related (oliguria) symptoms. |
| Radiological features | |||
| Lytic lesions | 82 | 87% | |
| Double cortical line | 64 | 68% | Cortical doubling; the expression of periosteum elevation became visible after 3 weeks from onset. |
| Soft tissue edema | 30 | 31% | Initially the only manifestation was discrete soft tissue edema. |
Local examination revealed pain of variable intensity in all patients, antalgic posture (90 patients), erythema (84 patients), increased local warmth (82 patients), partial functional impairment (73 sites), and complete functional impairment (42 sites) (Table 2).
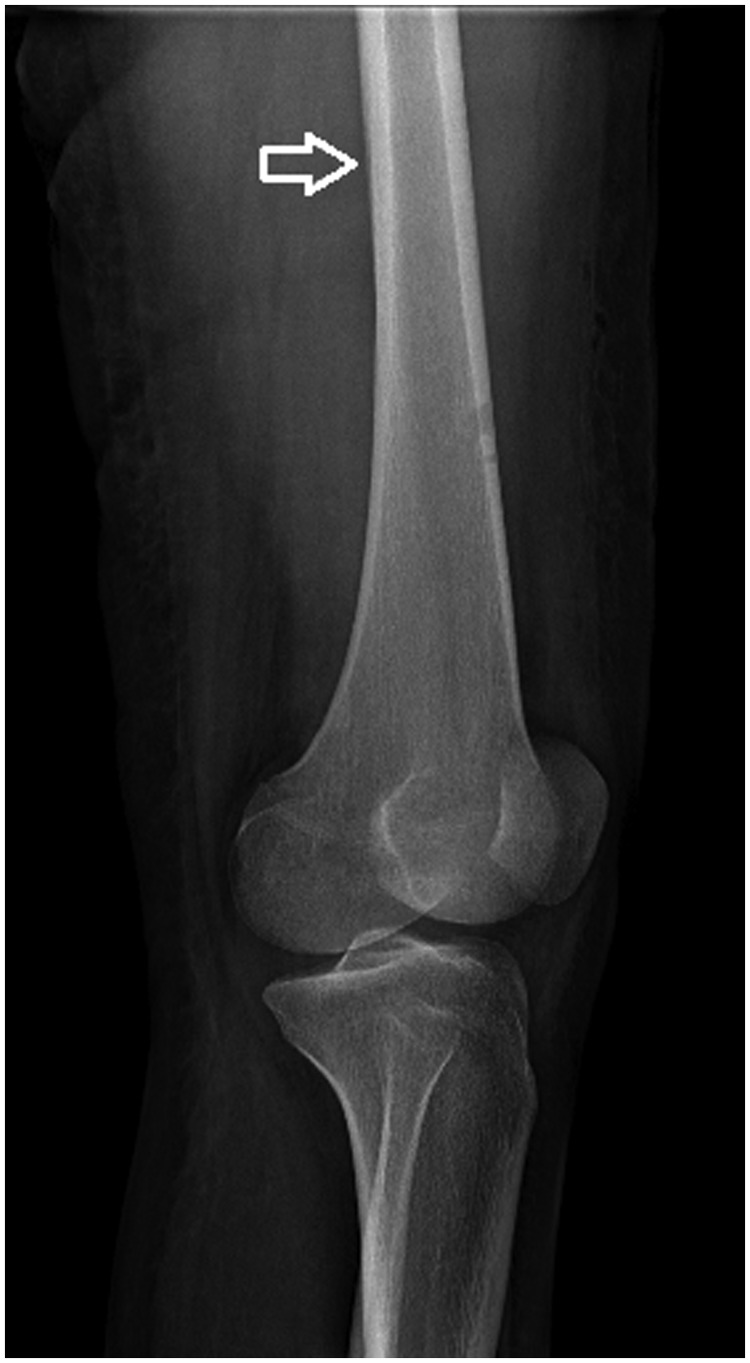
Most patients presented with anemia (RB < 10 g/L), leukocytosis (WBC > 15,000/mm3), and an ESR value of over 100 mm/h. Hemocultures were positive in 36 patients. Cultures from pus specimens and antibiotic sensitivity tests were performed, and the results showed that the most common pathogen was S. aureus (89%), followed by GABHS (8.5%). After 3 weeks from onset, radiological findings revealed the double cortical line sign and periosteum elevation (Figure 1). The earliest changes consisted of soft tissue edema (Table 2).
Figure 1.
Radiograph of the femur in a lateral view showing a double cortical line sign.
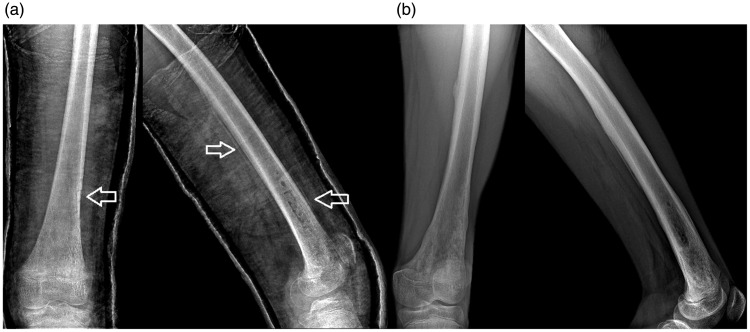
Casting and antibiotic treatment were administered for all 94 patients. In 65 patients, ultrasonography revealed subperiosteal abscess and surgical intervention was performed (Figure 2). After treatment administration, no sequelae healing was achieved in 58 patients (61.7%); 27 patients (28.7%) had a poor outcome that led to chronic infection. In the group age 0 to 2 years, one patient died because of sepsis.
Figure 2.
Radiograph of the femoral osteomyelitic site in an anteroposterior and lateral view, (a) 6 weeks post-surgery, where the double cortical line sign and drilling sites can be seen and (b) aspect of the affected femur at 5 months after surgery.
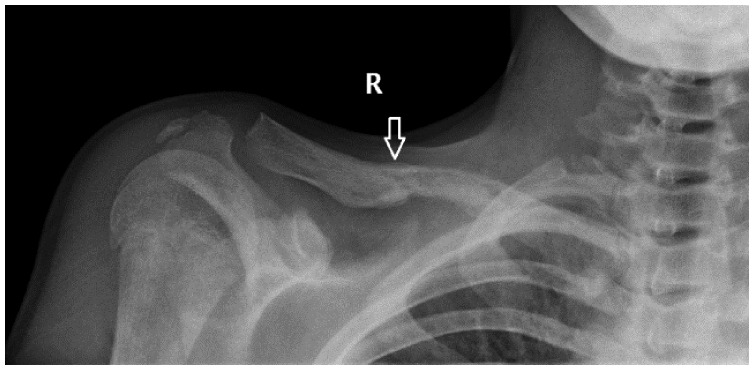
The most frequent late complication was severe sepsis (12 patients) followed by secondary septic localization (10 patients), pathological fracture (9 patients), septic arthritis (8 patients), and pathological dislocation (1 patient). Pathological fractures were mainly encountered in the lower limbs, mostly affecting the tibia but also occurring in rare sites, like the clavicle (Figure 3).
Figure 3.
Radiograph showing a pathological fracture of the clavicle as a late complication of osteomyelitis.
Discussion
The patient distribution by age showed a high incidence among 11- to 13-year olds, overlapping with the maximum skeletal growth period and abundant metaphyseal blood flow.19 Eleven patients were age 0 to 2 years. Unfortunately, most patients in this age group had negative outcomes because of loss of their maternal immunity and subtle symptoms at this age, which leads to late diagnosis.13 Moreover, because of the continuity of circulation across the physis until 18 months of age in infants, a pathogenic agent can reach the epiphysis and the joint, causing permanent epiphyseal damage and joint infection.20
Concerning the distribution by sex, we observed a predominance of male patients. This can be explained by a higher exposure to trauma among boys.21 The rural/urban distribution was comparable (26/32) in patients with good outcomes, but we noted a significant difference (30/6) in patients with negative outcomes (P=0.002) (Table 1). In Romania, boys from rural areas are more involved in activities that are prone to trauma and injuries, and they do not have proper access to emergency medical care. Negative outcomes are likely related to the fact that these patients are diagnosed and treated later.
The most frequent site of infection was the metaphysis of the long bones, which is consistent with the literature data.22 The femur and tibia were the most frequently affected bones, followed by the humerus, forearm bones, and fibula. Rare sites such as the calcaneus, pelvis, or scapula were also affected. Ten patients had more than one site of infection. The infection site did not influence the evolution of disease nor the outcome, but all 10 patients with multifocal osteomyelitis had a poor outcome (Table 1). Hematogenous multifocal osteomyelitis in children is a rare and dangerous form of osteomyelitis in which sepsis can develop quickly, requiring rapid treatment.23
Most patients reported pain, swelling, and partial or total functional impairment. These symptoms were associated with general signs of severe infection like shivering and septic fever. General impairment with circulatory (pallor, cyanosis, cold and sweaty extremities), urinary (oliguria), and digestive disorders has been reported. The intensity of general symptoms was in direct relation to the patient’s age, with infants presenting with less notable and often nonspecific symptoms.
Clinical examination revealed swelling, erythema, clinically apparent venous circulation, antalgic posture of the affected limb, increased local warmth, limited active range of motion, and pain at manipulation, with maximum intensity in the metaphyseal areas. Except for patients who had articular involvement, passive joint motion was relatively unaffected. Pain is the most common symptom in patients with bone or joint infection, but pain is frequently absent in small children. Rather than complaining of pain, children limp, refuse to walk, bear weight, or move a limb.9
Usually, children with AHO have a high fever, >38°C in 36% to 74% of patients.7,24,25 In our study the main symptoms were pain, functional impairment, swelling, and general signs. General symptoms were those of severe infection (fever, shivering, pallor, increased heart rate) and gastrointestinal (nausea, vomiting), and urinary tract-related (oliguria) symptoms (Table 2).
Standard X-ray shows bone changes when osteomyelitis is already established, appearing as a lytic metaphyseal lesion with periosteal elevation and new bone formation.11 Ultrasonography is a good method for identifying subperiosteal abscess in acute osteomyelitis. This procedure has been proven to be helpful in diagnosing AHO in small children with insidious symptoms and could serve as an indication for surgical treatment.23 For patients in our study who we considered to have late diagnosis, an abscess was ultrasonographically identified at hospital admission.
Computed tomography can help in determining the destruction of bone and soft tissue, and magnetic resonance imaging (MRI) can be used to differentiate osteomyelitis from cellulitis and identify abscesses.9,12 In acute osteomyelitis, the bone marrow is congested with fluid and pus, edema being one of the first changes that appear. This can be highlighted on MRI as early as 1–2 days after the onset of infection.15
In our patients, radiologic examination revealed AHO characteristic findings. The earliest changes were discreet, with soft tissue edema (Table 2). First, lytic lesions appeared on X-ray after 14 days, with conventional imaging having a sensitivity of 20% to 75% and a specificity of 75% to 83%, with a limited level of evidence (II–III).26,27
Most patients had anemia (RBC < 10 g/L), leukocytosis (WBC > 15,000/mm3), and an ESR value of over 100 mm/h. This value has great predictive value in establishing the diagnosis, as Riise et al. (2008)1 showed in their report that an ESR of more than 40 mm/h has the highest predictive value (26%).
S. aureus is one of the most common causes of bacteremia, with a high mortality rate of 65% to 70% in the pre-antibiotic era; currently, the 30-day mortality is 20% to 40%, despite adequate treatment.28,29 Human skin is optimized to prevent the entrance of and colonization by S. aureus, the most common species of Staphylococcus found on the skin. However, S. aureus infections occur in skin compromised by diseases or wounds.30 In our study, the most common etiological pathogen was S. aureus (89%), followed by GABHS (8.5%).
The therapeutic approach to osteomyelitis is a complex combination of antibiotherapy, surgical debridement, and limb immobilization.13 Considering the fact that the main pathogen incriminated in AHO was S. aureus in our patients, initial drug therapy consisted of intravenous administration of a combination of empiric anti-Staphylococcus antibiotics, mainly oxacillin and gentamicin. In some patients, cephalosporins were used. Antibiotic treatment was administered parenterally until normalization of CRP levels, and then switched to oral administration of oxacillin or erythromycin. Alongside antibiotic therapy, vitamins, blood transfusion, and immune stimulators (human immunoglobulins) were administered.
After treatment in our study, healing with no sequelae was achieved in 58 patients (61.7%) whereas 27 patients (28.7%) had a poor outcome that led to chronic infection. Most chronic patients presented late, with a developed abscess. One patient in the age group 0 to 2 years died from sepsis. This patient had late-diagnosed acute osteomyelitis and was admitted to the hospital when the disease was in an advanced stage of abscess formation.30
The limitations of our study include its retrospective nature and the lack of long-term follow-up in most patients. a lack of useful data concerning the onset of the symptoms associated with hospital presentation also constitutes a drawback.
Conclusions
In our study, AHO showed a high incidence among 11- to 13-years olds, overlapping the period of maximal osseous growth. Children age 0 to 2 years had negative outcomes owing to loss of maternal immunity and subtle symptoms at this age, which leads to late diagnosis. We observed a predominance of male patients, and boys from rural areas were more prone to have a poor outcome because they are more exposed to trauma; moreover, children in rural areas do not have proper access to emergency medical care.
The single-site form of AHO was the most frequent among our patients, with a predilection for long bones, especially the femur and tibia. In most patients, the entrance site was cutaneous, and the most common etiological pathogen was S. aureus.
Disease evolution was toward chronicity in cases where the diagnosis was established late. The most frequent complications were sepsis and pathological fractures. Patients who had a negative outcome were characterized by young age, repeated negative cultures, delayed surgery, rural residence, and male sex.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Madalina Carp https://orcid.org/0000-0002-0606-4975
Alexandru Ulici https://orcid.org/0000-0003-1018-1506
References
- 1.Riise ØR, Kirkhus E, Handeland KS, et al. Childhood osteomyelitis-incidence and differentiation from other acute onset musculoskeletal features in a population-based study. BMC Pediatr 2008; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krogstad P, Smigh AL. Osteomyelitis and septic arthritis In: Feigin RD, Cherry JD. (eds) Textbook of pediatric infectious disease. Philadelphia, PA: WB Saunders, 1998, pp. 683–704. [Google Scholar]
- 3.Gutierrez KM. Osteomyelitis In: Long SS, Pickering LK, Prober CG. (eds) Principles and practice of pediatric infectious disease. New York, NY: Churchill Livingstone, 1997, pp. 528–536. [Google Scholar]
- 4.Blyth MJ, Kincaid R, Craigen MA, et al. The changing epidemiology of acute and subacute haematogenous osteomyelitis in children. J Bone Joint Surg Br 2001; 83: 99–102. [DOI] [PubMed] [Google Scholar]
- 5.Craigen MA, Watters J, Hackett JS. The changing epidemiology of osteomyelitis in children. J Bone Joint Surg Br 1992; 74: 541–545. [DOI] [PubMed] [Google Scholar]
- 6.Street M, Puna R, Huang M, et al. Pediatric acute hematogenous osteomyelitis. J Pediatr Orthop 2015; 35: 634–639. [DOI] [PubMed] [Google Scholar]
- 7.Wang CL, Yang YJ, Liu CC, et al. Septic arthritis in children: relationship of causative: pathogens, complications and outcomes. J Microbiol Immunol Infect 2003; 36: 41–46. [PubMed] [Google Scholar]
- 8.Petola H, Kallio MJ, Unkila-Kallio L. Reduced incidence of septic arthritis in children by Haemophilus influenza type-b vaccination: implications for treatment. J Bone Joint Surg Br 1998; 80: 471–473. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein SL, Flynn JM. Lovell and Winter’s pediatric orthopedics. 7th ed Philadelphia: Ed Wolters Kluwer, 2013, pp. 369–386. [Google Scholar]
- 10.Calhoun JH, Manring MM. Adult osteomyelitis. Infect Dis Clin North Am 2005; 19: 765–786. [DOI] [PubMed] [Google Scholar]
- 11.van Schuppen J, van Doorn MM, van Rijn RR. Childhood osteomyelitis: imaging characteristics. Insights Imaging 2012; 3: 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song KM, Sloboda JF. Acute hematogenous osteomyelitis in children. J Am Acad Orthop Surg 2001; 9: 166–175. [DOI] [PubMed] [Google Scholar]
- 13.Herring JA. Tachdjian’s pediatric orthopaedics. 5th ed Philadelphia: Ed Elsevier, 2014, pp. 1042–1054. [Google Scholar]
- 14.Hobo T. Zur pathogencse de akuten haematogenen osteomyelitis, mit berucksichtigungder vitalfarbungs leher. Acta Scolar Met Kicto 1921; 4: 1–2. [Google Scholar]
- 15.Lee YJ, Sadigh S, Mankad K, et al. The imaging of osteomyelitis. Quant Imaging Med Surg 2016; 6: 184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaramillo D. Infection: musculoskeletal. Pediatr Radiol 2011; 41: S127–S134. [DOI] [PubMed] [Google Scholar]
- 17.Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther 2010; 8: 175–181. doi: 10.1586/eri.09.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krogstad P. Osteomyelitis In: Feigin RD, Cherry JD, Demmler-Harrison GD, Kaplan SL. (eds) Textbook of pediatric infectious diseases. 6th ed Philadelphia, PA: Saunders Elsevier, 2009, pp. 725–742. [Google Scholar]
- 19.Rauch F. The dynamics of bone structure development during pubertal growth. J Musculoskelet Neuronal Interact 2012; 12: 1–6. [PubMed] [Google Scholar]
- 20.Trueta J. The three types of acute haematogenous osteomyelitis. Bone Joint J 1959; 41: 671–680. [Google Scholar]
- 21.Chiappini E, Camposampiero C, Lazzeri S, et al. Epidemiology and management of acute haematogenous osteomyelitis in a tertiary paediatric center. Int J Environ Res Public Health 2017; 14: 477. doi: 10.3390/ijerph14050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinikumpu JJ, Tapiainen T, Korhonen J, et al. Acute hematogenous osteomyelitis in children. Duodecim 2014; 130: 1591–1598. [PubMed] [Google Scholar]
- 23.Sreenivas T, Nataraj AR, Menon J, et al. Acute multifocal haematogenous osteomyelitis in children. J Child Orthop 2011; 5: 231–235. doi: 10.1007/s11832-011-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott RJ, Christofersen MR, Robertson WW, Jr, et al. Acute osteomyelitis in children: a review of 116 cases. J Pediatr Orthop 1990; 10: 649–652. [DOI] [PubMed] [Google Scholar]
- 25.Klein DM, Barbera C, Gray ST, et al. Sensitivity of objective parameters in the diagnosis of pediatric septic hips. Clin Orthop Relat Res 1997; 338: 153–159. [DOI] [PubMed] [Google Scholar]
- 26.Kao HC, Huang YC, Chiu CH, et al. Acute hematogenous osteomyelitis and septic arthritis in children. J Microbiol Immunol Infect 2003; 36: 260–265. [PubMed] [Google Scholar]
- 27.Jaramillo D. Infection: musculoskeletal. Pediatr Radiol 2011; 41: S127–S134. doi: 10.1007/s00247-011-2001-y. [DOI] [PubMed] [Google Scholar]
- 28.MacNeal WJ, Frisbee FC, McRae MA. Staphylococcemia 1931-1940. Five hundred patients. Am J Clin Pathol 1942; 12: 6. [Google Scholar]
- 29.Melzer M, Welch C. Thirty-day mortality in UK patients with community-onset and hospital-acquired meticillin-susceptible Staphylococcus aureus bacteraemia. J Hosp Infect 2013; 84: 143–150. doi: 10.1016/j.jhin.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Choi JH, Seo HS, Lim SY, et al. Cutaneous immune defenses against staphylococcus aureus infections. J Lifestyle Med 2014; 4: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]