Abstract
Sepsis has been identified by the World Health Organization (WHO) as a global health priority. There has been a tremendous effort to decipher underlying mechanisms responsible for organ failure and death, and to develop new treatments. Despite saving thousands of animals over the last three decades in multiple preclinical studies, no new effective drug has emerged that has clearly improved patient outcomes. In the present review, we analyze the reasons for this failure, focusing on the inclusion of inappropriate patients and the use of irrelevant animal models. We advocate against repeating the same mistakes and propose changes to the research paradigm. We discuss the long‐term consequences of surviving sepsis and, finally, list some putative approaches—both old and new—that could help save lives and improve survivorship.
Keywords: animal models, cytokine storm, personalized medicine, reprogramming, sepsis
Subject Categories: Immunology; Microbiology, Virology & Host Pathogen Interaction; Molecular Biology of Disease
This review recapitulates our knowledge on sepsis and its long‐term consequences, the lack of therapeutic advances in the last decades, and proposes new approaches to improve sepsis survival.
Glossary
- Autophagy
A natural, regulated mechanism of the cells that removes unnecessary or dysfunctional components within the cytoplasm. It allows the orderly degradation and recycling of cellular components.
- Cecal Ligation and Puncture (CLP)
A common rodent model of sepsis following surgery with ligation and puncture of the cecum, which induces a polymicrobial septic insult (peritonitis).
- Compartmentalization of the immune response
A differential polarization of the immune response in different tissues and organs that is regulated by the local microenvironments ending to specific responses of cells.
- Endothelial permeability
An inflammation‐induced impairment of the dynamic barrier between endothelial cells embedding vessels that leads to the leak of small plasma molecules.
- Endotoxin tolerance
A state induced mainly in monocytes/macrophages by endotoxin or by exogenous or endogenous inflammatory stimuli. Cells become refractory to secondary challenge with endotoxin or other inflammatory stimuli. The phenomenon is regulated at multiple levels (epigenic, miRNA, negative signaling, cross‐talk with immunosuppressive cells…).
- Endotype
A subtype of a clinical syndrome that shares common pathophysiological mechanisms.
- Hibernation
A metabolic‐bioenergetic shutdown allowing the organs to retain the capacity to recover after the insult has passed.
- Immunosenescence
A natural decline of the immune system functions with aging.
- Microbiome
A collection of microorganisms that can be found in or on multicellular organism in the context of anatomical location (gut microbiome, skin microbiome, lung microbiome).
- Neutrophil Extracellular Traps (NETs)
DNA structures actively released during programmed death of neutrophils, which primary aim to entrap and kill the pathogens. A dysregulated production of NETs can be detrimental to the host and induce pathologies.
- Pathobiome
A disease‐related changed in microbiome.
- Persistent inflammation, immunosuppression, and catabolism syndrome (PICS)
A phenotype underlying the chronic critical illness, initiated early in the course of disease. This is perpetuated by the release of damage‐associated molecular patterns (DAMPs) associated with signs of immunosuppression and altered metabolism.
- Reprogramming
The modified responsiveness of innate immune cells in response to a second stimulus. Depending on the nature of the first challenge, it can be similar to endotoxin tolerance or to priming (also referred to as innate immune memory).
- Sepsis
A life‐threatening organ dysfunction caused by a dysregulated host response to infection.
- Septic shock
A subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone.
- Sequential Organ Failure Assessment Score (SOFA)
A simple tool to assess the degree of organ dysfunction, to track disease progression, and to predict outcome; it comprises six categories covering abnormalities in respiratory, cardiovascular, hepatic, coagulation, renal, and neurological systems.
- Sick euthyroid syndrome
A condition in which low levels of thyroid hormones are found in patients with non‐thyroid illness.
- Theranostics
An individualized medicine approach combining targeted therapeutics and diagnostic test
- Translational research
Preclinical research aiming at understanding the physiopathology and the molecular mechanisms of a human disease or at proposing new therapeutic approaches to improve health outcome. Laboratory animals are often used to mimic human diseases.
Sepsis: a new WHO global health priority
Sepsis was previously considered an infectious systemic inflammatory response syndrome (SIRS; Bone et al, 1992; Levy et al, 2003). However, recognizing that this host response is usually part and parcel of an individual's appropriate defense against infection, sepsis was re‐defined in 2016 (“Sepsis‐3”) as “a life‐threatening organ dysfunction caused by a dysregulated host response to infection” (Fig 1) (Singer et al, 2016). For clinical operationalization, organ dysfunction is identified by an increase from baseline of ≥ 2 points in the Sequential Organ Failure Assessment (SOFA) score. Patients are identified as having septic shock when vasopressors are required to maintain a mean arterial pressure ≥ 65 mmHg and when serum lactate levels remain > 2 mmol/l (>18 mg/dl) despite adequate volume replacement.
Figure 1. Summary of sepsis pathophysiology.
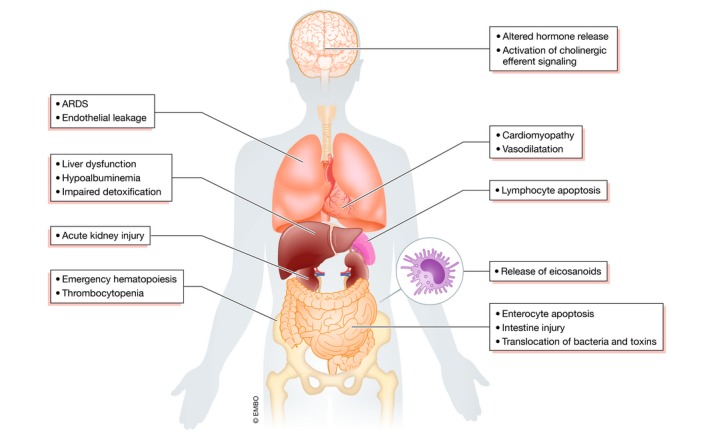
Upon direct activation of immune and endothelial cells by the pathogen‐associated molecular patterns, there is a massive release of inflammatory mediators which affect each body system. Inflammatory response activates the central nervous system, which acts by cholinergic anti‐inflammatory impulsion and altered neuroendocrine response to control the body response to infection and increase chances of survival. Cardiovascular dysfunction plays a central role in the pathogenesis of sepsis with the major role of vasoplegia, hypovolemia, microcirculation perturbations, and cardiomyopathy. Altered endothelium and inflammatory cells lead to the development of acute respiratory distress syndrome (ARDS). The direct action of cytokines and toxins, together with decreased blood flow, leads to acute kidney injury (AKI). Inflammatory response and ischemia alter gut permeability which enables entry of bacteria and their metabolites into the tissues. Both bacterial products and inflammatory mediators affect bone marrow progenitor cells enhancing the emergency myelopoiesis. Most often, the failure of multiple organs is present, which has significant consequences as there is a cross‐talk between injured organs which further perpetuates their dysfunction. For a more detailed perspective on organ failure in sepsis, we refer to a recent review (Lelubre & Vincent, 2018).
In 2017, the WHO passed a resolution, recognizing sepsis as a global health priority (Reinhart et al, 2017). A recent investigation estimated that in 2017, sepsis occurred in 48.9 million people worldwide, ending to the death of 11 million patients (Rudd et al, 2020). These estimates are more than double previous global figures (Fleischmann et al, 2016). This increase is probably attributable to inclusion of more data from low‐income and middle‐income countries, locations where sepsis incidence and mortality are considerably higher and for which data were previously under‐represented. It is also unclear how many people die “of” or “with” sepsis as the majority of deaths, at least in developed countries, occur in patients who are elderly, frail, and/or have significant underlying comorbidities. National Health Service data from England suggest that 77.5% of deaths occur in those aged ≥ 75 years (Singer et al, 2019). The incidence of sepsis is also reported to be rising at an alarming rate though such data should be treated cautiously. While in part due to an aging population and increasing invasive medical interventions, increased awareness of the condition and significant financial incentivizations to use administrative sepsis codes play an important part in the reported incidence increase. Indeed, Rhee et al (2017) indicated that sepsis incidence based on Sepsis‐3 clinical criteria was stable over a 5‐year period in 409 US hospitals (0.6% relative increase per year), yet had risen by 10.3% per year according to insurance claims data. By 2014, the incidence of sepsis was twice as high, affecting 12% of the total hospital cohort, using claims‐based data. This large rise in denominator also generated a spurious reduction in the rate of death or discharge to hospice, namely 4.5% per year using claims data compared to 1.3% using clinical data.
These conflicting data on incidence and outcomes reflect considerable inconsistencies within the literature, particularly as most of the data are extracted from hospital administrative databases or insurance claims data that are prone to the confounders described above. Nonetheless, it is fair to say that sepsis continues to be a significant healthcare issue with high mortality (approximately 25% for sepsis and 40–50% for septic shock (Shankar‐Hari et al, 2017) and morbidity, and with a major impact on resource utilization. This continues after discharge from hospital, as survivors often suffer post‐sepsis symptoms such as fatigue, neuromuscular weakness, chronic pain, post‐traumatic stress disorder, cognitive impairments, and depression. Furthermore, sepsis has a high price to insurance systems (Arefian et al, 2017).
Notwithstanding coding anomalies, mortality from sepsis does appear to be improving. This is mainly the consequence of improved health care with earlier recognition and treatment, and less iatrogenic harm. Multiple studies in intensive care patients have demonstrated an injurious impact from over‐exuberant use of mechanical ventilation, fluid therapy, sedation, nutrition, and inotropes, to name but some interventions. Unfortunately, these outcome improvements cannot be attributed to introduction of new therapies, nor to a better understanding of the pathophysiology. This is not for the lack of clinical trials undertaken over the last 30 years, started after successful outcomes in animal models that are often reported in high impact journals. The failure of this translational research should raise questions within the medical and scientific communities, and inspire them to avoid perpetuating expensive experimental animal studies and clinical trials without modifying the underlying paradigms. Several published articles have already explored the reasons for these failures (Rittirsch et al, 2007; Dyson & Singer, 2009); however, no significant changes have emerged.
Well‐established processes and new concepts
Molecular players
New findings are constantly enlightening our understanding of the pathophysiological processes involved in sepsis. Most of the players involved have likely been identified (Fig 2). The host response is initiated by both pathogen‐associated molecular patterns (PAMPs), such as endotoxin and lipoteichoic acid, and damage‐associated molecular patterns (DAMPs), such as heat shock proteins, high mobility group box 1, nucleotides, and mitochondria released from injured host cells.
Figure 2. Summary of the players and pathophysiological events occurring and influencing sepsis.
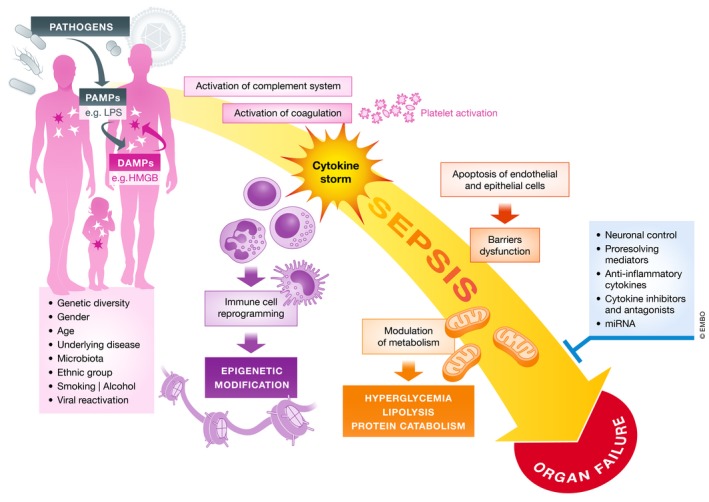
Complex interactions between genetic and chronic health status determine the host response to pathogens. The magnitude and variety of humoral and cellular response may lead to organ dysfunctions, which are a key denominator of sepsis in comparison with other forms of infection.
Activation of the complement system has long been recognized as a potentiator of the inflammatory response during sepsis, in particular the role played by anaphylatoxin C5a (Riedemann et al, 2002). An inhibitor of C5 cleavage showed reduced organ damage and better outcome in a baboon model of Escherichia coli sepsis (Keshari et al, 2017). Similarly, modifying the coagulation cascade has been advanced with numerous successful approaches in animal sepsis models targeting pro‐coagulant factors (Levi & van der Poll, 2017).
Various other molecular players have been identified as performing a beneficial role in murine cecal ligature and puncture (CLP) models of sepsis. These include IL‐33 and IL‐38 (Li et al, 2016; Xu et al, 2018), pro‐resolving mediators such as resolvin D2 (Spite et al, 2009), and the cell surface nucleotide‐metabolizing enzyme CD39 (Csóka et al, 2015). Acetylcholine produced by neurons and by a specific subset of T‐lymphocytes in response to norepinephrine is considered a significant component in the neuronal control of inflammation (Rosas‐Ballina et al, 2011), though the role of the vagus nerve in neuro‐immune cross‐talk is controversial (Martelli et al, 2014; Pavlov et al, 2018). In a case report of successful use of experimental electroacupuncture to protect against sepsis, dopamine was shown to be the primary beneficial mediator (Torres‐Rosas et al, 2014).
Circulating cells
Sepsis is associated with the reprogramming of circulating leukocytes (Cavaillon et al, 2005). The term “immunosuppression” is widely used to qualify this phenomenon, which is inappropriate and misleading (Cavaillon & Giamarellos‐Bourboulis, 2019). The ex vivo behavior of leukocytes is greatly influenced by the compartment they are derived from (Rasid & Cavaillon, 2018; Fig 3). Blood leukocytes display reduced capacities to proliferate and to produce cytokines and antibodies. Monocytes show reduced expression of HLA‐DR mRNA, while neutrophils show an increased expression of CD64 mRNA. Notably, recruited monocytes within the lungs express 3.5‐fold more membrane HLA‐DR compared to circulating monocytes (Skirecki et al, 2016). Lymphocytes exhibit enhanced spontaneous apoptosis while neutrophils’ anti‐CD24‐induced apoptosis is reduced (Parlato et al, 2014). These alterations occur rapidly and in proportion to the intensity of the insult, be it sepsis or other critical illnesses such as trauma, hemorrhagic shock, major surgery, resuscitation after cardiac arrest, and pancreatitis (Kim et al, 2010; Timmermans et al, 2016). As expected, the altered capacity of circulating monocytes is associated with an enhanced expression of inhibitory signaling molecules (Escoll et al, 2003; Adib‐Conquy et al, 2006), histone modifications (Bomsztyk et al, 2015), and specific miRNAs (Zhou et al, 2015; Reithmair et al, 2017). Some miRNAs can attenuate sepsis‐associated alterations in myeloid cells, endothelial cells, and in the myocardium (Zhou et al, 2017; Sisti et al, 2018). Circulating extracellular vesicles containing miRNAs may be a novel mechanism of intercellular communication during sepsis (Real et al, 2018). Whether these extracellular vesicles have an inhibitory role via delivery of miRNAs or display a pro‐inflammatory potential via their capacity to activate monocytes (Danesh et al, 2018) remains to be elucidated.
Figure 3. Compartment‐specific reprogramming of the macrophages in sepsis.
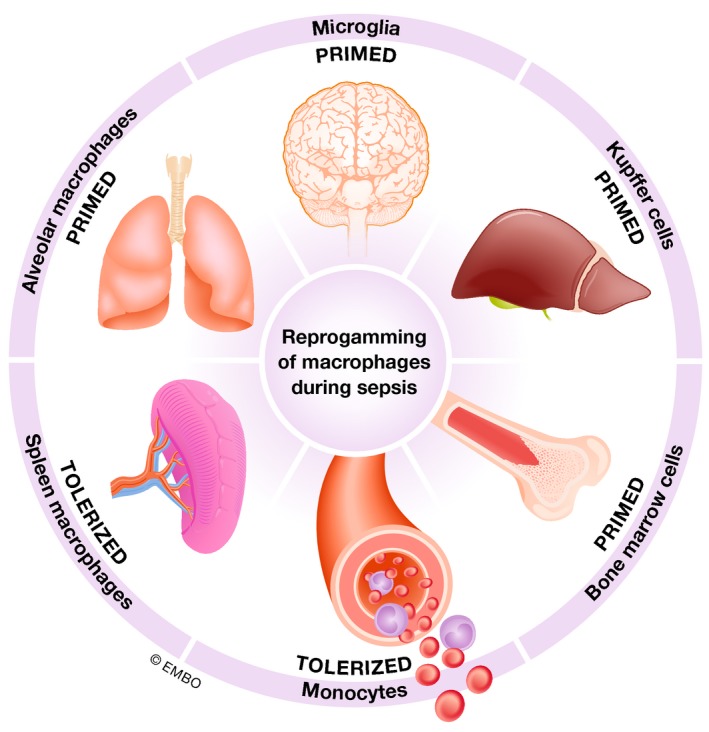
Microenvironment modulates the response of macrophages during sepsis. Therefore, the features of cells from one compartment cannot be generalized upon others.
In addition to their role in blood clotting, platelets play an important role in multiple biological functions. This diversity relates to their capacity to sense and respond to infectious agents and to release significant amounts of inflammatory mediators (Garraud et al, 2013). Platelets can bind to numerous circulating leukocytes, particularly neutrophils (Gawaz et al, 1995), favoring the formation of neutrophil extracellular traps (Clark & Coopersmith, 2007), neutrophil recruitment LPS‐induced lung injury (Grommes et al, 2012), and neutrophil rolling onto the endothelium and margination outside venules (Sreeramkumar et al, 2014). Thrombocytopenia is a hallmark of sepsis and may relate to decreased production and/or increased destruction. In a recent study, 38% of sepsis patients had low or very low platelet counts; such patients had a higher clinical illness severity score and an increased mortality (Claushuis et al, 2016). Of note, levels of circulating RANTES (CCL5), a chemokine mainly derived from platelets, were lower in the most severely ill patients with poor outcomes (Cavaillon et al, 2003).
It should be stressed that for most molecular and cellular players, conflicting contributions have been revealed. While platelets contribute to inflammation, they have also been shown to decrease organ damage in LPS‐treated mice (Xiang et al, 2013) through a C‐type‐lectin‐like‐2 (CLEC‐2)‐dependent process (Rayes et al, 2017).
Endothelium
The pivotal role of endothelial cells in the pathogenesis of sepsis is well recognized. Most endothelium physiological functions are disturbed, leading to increased vascular permeability, activation of coagulation, and participation in the inflammatory response (Opal & van der Poll, 2015). Some recently reported aspects of endothelial injury in sepsis may also contribute significantly to mortality (Johansen et al, 2015). The role in sepsis of the angiotensin‐1,‐2/Tie2 pathway, an important regulatory axis of the endothelium, was recently reviewed (Leligdowicz et al, 2018). High levels of angiopoietin‐2 in pneumonia patients contributed to endothelial permeability and were related to poor outcomes, while angiopoietin‐1 was shown to ameliorate vascular leak (Gutbier et al, 2018). Mechanistically, a reduction of the histone deacetylase, sirtuin 3, leads to the increase in angiopoietin‐2 and fall in angiotensin‐1. These changes also contribute to the loss of pericytes (Zeng et al, 2016). Furthermore, the Tie‐2 receptor is downregulated in sepsis at both transcriptional and protein levels (Thamm et al, 2018). Another mediator in sepsis‐induced endothelial dysfunction is microRNA‐155, which promotes bioenergetic deterioration, contractile dysfunction, pro‐inflammatory activation, and downregulation of the angiotensin type 1 receptor (Vasques‐Novoa et al, 2018). A novel insight into the mechanism of endothelial cell death in sepsis described an intracellular endotoxin‐sensing machinery in the induction of endothelial cell pyroptosis (Cheng et al, 2017).
Epithelium
Maintenance of the gut barrier can be severely impaired in sepsis by enterocyte loss from apoptosis and pyroptosis (Mandal et al, 2018), and a rapid decline of tight junctions following reduced expression of claudins and occludin (Yoseph et al, 2016). The observations of enterocyte injury and increased gut wall permeability led to the concept of the “leaky gut” as the motor of multi‐organ dysfunction in critical illness, acting as a source of bacteria and bacterial toxins (Carrico et al, 1986). Although this hypothesis has not gained sufficient traction in clinical studies, and patients do not seem to die from bacteremia (Savage et al, 2016), updated and modified insights offer attractive new pathophysiological concepts, in particular the protective role of the microbiome (Alverdy & Krezalek, 2017; Meng et al, 2017) and its modulation by probiotics (Angurana et al, 2018). However, dissemination of intestinal bacteria does not seem to be an essential event as gut‐derived DAMPs can travel through the lymph and cause distant organ injury (Reino et al, 2011).
Endocrinopathy
The central nervous system is stimulated during sepsis by efferent impulsion, inflammatory cytokines, and PAMPs that can enter the blood‐brain barrier at specific locations (Annane, 2016). These signals modify the central endocrine axes that orchestrate metabolic and immune responses, and these change over time. For a detailed description of endocrine alterations in sepsis, we recommend a recent review by Ingels et al (2018). Briefly, early endocrine responses to a critical insult are generally protective. However, the degree of activation of the hypothalamic–pituitary–adrenal (HPA) axis represents a significant stress response that correlates with poor outcomes (Vassiliadi et al, 2014). Release of adrenocorticotropic hormone is increased, while the high serum concentration of cortisol is also due to impaired clearance (Annane, 2016). The potentially advantageous effects of early high cortisol production are counteracted by peripheral glucocorticoid resistance mediated by pro‐inflammatory cytokines. However, a subgroup of patients could not mount an efficient cortisol response due to inhibition of the HPA axis and would potentially benefit from glucocorticoid supplementation (Annane, 2016).
Plasma catecholamine levels are also elevated, more so in eventual non‐survivors (Boldt et al, 1995), with multiple negative consequences including impaired myocardial function, inhibition of both innate and adaptive immunity yet enhanced pathogen virulence and growth, pro‐coagulopathic effects, altered gut motility, lipolysis, and insulin resistance (Andreis & Singer, 2016).
Sepsis also affects thyroid function by an early increase in thyrotropin (TSH) production but a reduction in both thyroxine and the more active triiodothyronine (Peeters et al, 2006). This condition, often called the sick euthyroid syndrome or the low T3 syndrome, also correlates with a poor outcome (Angelousi et al, 2011). A trial of thyroxine treatment in critically ill patients however resulted in higher mortality (Acker et al, 2000).
Another pituitary hormone, growth hormone (GH), is initially upregulated in septic patients and correlates with disease severity (Schuetz et al, 2009). However, its downstream effectors are decreased in the serum (Baxter et al, 1998). High levels of GH stimulate lipolysis and antagonize insulin. Yet, two multi‐centre studies of growth hormone treatment in critically ill patients showed a doubling in mortality rates (Takala et al, 1999).
Apart from disturbances in the central hormonal axes, there is a growing interest in the role of intestine‐released hormones and fat‐derived adipokines in the pathogenesis of sepsis. However, it remains unclear whether altered levels of these hormones and adipokines contribute to pathology, or are simply epiphenomenal and reflective of dysregulated homeostasis. Examples include insulin, the incretins (GLP‐1 and GIP), ghrelin, and leptin. High and persisting levels of glucagon‐like peptide‐1 in septic patients are associated with an increase in mortality and functional disability (Brakenridge et al, 2019). Ghrelin, involved in appetite stimulation, increases in the plasma of septic patients (Nikitopoulou et al, 2019), and active ghrelin levels are inversely correlated with the SOFA organ dysfunction score and length of ICU stay. In short‐term preclinical models of sepsis, ghrelin has been shown to decrease pro‐inflammatory cytokine levels, stimulate proliferation of T cells, and attenuate the decrease in serum levels of IGF‐1 (Faim et al, 2019); however, we found that ghrelin infusion in a long‐term rodent fecal peritonitis model had no impact on outcomes (Hill et al, 2017). Plasma insulin levels are variably reported as raised or depressed in sepsis, but insulin resistance is commonplace regardless, with subsequent hyperglycemia‐inducing toxicity. The optimal degree of glycemic control nonetheless remains controversial (Gunst et al, 2019). Peripheral resistance indicates abnormalities at, or distal to, the insulin receptor (Van Bogaert et al, 2011). Similar issues are likely to exist for other hormonal pathways, such as glucocorticoids and growth hormone.
Metabolism
The importance of alterations in metabolism during sepsis is well recognized and is part of the current definition of septic shock (Singer et al, 2016). With peripheral insulin resistance and increased lipolysis and proteolysis, there is a general shift toward fat and protein utilization. This in turn impacts upon immune functionality (Balmer & Hess, 2017) and metabolic efficiency. However, many questions remain unanswered (Van Wyngene et al, 2018). Many clinical and preclinical data indicate sepsis‐induced mitochondrial dysfunction in multiple tissues (Brealey et al, 2002; Singer, 2014) with a shift toward anaerobic (glycolytic) metabolism. While anaerobic metabolism can boost the antimicrobial capacities of immune cells, it may also contribute to their loss of function (Balmer & Hess, 2017). In the course of sepsis, monocytes develop broad defects in both glycolysis and oxidative phosphorylation that are related to the phenomenon of endotoxin tolerance and which can be restored by interferon‐gamma (IFNγ) treatment (Cheng et al, 2016). A recent genome‐wide array study (Davenport et al, 2016) proposed two signatures of gene expression patterns, with one related to higher mortality and features of immunosuppression. This subgroup showed upregulated expression of hypoxia‐inducible factor 1 alpha (HIF1α), HIF2α, and lactate dehydrogenase A, but downregulation of the mechanistic target of rapamycin (mTOR).
As failed organs show minimal to no evidence of cell death, a global metabolic shutdown is postulated as the primary mechanism of organ dysfunction in sepsis (Singer, 2014). This state, akin to hibernation, may even represent an adaptive mechanism, allowing restoration of organ function once the inflammatory process resolves (Singer et al, 2004; Stanzani et al, 2020). Notably, sepsis non‐survivors are characterized by subnormal levels of muscle ATP (Brealey et al, 2002), while maintenance of hepatic gluconeogenesis was shown to be crucial in establishing disease tolerance (Weis et al, 2017). These data suggest that adaptive pathways can spill over into maladaptation if sufficiently dysregulated.
Impaired bioenergetics under septic conditions have also been shown to affect energy‐consuming functions in multiple types of epithelial cells. For example, pneumocytes downregulate the Na+,K+‐ATPase pump, which is responsible for fluid clearance in alveoli (Vadasz et al, 2008), while renal tubular epithelial cells decrease sodium channel expression (Gomez et al, 2015). Controlled autophagy may be an important process required for epithelial cell survival under septic conditions (Oami et al, 2018).
Microbiome/Pathobiome
A growing body of evidence demonstrates that gut microbiota interacts with the gut epithelium and the host's immune system. The microbiome and its metabolites are essential regulators of health under steady‐state conditions. A severe insult such as infection or trauma rapidly induces profound changes in the intestinal ecosystem (dysbiosis), leading to loss of diversity of the physiological, mostly anaerobic flora (Ojima et al, 2016), unification of the microbiome composition at different sites (Rogers et al, 2016), and outgrowth of pathological species that are often nosocomial (Alverdy & Krezalek, 2017). The microbiome is compromised further by concurrent use of broad‐spectrum antibiotics, even with only a few doses (Iapichino et al, 2008; Ferrer et al, 2017).
The gut is not the only site harboring microbiota. For example, the skin and lung are also colonized with their respective microbiome, but their biology is less well understood (Gilbert et al, 2018). Recently, both intestine and upper airways microbiota were shown to regulate lung immunity (Brown et al, 2017). While the role of the microbiome in other tissues such as the kidney remains to be specified in the pathophysiology of sepsis, the skin microbiome has recently been shown to favor the pathogenicity of certain bacteria such as Staphylococcus aureus (Boldock et al, 2018).
Notwithstanding iatrogenic administration of antibiotics and gastric acid suppressants, the host's overall changes during critical illness promote the emergence of the pathobiome (disease‐related flora; Zaborin et al, 2014; Gilbert et al, 2016). This includes supranormal levels of catecholamines that induce pathogen growth, virulence, and biofilm formation, as well as directly impacting on the microbiome (Dickson et al, 2015; Sarkodie et al, 2019).
Numerous studies suggest that the relationship between the host and microbiome/pathobiome is bidirectional (Kelly et al, 2015; Sarkodie et al, 2019). Of note, gut‐specific bacteria can dominate the lung microbiome in septic patients, and this change correlates with alveolar TNF expression (Dickson et al, 2016). Preserving the physiological gut microbiome could protect the host from pneumonia (Schuijt et al, 2016), sepsis (Wilmore et al, 2018), or melioidosis (Lankelma et al, 2017) by maintaining the gut barrier (Fox et al, 2012), enhancing immunity, and protecting the brain (Li et al, 2018). Clinical data have indirectly shown the impact of the disturbed microbiome on susceptibility to sepsis (Prescott et al, 2015a; Baggs et al, 2018). Colonization with Enterococci at ICU admission was related to a higher risk of subsequent infection and mortality (Freedberg et al, 2018). The abovementioned findings shine a new light on the gut and the microbiome/pathobiome as significant players in the pathophysiology of sepsis. The development and decreasing cost of metagenomics and metabolomics will likely move this field rapidly. Recent murine trials showed the potential of modulating the microbiome with a high fiber diet (Morowitz et al, 2017), while the first successful fecal microbiota transplantation used as a salvage therapy in critically ill ICU patients has also been reported (Wei et al, 2016).
Short‐ and long‐term consequences of surviving sepsis
Surviving sepsis creates a plethora of new healthcare challenges. Up to 40% of patients who survive the early phase of sepsis develop chronic critical illness, a state defined as a prolonged (> 14 days) ICU stay with sustained organ dysfunction (Stortz et al, 2018). Many of these patients do not leave hospital alive or are not fit enough to return to their own homes. In the longer term, there is a higher mortality risk, poorer health status, and an increased requirement for more medical interventions and rehospitalizations (DeMerle et al, 2017). This so‐called post‐intensive care syndrome reflects new onset (or worsening of preexisting) neurocognitive deterioration, psychiatric complications, and/or physical impairments (Needham et al, 2012).
Neuropathy
Acute brain dysfunction is a frequent and severe early sign of sepsis present in about 50% of cases admitted to the ICU (Sonneville et al, 2017). It can manifest as confusion, delirium, drowsiness, coma, seizures, or with focal neurological signs. Sepsis‐associated encephalopathy (SAE) includes not only early brain dysfunction but also the longer‐term impairments seen in sepsis survivors. Brain lesions can be found in approximately a third of patients with clinically diagnosed SAE (Orhun et al, 2018). Two distinct patterns of neuroaxonal injury—ischemic and diffuse—were identified using magnetic resonance imaging and immunohistopathologic analysis of post‐mortem brains (Ehler et al, 2017). The pathophysiology of SAE is incompletely understood, but is driven by systemic inflammation, which leads to disruption of the blood–brain barrier and ingress of bacterial toxins, pro‐inflammatory monocytes, and neutrophils into the brain tissue (Kuperberg & Wadgaonkar, 2017; Tauber et al, 2017). Early infiltration of NK cells enhances monocyte recruitment (He et al, 2016). Pro‐inflammatory cytokines and toxins activate microglia and astrocytes (Hasegawa‐Ishii et al, 2016), and fueling local inflammation (Zrzavy et al, 2018). Together with afferent impulsion, the inflamed microenvironment induces neuronal changes. At the functional level, SAE is related to decreased brain oxygenation (Wood et al, 2016), metabolic shifts (Hara et al, 2015), and excitotoxicity (Mazeraud et al, 2016). The aforementioned mechanisms may be sustained in sepsis survivors with long‐term consequences such as cognitive and functional impairment (Annane & Sharshar, 2015), depression (Davydow et al, 2013), stress disorders (Wintermann et al, 2015), and neurological complications (Reznik et al, 2017). Recent animal studies support the causative link between sepsis and long‐term psychological dysfunction (Barichello et al, 2019). Other improvements of cognitive impairment after sepsis have been achieved with some of the treatments known to protect mice against sepsis‐induced death, such as anti‐HMGB‐1 antibodies (Chavan et al, 2012; Gentile & Moldawer, 2014; Stevens et al, 2017) or electroacupuncture (Han et al, 2018). Peripheral nerves can also be affected by sepsis inducing a critical illness polyneuropathy. Loss of axonal fibers affecting motor and/or sensory nerves can be observed in skin biopsies, with impairment of signal conduction (Hermans & Van den Berghe, 2015; Axer et al, 2016).
Myopathy
Similar mechanisms to neuropathy can also induce sepsis‐related myopathy. Either one and/or both conditions (collectively known as ICU‐acquired weakness) can worsen the patient's outcome, lead to prolonged mechanical ventilation (Sharshar et al, 2009), and carry long‐term consequences (Hermans & Van den Berghe, 2015). Sepsis‐related myopathy is characterized by the loss of muscle mass, a fall in force‐generating capacity, and altered bioenergetics (Hermans & Van den Berghe, 2015). Critically ill patients face multiple risk factors causing myopathies such as immobilization, local inflammation‐induced by systemic mediators, and nutritional defects (Friedrich et al, 2015). Mechanisms responsible for septic myopathy include sodium channel dysfunction with upregulation of non‐selective channels (Balboa et al, 2018), ubiquitin–proteasome pathway protein proteolysis, proteasome activation and increased autophagy (Wollersheim et al, 2014; Preau et al, 2019), changes in intracellular calcium levels leading to excitation‐contraction uncoupling (Batt et al, 2013), and mitochondrial derangements with an increase in free radical generation (Zolfaghari et al, 2015). Although autophagy is among the mechanisms of protein loss, its balanced activation can protect muscles from the accumulation of toxic proteins (Morel et al, 2017). Through activation of the TNF and mTORC1 pathways, sepsis impairs the physiological anabolic response of muscles to contraction, thereby slowing down recovery from atrophy (Steiner & Lang, 2015). Recovery from sepsis is related to increased muscle protein synthesis and decreased autophagy, but in juxtaposition, proteasome activity is upregulated, possibly hindering myosin restoration (Crowell et al, 2017). Interestingly, the satellite cells, which serve as muscle progenitor cells, are severely impaired by sepsis and are not able to regenerate injured muscle (Rocheteau et al, 2015) due to upregulated oxidative phosphorylation and loss of mitochondrial mass. In consequence, these alterations lead to the loss of satellite cells.
The incidence of ICU‐acquired weakness negatively affects the quality of life of survivors (Hermans & Van den Berghe, 2015), and recovery from ICU‐acquired weakness can take up to one year after discharge, or even be permanent, especially with neuropathy.
Altered immunity
Sepsis survivors are at higher risk of recurrent infection, and this is a major reason for re‐hospitalization (Prescott et al, 2015b). Whether recurrent infections contribute substantially to long‐term mortality is unclear, but they worsen quality of life and constitute an additional risk to survivors (Shankar‐Hari et al, 2016). Susceptibility to infection suggests sustained immune impairments in sepsis survivors, which are only now being identified. Currently recognized mechanisms of long‐term immune impairment include expansion of myeloid‐derived suppressor cells (Mathias et al, 2017), a high proportion of regulatory T cells (Cavassani et al, 2010), disturbed T‐cell recovery (Condotta et al, 2013), and multiple epigenetic modifications within various cell types (Hassan et al, 2018). A recent study also revealed persistently increased inflammatory mediators such as HMGB1, TNF, IL‐7, and resolvins, even at 1 year after hospital discharge (Riche et al, 2018).
These alterations in immune status do not necessarily reflect a global defect (Rubio et al, 2019). For example, sepsis could improve tumor‐specific CD8+ T‐cell responses (Danahy et al, 2019). TCR‐dependent T‐cell function is somewhat augmented, pointing against a paradigm of generalized immunosuppression in sepsis survivors (Borken et al, 2017). However, there is an incomplete recovery of the TCR reservoir after sepsis, and this process is in part dependent on the gut microbiome (Cabrera‐Perez et al, 2016). Importantly, these changes are accompanied by a smoldering low‐grade inflammation that induces a catabolic shift and may perpetuate disturbed myelopoiesis (Horiguchi et al, 2018). This phenotype has been coined “persistent inflammation, immunosuppression, and catabolism syndrome” (PICS) and can be viewed as a mechanism underlying chronic critical illness (Gentile et al, 2012; Horiguchi et al, 2018; Fig 4).
Figure 4. Long‐term sequel of sepsis.
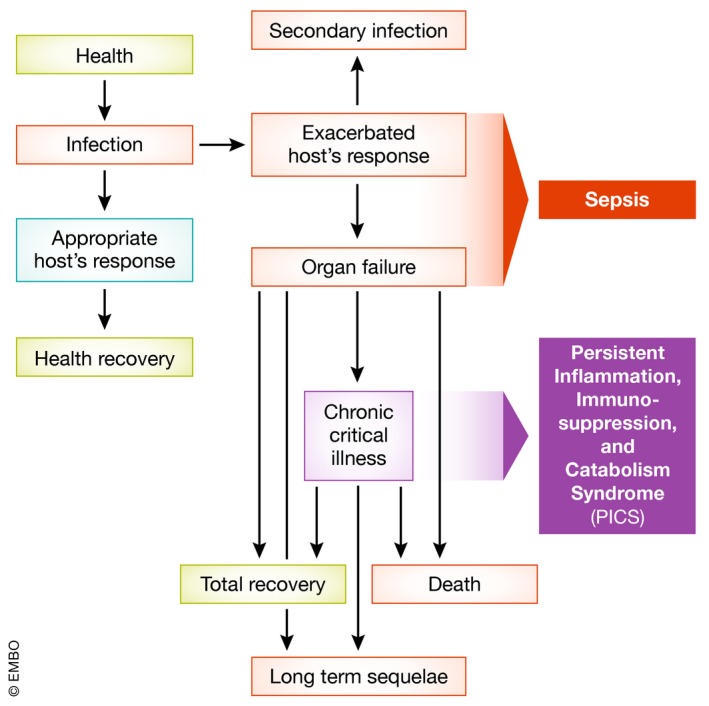
Most of the sepsis cases occur in patients with chronic comorbidities. Within days to weeks, some patients can be healed while some will succumb due to acute organ dysfunctions. However, a high frequency of patients develop chronic critical illness (ICU stay for more than 14 days). This condition is caused mechanistically by the persistent inflammation, immunosuppression, and catabolism syndrome (PICS). During this phase, some patients die due to organ dysfunctions, and some develop secondary infections. A group of chronic critical illness patients still can fully recover. However, most of the patients will experience the worsening of their chronic conditions and suffer from the onset of new ones.
What went wrong? Explaining 30 years of failure of translational research
Inappropriate animal models
For the last thirty years, using genetically engineered mice or animals treated with antibodies or drugs that target a given molecule, investigators have successfully demonstrated a deleterious contribution from a large number of molecular players and pathways during sepsis, and beneficial consequences following their neutralization. However, none have resulted in positive clinical trials. This may relate to the limited predictive value of preclinical models of disease (Plenge et al, 2013).
It is indeed essential to acknowledge significant differences that exist between mice and humans (Fig 5, Table 1), not only in terms of physiology but also with regard to the response to a septic insult. For instance, as opposed to humans, mice develop bradypnea rather than tachypnea, and bradycardia rather than tachycardia (Iskander et al, 2013; Hoover et al, 2015). When maintained at room temperature, septic mice are under cold temperature stress (Karp, 2012) and usually display hypothermia in line with illness severity (Zolfaghari et al, 2013). If their temperature is kept close to thermoneutrality, mice can generate a fever, and their ability to manage sepsis significantly improves (Jiang et al, 2000). The different circadian rhythm between mice and humans also impacts upon the immune response and the timing of expression of key regulators such as HIF1α (Zhao et al, 2017). While the gut is postulated to be the “motor” of multiple organ failure (MOF), allowing systemic ingress of gut bacteria and their cellular constituents (Klingensmith & Coopersmith, 2016), the surface pathophysiology involving the intestine may be different between species. Indeed, the surface ratio of the small intestine to the colon is 22‐fold lower in mice than humans (Nguyen et al, 2015). The immune system also differs in many ways (Mestas & Hughes, 2004), and the main acute phase proteins produced in sepsis vary between species. Human and murine neutrophils also display numerous differences (Table 1); most striking is the different effect of sepsis on NETosis, which is reduced in humans (Hashiba et al, 2015) but enhanced in mice (Meng et al, 2012). With respect to platelets, not only do number and size differ between humans and mice, but also their mRNA content (Schmitt et al, 2001; Rowley et al, 2012).
Figure 5. Some keys differences in murine and human physiology that affect the response to sepsis (CRP —C‐reactive protein, MAC—membrane attack complex, SAP—serum amyloid protein).
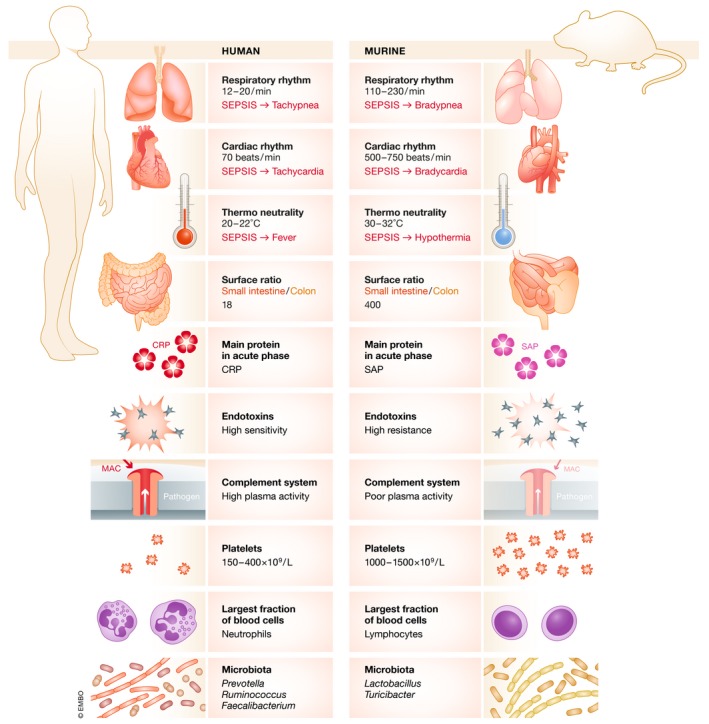
Table 1.
Some physiologic and immunologic differences between mice and humans that may affect the host response to infection, the development of sepsis, and its monitoring
| Mice | Humans | |
|---|---|---|
| Physiology | ||
| Circadian rhythm | Nocturnal | Diurnal |
| Nutrition | Standardized chow diet | Varied |
| Glucose levels | ↓ after sepsis | ↑ after sepsis |
| Temperature | ↓ after sepsis | ↑ after sepsis |
| Metabolic rate | ↓ after sepsis | ↑ with initial sepsis, normalizes with increasing severity |
| Immune system | ||
| Predominant white blood cell type | Lymphocyte | Neutrophil |
| Enzymatic content in neutrophils | Low | High |
| α‐defensin production by neutrophils | No | Yes |
| Expression of CXCR1 on neutrophils | No | Yes |
| NETosis after sepsis | Increased | Decreased |
| Missing genes | IL‐8, IL‐32, IL‐37, LFA‐3 | TLR11, TLR12 |
| TLR10, Caspase 10 | MCP‐5 | |
| Main inflammasome player in LPS sensing | Caspase 11 | Caspase 4 and 5 |
A well‐recognized difference between rodents and humans is the very high resistance of rodents to endotoxin and their greater resilience to infection (Warren et al, 2010) or sterile insults (Gentile et al, 2013). Genes encoding proteins that sense PAMPs and DAMPs, and coding for cytokines and chemokines are not strictly identical (Table 1). The transcriptomic expression of cells within human and murine blood following different severe inflammatory insults poorly correlates (Seok et al, 2013), even if an independent re‐analysis of the same dataset yields completely different results (Takao & Miyakawa, 2015).
Another thorny aspect of animal experimentation is that the experimental model of infection strongly influences the host response. Thus, findings from one model may not necessarily be generalizable. For example, the metabolomic signature differed in rats undergoing cecal ligation and puncture (CLP) compared to those receiving an intra‐peritoneal injection of S. aureus (Lin et al, 2016). Similarly, in two models of intra‐abdominal sepsis (CLP versus administration of a cecal slurry) with similar mortality, addition of tissue ischemia/infarction (cecal ligation) to an infectious process significantly modified the host transcriptomic response (Gentile et al, 2014).
Finally in most animal models, the treatment is administered before, concurrent, or soon after the initiation of the infectious insult. By contrast, patients usually develop sepsis over days; often by the time of presentation, organ dysfunction is already well established.
Inappropriate selection of patients
Agglomerating different types of severe infection under the “sepsis” umbrella has resulted in enrollment of a heterogeneous population of patients into clinical trials. In addition to intrinsic individual heterogeneity reflecting genetic and epigenetic diversity (Fig 2), specific patient characteristics such as underlying comorbidities, obesity, medications, reactivation of asymptomatic viral infections, age, and sex can all modify the overall host response to pathogens, and thus patient outcomes. Individual heterogeneity is impossible to avoid, though selecting patients according to the type of infection would be more feasible. Mechanisms underlying the host response are greatly influenced by the location of the infectious insult. This reflects the fact that immune cells reside within varied molecular and cellular microenvironments in different tissues and, as a consequence, display specific behavior. For example, murine intestinal macrophages are unresponsive to conventional stimuli (Smythies et al, 2005); peritoneal macrophages, alveolar macrophages, and blood monocytes are stimulated differently by Staphylococcus aureus (Kapetanovic et al, 2011); spleen, lung, peritoneal macrophages, and microglial cells express different patterns of transcriptomic and cell surface expression (Gautier et al, 2012); and alveolar macrophages fail to develop endotoxin tolerance (Philippart et al, 2012). This late phenomenon has also been observed with human alveolar macrophages (Smith et al, 1994). Studies in septic patients confirm how different sites of infection affect the systemic response (Gogos et al, 2010; Hoser et al, 2012). Thus, not surprisingly, specific pathophysiological mechanisms differ between compartments, and therapeutic intervention should be adapted accordingly with patient cohorts more homogenous in terms of the infection site.
Another cause of heterogeneity is the nature of the infectious agent. The PAMPs and toxins released by Gram‐positive and Gram‐negative bacteria are not identical. Most superantigens are derived from Gram‐positive bacteria and induce an unique cytokine profile, including lymphotoxin‐α derived from T‐lymphocytes (Müller‐Alouf et al, 1994). This can be observed particularly in patients with Streptococcal toxic‐shock syndrome (Sriskandan et al, 1996). Endotoxins from Gram‐negative bacteria are highly potent PAMPs inducing the greatest number of gene activations (Schmitz et al, 2004). Notably, endotoxin can also be present in patients with Gram‐positive infection due to gut translocation (Opal et al, 1999). A strong synergy has been regularly reported between Gram‐positive superantigens and endotoxin or other Toll‐like receptor agonists (Cavaillon, 2018). CD137, a member of the TNF receptor superfamily, is expressed on neutrophils and shown to be protective during Gram‐positive and deleterious during Gram‐negative infections (Nguyen et al, 2013).
Improving the chances of therapeutic success
Faster and more reliable diagnosis
Significant delays in initiating treatment will impact upon patient outcomes. Highly specific, sensitive, and rapid tests are urgently needed to avoid unnecessary, inappropriate, or ineffective use of antibiotics. Although many host biomarkers have been reported (Parlato & Cavaillon, 2015), none of them (alone or in combination) displays sufficiently high levels of specificity and sensitivity (Parlato et al, 2018 ). However, various novel approaches show promise in distinguishing sepsis from sterile inflammation using transcriptomics (Sweeney et al, 2018a), metabolomics (Mickiewicz et al, 2015), or microfluidics (Hassan et al, 2017; Ellett et al, 2018). Combined analysis of leukocyte biomarkers has also been investigated. Finally, proteomics and transcriptomics have also been proposed to allow discrimination between bacterial and viral infection (Oved et al, 2015; Miller et al, 2018).
As therapeutic approaches could differ depending on illness severity, signatures stratifying patients according to their risk of death could be of interest. Transcriptomic (Sweeney et al, 2018a, 2018b) and cell surface marker (Conway Morris et al, 2018) signatures have already been described. More desirably, patients could be identified based on the likelihood of response to a particular treatment. For example, very high serum levels of ferritin could be used as a marker of hemophagocytic lymphohistiocytosis, for which specific treatments such as an IL‐1 receptor antagonist (anakinra) may be suited (Lachmann et al, 2018).
Rapid identification of the infectious agent and of its antibiotic sensitivity is also of primary importance. While PCR assays offer good sensitivity and specificity (Salimnia et al, 2016), they require time‐consuming blood cultures, and direct identification of bacterial DNA within freshly isolated blood samples is still associated with too many false‐positive and false‐negative results (Fitting et al, 2012; Dark et al, 2015). A test combining culture‐independent PCR/electrospray ionization‐mass spectrometry technology was launched commercially but, alas, withdrawn as the cost was too prohibitive. However, it displayed 81% sensitivity, 69% specificity, and 97% negative predictive value at 6 hours from sample acquisition (Vincent et al, 2015). Post hoc analysis of this cohort revealed a higher mortality in patients with molecular test‐positive, culture‐negative samples (O'Dwyer et al, 2017).
Improving translational research
Refinement, reduction, and replacement of animal models should be strongly encouraged. In vitro or ex vivo studies can be performed directly on human cells and tissues, and these may be complemented by more sophisticated and robust human organoid and organ‐on‐chip models. Experiments using rodent models remain useful and informative; however, their limitations should be humbly recognized. Improvements have been proposed (Osuchowski et al, 2018), such as the use of antibiotics, fluids, and clinical isolates instead of laboratory bacterial strains, which may fail to mimic real‐life pathogenesis due to their reduced capacity to make biofilms (Fux et al, 2005). The role of wild‐type microbial flora in influencing outcome has only recently been appreciated (Velazquez et al, 2019). The wild‐type microbiome acquired by co‐housing specific pathogen‐free mice with their pet‐shop mates allowed the mice to develop a mature immunity capable of generating responses more similar to those of humans (Beura et al, 2016). Colonizing experimental mice with a human patient‐derived pathobiome that emerges in critical illness would further allow to better mimic pivotal host–pathobiome interactions (Alverdy & Krezalek, 2017). An appropriate choice of infection model (pneumonia, CLP, or soft tissue infection) with a relevant infection route and pathogen load would also better reflect a given human sepsis type.
Stratification of septic animals according to their predicted probability of survival may help discriminate the effectiveness of novel treatments (Osuchowski et al, 2009). Telemetry techniques reveal a variable physiological response of mice subjected to a similar septic insult (Lewis et al, 2016); this could be utilized to select only significantly affected individuals for enrollment into a treatment study, to increase homogeneity and improve assessment of the treatment effects. More “real‐life” conditions could be achieved, for instance by the use of aged animals or animals with comorbidities. Two‐hit models, where an initial trauma or infectious episode is followed by induction of a secondary infection, resemble a frequent clinical scenario, and may recapitulate the human host responses better than single hit models (Muenzer et al, 2006). Finally, specific aspects of the human immune response could be examined within humanized mice models with added genetic variability from transplantation of stem cells originating from different donors (Skirecki et al, 2015; Prince et al, 2017).
Non‐rodent models such as rabbits and pigs have some advantages, for example, greater sensitivity to bacterial infection and responses resembling better those observed in humans (Fairbairn et al, 2013; Salgado‐Pabon et al, 2013; Waterhouse et al, 2018). While rhesus monkeys and baboons are highly resistant to endotoxin and have a separate pattern of host response (Barreiro et al, 2010), squirrel monkeys have a relatively high sensitivity (Lipton & Fossler, 1974). Studying naturally sick animals (Werners, 2017) or non‐laboratory‐bred animals could also be informative as their immunity and host response will differ.
Should the main goal be to save life or to improve quality of life after sepsis?
With such a provocative question, we want to address whether trials should aim at improving mortality benefit and/or improving patient selection. With a current hospital mortality rate of 25%, achieving a 20% improvement in mortality with a P value below 0.05 requires inclusion of 1,191 patients. Arguments are however made to increase the default P value threshold to 0.005 for claims of discovery (Benjamin et al, 2018), thus requiring many more patients, or reducing the sample size by a Bayesian approach (Goligher et al, 2018). Others argue for adaptive trial designs with simultaneous inclusion of several treatment arms and modifications justified by findings emerging during the trial (Talisa et al, 2018). However, adequate group sizes in such a heterogenous critical care population may prove to be problematic.
Another challenge lies in accurate selection of therapeutic targets that have a realistic probability of improving survival from sepsis. Possible ways to improve translational research are discussed below. Furthermore, selection of targets based upon observational clinical trials can also be misleading. There is a confounding effect of illness severity that may not be adequately adjusted for in post hoc analyses. For example, a positive fluid balance is linked to worse outcomes, yet sicker patients generally need more fluid replacement and are more likely to have concurrent renal dysfunction. Thus a positive fluid balance may be epiphenomenal rather than necessarily causative of a higher mortality. Furthermore, changes in a blood mediator level linked to a poor outcome can be part of the host response to injury, or even a protective element related to disease tolerance (Medzhitov et al, 2012; Bauer et al, 2018).
Enhancing survivorship is gaining increasing attention as many of the 70% of septic patients discharged from hospital will suffer from post‐sepsis physical, psychological, and/or cognitive impairments, with consequences for the patients, their family and the healthcare system (Prescott & Angus, 2018). Contributing factors to poor survivorship comprise underlying frailty and comorbidities, degree and duration of illness severity, lack of early rehabilitation, and drugs, such as corticosteroids, sedatives and paralyzing agents. While frailty and underlying comorbidities are not usually correctable, identification of interventions for reversible conditions will hopefully lead to enhanced recovery (Schweickert et al, 2009; Wade et al 2019).
As described at the beginning of this article, we pointed out that many people die “with” rather than “from” sepsis because of their significant underlying comorbidities, which both compromise their initial host response and/or the subsequent recovery pathway. A treatment may be effective in reversing the acute septic illness, yet the patient still dies in the hospital because of frailty and/or preexisting organ dysfunction. Length of stay and duration of mechanical support will also be longer in such patients, confounding any beneficial effect on time to recovery and ICU or hospital discharge in less sick survivors. In view of the many available prognosticators—physiological, biochemical, and molecular—that can identify likely survival at an early stage in the patient's hospital admission, the possibility of better trial stratification is intriguing. Predicted survivors can be separated from predicted non‐survivors. In the former, the focus could be on time to recovery and quality of survivorship with unanticipated death used as a safety signal. On the other hand, a mortality signal can be primarily sought in predicted non‐survivors, with a greater treatment effect being perhaps more likely in this sicker cohort.
A personalized medicine approach
Increasing emphasis is being placed on personalized medicine (Fig 6), and an important aspect to consider is aging. Indeed, the links between immunosenescence and sepsis have been poorly investigated (Martín et al, 2017), although immunosenescence is associated with increased sensitivity of aged people to infection (Fulop et al, 2018; Mannick et al, 2018). However, most experimental sepsis models are performed on murine teenagers. Mortality is far greater in aged mice following CLP (Saito et al, 2003) or with polytrauma with pneumonia (Nacionales et al, 2015). The elderly animals show enhanced coagulopathy, decreased generation of activated protein C (Starr et al, 2015), and a more pronounced cytokine storm.
Figure 6. Strategies to enrich treatment‐sensitive subpopulations of patients.
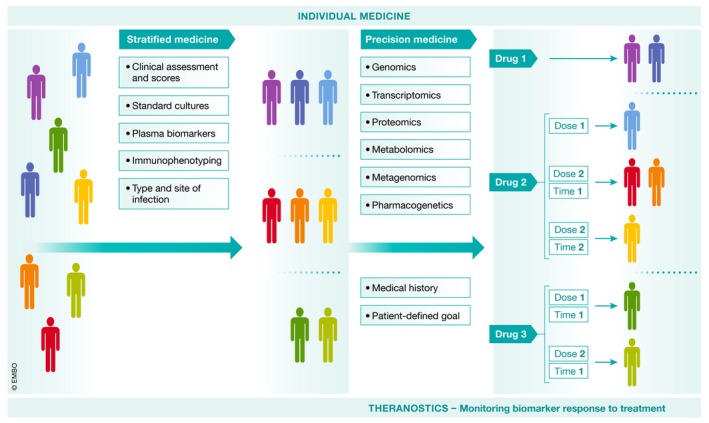
At the admission, patient is subjected to supportive therapies and samples are taken for laboratory analyses. The flowchart on the left side shows current approach to stratify the patients using relatively simple methods such as clinical scales, mediator concentrations, or activation of immune cells. Then basing on the thresholds patients are qualified or not to a given specific therapy. This approach is already used in some clinical trials and for some biomarkers feasible at the bedside. On the right, a procedure of future individual medicine is presented. It involves a more complicated approach which applies potent analytical platforms to assess the genome, transcriptome, proteinome, and metabolome of the patient. Together with metagenome of the pathogen, the decision is made on the drug to prescribe as well as its dose and timing.
Blocking or activating specific pathways may be potentially beneficial when a pathway is over‐ or under‐expressed in patients who fare badly, albeit with the caveat that this may represent an adaptive rather than detrimental response. This may apply to the use of IL‐1 receptor antagonism (Shakoory et al, 2016), anti‐TNF therapies (Panacek et al, 2004), or corticosteroids (König et al, 2018), where post hoc analyses suggest benefit in those with raised ferritin, raised IL‐6 levels and low IFN‐γ/IL10, respectively (Table 2). On the other hand, a post hoc analysis of a clinical trial showed that a transcriptomic signature suggestive of a more immunocompetent profile fared significantly worse with steroid therapy (Antcliffe et al, 2019).
Table 2.
Examples of clinical trials that showed benefits in subgroups of septic patients
| Drug/intervention | Subgroups | Benefit | Mode of analysis | References |
|---|---|---|---|---|
| Afelimomab (anti‐tumor necrosis factor F(ab’)2 monoclonal antibody fragment) | IL‐6 > 1,000 pg/ml | 28‐day mortality 43.6% vs. 47.6% placebo | Prospective | Panacek et al (2004) |
| GM‐CSF | Monocytic HLA‐DR < 8,000 antibodies per cell | Time of mechanical ventilation 148 ± 103 vs. 207 ± 58 h (placebo), P = 0.04 | Prospective | Meisel et al (2009) |
| Anakinra (IL‐1 receptor antagonist) | Features of hemophagocytic lymphohistiocytosis (disseminated intravascular coagulation (DIC), thrombocytopenia and hepatobiliary dysfunction) | 28‐day mortality 34.6% vs. 64.7% placebo | Re‐analysis of de‐identified data from the phase III randomized interleukin‐1 receptor antagonist trial in severe sepsis | Shakoory et al (2016) |
| Trimodulin (polyclonal immunoglobulin preparation) | CRP ≥ 70 mg/l and IgM ≤ 0.8 g/l | 28‐day mortality 11.8% vs. 36.6% placebo (P = 0.006) | Exploratory post hoc | Welte et al (2018) |
Analysis of clinical data from more than 20,000 patients identified four distinct clinical phenotypes with different host‐response patterns (Seymour et al, 2019). Computer simulations suggested that the distribution of particular phenotypes could affect the trial outcome. Others have suggested identifying patients with a major immunosuppressive profile (Hotchkiss et al, 2013; Bermejo‐Martin et al, 2016) though this may be less promising than anticipated (Cavaillon et al, 2014). A recent study on 700 transcriptomic profiles suggested that patients with bacterial sepsis could be divided into “inflammopathic”, “adaptive”, and “coagulopathic” clusters (Sweeney et al, 2018a), with each state potentially corresponding to different therapeutic approaches. A summary of transcriptomic studies exploring subgroup responses can be found in Table 3. While intriguing, such approaches have yet to be tested prospectively, let alone validated.
Table 3.
Described endotypes of sepsis. Heterogeneity of the host response to sepsis is a major cause of difficulties in the development of effective targeted therapies. By the use of genome‐wide expression assays, different patterns of transcriptomic response (of blood leukocytes) in sepsis are distinguished. Unraveling these patterns creates opportunities to find new pathways that can be targeted in a given subgroup. The term endotype is used to distinguish the transcriptome‐based diversity from the classical phenotypic description
| Endotypes | Methodology | Studied group | Implications | References |
|---|---|---|---|---|
|
Subclass A: repression of adaptive immunity and zinc‐related biology Subclass B Subclass C |
Genome‐wide expression profiling, unsupervised hierarchical clustering of genes which expression was ≥ 2‐fold changed (comparing to controls) in 25–50% of patients | Children with septic shock (n = 98) | Identification of high‐risk subpopulation by subclass An assessment identification of novel therapeutic targets | Wong et al (2009) |
|
Subclass A Subclass B |
Multiplex mRNA quantification platform to analyze the expression of the 100 subclass‐defining genes | Children with septic shock (n = 168) |
Development of a method for endotyping pediatric septic shock Identification of endotype (A) associated with the harmful effects of glucocorticosteroids |
Wong et al (2015) |
|
Mars1: immunosuppression, increase in heme biosynthesis pathway components Mars2: increased expression of genes related to pattern recognition, cytokines, cell growth Mars3: adaptive immunity; IL‐4, NK‐cell signaling Mars4: interferon signaling, pattern recognition, TREM1 signaling |
Genome‐wide expression | Sepsis (n = 306), validation cohort (n = 216), second validation cohort (CAP sepsis n = 265) | Mars1 type response is related to poor early‐ and long‐term outcome | Sclicluna et al (2017) |
|
SRS1 (Sepsis Response Signature 1): immunosuppression, T‐cell exhaustion, endotoxin tolerance SRS2: proliferation, immune response, cell adhesion |
Genome‐wide microchip array, variation in global gene expression by unsupervised hierarchical clustering | Sepsis due to CAP (n = 265 and validation cohort n = 106) | SRS1 is a predictor of high early mortality | Davenport et al (2016) |
|
SRS1: cell death, apoptosis, endotoxin tolerance SRS2: cell adhesion, differentiation, proliferation, immune response |
Genome‐wide Microarray, variation in global gene expression | Fecal peritonitis sepsis(n = 117) (also comparison with CAP; n = 126) | SRS1 is a denominator of high early mortality, but the shift to SRS2 pattern is a marker of favorable prognosis | Burnham et al (2017) |
|
Endotype A Endotype B |
Retrospective analysis of transcriptomic data using pattern of 100 genes expression | Sepsis (n = 549) | Highest mortality in patients < 40 y.o. co‐allocated into endotype A/SRS1. Suggestion of relationship between immunosuppressive response and mortality | Wong et al (2017a) |
|
Endotype A Endotype B |
Retrospective classification and regression tree analysis of retrospective data to find the smallest discriminatory set of genes | Septic children (n = 300); validation group (n = 43) |
Development of four‐gene based protocol for endotyping of septic children. Potential to identify glucocorticoid responses |
Wong et al (2017b) |
|
SRS1 SRS2 |
Genome‐wide microarray, allocation based on the generalized linear model based on 7 genes (from Davenport et al, 2016) | Sepsis (n = 177) | Hydrocortisone treatment increases mortality in SRS2 | Antcliffe et al (2019) |
|
Inflammopathic: pro‐inflammatory, complement pathways Adaptive: adaptive immunity and interferon signaling Coagulopathic: platelet degranulation, coagulation cascade |
Genome‐wide expression | Retrospective analysis of septic patients (n = 700) from 14 trials | Identification of major deregulated pathways in endotypes that can direct selective treatment | Sweeney et al (2018a) |
CAP, community acquired pneumonia; SRS, sepsis response signature.
Clearly, this branch of diagnostics is still in its relative infancy. Advances in platform technology such as next‐generation sequencing with interrogation of the whole genome will open up important new insights, as will the rapidly advancing areas of metagenomics, metabolomics, and proteomics. The time to access data will be dramatically reduced, enabling results within minutes to hours, a timeline that is crucial for intervention in a critically ill patient. PCR and multiplex protein point‐of‐care technologies already exist and yield results within this timeline; such devices will continue to become more refined and sophisticated. Crucially, standardization, or at least cross‐validation, of the different platforms and computer analytic techniques will be needed to ensure consistency. A further point to consider is the current reliance on blood sampling and extrapolation of results from either predominantly white cells, or circulating humoral mediators (e.g., cytokines, hormones, metabolites), to changes at the organ level. An excellent but sadly overlooked rat CLP study showed markedly different transcriptomic changes between lung, liver, kidney, thymus, spleen, and brain, both spatially and temporally (Chinnaiyan et al, 2001). Pathophysiological assumptions made from circulating blood cells may not hold true in the overall scheme.
Revisiting old strategies or new approaches?
As mentioned earlier, many approaches that were successful in preclinical models and Phase II clinical trials have failed at the larger multi‐centre trial stage. The increasing appreciation of different phenotypes/endotypes within the syndrome of sepsis and post hoc analyses of trial data suggesting outcome benefit (e.g., Calfee et al, 2018 and statins), or detriment (Antcliffe et al, 2019, and steroids) in specific subsets, does suggest that these old agents could be gainfully revisited, especially with the advent of diagnostics enabling rapid subset identification. IL‐1Ra therapy could be considered for patients with a hemophagocytosis profile (Shakoory et al, 2016), recombinant soluble thrombomodulin in those with a coagulopathic profile (Kato & Matsuura, 2018), or intravenous immunoglobulin (IVIg) in those with a hyperinflammatory profile (Welte et al, 2018). Immunostimulant drugs such as interferon‐gamma and GM‐CSF are also being re‐explored, using lymphopenia as a surrogate for immunosuppression. Non‐drug approaches, such as hemofiltration and blood purification techniques, may also show benefit when targeting specific subsets rather than general populations (e.g., Payen et al, 2009; Gaudry et al, 2016; Dellinger et al, 2018; Hawchar et al, 2019).
The same argument should be applied to novel investigational drugs. Many are in a clinical testing phase, but it is imperative that they be tested in optimal population subsets. For example, a dose‐finding phase II trial (Adrenoss‐2) has just been completed for adrecizumab in patients with septic shock and elevated concentrations of circulating bio‐adrenomedullin (Geven et al., 2019). Other approaches are also being studied, including electroacupuncture, which showed benefit in endotoxic mice (Torres‐Rosas et al, 2014) and more recently, anti‐inflammatory bowel‐protective effects on intestinal functions in patients with sepsis‐induced intestinal dysfunction (Meng et al, 2018).
There are multiple other agents being studied at present in preclinical models targeting a wide range of inflammatory, immune, metabolic, bioenergetic, and hormonal pathways (Dalli et al, 2014; Tancevski et al, 2014; Winkler et al, 2017; Rathkey et al, 2018; Sham et al, 2018). Transfer of mesenchymal stem cells appeared promising in preclinical studies, and phase 1 and 2 clinical trials are ongoing (Keane et al, 2017). Preparations of exosomes from mesenchymal stem cells (Chang et al, 2018) or endothelial progenitor cells (Zhou et al, 2018) also show early promise. How many of these approaches will proceed to clinical trials and then clinical use remains open to question. There may be no magic target to cure sepsis, and a combined approach could be more beneficial than targeting a single molecule, as shown for IL‐1 and IL‐18 in murine sepsis (Vanden Berghe et al, 2014), IL‐1 and TNF in a rat model (Russel et al 1995), or CD14 and factor XIa in rabbits (Nakamura et al, 2017).
Improving survivorship
While the therapeutic emphasis has long been focused on survival, increasing effort is now being placed on enhancing survivors’ quality of life. Below are a few approaches being examined to improve functionality in different organs.
Protecting the brain
Minimization or avoidance of iatrogenic factors related to treatment (e.g., prolonged use of benzodiazepine sedation, and adequate pain treatment) and non‐pharmacological modalities (e.g., physiological light cycle, cognitive stimulation, and early mobilization) may prove useful (Souza‐Dantas et al, 2016). Existing drugs such as metformin (Tang et al, 2017) and minocycline (Adembri et al, 2014) show brain protective effects in experimental sepsis. The natural antioxidant berberine improved survival in a rat CLP model but also motor and cognitive functions (Shi et al, 2018).
Improving muscle function
An adequate diet with high protein intake may be pivotal in dealing with critical illness‐induced sarcopenia (Wischmeyer & San‐Millan, 2015), perhaps in conjunction with an individualized physical rehabilitation regimen. Early rehabilitation appears to improve short‐term ICU‐acquired weakness, but not long‐term weakness nor mental status (Fuke et al, 2018). Despite contradictory clinical data, neuromuscular electrostimulation reduced muscle atrophy in endotoxemia models (Poulsen et al, 2011; Rodriguez et al, 2012; Tanaka et al, 2016). Use of beta‐blockers to inhibit catabolism was proved to be efficient in burn patients (Herndon et al, 2001). Anabolic hormones such as testosterone or growth hormone are also of interest, despite their potential side effects (Rosenthal & Moore, 2015). However, growth hormone increased mortality in critically ill patients (Takala et al, 1999).
Preserving immune functionality
Different strategies are proposed, ranging from boosting reagents (Hotchkiss et al, 2013) to agents limiting the inflammatory reaction, such as myeloid suppressor cells (McPeak et al, 2017) or regulatory T cells (Heuer et al, 2005). Preventing T‐cell exhaustion by anti‐PD‐1/PD‐L1, anti‐CTLA‐4 treatments, or IL‐7 therapy, has also been suggested (Hotchkiss et al, 2019a,b; Francois et al, 2018). Another approach is to boost immune function by vaccination; a multi‐centre trial using a polyvalent conjugate pneumococcal vaccine is ongoing in the UK (https://clinicaltrials.gov/ct2/show/NCT03565159).
Conclusions
Tremendous progress in basic science together with numerous clinical/epidemiological studies has increased our knowledge of sepsis. However, these advances have not yet translated into the development of effective new treatments. Rather, they have demonstrated the complexity and heterogeneity of the syndrome, and the need to better target interventions to the right patient subset, at the right time, at an optimal dose and for an optimal duration. This requires better diagnostics and theranostics to first identify suitable patients, and then titrate treatment to an optimal endpoint, rather than adopt a rather naïve and simplistic “one‐size‐fits‐all” philosophy. Such diagnostics and theranostics need to be measured rapidly to enable timely intervention, and suitable targets where modification will impact the outcome need to be identified. These should be tested in improved translational preclinical models that better reflect the human illness with appropriate timing of treatment.
Without such a change in the approach, it is unlikely that the battle against sepsis will succeed. It is also important to recognize that, even with the best care, only a proportion of septic patients can be rescued.
It is also important to rethink treatment goals for survivors as many will suffer from multiple physical, psychological, and cognitive dysfunctions that may be permanent (Tinetti et al, 2016). This requires expansion of our understanding of the disease at the molecular and cellular levels. We advocate for clinical studies that have hypotheses that can be carefully tested in relevant long‐term animal models (Efron et al, 2015). The bridge between clinical and basic studies in sepsis needs to be rebuilt and made two‐way.
Pending issues.
-
(i)
Identification of critical pathophysiological events in development of sepsis and distinction between causative, adaptive or bystander phenomena.
-
(ii)
Better understanding of the local tissue‐specific responses during sepsis.
-
(iii)
Improvement of the preclinical models for both basic and translation research: animal models more relevant than rodents (e.g., rabbits, squirrel monkeys, pigs), site of infection frequently found in human sepsis (e.g., lungs…), clinical isolates.
-
(iv)
Development of integrated personalized approach that combines clinical phenotypes, biomarkers, and endotypes unraveled by ‐omics technologies, microfluidic approaches, and artificial intelligence technologies.
-
(v)
Better design of clinical trials to stratify or identify patient populations that may benefit from treatment (prognostic or predictive enrichment) and thus decrease heterogeneity; use of more relevant endpoint (such as quality‐of‐life after sepsis).
Author contributions
J‐MC, MS, and TS contributed to the conception, the writing, and the editing of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
For more information
Acknowledgements
T. Skirecki was supported by the Polish National Science Centre (Grant no. 2013/11/N/NZ6/02581 and UMO‐2016/23/D/NZ6/02554).
EMBO Mol Med (2020) 12: e10128
See the Glossary for abbreviations used in this article.
References
- Acker CG, Singh AR, Flick RP, Bernardini J, Greenberg A, Johnson JP (2000) A trial of thyroxine in acute renal failure. Kidney Int 57: 293–298 [DOI] [PubMed] [Google Scholar]
- Adembri C, Selmi V, Vitali L, Tani A, Margheri M, Loriga B, Carlucci M, Nosi D, Formigli L, De Gaudio AR (2014) Minocycline but not tigecycline is neuroprotective and reduces the neuroinflammatory response induced by the superimposition of sepsis upon traumatic brain injury. Crit Care Med 42: e570–e582 [DOI] [PubMed] [Google Scholar]
- Adib‐Conquy M, Adrie C, Fitting C, Gattoliat O, Beyaert R, Cavaillon JM (2006) Up‐regulation of MyD88s and SIGIRR, molecules inhibiting Toll‐like receptor signaling, in monocytes from septic patients. Crit Care Med 34: 2377–2385 [DOI] [PubMed] [Google Scholar]
- Alverdy JC, Krezalek MA (2017) Collapse of the microbiome, emergence of the pathobiome, and the Immunopathology of Sepsis. Crit Care Med 45: 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreis DT, Singer M (2016) Catecholamines for inflammatory shock: a Jekyll‐and‐Hyde conundrum. Intensive Care Med 42: 1387–1397 [DOI] [PubMed] [Google Scholar]
- Angelousi AG, Karageorgopoulos DE, Kapaskelis AM, Falagas ME (2011) Association between thyroid function tests at baseline and the outcome of patients with sepsis or septic shock: a systematic review. Eur J Endocrinol 164: 147–155 [DOI] [PubMed] [Google Scholar]
- Angurana SK, Bansal A, Singhi S, Aggarwal R, Jayashree M, Salaria M, Mangat NK (2018) Evaluation of effect of probiotics on cytokine levels in critically ill children with severe sepsis: a double‐blind, placebo‐controlled trial. Crit Care Med 46: 1656–1664 [DOI] [PubMed] [Google Scholar]
- Annane D, Sharshar T (2015) Cognitive decline after sepsis. Lancet Respir Med 3: 61–69 [DOI] [PubMed] [Google Scholar]
- Annane D (2016) The role of ACTH and corticosteroids for sepsis and septic shock: an update. Front Endocrinol (Lausanne) 7: 70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antcliffe DB, Burnham KL, Al‐Beidh F, Santhakumaran S, Brett SJ, Hinds CJ, Ashby D, Knight JC, Gordon AC (2019) Transcriptomic signatures in sepsis and a differential response to steroids. From the VANISH randomized trial. Am J Respir Crit Care Med 199: 980–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arefian H, Heublein S, Scherag A, Brunkhorst FM, Younis MZ, Moerer O, Fischer D, Hartmann M (2017) Hospital‐related cost of sepsis: a systematic review. J Infection 74: 107–117 [DOI] [PubMed] [Google Scholar]
- Axer H, Grimm A, Pausch C, Teschner U, Zinke J, Eisenach S, Beck S, Guntinas‐Lichius O, Brunkhorst FM, Witte OW (2016) The impairment of small nerve fibers in severe sepsis and septic shock. Crit Care 20: 64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggs J, Jernigan JA, Halpin AL, Epstein L, Hatfield KM, McDonald LC (2018) Risk of subsequent sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin Infect Dis 66: 1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa E, Saavedra‐Leiva F, Cea LA, Vargas AA, Ramirez V, Escamilla R, Saez JC, Regueira T (2018) Sepsis‐induced channelopathy in skeletal muscles is associated with expression of non‐selective channels. Shock 49: 221–228 [DOI] [PubMed] [Google Scholar]
- Balmer ML, Hess C (2017) Starving for survival‐how catabolic metabolism fuels immune function. Curr Opin Immunol 46: 8–13 [DOI] [PubMed] [Google Scholar]
- Barichello T, Sayana P, Giridharan VV, Arumanayagam AS, Narendran B, Della Giustina A, Petronilho F, Quevedo J, Dal‐Pizzol F (2019) Long‐term cognitive outcomes after sepsis: a translational systematic review. Mol Neurobiol 56: 186–251 [DOI] [PubMed] [Google Scholar]
- Barreiro LB, Marioni JC, Blekhman R, Stephens M, Gilad Y (2010) Functional comparison of innate immune signaling pathways in primates. PLoS Genet 6: e1001249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt J, dos Santos CC, Cameron JI, Herridge MS (2013) Intensive care unit‐acquired weakness: clinical phenotypes and molecular mechanisms. Am J Respir Crit Care Med 187: 238–246 [DOI] [PubMed] [Google Scholar]
- Bauer M, Coldewey SM, Leitner M, Löffler B, Weis S, Wetzker R (2018) Deterioration of organ function as a hallmark in sepsis: the cellular perspective. Front Immunol 9: 1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC, Hawker FH, To C, Stewart PM, Holman SR (1998) Thirty‐day monitoring of insulin‐like growth factors and their binding proteins in intensive care unit patients. Growth Horm IGF Res 8: 455–463 [DOI] [PubMed] [Google Scholar]
- Benjamin DJ, Berger JO, Johannesson M, Nosek BA, Wagenmakers E‐J, Berk R, Bollen KA, Brembs B, Brown L, Camerer C et al (2018) Redefine statistical significance. Nat Hum Behav 2: 6–10 [DOI] [PubMed] [Google Scholar]
- Bermejo‐Martin JF, Andaluz‐Ojeda D, Almansa R, Gandía F, Gómez‐Herreras JI, Gomez‐Sanchez E, Heredia‐Rodríguez M, Eiros JM, Kelvin DJ, Tamayo E (2016) Defining immunological dysfunction in sepsis: a requisite tool for precision medicine. J infection 72: 525–536 [DOI] [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali‐Mouhim A et al (2016) Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532: 512–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldock E, Surewaard BGJ, Shamarina D, Na M, Fei Y, Ali A, Williams A, Pollitt EJG, Szkuta P, Morris P et al (2018) Human skin commensals augment Staphylococcus aureus pathogenesis. Nat Microbiol 3: 881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt J, Menges T, Kuhn D, Diridis C, Hempelmann G (1995) Alterations in circulating vasoactive substances in the critically ill–a comparison between survivors and non‐survivors. Intensive Care Med 21: 218–225 [DOI] [PubMed] [Google Scholar]
- Bomsztyk K, Mar D, An D, Sharifian R, Mikula M, Gharib SA, Altemeier WA, Liles WC, Denisenko O (2015) Experimental acute lung injury induces multi‐organ epigenetic modifications in key angiogenic genes implicated in sepsis‐associated endothelial dysfunction. Crit Care 19: 225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101: 1644–1655 [DOI] [PubMed] [Google Scholar]
- Borken F, Markwart R, Requardt RP, Schubert K, Spacek M, Verner M, Rückriem S, Scherag A, Oehmichen F, Brunkhorst FM et al (2017) Chronic critical illness from sepsis is associated with an enhanced TCR response. J Immunol 198: 4781–4791 [DOI] [PubMed] [Google Scholar]
- Brakenridge SC, Moore FA, Mercier NR, Cox M, Wu Q, Moldawer LL, Mohr AM, Efron PA, Smith RS (2019) Persistently elevated glucagon‐like peptide‐1 levels among critically Ill surgical patients after sepsis and development of chronic critical illness and dismal long‐term outcomes. J Am Coll Surg 229: 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223 [DOI] [PubMed] [Google Scholar]
- Brown RL, Sequeira RP, Clarke TB (2017) The microbiota protects against respiratory infection via GM‐CSF signaling. Nat Commun 8: 1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KL, Davenport EE, Radhakrishnan J, Humburg P, Gordon AC, Hutton P, Svoren‐Jabalera E, Garrard C, Hill AVS, Hinds CJ et al (2017) Shared and distinct aspects of the sepsis transcriptomic response to fecal peritonitis and pneumonia. Am J Respir Crit Care Med 196: 328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera‐Perez J, Babcock JC, Dileepan T, Murphy KA, Kucaba TA, Badovinac VP, Griffith TS (2016) Gut microbial membership modulates CD4 T cell reconstitution and function after sepsis. J Immunol 197: 1692–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar‐Hari M, McDowell C, Laffey JG, O'Kane CM, McAuley DF et al (2018) Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med 6: 691–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV (1986) Multiple‐organ‐failure syndrome. Arch Surg 121: 196–208 [DOI] [PubMed] [Google Scholar]
- Cavaillon JM, Adib‐Conquy M, Fitting C, Adrie C, Payen D (2003) Cytokine cascade in sepsis. Scand J Infect Dis 35: 535–544 [DOI] [PubMed] [Google Scholar]
- Cavaillon JM, Adrie C, Fitting C, Adib‐Conquy M (2005) Reprogramming of circulatory cells in sepsis and SIRS. J Endotoxin Res 11: 311–320 [DOI] [PubMed] [Google Scholar]
- Cavaillon J‐M, Eisen D, Annane D (2014) Is boosting the immune system in sepsis appropriate? Crit Care 18: 216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J‐M (2018) Exotoxins and endotoxins: inducers of inflammatory cytokines. Toxicon 149: 45–53 [DOI] [PubMed] [Google Scholar]
- Cavaillon J‐M, Giamarellos‐Bourboulis EJ (2019) “Immunosuppression” is inappropriately qualifying the immune status of septic and SIRS patients. Shock 52: 307–317 [DOI] [PubMed] [Google Scholar]
- Cavassani KA, Carson WF IV, Moreira AP, Wen H, Schaller MA, Ishii M, Lindell DM, Dou Y, Lukacs NW, Keshamouni VG et al (2010) The post sepsis‐induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood 115: 4403–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CL, Sung PH, Chen KH, Shao PL, Yang CC, Cheng BC, Lin KC, Chen CH, Chai HT, Chang HW et al (2018) Adipose‐derived mesenchymal stem‐cell derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am J Transl Res 10: 1053–1070 [PMC free article] [PubMed] [Google Scholar]
- Chavan SS, Huerta PT, Robbiati S, Valdes‐Ferrer SI, Ochani M, Dancho M, Frankfurt M, Volpe BT, Tracey KJ, Diamond B (2012) HMGB1 mediates cognitive impairment in sepsis survivors. Mol Med 18: 930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SC, Scicluna BP, Arts RJ, Gresnigt MS, Lachmandas E, Giamarellos‐Bourboulis EJ, Kox M, Manjeri GR, Wagenaars JA, Cremer OL et al (2016) Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 17: 406–413 [DOI] [PubMed] [Google Scholar]
- Cheng KT, Xiong S, Ye Z, Hong Z, Di A, Tsang KM, Gao X, An S, Mittal M, Vogel SM et al (2017) Caspase‐11‐mediated endothelial pyroptosis underlies endotoxemia‐induced lung injury. J Clin Invest 127: 4124–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan AM, Huber‐Lang M, Kumar‐Sinha C, Barrette TR, Shankar‐Sinha S, Sarma VJ, Padgaonkar VA, Ward PA (2001) Molecular signatures of sepsis: multiorgan gene expression profiles of systemic inflammation. Am J Pathol 159: 1199–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JA, Coopersmith CM (2007) Intestinal crosstalk: a new paradigm for understanding the gut as the ‘motor’ of critical illness. Shock 28: 384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claushuis TAM, van Vught LA, Scicluna BP, Wiewel MA, Klein Klouwenberg PMC, Hoogendijk AJ, Ong DSY, Cremer OL, Horn J, Franitza M et al (2016) Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood 127: 3062–3072 [DOI] [PubMed] [Google Scholar]
- Condotta SA, Rai D, James BR, Griffith TS, Badovinac VP (2013) Sustained and incomplete recovery of naive CD8+ T cell precursors after sepsis contributes to impaired CD8+ T cell responses to infection. J Immunol 190: 1991–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway Morris A, Datta D, Shankar‐Hari M, Stephen J, Weir CJ, Rennie J, Antonelli J, Bateman A, Warner N, Judge K et al (2018) Cell‐surface signatures of immune dysfunction risk‐stratify critically ill patients: INFECT study. Intensive Care Med 44: 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell KT, Soybel DI, Lang CH (2017) Restorative mechanisms regulating protein balance in skeletal muscle during recovery from sepsis. Shock 47: 463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csóka B, Németh ZH, Törő G, Koscsó B, Kókai E, Robson SC, Enjyoji K, Rolandelli RH, Erdélyi K, Pacher P et al (2015) CD39 improves survival in microbial sepsis by attenuating systemic inflammation. FASEB J 29: 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Norling LV, Montero‐Melendez T, Canova DF, Lashin H, Pavlov AM, Sukhorukov GB, Hinds CJ, Perretti M (2014) Microparticle alpha‐2‐macroglobulin enhances pro‐resolving responses and promotes survival in sepsis. EMBO Mol Med 6: 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danahy DB, Jensen IJ, Griffith TS, Badovinac VP (2019) Polymicrobial sepsis has the capacity to reinvigorate tumor‐infiltrating CD8 T cells and prolong host survival. J Immunol 202: 2843–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh A, Inglis HC, Abdel‐Mohsen M, Deng X, Adelman A, Schechtman KB, Heitman JW, Vilardi R, Shah A, Keating SM et al (2018) Granulocyte‐derived extracellular vesicles activate monocytes and are associated with mortality in intensive care unit patients. Front Immunol 9: 956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dark P, Blackwood B, Gates S, McAuley D, Perkins GD, McMullan R, Wilson C, Graham D, Timms K, Warhurst G (2015) Accuracy of LightCycler® SeptiFast for the detection and identification of pathogens in the blood of patients with suspected sepsis: a systematic review and meta‐analysis. Intensive Care Med 41: 21–33 [DOI] [PubMed] [Google Scholar]
- Davenport EE, Burnham KL, Radhakrishnan J, Humburg P, Hutton P, Mills TC, Rautanen A, Gordon AC, Garrard C, Hill AV et al (2016) Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med 4: 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydow DS, Hough CL, Langa KM, Iwashyna TJ (2013) Symptoms of depression in survivors of severe sepsis: a prospective cohort study of older Americans. Am J Geriatr Psychiatry 21: 887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger RP, Bagshaw SM, Antonelli M, Foster DM, Klein DJ, Marshall JC, Palevsky PM, Weisberg LS, Schorr CA, Trzeciak S et al (2018) Effect of targeted polymyxin B hemoperfusion on 28‐day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA 320: 1455–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMerle KM, Vincent BM, Iwashyna TJ, Prescott HC (2017) Increased healthcare facility use in veterans surviving sepsis hospitalization. J Crit Care 42: 59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP, Erb‐Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB (2015) Intraalveolar catecholamines and the human lung microbiome. Am J Respir Crit Care Med 192: 257–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb‐Downward JR, Standiford TJ, Huffnagle GB (2016) Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol 1: 16113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson A, Singer M (2009) Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med 37: S30–S37 [DOI] [PubMed] [Google Scholar]
- Efron PA, Mohr AM, Moore FA, Moldawer LL (2015) The future of murine sepsis and trauma research models. J Leukoc Biol 98: 945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler J, Barrett LK, Taylor V, Groves M, Scaravilli F, Wittstock M, Kolbaske S, Grossmann A, Henschel J, Gloger M et al (2017) Translational evidence for two distinct patterns of neuroaxonal injury in sepsis: a longitudinal, prospective translational study. Crit Care 21: 262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett F, Jorgensen J, Marand AL, Liu YM, Martinez MM, Sein V, Butler KL, Lee J, Irimia D (2018) Diagnosis of sepsis from a drop of blood by measurement of spontaneous neutrophil motility in a microfluidic assay. Nat Biomed Eng 2: 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoll P, del Fresno C, Garcia L, Valles G, Lendinez MJ, Arnalich F, Lopez‐Collazo E (2003) Rapid up‐regulation of IRAK‐M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem Biophys Res Commun 311: 465–472 [DOI] [PubMed] [Google Scholar]
- Faim F, Passaglia P, Batalhao M, Lacchini R, Stabile AM, Carnio EC (2019) Role of ghrelin on growth hormone/insulin‐like growth factor‐1 axis during endotoxemia. Growth Horm IGF Res 48–49: 36–44 [DOI] [PubMed] [Google Scholar]
- Fairbairn L, Kapetanovic R, Beraldi D, Sester DP, Tuggle CK, Archibald AL, Hume DA (2013) Comparative analysis of monocyte subsets in the pig. J Immunol 190: 6389–6396 [DOI] [PubMed] [Google Scholar]
- Ferrer M, Méndez‐García C, Rojo D, Barbas C, Moya A (2017) Antibiotic use and microbiome function. Biochem Pharmacol 134: 114–126 [DOI] [PubMed] [Google Scholar]
- Fitting C, Parlato M, Adib‐Conquy M, Memain N, Philippart F, Misset B, Monchi M, Cavaillon JM, Adrie C (2012) DNAemia detection by multiplex PCR and biomarkers for infection in systemic inflammatory response syndrome patients. PLoS One 7: e38916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann C, Thomas‐Rueddel DO, Hartmann M, Hartog CS, Welte T, Heublein S, Dennler U, Reinhart K (2016) Hospital incidence and mortality rates of sepsis. Dtsch Arztebl Int 113: 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AC, McConnell KW, Yoseph BP, Breed E, Liang Z, Clark AT, O'Donnell D, Zee‐Cheng B, Jung E, Dominguez JA et al (2012) The endogenous bacteria alter gut epithelial apoptosis and decrease mortality following Pseudomonas aeruginosa pneumonia. Shock 38: 508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmelé T, Blood T, Morre M et al (2018) Interleukin‐7 restores lymphocytes in septic shock: the IRIS‐7 randomized clinical trial. JCI Insight 3: 98960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg DE, Zhou MJ, Cohen ME, Annavajhala MK, Khan S, Moscoso DI, Brooks C, Whittier S, Chong DH, Uhlemann AC et al (2018) Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med 44: 1203–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich O, Reid MB, Van den Berghe G, Vanhorebeek I, Hermans G, Rich MM, Larsson L (2015) The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev 95: 1025–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke R, Hifumi T, Kondo Y, Hatakeyama J, Takei T, Yamakawa K, Inoue S, Nishida O (2018) Early rehabilitation to prevent postintensive care syndrome in patients with critical illness: a systematic review and meta‐analysis. BMJ Open 8: e019998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C (2018) Immunosenescence and inflamm‐aging as two sides of the same coin: friends or foes? Front Immunol 8: 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fux CA, Shirtliff M, Stoodley P, Costerton JW (2005) Can laboratory reference strains mirror ‘real‐world’ pathogenesis? Trends Microbiol 13: 58–63 [DOI] [PubMed] [Google Scholar]
- Garraud O, Hamzeh‐Cognasse H, Pozzetto B, Cavaillon J‐M, Cognasse F (2013) Bench‐to‐bedside review: platelets and active immune functions ‐ new clues for immunopathology? CritCare 17: 236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry S, Hajage D, Schortgen F, Martin‐Lefevre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N, Carpentier D et al (2016) Initiation strategies for renal‐replacement therapy in the intensive care unit. N Engl J Med 375: 122–133 [DOI] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S et al (2012) Gene‐expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13: 1118–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawaz M, Fateh‐Moghadam S, Pilz G, Gurland HJ, Werdan K (1995) Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. EurJ Clin Invest 25: 843–851 [DOI] [PubMed] [Google Scholar]
- Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA (2012) Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 72: 1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile LF, Nacionales DC, Cuenca AG, Armbruster M, Ungaro RF, Abouhamze AS, Lopez C, Baker HV, Moore FA, Ang DN et al (2013) Identification and description of a novel murine model for polytrauma and shock. Crit Care Med 41: 1075–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile LF, Moldawer LL (2014) HMGB1 as a therapeutic target for sepsis: it's all in the timing!. Expert Opin Ther Targets 18: 243–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Szpila BE, Cuenca AG, Joseph A, Moore FA, Leeuwenburgh C et al (2014) Host responses to sepsis vary in different low‐lethality murine models. PLoS One 9: e94404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geven C, Blet A, Kox M, Hartmann O, Scigalla P, Zimmermann J, Marx G, Laterre PF, Mebazaa A, Pickkers P (2019) A double‐blind, placebo‐controlled, randomised, multicentre, proof‐of‐concept and dose‐finding phase II clinical trial to investigate the safety, tolerability and efficacy of adrecizumab in patients with septic shock and elevated adrenomedullin concentration (AdrenOSS‐2). BMJ Open 9: e024475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geven C, van Lier D, Blet A, Peelen R, Ten Elzen B, Mebazaa A, Kox M, Pickkers P (2018) Safety, tolerability and pharmacokinetics/pharmacodynamics of the adrenomedullin antibody adrecizumab in a first‐in‐human study and during experimental human endotoxaemia in healthy subjects. Brit J Clin Pharmacol 84: 2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, Jansson JK, Dorrestein PC, Knight R (2016) Microbiome‐wide association studies link dynamic microbial consortia to disease. Nature 535: 94–103 [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R (2018) Current understanding of the human microbiome. Nat Med 24: 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos C, Kotsaki A, Pelekanou A, Giannikopoulos G, Vaki I, Maravitsa P, Adamis S, Alexiou Z, Andrianopoulos G, Antonopoulou A et al (2010) Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care 14: R96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Jüni P, Brodie D, Slutsky AS, Combes A (2018) Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc bayesian analysis of a randomized clinical trial. JAMA 320: 2251–2259 [DOI] [PubMed] [Google Scholar]
- Gomez H, Jin K, Kellum JA (2015) The role of energy regulation in the tubular epithelial cell response to sepsis. Nephron 131: 255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes J, Alard J‐E, Drechsler M, Wantha S, Mörgelin M, Kuebler WM, Jacobs M, von Hundelshausen P, Markart P, Wygrecka M et al (2012) Disruption of platelet‐derived chemokine heteromers prevents neutrophil extravasation in acute lung injury. Am J Respir Crit Care Med 185: 628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunst J, De Bruyn A, Van den Berghe G (2019) Glucose control in the ICU. Curr Opin Anaesthesiol 32: 156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutbier B, Neuhauß AK, Reppe K, Ehrler C, Santel A, Kaufmann J, Scholz M, Weissmann N, Morawietz L, Mitchell TJ et al (2018) Prognostic and pathogenic role of angiopoietin‐1 and ‐2 in pneumonia. Am J Respir Crit Care Med 198: 220–231 [DOI] [PubMed] [Google Scholar]
- Han Y‐G, Qin X, Zhang T, Lei M, Sun F‐Y, Sun J‐J, Yuan W‐F (2018) Electroacupuncture prevents cognitive impairment induced by lipopolysaccharide via inhibition of oxidative stress and neuroinflammation. Neurosci Lett 683: 190–195 [DOI] [PubMed] [Google Scholar]
- Hara N, Chijiiwa M, Yara M, Ishida Y, Ogiwara Y, Inazu M, Kuroda M, Karlsson M, Sjovall F, Elmér E, Uchino H (2015) Metabolomic analyses of brain tissue in sepsis induced by cecal ligation reveal specific redox alterations‐protective effects of the oxygen radical scavenger edaravone. Shock 44: 578–584 [DOI] [PubMed] [Google Scholar]
- Hasegawa‐Ishii S, Inaba M, Umegaki H, Unno K, Wakabayashi K, Shimada A (2016) Endotoxemia‐induced cytokine‐mediated responses of hippocampal astrocytes transmitted by cells of the brain‐immune interface. Sci Rep 6: 25457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiba M, Huq A, Tomino A, Hirakawa A, Hattori T, Miyabe H, Tsuda M, Takeyama N (2015) Neutrophil extracellular traps in patients with sepsis. J Surg Res 194: 248–254 [DOI] [PubMed] [Google Scholar]
- Hassan U, Ghonge T, Reddy B, Patel M, Rappleye M, Taneja I, Tanna A, Healey R, Manusry N, Price Z et al (2017) A point‐of‐care microfluidic biochip for quantification of CD64 expression from whole blood for sepsis stratification. Nat Commun 8: 15949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan FI, Didari T, Khan F, Mojtahedzadeh M, Abdollahi M (2018) The role of epigenetic alterations involved in sepsis: an overview. Curr Pharm Des 24: 2862–2869 [DOI] [PubMed] [Google Scholar]
- Hawchar F, László I, Öveges N, Trásy D, Ondrik Z, Molnar Z (2019) Extracorporeal cytokine adsorption in septic shock: a proof of concept randomized, controlled pilot study. J Crit Care 49: 172–178 [DOI] [PubMed] [Google Scholar]
- He H, Geng T, Chen P, Wang M, Hu J, Kang L, Song W, Tang H (2016) NK cells promote neutrophil recruitment in the brain during sepsis‐induced neuroinflammation. Sci Rep 6: 27711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans G, Van den Berghe G (2015) Clinical review: intensive care unit acquired weakness. Crit Care 19: 274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR (2001) Reversal of catabolism by beta‐blockade after severe burns. N Engl J Med 345: 1223–1229 [DOI] [PubMed] [Google Scholar]
- Heuer JG, Zhang T, Zhao J, Ding C, Cramer M, Justen KL, Vonderfecht SL, Na S (2005) Adoptive transfer of in vitro‐stimulated CD4+CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J Immunol 174: 7141–7146 [DOI] [PubMed] [Google Scholar]
- Hill NE, Murphy KG, Saeed S, Phadke R, Chambers D, Wilson DR, Brett SJ, Singer M (2017) Impact of ghrelin on body composition and muscle function in a long‐term rodent model of critical illness. PLoS One 12: e0182659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DB, Ozment TR, Wondergem R, Li C, Williams DL (2015) Impaired heart rate regulation and depression of cardiac chronotropic and dromotropic function in polymicrobial sepsis. Shock 43: 185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi H, Loftus TJ, Hawkins RB et al (2018) Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol 9: 595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoser GA, Skirecki T, Złotorowicz M, Zielińska‐Borkowska U, Kawiak J (2012) Absolute counts of peripheral blood leukocyte subpopulations in intraabdominal sepsis and pneumonia‐derived sepsis: a pilot study. Folia Histochem Cytobiol 50: 420–426 [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Monneret G, Payen D (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13: 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED, Martin GS, Coopersmith CM, Brakenridge S, Mayr FB et al (2019a) Immune checkpoint inhibition in sepsis: a phase 1b randomized, placebo‐controlled, single ascending dose study of antiprogrammed cell death‐ligand 1 antibody. Crit Care Med 47: 632–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Colston E, Yende S, Crouser ED, Martin GS, Albertson T, Bartz RR, Brakenridge SC, Delano MJ, Park PK et al (2019b) Immune checkpoint inhibition in sepsis: a Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med 45: 1360–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iapichino G, Callegari ML, Marzorati S, Cigada M, Corbella D, Ferrari S, Morelli L (2008) Impact of antibiotics on the gut microbiota of critically ill patients. J Med Microbiol 57: 1007–1014 [DOI] [PubMed] [Google Scholar]
- Ingels C, Gunst J, Van den Berghe G (2018) Endocrine and metabolic alterations in sepsis and implications for treatment. Crit Care Clin 34: 81–96 [DOI] [PubMed] [Google Scholar]
- Iskander KN, Craciun FL, Stepien DM, Duffy ER, Kim J, Moitra R, Vaickus LJ, Osuchowski MF, Remick DG (2013) Cecal ligation and puncture‐induced murine sepsis does not cause lung injury. Crit Care Med 41: 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD (2000) Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun 68: 1265–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen ME, Johansson PI, Ostrowski SR, Bestle MH, Hein L, Jensen AL, Søe‐Jensen P, Andersen MH, Steensen M, Mohr T et al (2015) Profound endothelial damage predicts impending organ failure and death in sepsis. Semin Thromb Hemost 41: 16–25 [DOI] [PubMed] [Google Scholar]
- Kapetanovic R, Parlato M, Fitting C, Quesniaux V, Cavaillon JM, Adib‐Conquy M (2011) Mechanisms of TNF induction by heat‐killed Staphylococcus aureus differ upon the origin of mononuclear phagocytes. Am J Physiol Cell Physiol 300: C850–C859 [DOI] [PubMed] [Google Scholar]
- Karp CL (2012) Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med 209: 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Matsuura K (2018) Recombinant human soluble thrombomodulin improves mortality in patients with sepsis especially for severe coagulopathy: a retrospective study. Thromb J 16: 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane C, Jerkic M, Laffey JG (2017) Stem cell‐based therapies for sepsis. Anesthesiology 127: 1017–1034 [DOI] [PubMed] [Google Scholar]
- Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A et al (2015) Crosstalk between microbiota‐derived short‐chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17: 662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshari RS, Silasi R, Popescu NI, Patel MM, Chaaban H, Lupu C, Coggeshall KM, Mollnes TE, DeMarco SJ, Lupu F (2017) Inhibition of complement C5 protects against organ failure and reduces mortality in a baboon model of Escherichia coli sepsis. Proc Natl Acad Sci USA 114: E6390–E6399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OY, Monsel A, Bertrand M, Coriat P, Cavaillon JM, Adib‐Conquy M (2010) Differential down‐regulation of HLA‐DR on monocyte subpopulations during systemic inflammation. Crit Care 14: R61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith NJ, Coopersmith CM (2016) The Gut as the motor of multiple organ dysfunction in critical illness. Crit Care Clin 32: 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König R, Kolte A, Ahlers O, Oswald M, Röll D, Dimopoulos G, Tsangaris I, Antoniadou E, Bogatsch H, Löffler M et al (2018) Use of IFNγ/IL10 ratio for stratification of hydrocortisone therapy in patients with septic shock. bioRxiv 10.1101/502864 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg SJ, Wadgaonkar R (2017) Sepsis‐associated encephalopathy: the blood‐brain barrier and the sphingolipid rheostat. Front Immunol 8: 597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann G, Spies C, Schenk T, Brunkhorst FM, Balzer F, La Rosée P (2018) Hemophagocytic lymphohistiocytosis. Shock 50: 149–155 [DOI] [PubMed] [Google Scholar]
- Lankelma JM, Birnie E, Weehuizen TAF, Scicluna BP, Belzer C, Houtkooper RH, Roelofs JJTH, de Vos AF, van der Poll T, Budding AE, Wiersinga WJ (2017) The gut microbiota as a modulator of innate immunity during melioidosis. PLoS Negl Trop Dis 11: e0005548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leligdowicz A, Richard‐Greenblatt M, Wright J, Crowley VM, Kain KC (2018) Endothelial activation: the Ang/Tie axis in sepsis. Front Immunol 9: 838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelubre C, Vincent JL (2018) Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol 14: 417–427 [DOI] [PubMed] [Google Scholar]
- Levi M, van der Poll T (2017) Coagulation and sepsis. Thromb Res 149: 38–44 [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent J‐L, Ramsay G, International Sepsis Definitions Conference (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International sepsis definitions conference. Intensive Care Med 29: 530–538 [DOI] [PubMed] [Google Scholar]
- Lewis AJ, Yuan D, Zhang X, Angus DC, Rosengart MR, Seymour CW (2016) Use of biotelemetry to define physiology‐based deterioration thresholds in a murine cecal ligation and puncture model of sepsis. Crit Care Med 44: e420–e431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhu F‐X, Zhao X‐J, An Y‐Z (2016) The immunoprotective activity of interleukin‐33 in mouse model of cecal ligation and puncture‐induced sepsis. Immunol lett 169: 1–7 [DOI] [PubMed] [Google Scholar]
- Li S, Lv J, Li J, Zhao Z, Guo H, Zhang Y, Cheng S, Sun J, Pan H, Fan S, Li Z (2018) Intestinal microbiota impact sepsis associated encephalopathy via the vagus nerve. Neurosci Lett 662: 98–104 [DOI] [PubMed] [Google Scholar]
- Lin Z, Liu X, Sun L, Li J, Hu Z, Xie H, Zu X, Deng X, Zhang W (2016) Comparison of sepsis rats induced by caecal ligation puncture or Staphylococcus aureus using a LC‐QTOF‐MS metabolomics approach. Infect Gen Evol 43: 86–93 [DOI] [PubMed] [Google Scholar]
- Lipton J, Fossler D (1974) Fever produced in the squirrel monkey by intravenous and intracerebral endotoxin. Am J Physiol 226: 1022–1027 [DOI] [PubMed] [Google Scholar]
- Mandal P, Feng Y, Lyons JD, Berger SB, Otani S, DeLaney A, Tharp GK, Maner‐Smith K, Burd EM, Schaeffer M et al (2018) Caspase‐8 collaborates with caspase‐11 to drive tissue damage and execution of endotoxic shock. Immunity 49: 42–55.e46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannick JB, Morris M, Hockey H‐UP, Roma G, Beibel M, Kulmatycki K, Watkins M, Shavlakadze T, Zhou W, Quinn D et al (2018) TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med 10: eaaq1564 [DOI] [PubMed] [Google Scholar]
- Martelli D, Yao ST, McKinley MJ, McAllen RM (2014) Reflex control of inflammation by sympathetic nerves, not the vagus. J Physiol 592: 1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín S, Pérez A, Aldecoa C (2017) Sepsis and immunosenescence in the elderly patient: a review. Front Med 4: 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias B, Delmas AL, Ozrazgat‐Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL et al (2017) Human myeloid‐derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg 265: 827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazeraud A, Pascal Q, Verdonk F, Heming N, Chretien F, Sharshar T (2016) Neuroanatomy and physiology of brain dysfunction in sepsis. Clin Chest Med 37: 333–345 [DOI] [PubMed] [Google Scholar]
- McPeak MB, Youssef D, Williams DA, Pritchett CL, Yao ZQ, McCall CE, El Gazzar M (2017) Myeloid cell‐specific deletion of Cebpb decreases sepsis‐induced immunosuppression in mice. J Leukoc Biol 102: 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS, Soares MP (2012) Disease tolerance as a defense strategy. Science 335: 936–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber‐Carstens S, Hasper D, Keh D, Zuckermann H et al (2009) Granulocyte‐macrophage colony‐stimulating factor to reverse sepsis‐associated immunosuppression: a double‐blind, randomized, placebo‐controlled multicenter trial. Am J Respir Crit Care Med 180: 640–648 [DOI] [PubMed] [Google Scholar]
- Meng W, Paunel‐Görgülü A, Flohé S, Hoffmann A, Witte I, MacKenzie C, Baldus SE, Windolf J, Lögters TT (2012) Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit Care 16: R137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng M, Klingensmith NJ, Coopersmith CM (2017) New insights into the gut as the driver of critical illness and organ failure. Curr Opin Crit Care 23: 143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Jiao Y, Zhang G, Xu X, Ji C, Hu M, Lai Z, Zhang M (2018) Electroacupuncture improves intestinal dysfunction in septic patients: a randomised controlled trial. Biomed Res Int 2018: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW (2004) Of mice and not men: differences between mouse and human immunology. J Immunol 172: 2731–2738 [DOI] [PubMed] [Google Scholar]
- Mickiewicz B, Tam P, Jenne CN, Leger C, Wong J, Winston BW, Doig C, Kubes P, Vogel H, Alberta Sepsis Network (2015) Integration of metabolic and inflammatory mediator profiles as a potential prognostic approach for septic shock in the intensive care unit. Crit Care 19: 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Lopansri BK, Burke JP, Levy M, Opal S, Rothman RE, D'Alessio FR, Sidhaye VK, Aggarwal NR, Balk R et al (2018) Validation of a host response assay, SeptiCyte LAB, for discriminating sepsis from systemic inflammatory response syndrome in the ICU. Am J Respir Crit Care Med 198: 903–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel J, Palao JC, Castells J, Desgeorges M, Busso T, Molliex S, Jahnke V, Del Carmine P, Gondin J, Arnould D et al (2017) Regulation of Akt‐mTOR, ubiquitin‐proteasome and autophagy‐lysosome pathways in locomotor and respiratory muscles during experimental sepsis in mice. Sci Rep 7: 10866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morowitz MJ, Di Caro V, Pang D, Cummings J, Firek B, Rogers MB, Ranganathan S, Clark RS, Aneja RK (2017) Dietary supplementation with nonfermentable fiber alters the gut microbiota and confers protection in murine models of sepsis. Crit Care Med 45: e516–e523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzer JT, Davis CG, Dunne BS, Unsinger J, Dunne WM, Hotchkiss RS (2006) Pneumonia after cecal ligation and puncture: a clinically relevant two hit model of sepsis. Shock 26: 565–570 [DOI] [PubMed] [Google Scholar]
- Müller‐Alouf H, Alouf JE, Gerlach D, Ozegowski JH, Fitting C, Cavaillon J‐M (1994) Comparative study of cytokine release by human peripheral blood mononuclear cells stimulated with Streptococcus pyogenes superantigen erythrogenic toxins, heat‐killed streptococci and lipopolysaccharide. Infect Immun 62: 4915–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacionales DC, Szpila B, Ungaro R, Lopez MC, Zhang J, Gentile LF, Cuenca AL, Vanzant E, Mathias B, Jyot J et al (2015) A detailed characterization of the dysfunctional immunity and abnormal myelopoiesis induced by severe shock and trauma in the aged. J Immunol 195: 2396–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Takeuchi T, Kawahara T, Hirose J, Nakayama K, Hosaka Y, Furusako S (2017) Simultaneous targeting of CD14 and factor XIa by a fusion protein consisting of an anti‐CD14 antibody and the modified second domain of bikunin improves survival in rabbit sepsis models. Eur J Pharmacol 802: 60–68 [DOI] [PubMed] [Google Scholar]
- Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis‐Dougherty A, Berney SC, Bienvenu OJ et al (2012) Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 40: 502–509 [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Nguyen TH, Ju SA, Lee YS, Han SH, Lee SC, Kwon BS, Yu R, Kim GY, Lee BJ et al (2013) CD137 expressed on neutrophils plays dual roles in antibacterial responses against Gram‐positive and Gram‐negative bacterial infections. Infect Immun 81: 2168–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TLA, Vieira‐Silva S, Liston A, Raes J (2015) How informative is the mouse for human gut microbiota research? Dis Model Mech 8: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitopoulou I, Kampisiouli E, Jahaj E, Vassiliou AG, Dimopoulou I, Mastora Z, Tsakiris S, Perreas K, Tzanela M, Routsi C et al (2019) Ghrelin alterations during experimental and human sepsis. Cytokine 127: 154937 [DOI] [PubMed] [Google Scholar]
- Oami T, Watanabe E, Hatano M, Teratake Y, Fujimura L, Sakamoto A, Ito C, Toshimori K, Swanson PE, Oda S (2018) Blocking liver autophagy accelerates apoptosis and mitochondrial injury in hepatocytes and reduces time to mortality in a murine sepsis model. Shock 50: 427–434 [DOI] [PubMed] [Google Scholar]
- O'Dwyer MJ, Starczewska MH, Schrenzel J, Zacharowski K, Ecker DJ, Sampath R, Brealey D, Singer M, Libert N, Wilks M et al (2017) The detection of microbial DNA but not cultured bacteria is associated with increased mortality in patients with suspected sepsis‐a prospective multi‐centre European observational study. Clin Microbiol Infect 23: 208.e1–208.e6 [DOI] [PubMed] [Google Scholar]
- Ojima M, Motooka D, Shimizu K, Gotoh K, Shintani A, Yoshiya K, Nakamura S, Ogura H, Iida T, Shimazu T (2016) Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig Dis Sci 61: 1628–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH (1999) Relationship between plasma levels of lipopolysaccharide (LPS) and LPS‐binding protein in patients with severe sepsis and septic shock. J Infect Dis 180: 1584–1598 [DOI] [PubMed] [Google Scholar]
- Opal SM, van der Poll T (2015) Endothelial barrier dysfunction in septic shock. J Intern Med 277: 277–293 [DOI] [PubMed] [Google Scholar]
- Orhun G, Esen F, Özcan PE, Sencer S, Bilgiç B, Ulusoy C, Noyan H, Küçükerden M, Ali A, Barburoğlu M et al (2018) Neuroimaging findings in sepsis‐induced brain Dysfunction: Association with Clinical and Laboratory Findings. Neurocrit Care 30: 106–117 [DOI] [PubMed] [Google Scholar]
- Osuchowski MF, Connett J, Welch K, Granger J, Remick DG (2009) Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit Care Med 37: 1567–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuchowski MF, Ayala A, Bahrami S, Bauer M, Boros M, Cavaillon J‐M, Chaudry IH, Coopersmith CM, Deutschman C, Drechsler S et al (2018) Minimum quality threshold in pre‐clinical sepsis studies (MQTIPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Shock 50: 377–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oved K, Cohen A, Boico O, Navon R, Friedman T, Etshtein L, Kriger O, Bamberger E, Fonar Y, Yacobov R et al (2015) A novel host‐proteome signature for distinguishing between acute bacterial and viral infections. PLoS One 10: e0120012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, Miller M, Barchuk WT, Fischkoff S, Kaul M et al (2004) Efficacy and safety of the monoclonal anti‐tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin‐6 levels. Crit Care Med 32: 2173–2182 [DOI] [PubMed] [Google Scholar]
- Parlato M, Souza‐Fonseca‐Guimaraes F, Philippart F, Misset B, Adib‐Conquy M, Cavaillon J‐M (2014) CD24‐triggered caspase‐dependent apoptosis via mitochondrial membrane depolarization and reactive oxygen species production of human neutrophils is impaired in sepsis. J Immunol 192: 2449–2459 [DOI] [PubMed] [Google Scholar]
- Parlato M, Cavaillon JM (2015) Host response biomarkers in the diagnosis of sepsis: a general overview. Methods Mol Biol 1237: 149–211 [DOI] [PubMed] [Google Scholar]
- Parlato M, Philippart F, Rouquette A, Moucadel V, Puchois V, Blein S, Bedos J‐P, Diehl J‐L, Hamzaoui O, Annane D et al (2018) Circulating biomarkers may be unable to detect infection at the early phase of sepsis in ICU patients: the CAPTAIN prospective multicenter cohort study. Intensive Care Med 44: 1061–1070 [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Chavan SS, Tracey KJ (2018) Molecular and functional neuroscience in immunity. Annu Rev Immunol 36: 783–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payen D, Mateo J, Cavaillon JM, Fraisse F, Floriot C, Vicaut E (2009) Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med 37: 803–810 [DOI] [PubMed] [Google Scholar]
- Peeters RP, Debaveye Y, Fliers E, Visser TJ (2006) Changes within the thyroid axis during critical illness. Crit Care Clin 22: 41–55 [DOI] [PubMed] [Google Scholar]
- Philippart F, Fitting C, Cavaillon JM (2012) Lung microenvironment contributes to the resistance of alveolar macrophages to develop tolerance to endotoxin*. Crit Care Med 40: 2987–2996 [DOI] [PubMed] [Google Scholar]
- Plenge RM, Scolnick EM, Altshuler D (2013) Validating therapeutic targets through human genetics. Nat Rev Drug Discovery 12: 581–594 [DOI] [PubMed] [Google Scholar]
- Poulsen JB, Moller K, Jensen CV, Weisdorf S, Kehlet H, Perner A (2011) Effect of transcutaneous electrical muscle stimulation on muscle volume in patients with septic shock. Crit Care Med 39: 456–461 [DOI] [PubMed] [Google Scholar]
- Preau S, Ambler M, Sigurta A, Kleyman A, Dyson A, Hill NE, Boulanger E, Singer M (2019) Protein recycling and limb muscle recovery after critical illness in slow‐ and fast‐twitch limb muscle. Am J Physiol Regul Integr Comp Physiol 316: R584–R593 [DOI] [PubMed] [Google Scholar]
- Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ (2015a) Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med 192: 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott HC, Langa KM, Iwashyna TJ (2015b) Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA 313: 1055–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott HC, Angus DC (2018) Enhancing recovery from sepsis: a review. JAMA 319: 62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A, Wang H, Kitur K, Parker D (2017) Humanized mice exhibit increased susceptibility to staphylococcus aureus pneumonia. J Infect Dis 215: 1386–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasid O, Cavaillon J‐M (2018) Compartment diversity in innate immune reprogramming. Microbes Infect 20: 156–165 [DOI] [PubMed] [Google Scholar]
- Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, Benson BL, Chirieleison SM, Huang AY, Dubyak GR et al (2018) Chemical disruption of the pyroptotic pore‐forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol 3: eaat2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayes J, Lax S, Wichaiyo S, Watson SK, Di Y, Lombard S, Grygielska B, Smith SW, Skordilis K, Watson SP (2017) The podoplanin‐CLEC‐2 axis inhibits inflammation in sepsis. Nat Commun 8: 2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real JM, Ferreira LRP, Esteves GH, Koyama FC, Dias MVS, Bezerra‐Neto JE, Cunha‐Neto E, Machado FR, Salomão R, Azevedo LCP (2018) Exosomes from patients with septic shock convey miRNAs related to inflammation and cell cycle regulation: new signaling pathways in sepsis? Crit Care 22: 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S (2017) Recognizing sepsis as a global health priority – a WHO resolution. N Engl J Med 377: 414–417 [DOI] [PubMed] [Google Scholar]
- Reino DC, Pisarenko V, Palange D, Doucet D, Bonitz RP, Lu Q, Colorado I, Sheth SU, Chandler B, Kannan KB et al (2011) Trauma hemorrhagic shock‐induced lung injury involves a gut‐lymph‐induced TLR4 pathway in mice. PLoS One 6: e14829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmair M, Buschmann D, Märte M, Kirchner B, Hagl D, Kaufmann I, Pfob M, Chouker A, Steinlein OK, Pfaffl MW et al (2017) Cellular and extracellular miRNAs are blood‐compartment‐specific diagnostic targets in sepsis. J Cell Mol Med 21: 2403–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik ME, Merkler AE, Mahta A, Murthy SB, Claassen J, Kamel H (2017) Long‐term risk of seizures in adult survivors of sepsis. Neurology 89: 1476–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA et al (2017) Incidence and trends of sepsis in US hospitals using clinical vs. claims data, 2009‐2014. JAMA 318: 1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riche F, Chousterman BG, Valleur P, Mebazaa A, Launay JM, Gayat E (2018) Protracted immune disorders at one year after ICU discharge in patients with septic shock. Crit Care 22: 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Neff TA, Laudes IJ, Keller KA, Sarma VJ, Markiewski MM, Mastellos D, Strey CW, Pierson CL et al (2002) Increased C5a receptor expression in sepsis. J Clin Invest 110: 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D, Hoesel LM, Ward PA (2007) The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol 81: 137–143 [DOI] [PubMed] [Google Scholar]
- Rocheteau P, Chatre L, Briand D, Mebarki M, Jouvion G, Bardon J, Crochemore C, Serrani P, Lecci PP, Latil M et al (2015) Sepsis induces long‐term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun 6: 10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PO, Setten M, Maskin LP, Bonelli I, Vidomlansky SR, Attie S, Frosiani SL, Kozima S, Valentini R (2012) Muscle weakness in septic patients requiring mechanical ventilation: protective effect of transcutaneous neuromuscular electrical stimulation. J Crit Care 27: 319.e1–8 [DOI] [PubMed] [Google Scholar]
- Rogers MB, Firek B, Shi M, Yeh A, Brower‐Sinning R, Aveson V, Kohl BL, Fabio A, Carcillo JA, Morowitz MJ (2016) Disruption of the microbiota across multiple body sites in critically ill children. Microbiome 4: 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas‐Ballina M, Olofsson PS, Ochani M, Valdes‐Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S et al (2011) Acetylcholine‐synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal MD, Moore FA (2015) Persistent inflammatory, immunosuppressed, catabolic syndrome (PICS): a new phenotype of multiple organ failure. J Adv Nutr Hum Metab 1: e784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JW, Schwertz H, Weyrich AS (2012) Platelet mRNA: the meaning behind the message. Curr Opin Hematol 19: 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio I, Osuchowski MF, Shankar‐Hari M, Skirecki T, Winkler MS, Lachmann G, La Rosée P, Monneret G, Venet F, Bauer M et al (2019) Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis 19: e422–e436 [DOI] [PubMed] [Google Scholar]
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S et al (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395: 200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DA, Tucker KK, Chinookoswong N, Thompson RC, Kohno T (1995) Combined inhibition of interleukin‐1 and tumor necrosis factor in rodent endotoxemia: improved survival and organ function. J Infect Dis 171: 1528–1538 [DOI] [PubMed] [Google Scholar]
- Saito H, Sherwood ER, Varma TK, Evers BM (2003) Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev 124: 1047–1058 [DOI] [PubMed] [Google Scholar]
- Salgado‐Pabon W, Breshears L, Spaulding AR, Merriman JA, Stach CS, Horswill AR, Peterson ML, Schlievert PM (2013) Superantigens are critical for Staphylococcus aureus infective endocarditis, sepsis, and acute kidney injury. mBio 4: e00494‐13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimnia H, Fairfax MR, Lephart PR, Schreckenberger P, DesJarlais SM, Johnson JK, Robinson G, Carroll KC, Greer A, Morgan M et al (2016) Evaluation of the filmarray blood culture identification panel: results of a multicenter controlled trial. J Clin Microbiol 54: 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkodie EK, Zhou S, Baidoo SA, Chu W (2019) Influences of stress hormones on microbial infections. Microb Pathog 131: 270–276 [DOI] [PubMed] [Google Scholar]
- Savage RD, Fowler RA, Rishu AH, Bagshaw SM, Cook D, Dodek P, Hall R, Kumar A, Lamontagne F, Lauzier F et al (2016) The effect of inadequate initial empiric antimicrobial treatment on mortality in critically ill patients with bloodstream infections: a multi‐centre retrospective cohort study. PLoS One 11: e0154944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Guichard J, Massé JM, Debili N, Cramer EM (2001) Of mice and men: comparison of the ultrastructure of megakaryocytes and platelets. Exp Hematol 29: 1295–1302 [DOI] [PubMed] [Google Scholar]
- Schmitz F, Mages J, Heit A, Lang R, Wagner H (2004) Transcriptional activation induced in macrophages by Toll‐like receptor (TLR) ligands: from expression profiling to a model of TLR signaling. Eur J Immunol 34: 2863–2873 [DOI] [PubMed] [Google Scholar]
- Schuetz P, Müller B, Nusbaumer C, Wieland M, Christ‐Crain M (2009) Circulating levels of GH predict mortality and complement prognostic scores in critically ill medical patients. Eur J Endocrinol 160: 157–163 [DOI] [PubMed] [Google Scholar]
- Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C et al (2016) The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65: 575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D et al (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 373: 1874–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclicluna BP, van Vught LA, Zwinderman AH, Wiewel MA, Davenport EE, Burnham KL, Nürnberg P, Schultz MJ, Horn J, Cremer OL et al (2017) Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respir Med 5: 816–826 [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald‐Smith GP, Gao H, Hennessy L et al (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H et al (2019) Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 321: 2003–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, Opal SM (2016) Interleukin‐1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 44: 275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham HP, Walker KH, Abdulnour R‐EE, Krishnamoorthy N, Douda DN, Norris PC, Barkas I, Benito‐Figueroa S, Colby JK, Serhan C et al (2018) 15‐epi‐lipoxin A4, resolvin D2, and resolvin D3 induce NF‐κB regulators in bacterial pneumonia. J Immmunol 200: 2757–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar‐Hari M, Ambler M, Mahalingasivam V, Jones A, Rowan K, Rubenfeld GD (2016) Evidence for a causal link between sepsis and long‐term mortality: a systematic review of epidemiologic studies. Crit Care 20: 101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar‐Hari M, Harrison DA, Rubenfeld GD, Rowan K (2017) Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis‐2 and sepsis‐3 populations using a national critical care database. Br J Anaesth 119: 626–636 [DOI] [PubMed] [Google Scholar]
- Sharshar T, Bastuji‐Garin S, Stevens RD, Durand MC, Malissin I, Rodriguez P, Cerf C, Outin H, De Jonghe B (2009) Presence and severity of intensive care unit‐acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med 37: 3047–3053 [DOI] [PubMed] [Google Scholar]
- Shi M, Zhao C, Ge X, Yang H, Ge L, Zhu GXW (2018) Berberine prevents cognitive disorders induced by sepsis by regulating the inflammatory cytokines, oxidative stress and neuronal apoptosis in rat brain. Neuropsychiatry (London) 8: 24–33 [Google Scholar]
- Singer M, De Santis V, Vitale D, Jeffcoate W (2004) Multiorgan failure is an adaptive, endocrine‐mediated, metabolic response to overwhelming systemic inflammation. Lancet 364: 545–548 [DOI] [PubMed] [Google Scholar]
- Singer M (2014) The role of mitochondrial dysfunction in sepsis‐induced multi‐organ failure. Virulence 5: 66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Deutschman CS, Seymour CW, Shankar‐Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J‐D et al (2016) The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA 315: 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Inada‐Kim M, Shankar‐Hari M (2019) Sepsis hysteria: excess hype and unrealistic expectations. Lancet 394: 1513–1514 [DOI] [PubMed] [Google Scholar]
- Sisti F, Wang S, Brandt SL, Glosson‐Byers N, Mayo LD, Son YM, Sturgeon S, Filgueiras L, Jancar S, Wong H et al (2018) Nuclear PTEN enhances the maturation of a microRNA regulon to limit MyD88‐dependent susceptibility to sepsis. Sci Signal 11: eaai9085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirecki T, Kawiak J, Machaj E, Pojda Z, Wasilewska D, Czubak J, Hoser G (2015) Early severe impairment of hematopoietic stem and progenitor cells from the bone marrow caused by CLP sepsis and endotoxemia in a humanized mice model. Stem Cell Res Ther 6: 142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirecki T, Mikaszewska‐Sokolewicz M, Hoser G, Zielińska‐Borkowska U (2016) The early expression of HLA‐DR and CD64 myeloid markers is specifically compartmentalized in the blood and lungs of patients with septic shock. Mediators Inflamm 2016: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Suffredini AF, Allen JB, Wahl LM, Parrillo JE, Wahl SM (1994) Endotoxin administration to humans primes alveolar macrophages for increased production of inflammatory mediators. J Clin Immunol 14: 141–148 [DOI] [PubMed] [Google Scholar]
- Smythies LE, Sellers M, Clements RH, Mosteller‐Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD (2005) Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 115: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneville R, de Montmollin E, Poujade J, Garrouste‐Orgeas M, Souweine B, Darmon M, Mariotte E, Argaud L, Barbier F, Goldgran‐Toledano D et al (2017) Potentially modifiable factors contributing to sepsis‐associated encephalopathy. Intensive Care Med 43: 1075–1084 [DOI] [PubMed] [Google Scholar]
- Souza‐Dantas VC, Povoa P, Bozza F, Soares M, Salluh J (2016) Preventive strategies and potential therapeutic interventions for delirium in sepsis. Hosp Pract (1995) 44: 190–202 [DOI] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN (2009) Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461: 1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, Nácher M, Pitaval C, Radovanovic I, Fukui Y et al (2014) Neutrophils scan for activated platelets to initiate inflammation. Science 346: 1234–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriskandan S, Moyes D, Cohen J (1996) Detection of circulating bacterial superantigen and lymphotoxin‐alpha in patients with streptococcal toxic‐shock syndrome. Lancet 348: 1315–1316 [DOI] [PubMed] [Google Scholar]
- Stanzani G, Tidswell R, Singer M (2020) Do critical care patients hibernate? Theoretical support for less is more Intensive Care Med 46: 495–497 [DOI] [PubMed] [Google Scholar]
- Starr ME, Saito M, Evers BM, Saito H (2015) Age‐associated increase in cytokine production during systemic inflammation—ii: the role of IL‐1β in age‐dependent il‐6 upregulation in adipose tissue. J Gerontol A Biol Sci Med Sci 70: 1508–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Lang CH (2015) Sepsis attenuates the anabolic response to skeletal muscle contraction. Shock 43: 344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens NE, Chapman MJ, Fraser CK, Kuchel TR, Hayball JD, Diener KR (2017) Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci Rep 7: 5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stortz JA, Murphy TJ, Raymond SL, Mira JC, Ungaro R, Dirain ML, Nacionales DC, Loftus TJ, Wang Z, Ozrazgat‐Baslanti T et al (2018) Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock 49: 249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney TE, Azad TD, Donato M, Haynes WA, Perumal TM, Henao R, Bermejo‐Martin JF, Almansa R, Tamayo E, Howrylak JA et al (2018a) Unsupervised analysis of transcriptomics in bacterial sepsis across multiple datasets reveals three robust clusters. Crit Care Med 46: 915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney TE, Perumal TM, Henao R, Nichols M, Howrylak JA, Choi AM, Bermejo‐Martin JF, Almansa R, Tamayo E, Davenport EE et al (2018b) A community approach to mortality prediction in sepsis via gene expression analysis. Nat Commun 9: 694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ (1999) Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 341: 785–792 [DOI] [PubMed] [Google Scholar]
- Takao K, Miyakawa T (2015) Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci USA 112: 1167–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talisa VB, Yende S, Seymour CW, Angus DC (2018) Arguing for adaptive clinical trials in sepsis. Front Immunol 9: 1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Tanaka K, Tategaki J, Fujino H (2016) Preventive effects of kilohertz frequency electrical stimulation on sepsis‐induced muscle atrophy. J Musculoskelet Neuronal Interact 16: 152–160 [PMC free article] [PubMed] [Google Scholar]
- Tancevski I, Nairz M, Duwensee K, Auer K, Schroll A, Heim C, Feistritzer C, Hoefer J, Gerner RR, Moschen AR et al (2014) Fibrates ameliorate the course of bacterial sepsis by promoting neutrophil recruitment via CXCR2. EMBO Mol Med 6: 810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Yang H, Chen J, Shi M, Ge L, Ge X, Zhu G (2017) Metformin ameliorates sepsis‐induced brain injury by inhibiting apoptosis, oxidative stress and neuroinflammation via the PI3K/Akt signaling pathway. Oncotarget 8: 97977–97989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber SC, Eiffert H, Bruck W, Nau R (2017) Septic encephalopathy and septic encephalitis. Expert Rev Anti Infect Ther 15: 121–132 [DOI] [PubMed] [Google Scholar]
- Thamm K, Schrimpf C, Retzlaff J, Idowu TO, van Meurs M, Zijlstra JG, Ghosh CC, Zeitvogel J, Werfel TA, Haller H et al (2018) Molecular regulation of acute Tie2 suppression in sepsis. Crit Care Med 46: e928–e936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans K, Kox M, Vaneker M, van den Berg M, John A, van Laarhoven A, van der Hoeven H, Scheffer GJ, Pickkers P (2016) Plasma levels of danger‐associated molecular patterns are associated with immune suppression in trauma patients. Intensive Care Med 42: 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, Naik AD, Dodson JA (2016) Moving from disease‐centered to patient goals‐directed care for patients with multiple chronic conditions: patient value‐based care. JAMA Cardiol 1: 9–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres‐Rosas R, Yehia G, Peña G, Mishra P, del Rocio Thompson‐Bonilla M, Moreno‐Eutimio MA, Arriaga‐Pizano LA, Isibasi A, Ulloa L (2014) Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 20: 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadász I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Tóth PT, Lecuona E, Witters LA, Schumacker PT et al (2008) AMP‐activated protein kinase regulates CO2‐induced alveolar epithelial dysfunction in rats and human cells by promoting Na, K‐ATPase endocytosis. J Clin Invest 118: 752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bogaert T, Vandevyver S, Dejager L, Van Hauwermeiren F, Pinheiro I, Petta I, Engblom D, Kleyman A, Schütz G, Tuckermann J et al (2011) Tumor necrosis factor inhibits glucocorticoid receptor function in mice: a strong signal toward lethal shock. J Biol Chem 286: 26555–26567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wyngene L, Vandewalle J, Libert C (2018) Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol Med 10: e8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Berghe T, Demon D, Bogaert P, Vandendriessche B, Goethals A, Depuydt B, Vuylsteke M, Roelandt R, Van Wonterghem E, Vandenbroecke J et al (2014) Simultaneous targeting of IL‐1 and IL‐18 is required for protection against inflammatory and septic shock. Am J Respir Crit Care Med 189: 282–291 [DOI] [PubMed] [Google Scholar]
- Vasques‐Nóvoa F, Laundos TL, Cerqueira RJ, Quina‐Rodrigues C, Soares‐Dos‐Reis R, Baganha F, Ribeiro S, Mendonça L, Gonçalves F, Reguenga C et al (2018) MicroRNA‐155 amplifies nitric oxide/cGMP signaling and impairs vascular angiotensin ii reactivity in septic shock. Crit Care Med 46: e945–e954 [DOI] [PubMed] [Google Scholar]
- Vassiliadi DA, Dimopoulou I, Tzanela M, Douka E, Livaditi O, Orfanos SE, Kotanidou A, Tsagarakis S (2014) Longitudinal assessment of adrenal function in the early and prolonged phases of critical illness in septic patients: relations to cytokine levels and outcome. J Clin Endocrinol Metab 99: 4471–4480 [DOI] [PubMed] [Google Scholar]
- Velazquez EM, Nguyen H, Heasley KT, Saechao CH, Gil LM, Rogers AWL, Miller BM, Rolston MR, Lopez CA, Litvak Y et al (2019) Endogenous Enterobacteriaceae underlie variation in susceptibility to Salmonella infection. Nat Microbiol 4: 1057–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Brealey D, Libert N, Abidi NE, O'Dwyer M, Zacharowski K, Mikaszewska‐Sokolewicz M, Schrenzel J, Simon F, Wilks M et al (2015) Rapid diagnosis of infection in the critically ill, a multicenter study of molecular detection in bloodstream infections, pneumonia, and sterile site infections. Crit Care Med 43: 2283–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade DM, Mouncey PR, Richards‐Belle A, Wulff J, Harrison DA, Sadique MZ, Grieve RD, Emerson LM4, Mason AJ, Aaronovitch D et al (2019) Effect of a nurse‐led preventive psychological intervention on symptoms of posttraumatic stress disorder among critically ill patients: a randomized clinical trial. JAMA 321: 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren HS, Fitting C, Hoff E, Adib‐Conquy M, Beasley‐Topliffe L, Tesini B, Liang X, Valentine C, Hellman J, Hayden D et al (2010) Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis 201: 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A, Leslie DC, Bolgen DE, Lighbown S, Dimitrakakis N, Cartwright MJ, Seiler B, Lighbown K, Smith K, Lombardo P et al (2018) Modified clinical monitoring assessment criteria for multi‐organ failure during bacteremia and sepsis progression in a pig model. Advan Crit Care Med 1: 002 [Google Scholar]
- Wei Y, Yang J, Wang J, Yang Y, Huang J, Gong H, Cui H, Chen D (2016) Successful treatment with fecal microbiota transplantation in patients with multiple organ dysfunction syndrome and diarrhea following severe sepsis. Crit Care 20: 332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Carlos AR, Moita MR, Singh S, Blankenhaus B, Cardoso S, Larsen R, Rebelo S, Schäuble S, Del Barrio L et al (2017) Metabolic adaptation establishes disease tolerance to sepsis. Cell 169: 1263–1275.e1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T, Dellinger P, Ebelt H, Ferrer M, Opal SM, Singer M, Vincent JL, Werdam K, Martin‐Loeches I, Almirall J et al (2018) Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community‐acquired pneumonia: a randomized, placebo‐controlled, double‐blind, multicenter, phase II trial (CIGMA study). Intensive Care Med 44: 438–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werners AH (2017) Treatment of endotoxaemia and septicaemia in the equine patient. J Vet Pharmacol Ther 40: 1–15 [DOI] [PubMed] [Google Scholar]
- Wilmore JR, Gaudette BT, Gomez Atria D, Hashemi T, Jones DD, Gardner CA, Cole SD, Misic AM, Beiting DP, Allman D (2018) Commensal microbes induce serum IgA responses that protect against polymicrobial sepsis. Cell Host Microbe 23: 302–311.e303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler MS, Nierhaus A, Poppe A, Greiwe G, Gräler MH, Daum G (2017) Sphingosine‐1‐phosphate: a potential biomarker and therapeutic target for endothelial dysfunction and sepsis? Shock 47: 666–672 [DOI] [PubMed] [Google Scholar]
- Wintermann GB, Brunkhorst FM, Petrowski K, Strauss B, Oehmichen F, Pohl M, Rosendahl J (2015) Stress disorders following prolonged critical illness in survivors of severe sepsis. Crit Care Med 43: 1213–1222 [DOI] [PubMed] [Google Scholar]
- Wischmeyer PE, San‐Millan I (2015) Winning the war against ICU‐acquired weakness: new innovations in nutrition and exercise physiology. Crit Care 19(Suppl 3): S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollersheim T, Woehlecke J, Krebs M, Hamati J, Lodka D, Luther‐Schroeder A, Langhans C, Haas K, Radtke T, Kleber C et al (2014) Dynamics of myosin degradation in intensive care unit‐acquired weakness during severe critical illness. Intensive Care Med 40: 528–538 [DOI] [PubMed] [Google Scholar]
- Wong HR, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Willson DF, Freishtat RJ, Anas N, Meyer K, Checchia PA et al (2009) Identification of pediatric septic shock subclasses based on genome‐wide expression profiling. BMC Med 7: 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HR, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, Weiss SL, Fitzgerald J, Checchia PA, Meyer K et al (2015) Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med 191(3): 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HR, Sweeney TE, Hart KW, Khatri P, Lindsell CJ (2017a) Pediatric sepsis endotypes among adults with sepsis. Crit Care Med 45: e1289–e1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HR, Sweeney TE, Lindsell CJ (2017b) Simplification of a septic shock endotyping strategy for clinical application. Am J Respir Crit Care Med 195: 263–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood M, Song A, Maslove D, Ferri C, Howes D, Muscedere J, Boyd JG (2016) Brain tissue oxygenation in patients with septic shock: a feasibility study. Can J Neurol Sci 43: 65–73 [DOI] [PubMed] [Google Scholar]
- Xiang B, Zhang G, Guo L, Li X‐A, Morris AJ, Daugherty A, Whiteheart SW, Smyth SS, Li Z (2013) Platelets protect from septic shock by inhibiting macrophage‐dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat Commun 4: 2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Lin S, Yan X, Wang C, Tu H, Yin Y, Cao J (2018) Interleukin‐38 is a protective cytokine for lethal sepsis. J Infect Dis 218: 1175–1184 [DOI] [PubMed] [Google Scholar]
- Yoseph BP, Klingensmith NJ, Liang Z, Breed ER, Burd EM, Mittal R, Dominguez JA, Petrie B, Ford ML, Coopersmith CM (2016) Mechanisms of intestinal barrier dysfunction in sepsis. Shock 46: 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborin A, Smith D, Garfield K, Quensen J, Shakhsheer B, Kade M, Tirrell M, Tiedje J, Gilbert JA, Zaborina O, Alverdy JC (2014) Membership and behavior of ultra‐low‐diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio 5: e01361‐01314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, He X, Tuo QH, Liao DF, Zhang GQ, Chen JX (2016) LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie‐2 and HIF‐2alpha/Notch3 pathways. Sci Rep 6: 20931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liu M, Chan XY, Tan SY, Subramaniam S, Fan Y, Loh E, Tou En Chang K, Tan TC, Chen Q (2017) Uncovering the mystery of opposite circadian rhythms between mouse and human leukocytes in humanized mice. Blood 130: 1995–2005 [DOI] [PubMed] [Google Scholar]
- Zhou J, Chaudhry H, Zhong Y, Ali MM, Perkins LA, Owens WB, Morales JE, McGuire FR, Zumbrun EE, Zhang J et al (2015) Dysregulation in microRNA expression in peripheral blood mononuclear cells of sepsis patients is associated with immunopathology. Cytokine 71: 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Song Y, Shaikh Z, Li H, Zhang H, Caudle Y, Zheng S, Yan H, Hu D, Stuart C et al (2017) MicroRNA‐155 attenuates late sepsis‐induced cardiac dysfunction through JNK and β‐arrestin 2. Oncotarget 8: 47317–47329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Chang E, Fan H (2018) Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol Ther 26: 1375–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari PS, Pinto BB, Dyson A, Singer M (2013) The metabolic phenotype of rodent sepsis: cause for concern? Intensive Care Med Exp 1: 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari PS, Carre JE, Parker N, Curtin NA, Duchen MR, Singer M (2015) Skeletal muscle dysfunction is associated with derangements in mitochondrial bioenergetics (but not UCP3) in a rodent model of sepsis. Am J Physiol Endocrinol Metab 308: E713–E725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrzavy T, Hoftberger R, Berger T, Rauschka H, Butovsky O, Weiner H, Lassmann H (2018) Pro‐inflammatory activation of microglia in the brain of patients with sepsis. Neuropathol Appl Neurobiol 45: 278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]


