Abstract
Background: RECK, as a negative MMP regulator, is extensively expressed in normal cells but decreased in tumors. In OSCC, the relationship between RECK and MMPs and the potential prognostic impact remains unclear. In this research, for the first time, we investigated the expression of RECK associated with MMPs during OSCC carcinogenesis in a large sample and its association with 5-year survival rate. Material and Methods: Immunohistochemical SP technique was applied to study the expression of RECK and MMP-2 and MMP-9 in 108 cases of OSCC and 30 normal oral mucosae. Univariate and multivariate Cox regression analysis was utilized for disease-free survival and overall survival, and analyzed by Kaplan-Meier method regarding RECK expression in patients of OSCC. Results: We found lower expression of RECK in OSCC was 51.85% (56/108) compared with 93.33% (28/30) in the control group. However, the higher expression of MMP-2 and MMP-9 was 74.07% (80/108) and 70.37% (76/108) in OSCC, respectively, compared with 20% (6/30) and 13.3% (4/30) in the control group. Furthermore, the decrease of RECK expression and the increase of MMP-2, and -9 expression were significantly correlated with the loss of histologic differentiation, the occurrence of lymphatic metastasis, and the increase of OSCC clinical stage (P<0.05). OSCC patients with a low level of RECK expression had a lower rate of 5-year survival. Conclusion: RECK may prevent metastasis and improve OSCC patients’ prognosis through a RECK/MMP-2, and -9 imbalance. Furthermore, RECK is a prospective prognostic indicator and therapeutic target for cancer molecular targeting therapy. Low expression of RECK may be a significant negative prognostic predictor.
Keywords: RECK, MMP-2, MMP-9, OSCC
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most invasive tumors associated with a low survival rate, and ranks sixth in cancer incidence in the world. It has a higher rate of lymph node metastasis which is caused by adhesion molecules and matrix-degrading enzymes. In a series of malignant tumor invasion and metastasis processes, matrix metalloproteinases (MMPs) take a vital role in the decomposition of extracellular matrix (ECM). MMPs come from a wide range of sources that can be produced by almost all cells, and participate in diversified physiologic and pathologic procedures, such as tissue formation, angiogenesis, and the inflammatory response [1,2]. In addition, MMPs interact with the ECM in tumor cells, such as by destroying the connective tissue matrix, resulting in tumor invasion, invasion through the basement membrane into small blood and lymphatic vessels, and tumor metastasis [3]. Researchers have confirmed that MMPs expressed in tumors play important roles in tumor invasion and metastasis through uncontrolled decomposition of tissue basement membranes and the ECM, providing a prerequisite for tumor invasion and migration [4]. Clinical studies have also reported that high expression of MMP-2 and MMP-9 exist in patients with OSCC [5]. Additionally, the activities of MMPs can be controlled by MMP inhibitors such as reversion-inducing cysteine-rich protein with Kazal motifs (RECK). RECK is a type of tumor suppressor, which is involved in the negative regulation of MMP-2 and MMP-9 activities by preventing the breakdown of the ECM [6]. Previous studies have confirmed that RECK is extensively expressed in normal cells and tissues but reduced in many tumor types [6-9]. However, related studies in OSCC are limited, especially as to five-year survival rate.
MMP inhibitors work together with MMPs to maintain a dynamic balance between ECM degradation and synthesis, and play a decisive role in the process of tumor cell invasion and metastasis [10]. A significant high expression of MMP-2, and -9 and low expression of RECK have been confirmed in squamous cell carcinoma [11]. Therefore, RECK may produce a marked effect in inhibiting the destruction of ECM components through the dynamic imbalance of RECK/MMP-2, and -9 in the occurrence and metastasis of OSCC. This view is further demonstrated in our study.
In patients with OSCC, due to a lack of available tumor proliferation and invasion factors, tumor progression is unpredictable. Therefore, searching for specific markers can help us evaluate the prognosis. Moreover, it may be helpful for cancer molecular targeting therapy of OSCC in the future. In this study, we analyzed the expression of RECK and MMP-2, and -9 in OSCC and normal tissues to assess their effect and interrelationships. This is the first study to explore the RECK immunohistochemical expression in connection with clinicopathologic characteristics and five-year survival rate in a large sample of OSCC patients.
Materials and methods
Materials
Tumor tissues were acquired from 108 patients who underwent OSCC surgical resection and complete resection with lymphadenectomy immediately after surgery from the Center of Stomatology, Tongji Hospital of Tongji Medical College from August 2006 to September 2009. And normal oral mucosa was obtained during extraction of lower impacted mandibular third molars. Ethics Committee approval was obtained from Tongji Hospital of Tongji Medical College. All patients denied preoperative radiotherapy and chemotherapy. A total of 64 males and 44 females aged 27-72 years (average 52.4 years) were included in this study. Among them, 62 cases were well-differentiated, 46 were moderately-differentiated or poorly-differentiated. There were 50 cases of cervical lymphatic metastasis and 58 cases without cervical metastasis. Of these patients, 62 cases were stage I and II, 46 cases were stage III and IV on the basis of clinical stages. 30 cases of normal oral mucosa were also obtained, which included 18 men and 12 women. According to the Helsinki Declaration and our agency’s guidelines, all samples were obtained after patients signed informed consent.
Immunohistochemical staining
The specimens were fixed by 4% formaldehyde solution, dehydrated conventionally and embedded in paraffin. Then cut into 4 µm sections, and used for immunohistochemistry (IHC). Immunohistochemistry was conducted by the streptavidin-peroxidase method following the manufacturer’s guidelines to assess the expression level of RECK and MMPs. Immunohistochemical reagents include the goat anti-human polyclonal RECK antibody (Santa Cruz, 1:75) and the mouse anti-human monoclonal MMP-2 and MMP-9 antibodies (Boshide, China, 1:100). DAB (diaminobenzidine) staining incubation at room temperature for 2-5 minutes until brown precipitation occurs. Substitution of PBS for primary antibody as negative control.
The positive staining of RECK and MMP-2, 9 located in intra cytoplasm of cells. The deposits were straw yellow, or brown; IHC staining of RECK and MMP-2, -9 were estimated according to the score of intensity and proportion. At 200× magnification, at least three regions were randomly selected to analyze the staining results, which were calculated independently and blindly by two investigators. The final scores are calculated by the sum of intensity score and proportion score, which is between 0 and 6. The intensity score was as below: 0 = no staining; +1 = weak staining; +2 = moderate staining; and +3 = strong staining. The proportion score based on the percentage of staining was as below: 0 = no positivity tumor cell; +1 = <25% positivity; +2 = 26-50% tumor cell positivity; and +3 = >50% tumor cell positivity. Then the positive species of RECK and MMP-2, -9 were divided into low expression and high expression based on the sum of the score [8].
Statistical analyses
All statistical data were analyzed by SPSS 23.0 software. Chi-square test was used to analyze the relation between the expression of RECK, MMP-2, -9 and clinicopathological features. Fisher’s exact test for not applicable Chi-Square Test. Spearman’s rank correlation was used to appraise the correlation between variables. Disease-free survival (DFS) or overall survival (OS) was analyzed by univariate and multivariate Cox regression. Survival curves were calculated by Kaplan-Meier method and analyzed by logistic rank test.
Follow-up
Patients in this study were regularly followed for 5 years. All patients in our study were routinely followed up until death or study deadline (December 8, 2016). DFS and OS are two evaluation methods for prognostic analysis. The former measures the first recurrence at any site, and the latter measures the death in any case. During the follow-up period, patients with other malignant tumors were not considered to have recurrent cancer.
Results
We explored the expression levels of RECK, MMP-2, and MMP-9 in OSCC patients, and their relationships with the clinicopathologic findings of the patients. Furthermore, we elucidated the effect of RECK expression level on 5-year survival in OSCC patients.
Immunohistochemical expression of RECK, MMP-2, and MMP-9 in OSCC and normal tissue
Immunohistochemical staining for RECK, MMP-2, and MMP-9 are shown in (Figure 1). IHC staining of RECK, MMP-2, and MMP-9 were mainly located in the cytoplasm, with a straw yellow, or brown color. The expression of RECK in OSCC was significantly lower than that in the control, while the expression of MMP-2, or MMP-9 in OSCC was increased. The positive expression levels of MMP-2 and MMP-9 increased with the loss of histologic differentiation, the occurrence of lymphatic metastasis, and the increase of OSCC clinical stage (P<0.05) (Table 2). A high expression level of RECK was negatively correlated with a high level of MMP-2 and MMP-9 (P<0.001, r = -0.653) (Table 1).
Figure 1.
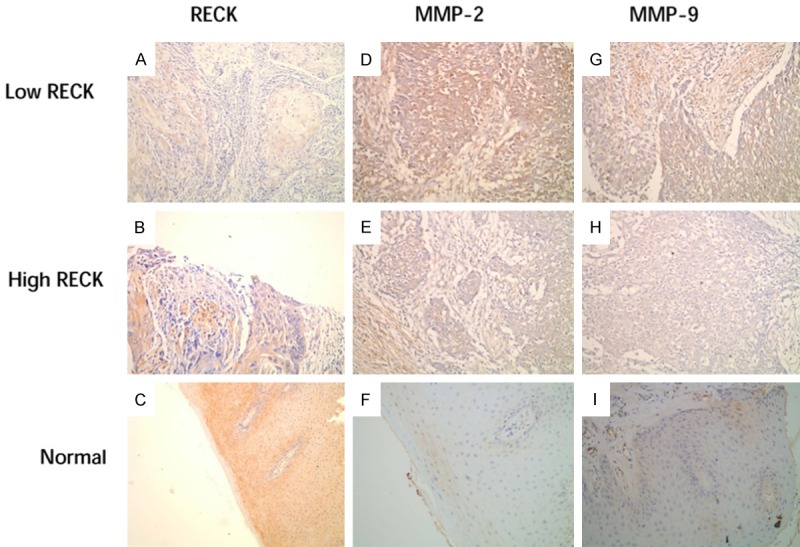
Immunohistochemical staining of OSCC and control group. IHC staining of RECK is shown in (A and B); MMP-2 in OSCC tissues is shown in (D and E); and MMP-9 in OSCC tissues is shown in (G and H). IHC staining of RECK, MMP-2 and MMP-9 in normal oral mucosa is exhibited in (C, F and I).
Table 2.
Relationship between expression of RECK, MMP-2, MMP-9 and clinicopathologic features
| Variables | Category | RECK | P | MMP-2 | P | MMP-9 | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| low | high | low | high | low | high | ||||||
| Gender | male | 64 | 30 | 34 | 0.94 | 14 | 50 | 0.247 | 12 | 52 | 0.012 |
| female | 44 | 22 | 22 | 14 | 30 | 18 | 26 | ||||
| Age | <60 | 44 | 20 | 24 | 0.642 | 9 | 35 | 0.164 | 16 | 28 | 0.099 |
| ≥60 | 64 | 32 | 32 | 19 | 45 | 14 | 50 | ||||
| Differentiation | well | 62 | 18 | 44 | 0 | 24 | 38 | 0.001 | 28 | 34 | 0 |
| mod/poor | 46 | 34 | 12 | 4 | 42 | 2 | 44 | ||||
| Lymph node metastasis | yes | 50 | 38 | 12 | 0.019 | 6 | 44 | 0.002 | 7 | 43 | 0.003 |
| no | 58 | 14 | 44 | 22 | 36 | 23 | 35 | ||||
| T stage | T1, T2 | 62 | 18 | 44 | 0 | 26 | 36 | 0 | 28 | 34 | 0 |
| T3, T4 | 23 | 34 | 12 | 2 | 44 | 2 | 44 | ||||
P<0.05.
Table 1.
The expression of RECK, MMP-2, and MMP-9 in OSCC
P<0.0001.
Correlation of RECK expression with clinicopathologic characteristics in OSCC patients
In our total of 108 OSCC cases, 56 (51.9%) had high expression of RECK and 52 (48.1%) were low. The high expression rate was reduced with the loss of histologic differentiation, occurrence of lymphatic metastasis, and increase in clinical stage (P<0.05). The correlations between RECK expression and clinicopathologic characteristics in OSCC patients are shown in (Table 2). The expression level of RECK was significantly correlated with the loss of histologic differentiation (P<0.000), occurrence of lymphatic metastasis (P<0.000), and increase of clinical stage (P<0.05).
Clinical results
In our study, 108 OSCC patients with an average age of 52 years (ranging from 27 to 72 years, with median age of 48 years) were included. The average follow-up period was 58 months (median, 59 months; range, 4-156 months). By the time our study was completed, 38 (35.2%) patients had died and 70 (64.8%) were still alive.
Univariate analysis for DFS
Univariate analysis and clinicopathologic features of RECK, MMP-2, and MMP-9 expressions are shown in (Table 3). Univariate analysis for DFS using Cox regression analysis was used to validate the univariate analysis for DFS which proved low level expression of RECK (P<0.05), high level of MMP-9 (P<0.05), loss of histologic differentiation (P<0.05), occurrence of lymphatic metastasis (P<0.05), and increase of clinical stage (P<0.05) were significant negative prognostic predictors in OSCC patients.
Table 3.
Univariate analysis of prognostic factors in the Cox proportional hazards model
| Variable | Category | Risk ratio | Univariate | P |
|---|---|---|---|---|
|
| ||||
| 95% confidence interval | ||||
| Gender | male/female | 1.923 | 0.676-5.989 | 0.194 |
| Age | <60/≥60 | 0.449 | 0.067-3.987 | 0.542 |
| Differentiation | well/mod, poor | 0.675 | 0.257-4.743 | 0.018 |
| Lymph node metastasis | yes/no | 2.334 | 0.908-6.556 | 0.039 |
| T stage | T1, T2/T3, T4 | 0.656 | 0.584-6.981 | 0.027 |
| MMP-2 | high/low | 2.113 | 0.711-6.918 | 0.063 |
| MMP-9 | high/low | 2.409 | 0.809-5.878 | 0.040 |
| RECK | positive/negative | 0.494 | 0.278-0.878 | 0.016 |
P<0.05.
Cox multiple regression analysis
Cox multiple regression analysis was used to evaluate the prognostic value of all variables in patients with OSCC. We concluded that low RECK expression and histologic differentiation were independent prognostic factors, indicating a worse prognosis (P<0.05) (Table 4).
Table 4.
Multivariate regression analysis
| Variable | B | OS | P | B | DFS | P | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Hazard Ratio | 95% Confidence Interval | Hazard Ratio | 95% Confidence Interval | |||||
| RECK expression | -1.785 | 9.564 | 0.047-0.510 | 0.002 | -1.438 | 4.564 | 0.064-0.9864 | 0.037 |
| Histologic differentiation | -2.397 | 13.754 | 0.028-0.343 | 0.000 | -1.556 | 4.987 | 0.049-0.898 | 0.030 |
P<0.05.
Kaplan-Meier survival analysis
According to the expression level of RECK in OSCC, Kaplan-Meier analysis was used for DFS and OS, as shown in (Figures 2 and 3). Patients with high RECK expression (n = 56) showed higher DFS (P = 0.013, log-rank test) and OS (P = 0.008, log-rank test) rates than patients with low RECK expression (n = 52).
Figure 2.
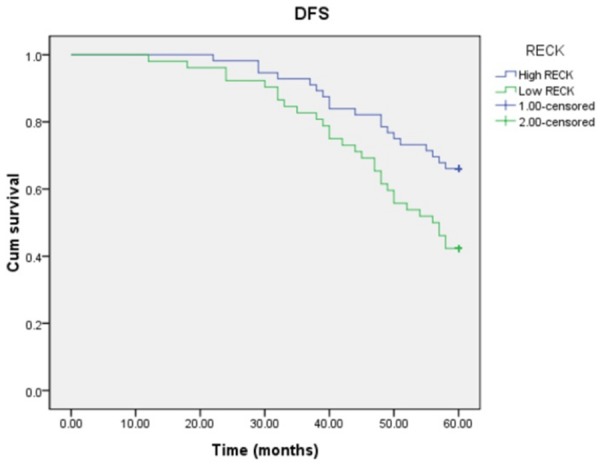
Kaplan-Meier methods for DFS analysis (P = 0.013, log-rank test).
Figure 3.
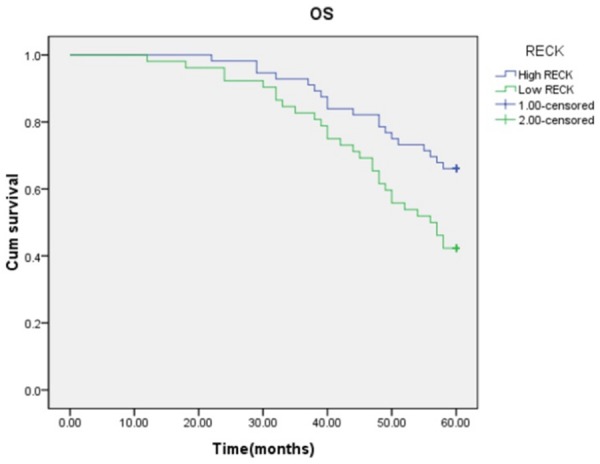
Kaplan-Meier methods for OS analysis (P = 0.008, log-rank test). Patients with high RECK expression had a significantly higher survival rate than those with low RECK expression (dotted line) (P = 0.016, Log-rank test).
Discussion
We discovered an imbalance of RECK and MMPs expression in OSCC patients. Immunohistochemistry showed that the expression of RECK was significantly inhibited in OSCC tissues compared to the normal control group. In contrast, the expression of MMP-2 and MMP-9 was significantly increased in OSCC. The results of the univariate survival analysis showed that the low expression of RECK, the high expression of MMP-9, the loss of histologic differentiation, the occurrence of lymphatic metastasis, and the increase of clinical stage served as significant negative prognostic predictors in OSCC patients. Cox multiple regression analysis was used to evaluate the prognostic value of all variables in patients with OSCC. Thus, we concluded that low RECK expression and the loss of differentiation degree were independent important prognostic factors, indicating a worse prognosis.
RECK expression is significantly downregulated in different types of tumors, such as non-small cell lung cancer, colorectal cancer, and esophageal cancer, and is closely related to high expression of MMP-2 and MMP-9 [12-14]. Increasing the expression of RECK to inhibit metastasis and progression of tumors seems to yield a better prognosis. Recent studies have also shown that there are several ways to up-regulate RECK expression such as, inhibiting RECK methylation to abate the expression of MMPs, and ultimately inhibiting the invasion and metastasis of tumors [15,16]. By up-regulation of RECK, AKT and other signaling pathways can be inactivated, ultimately inhibiting the proliferation and invasion of cancer cells [10].
Down-regulation of RECK leads to the overactivation of MMP-2 and MMP-9, which are secreted by cancer cells and are positively correlated with tumorigenesis and progression, including invasiveness of angiogenesis, metastasis, and shortened survival time of patients [17-19]. This idea is further supported by our findings. In our study, the immunohistochemical results indicated that RECK was down-regulated. In addition, we also found that patients with low expression of RECK had a lower 5-year survival rate than those pwith high RECK expression for OS and DFS. This suggests that the level of RECK expression of OSCC may play an important role in predicting cancer outcome and the recurrence risk.
Since RECK is a recognized tumor suppressor, exploring the mechanism of its downregulation is very important to tumor treatment. The mechanism might be downregulation of RECK-activated oncogenic P53 or Ras signaling, closely associated with the presence of the rs16932912 (G/A) SNP, including ERK1/2, Mekk/Sek/Jnk, and Raf/Mek/Erk pathways [20-24]. Many recent studies indicate that microRNAs can accelerate tumor proliferation and metastasis by targeting RECK [25,26]. Therefore, the tumor signaling pathway activated by RECK and microRNA targeting RECK to inhibit the proliferation of OSCC also deserve further verification.
In a number of clinical samples, it was found that the RECK/MMPs imbalance was already present in other types of tumors [27,28]. Our research showed low expression of RECK and high expression of MMP-2, and MMP-9 were positively correlated with loss of histological differentiation, occurrence of lymphatic and increased clinical stage of OSCC. We proposed that, for the first time to our knowledge, low RECK expression is a poor prognostic factor in OSCC patients. RECK may inhibit MMP-2, and MMP-9 through the imbalance of the ratio of RECK/MMP-2, MMP-9 in the occurrence and metastasis of OSCC, providing a prognostic factor for the prognosis of OSCC. The imbalance of RECK/MMPs in OSCC, provides a new research prospect for the expression and regulation of MMPs. The regulation of the RECK/MMPs imbalance could be a vital new approach for the prevention and treatment of OSCC, as well as a therapeutic target for novel drug development.
Conclusion
RECK may prevent metastasis through a RECK/MMP-2, -9 imbalance and improve OSCC patients’ prognosis. Low expression of RECK presents a significant negative prognostic predictor. In addition, RECK may be a promising prognostic indicator and a potential therapeutic target for cancer molecular targeted therapy.
Acknowledgements
Thanks to the Department of Pathology, Tongji Hospital Tongji Medical College to provide tumor specimens for our research.
Disclosure of conflict of interest
None.
References
- 1.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodeling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 3.Egeblad M, Werb Z. New roles for matrix metalloproteinases in cancer. Nat Rev Cancer. 2002;2:163–176. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 4.Thammineni KL, Thakur GK, Kaur N, Banerjee BD. Significance of MMP-9 and VEGF-C expression in North Indian women with breast cancer diagnosis. Mol Cell Biochem. 2019;457:93–103. doi: 10.1007/s11010-019-03515-w. [DOI] [PubMed] [Google Scholar]
- 5.Nishio K, Motozawa K, Omagari D, Gojoubori T, Ikeda T, Asano M, Gionhaku N. Comparison of MMP2 and MMP9 expression levels between primary and metastatic regions of oral squamous cell carcinoma. J Oral Sci. 2016;58:59–65. doi: 10.2334/josnusd.58.59. [DOI] [PubMed] [Google Scholar]
- 6.Noda M, Oh J, Takahashi R, Kondo S, Kitayama H, Takahashi C. RECK: a novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis Rev. 2003;22:167–175. doi: 10.1023/a:1023043315031. [DOI] [PubMed] [Google Scholar]
- 7.Alexius-Lindgren M, Andersson E, Lindstedt I, Engström W. The RECK gene and biological malignancy-its significance in angiogenesis and inhibition of matrix metalloproteinases. Anticancer Res. 2014;34:3867–3873. [PubMed] [Google Scholar]
- 8.Discacciati MG, Gimenes F, Pennacchi PC, Faião-Flores F, Zeferino LC, Derchain SM, Teixeira JC, Costa MC, Zonta M, Termini L, Boccardo E, Longatto-Filho A, Consolaro ME, Villa LL, Maria-Engler SS. MMP-9/RECK imbalance: a mechanism associated with high-grade cervical lesions and genital infection by human papillomavirus and chlamydia trachomatis. Cancer Epidemiol Biomarkers Prev. 2015;24:1539–47. doi: 10.1158/1055-9965.EPI-15-0420. [DOI] [PubMed] [Google Scholar]
- 9.Lee HN, Mitra M, Bosompra O, Corney DC, Johnson EL, Rashed N, Ho LD, Coller HA. RECK isoforms have opposing effects on cell migration. Mol Biol Cell. 2018;29:1825–1838. doi: 10.1091/mbc.E17-12-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jian Y, Xu CH, Li YP, Tang B, Xie SH, Zeng EM. Downregulated microRNA-30b-3p inhibits proliferation, invasion and migration of glioma cells via inactivation of the AKT signaling pathway by upregulating RECK. Biosci Rep. 2019;39 doi: 10.1042/BSR20182226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Wang N, Wang Q, Chi J, Xiang F, Lin M, Wang W, Wei F, Feng Y. Carcinoembryonic antigen cell adhesion molecule 1 inhibits the antitumor effect of neutrophils in tongue squamous cell carcinoma. Cancer Sci. 2019;110:519–529. doi: 10.1111/cas.13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen J, Wang B, Zhang T, Zhu N, Wang Z, Jin J, He Y, Hu M. Suppression of non-small cell lung cancer growth and metastasis by a novel small molecular activator of RECK. Cell Physiol Biochem. 2018;45:1807–1817. doi: 10.1159/000487872. [DOI] [PubMed] [Google Scholar]
- 13.Iseki Y, Shibutani M, Maeda K, Nagahara H, Fukuoka T, Matsutani S, Hirakawa K, Ohira M. MicroRNA-96 promotes tumor invasion in colorectal cancer via RECK. Anticancer Res. 2018;38:2031–2035. doi: 10.21873/anticanres.12442. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, Zabihula B, Yibulayin W, Liu X. Methylation and expression of RECK, P53 and RUNX genes in patients with esophageal cancer. Oncol Lett. 2017;14:5293–5298. doi: 10.3892/ol.2017.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang F, He K, Huang L, Zhang L, Liu A, Zhang J. Casticin inhibits the activity of transcription factor Sp1 and the methylation of RECK in MGC803 gastric cancer cells. Exp Ther Med. 2017;13:745–750. doi: 10.3892/etm.2016.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia W, Deng F, Fu W, Hu J, Chen G, Gao X, Tan X, Li G, Liu G, Zhu S. Curcumin suppresses wilms’ tumor metastasis by inhibiting RECK methylation. Biomed Pharmacother. 2019;111:1204–1212. doi: 10.1016/j.biopha.2018.12.111. [DOI] [PubMed] [Google Scholar]
- 17.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 18.Noda M, Oh J, Takahashi R, Kondo S, Kitayama H, Takahashi C. RECK: a novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis Rev. 2003;22:167–175. doi: 10.1023/a:1023043315031. [DOI] [PubMed] [Google Scholar]
- 19.Levin G, Coelho TM, Nóbrega NG, Trombetta-Lima M, Sogayar MC, Carreira ACO. Spatio-temporal expression profile of matrix metalloproteinase (MMP) modulators RECK and Sparc during the rat ovarian dynamics. Reprod Biol Endocrinol. 2018;16:116. doi: 10.1186/s12958-018-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Wang J, Liu H, Zhang Y, Dong F. The clinicopathologic relevance of RECK gene polymorphisms in ameloblastoma. Arch Oral Biol. 2017;79:77–86. doi: 10.1016/j.archoralbio.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Chang HC, Liu LT, Hung WC. Involvement of histone deacetylation in ras-induced down-regulation of the metastasis suppressor RECK. Cell Signal. 2004;16:675–9. doi: 10.1016/j.cellsig.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Hsu MC, Chang HC, Hung WC. HER-2/neu represses the metastasis suppressor RECK via ERK and Sp transcription factors to promote cell invasion. J Biol Chem. 2006;281:4718–25. doi: 10.1074/jbc.M510937200. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Li L, Liu Y, Geng P, Li G, Yang Y, Song H. RECK inhibits cervical cancer cell migration and invasion by promoting p53 signaling pathway. J Cell Biochem. 2018;119:3058–3066. doi: 10.1002/jcb.26441. [DOI] [PubMed] [Google Scholar]
- 24.Ning S, Ma X. Dephosphorylation-induced EZH2 activation mediated RECK downregulation by ERK1/2 signaling. J Cell Physiol. 2019;234:19010–19018. doi: 10.1002/jcp.28540. [DOI] [PubMed] [Google Scholar]
- 25.Pan Y, Liang H, Chen W, Zhang H, Wang N, Wang F, Zhang S, Liu Y, Zhao C, Yan X, Zhang J, Zhang CY, Gu H, Zen K, Chen X. microRNA-200b and microRNA-200c promote colorectal cancer cell proliferation via targeting the reversion-inducing cysteine-rich protein with Kazal motifs. RNA Biol. 2015;12:276–89. doi: 10.1080/15476286.2015.1017208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen B, Yu S, Zhang Y, Yuan Y, Li X, Zhong J, Feng J. miR-590-5p regulates gastric cancer cell growth and chemosensitivity through RECK and the AKT/ERK pathway. Onco Targets Ther. 2016;9:6009–6019. doi: 10.2147/OTT.S110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacomasso T, Trombetta-Lima M, Sogayar MC, Winnischofer SM. Down-regulation of reversion-inducing cysteine-rich protein with Kazal motifs in malignant melanoma: inverse correlation with membrane-type 1-matrix metalloproteinase and tissue inhibitor of metalloproteinase 2. Melanoma Res. 2014;24:32–9. doi: 10.1097/CMR.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 28.Reis ST, Pontes-Junior J, Antunes AA, de Sousa-Canavez JM, Dall’Oglio MF, Passerotti CC, Abe DK, Crippa A, da Cruz JA, Timoszczuk LM, Srougi M, Leite KR. MMP-9 overexpression due to TIMP-1 and RECK underexpression is associated with prognosis in prostate cancer. Int J Biol Markers. 2011;26:255–61. doi: 10.5301/JBM.2011.8831. [DOI] [PubMed] [Google Scholar]


