Abstract
This study aims to study the protective effect and mechanism of carnosol on intestinal oxidative stress. Porcine intestinal epithelial cells (ZYM-SIEC02) were pretreated with carnosol. Tert-butyl hydroperoxide (t-BHP) was added to stimulate the cells. The cell colonization and viability were detected by Edu staining, MTT, and cell counting kit-8 (CCK8) assays. The expressions of reactive oxygen species (ROS), nitric oxide (NO), superoxide dismutase (SOD), and malondialdehyde (MDA) in intracellular and oxidative stress were detected. The expression of related genes and proteins in cells was detected by real-time PCR and western blot. The regulatory mechanisms were identified by co-immunoprecipitation (Co-IP) and chromatin immunoprecipitation (CHIP) assays. The results showed that t-BHP reduced cell proliferation and viability, while cells pretreated with carnosol had resistance to t-BHP, decreased intracellular ROS, MDA and NO levels, and increased SOD content. The mRNA and protein levels of heme oxygenase 1/Nuclear respiratory factor 2 (HO-1/Nrf-2) in ZYM-SIEC02 cells treated with carnosol were significantly increased. Nrf2 was able to bind to cell cycle negative regulatory protein p21 Nrf2 could bind to the promoter regions of cyclin D1 (CCND1) and SOD genes. In conclusion, carnosol has a protective effect on intestinal epithelial cells by up-regulating the expression of Nrf2 and inhibiting p21 protein to promote the expression of CCND1 and SOD.
Keywords: Carnosol, intestinal oxidative stress, Nrf2, ZYM-SIEC02 cells
Introduction
Carnosol is a major compounds of rosemary and was first isolated from sage in 1942 [1]. In recent decades, a number of attention for carnosol shows activity in anti-oxidant [2], anti-cancer [3], anti-inflammatory [4], and neuroprotective [5]. Anti-oxidative activity of carnosol improved lifespan and health span in caenorhabditieselegans [6]. Moreover, carnosol also significantly protects the brain from chronic stress in rats through enhancing antioxidant defenses and decreasing oxidative injury [7].
As we all know, the accumulation of ROS can cause many diseases including cardiovascular disease [8], aging-related diseases [9], and metabolic disease [10], therefore, eliminating the production of ROS was important for maintaining cell function. In addition, SOD shows strong activity of antioxidant and reflects the extent of oxidative damage. Cells were more sensitive to oxygen radical with a low level of SOD. Furthermore, accumulations of oxidant produces exacerbations of the damage induced by oxidative stress. Therefore, supplementing the diet with natural antioxidants is very important to protect cells from free radical attacks.
Reports found that the activation of Nrf2 of microvascular endothelial cells improve endothelial dysfunction [11]. Nrf2 uncouples from Keap1; Keap1 releases Nrf2; and Nrf2 enters the nucleus. Nrf2 can bind its cognate response element [12]. Heme oxygenase 1 (HO-1) had antioxidative activity in acute cerebral ischemic injury via the activation of BDNF-TrkB-PI3K/AKT signaling pathway [13]. Thus, we analyzed the role of carnosol through anti-oxidative mechanism by regulating expression level of Nrf2 and HO-1. We explored the mechanisms underlying the actions of carnosol in HO-1 and AKT.
We have found in previous feeding experiments that piglets fed rosemary extract have obvious resistance to weaning stress. Growth indicators were also significantly superior to the control group. As a main component of rosemary extract, carnosol is presumed to have antioxidant, anti-stress, and growth-promoting effects. In this study, we pretreated pig intestinal epithelial cells with carnosol, stimulated cells with t-BHP to produce stress, and observed the resistance of cells pretreated with carnosol to t-BHP-induced stimulation. We examined the proliferation of cells, the expression of oxidative stress factors, and transcription factors. We also studied the binding regulation mechanism of Nrf2. Through this study, we wanted to clarify the protective effect of carnosol on intestinal cells and explore the molecular regulation mechanism.
Materials and method
Chemicals
ZYM-SIEC02 was obtained from American Type Culture Collection (ATCC). Dulbecco’s modified Eagle medium (DMEM), penicillin with streptomycin and trypsin with ethylenediaminetetraacetic acid (EDTA) were purchased from Hyclone (USA). Fetal bovine serum (FBS) was obtained from Gibco (USA). Carnosol and Tert-Butyl hydroperoxide solution (t-BHP) purchased from Sigma (USA). PBS was obtained from Solarbio (PH 7.2-7.4) (China). DCFH-DA was purchased from Beyotime (China). Cell Counting Kit-8 (CCK8) obtained from Dojido (Japan). NO kit was purchased from Beyotime (China).
Cell culture and treatment
ZYM-SIEC02 cells were maintained in DMEM with 10% FBS and 1% penicillin/streptomycin, in a humidified incubator at 37°C and 5% CO2. ZYM-SIEC02 cells pretreated with 10 μM carnosol for 24 h, and 200 μM t-BHP for an additional 3 h.
Cell viability
ZYM-SIEC02 cells (1×104 cells/well) were seeded in 96-well plates and incubated at 37°C for 24 h, pretreated with 10 μM carnosol for 24 h and then treated with 200 μM t-BHP for 3 h. The cells were incubated with CCK-8 reagent (100 µL/mL) for 2 h. The end result of the absorbance was read at 450 nm.
Measurement of ROS and NO generation
ZYM-SIEC02 cells were seeded in 6-well plate and pretreated with 24 h, then they were treated with t-BHP for 3 h. The intercelluar generation of ROS was measured by using DCFH-DA fluorescence assay and NO was detected by NO kit. Flow cytometer was employed to analyze ROS production by emission at 525 nm after excitation at 488 nm. The NO was detected by loaded with 5 μM DAF-FM DA.
SOD and MDA measurement
ZYM-SIEC02 cells were collected after treatment of carnosol for 24 h and 3 h for t-BHP. The SOD and MDA activity was measured by using the SOD assay kit and MDA assay kit according to the manufacturer’s instructions (Jiancheng, nanjing).
Real-time PCR
Total RNA was isolated with TRIzol reagent (Invitrogen) from ZYM-SIEC02 cells according to the manufacturer’s instructions. Then qRT-PCR was performed using a Real-time PCR System (Bio-rad, USA) with a SYBR Green PCR Kit (Takara, Otsu, Japan). The expression levels of nrf1 were normalized to GAPDH.
Western blot analysis
Total proteins were extracted from ZYM-SIEC02 cells using ice-cold lysis buffer, the extracted protein was quantified by BCA kit (Beyotime). Briefy, 30 ng/well proteins were separated by SDS-PAGE gels, then transferred into polyvinylidenedifuoride (PVDF) membranes. The PVDF membranes were blocked with 5% non-fat powdered milk for 2 h at room temperature and incubated with primary antibodies overnight at 4°C. After washing the PVDF membranes with TBST for 3 times (5 min per wash), the membranes were incubated with the secondary antibodies for 1.5 h-2 h at room temperature. The membranes were then washed 3 times again with TBST and washed with TBS for the last time. Protein expression levels were visualized using an enhanced chemiluminescence (ECL) detection system.
Co-immunoprecipitation (Co-IP) assay
After treatment, the cells were collected and incubated with WB and IP buffer at 4°C for 30 min. Then the lysate was centrifuged at 15,000 rpm, 4°C for 20 min. The supernatants containing 1 mg protein were first precleared with 1μg rabbit IgG antibody and 20 μl Protein A+G agarose beads (Beyotime, Shanghai, China) with gentle rocking for 1 h at 4°C. After centrifugation, the supernatants were incubated with rabbit anti-HO-1 (1:100 dilution) or anti-AKT (1:100 dilution) antibody and swung slowly overnight at 4°C. Then immunocomplexes were incubated with 40 μl protein A+G agarose beads for 2 h at 4°C. Protein A+G agarose beads were washed 5 times with PBS and washed beads were re-suspended in 1x electrophoresis sample buffer, boiled at 100°C for 10 min. After centrifugation, the supernatants were obtained as immunoprecipitates for western blotting analysis.
Chromatin immunoprecipitation (CHIP) assy
Cells were grown in a dish and were crosslinked by adding formaldehyde to final concentration of 1% and incubated at room temperature for 10 mins. After treatment, cells were washed with cold PBS and cells were scraped to collect through centrifugation at 2000 rpm, 4°C for 5 min. Then cells were washed with PBS and re-suspended in CHIP lysis buffer and incubated on ice for 10 min. The lysates were sonicated then diluted with CHIP diluted buffer, protein A agarose deads was added to the sonicated lysates and rotated at 4°C for 1 h to reduce the non-specific binding. The supernatants were centrifuged and collected, 20 ul of lysates were taken out as input control other 20 ul lysates were add to the antibody to be incubated at 4°C overnight. Add the immunoprecipitating antibody to the supernatants and incubated at 4°C for 2 h and pellet agarose was centrifuged softly. Carefully remove the supernatant and wash the agarose complex for 3-5 min on a rotating platform with low salt wash buffer, high salt wash buffer, LiCl buffer and TE buffer respectively. Elution buffer were add to the pellet agarose complex to elute the agarose complex from antibody. Vortex briefly to mix and incubated at room temperature for 15 min with rotation. Spin down agarose and transfer the supernatant to another tube and repeat elution. The combined elutes was added NaCl to incubated at 65°C for 4 h to reverse crosslink. The eluates were added with MBI to incubate in 37°C for 1 h and then added with EDTA, Tris-HCl and proteinase K to the elutates and incubated at 45°C for 2 h. Recover the DNA and diluted into ddH2O for later analysis.
Statistical analyses
Data is expressed as mean with standard deviation (SD). Analysis of variance (ANOVA) using Tukey’s test was applied to compare the mean of each group with that of the control group, and P < 0.05 was considered to be statistically significant.
Results
Carnosl protect ZYM-SIEC02 cells against t-BHP induced cell injury
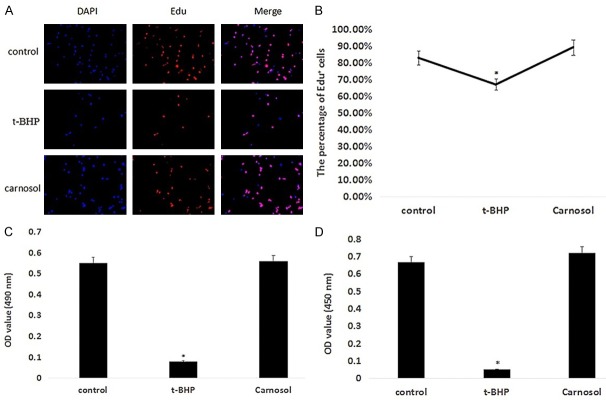
To measure the extent of t-BHP-induced damage to ZYM-SIEC02 cells, cell viability was detected. As shown in Figure 1A and 1B, the percentage of Edu positive cells in t-BHP treated group was lower than that of the control group (P < 0.05). In carnosol treated group, ZYM-SIEC02 cells were pretreated with 10 μM carnosol for 24 h and then treated with 200 μM t-BHP for 3 h. The percentage of Edu positive cells in carnosol treated group was 88.95%, which was higher than that in the control group (82.64%) and t-BHP treated group (66.67%).
Figure 1.
Carnosol protects ZYM-SIEC02 cells and reduces the effects of t-BHP on cell proliferation and viability. A. Edu staining results; B. Edu positive cell percentage statistics; C. MTT detection; D. CCK8 detection.
We further verified the effects of t-BHP and carnosol on cells by MTT and CCK8 assays. The MTT test results showed that the OD values of the control group, t-BHP group, and carnosol group were 0.55, 0.08, and 0.56, respectively (Figure 1C). The CCK8 test results showed that the OD values of the control group, t-BHP group and carnosol group were 0.66, 0.05 and 0.72, respectively (Figure 1D). These results showed that t-BHP reduces cell proliferation and reduces cell viability, while carnosol protects cells against t-BHP induced damage.
Carnosol enhanced the ability of antioxidant in ZYM-SIEC02 cells
Oxidative stress is an important mechanism of different type of cell damage. To clear the effect of carnosol on cellular oxidative stress, we examined the expression levels of ROS, MDA, SOD, and NO in three groups of cells. The results showed that the expression levels of ROS in the control group, t-BHP treatment group, and carnosol group were 24.32 RFU, 57.66 RFU, and 25.11 RFU, respectively; the MDA expression levels were 0.2145 nM, 0.8744 nM, and 0.2454 nM; The expression levels of SOD were 50.57 U, 26.22 U, and 58.56 U, respectively; the expression levels of NO were 0.45 μM, 0.95 μM, and 0.47 μM, respectively (Figure 2A-D). Our results showed that there was a significant increase in level of ROS, MDA, NO and decreased the production of SOD after treatment of t-BHP compared with the control group (P < 0.05). We found that oxidative stress in ZYM-SIEC02 induced by t-BHP caused a cell damage and this condition alleviated by carnosol through regulating the content of several key antioxidant enzyme activities and key factors.
Figure 2.
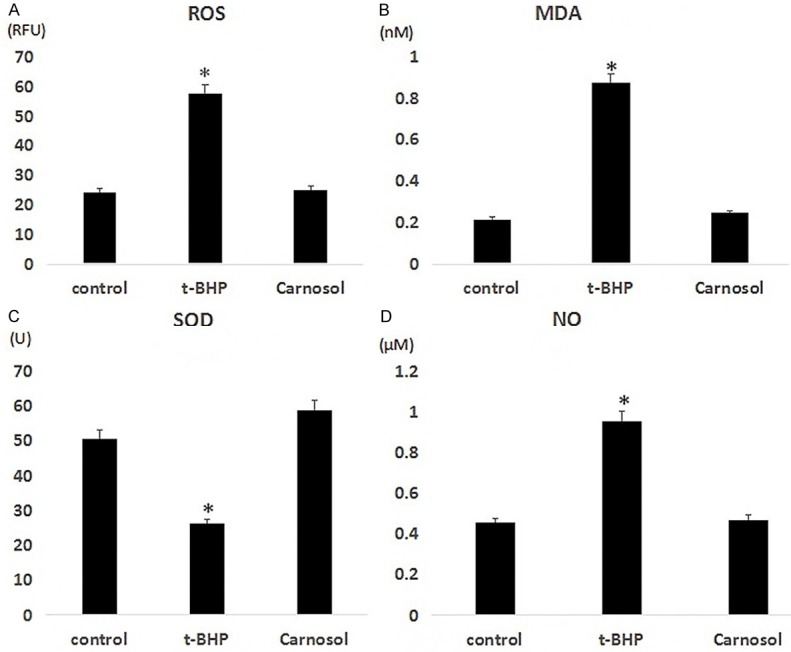
Carnosic acid increases the antioxidant capacity of ZYM-SIEC02 cells. A. ROS; B. MDA; C. SOD; D. NO.
Carnosol suppressed the oxidative stress by up-regulating the expression of Nrf2 and HO-1
We examined the expression of transcription factors related to oxidative stress, and detected the expression levels of HO-1, FoxO3a, FoxM1, FoxO1, CDX2, E2F1, Nrf-2, and NF-κB by q-PCR. The mRNA level of HO-1, Nrf2 were down-regulated after treatment of t-BHP compared with the control group (P < 0.05). Moreover, pretreatment with carnosol could increase the expression level of HO-1, Nrf2 compared with the t-BHP group (P < 0.05). These results showed that carnosol plays an anti-oxidative role against t-BHP possibly through up-regulating the expression of HO-1, Nrf2 to enhance antioxidant activities (Figure 3A).
Figure 3.
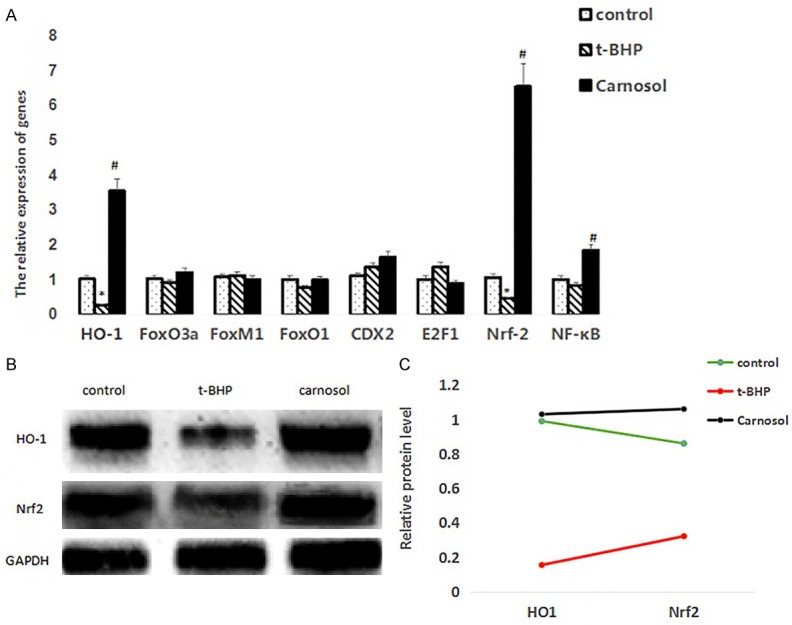
Detection of gene and protein expression by qPCR and western blot. A. qPCR results, * represents a significant difference between the t-BHP group and the control group; # represents a significant difference between the carnosol group and the t-BHP group; B. Western blot results; C. Statistical results of western blot.
To confirme whether both Nrf2 and HO-1 in response to the carnosol treatment for 24 h under and then BHP treatment for 3 h, the protein level of Nrf2 and HO-1 were decreased in t-BHP group compare with control group (P < 0.05). Carnosol treatment up-regulated the protein expression of Nrf2 and HO-1 significantly in ZYM-SIEC02 cells to antagonism oxidative stress caused by ROS (Figure 3B, 3C). Those results implicated that carnosol showed the anti-oxidative activity by inhibiting the decreasing expression level of Nrf2 and HO-1 induced by t-BHP.
Co-IP and CHIP detect the binding of Nrf2 to proteins and genes
In the above results, we observed that carnosol significantly up-regulated the expression levels of HO-1 and Nrf2. The expression of anti-stress factors in ZyM-SIEC02 cells treated with carnosol was significantly increased, and cell proliferation and viability were improved. Therefore, we wanted to further explore the regulatory mechanisms. The protein binding to Nrf2 was predicted in the HitPredict database, and the gene containing the Nrf2 binding site in the promoter region was predicted in the Jaspar database. The comparison revealed that Nrf2 may bind to the cyclin-dependent kinase inhibitor p21, and was found to be transcribed. The Nrf2 binding site was present at the -906~-892 locus of CCND1 gene transcription initiation site and the -1927~-1913 locus of SOD gene transcription initiation site. Next, we validated this prediction by Co-IP and CHIP. Co-IP results showed that Nrf2 protein and p21 protein were detected in the protein complex pulled down by Nrf2 antibody, but not in the control antibody IgG group (Figure 4A, 4B). The CHIP results indicated that the upstream sequences of CCND1 and SOD could be cloned in the nucleic acid pulled down with the Nrf2 antibody, but not in the control antibody IgG group (Figure 4C, 4D). The above results indicated that Nrf2 can bind to p21 protein, and Nrf2 can bind to the promoter regions of CCND1 and SOD genes.
Figure 4.
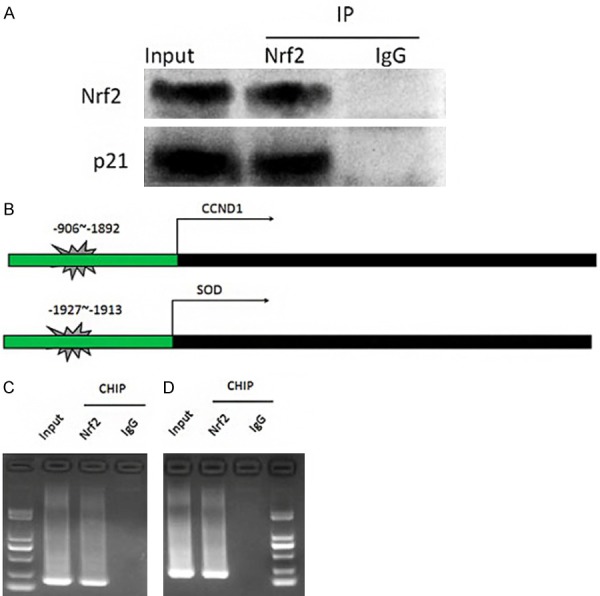
Co-IP and CHIP detect the binding of Nrf2 to proteins and genes. A. Co-IP detects the binding of Nrf2 to p21 protein; B. CCND1 and SOD gene promoter region Nrf2 binding site prediction schematic; C. CHIP test to verify the binding of Nrf2 and CCND1 promoter region. D. CHIP test to verify the binding of Nrf2 and SOD promoter region.
Discussion
Carnosol is a major bioactive compound from rosemary which shows strong activity of anti-oxidant, anti-cancer, and anti-inflammatory [1,14]. Intestinal injury caused disordered absorption of nutritions which contribute to malnutrition even metabolic disease. Most of intestinal injury caused by oxidative stress and low ability of anti-oxidant [15]. Here, we established intestinal injury model t-BHP in ZYM-SIEC02 cells.
It has been shown that t-BHP can cause oxidative stress in different pathways [16]. Alkaline phosphatase (ALP) as the maker of intestinal epithelial cells and expressed at a high concentration in he brush border membrane [17]. In our work, t-BHP led to a decrease of cell proliferation and expression of ALP, differently, carnosol suppressed the decreased cell proliferation and protein level of ALP in ZYM-SIEC02 cells. Report showed that the level of ALP activity and expression of ALP messenger RNA in ZYM-SIEC02 cells were up-regulated by vitamin K2 [17]. Meanwhile, cell proliferation related to the extent of damage caused by anti-oxidative stress, we found that carnosol not only increased the cell proliferation but also up-regulated protein level of ALP in ZYM-SIEC02 cells. Researchers found that carnosol have an anti-proliferative activity in prostate cancer cells [18]. Thus, we speculated that carnosol may protect ZYM-SIEC02 cells from t-BHP, the function of carnosol depend on the type of cell line.
Oxidative stress is a main cause of the development of intestinal injury, over accumulation of ROS and production of oxidation exceeds damage which leads to intercellular oxidative stress. At same time, low levels of anti-oxidant enzyme break down the balance between oxidant and antioxidant. Our results demonstrated that t-BHP induced ROS and MDA generation, inhibited the production of SOD and NO contributed to the promotion of the oxidative stress. Carnosol can significantly reduce the production of ROS and MDA, enhancing the content of SOD and NO to respond oxidative stress of t-BHP. Researchers discovered that carnosol also promote the release of NO in HMVEC cells [19]. Reports showed that carnosol induced the apoptosis of HCT116 through generation of ROS [20]. Moreover, NO play crucial roles in regulating vascular function, endothelium inflammatory reaction, as well as vascular growth and regeneration [21], therefore, the release of NO promoted by carnosol in ZYM-SIEC02 cells might be an important mechanism for carnosol protect from oxidative stress. SOD has been proved to play a strong antioxidative effect under oxidative stress and accumulation of the production of MDA of oxidant aggravated the damage of ZYM-SIEC02 cells. Based our work, we found that carnosol can relieve the damage via enhancing activity of antioxidant enzyme and eliminating production of oxidation.
HO-1 exerts a crucial role in restoring redox balance. Significant induction of HO-1 expression showed to protect neurons against oxidative stress [22]. Moreover, HO-1 was reported to improve SOD activity which suggested the potent effect on HO-1 in intestinal injury induced by oxidative stress. Additionally, Nrf2 as a key factor for oxidative stress, and Keap1/Nrf2 system play an important role in the study of intestinal injury. Those results showed that t-BHP caused oxidative stress through down-regulating expression of HO-1 and Nrf2 in mRNA and protein levels. Carnosol promoted the antioxidative activity by up-regulated the level of HO-1 and Nrf2. Previous studies report that carnosol can regulate the maturation and function of human dendritic cells (DC) through up-regulation activity of the HO-1 to enhance the immunocompetence [23]. In lung form mice, carnosol induced HO-1 expression, and this result showed potent success rate of lung transplantation through ameliorate antioxidative stress [24]. In particular, carnosol play a antioxidative activity may depend on Nrf2 signaling [25]. Reports showed that carnosol against (spinal cord injury) SCI-induced oxidative stress and inflammation through modulating NF-kappaB, COX-2, and Nrf-2 levels in Wistar rats, especially Nrf-2 up-regulation [26]. Therefore, we focused on the capability of carnosol on up-regulating HO-1 and Nrf2 gene and protein expression in ZYM-SIEC02 cell, the two factors have a potential interaction with other factors.
AKT is a serine/threonine kinase and it can be activated by a wide range of growth signals and the biochemical mechanisms [27]. Moreover, AKT participates in PI3K/mTOR signaling pathway to respond to extracellular stimulators [28]. In recent decades, AKT was dysregulated in different type of cancer and AKT considered as a therapeutic target of cancer [29,30]. Our results show that t-BHP induced ZYM-SIEC02 cells damage through disrupting the interaction of HO-1 and AKT. Carnosol shows an antioxidative effect on ZYM-SIEC02 cells against t-BHP treatment by promoting interaction of HO-1 with AKT. Researchers discovered that HO-1 interact with EBP plays a protective role in alleviating the dysfunction of oxidative stress and cardiac systolic function induced by cholesterol stimulation [31]. Moreover, pretreatment with BME activated survival-promoting signals through ERK and Akt activated Nrf2/HO-1 signaling pathway, resulting in decrease in cytotoxicity induced by the OGD [32]. These results suggested the involvement of HO-1 and AKT in oxidative stress is induced by t-BHP regulated by carnosol.
Carnosol has a protective effect on intestinal epithelial cells, which can up-regulate the expression of Nrf2, inhibit the cell cycle negative regulatory protein p21, and promote the expression of CCND1 and SOD.
Acknowledgements
This work was supported by Provincial Agricultural Science Innovation and Promotion Project (2018LM2150) and the China Postdoctoral Science Foundation (2018M640789).
Disclosure of conflict of interest
None.
References
- 1.Johnson JJ. Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer Lett. 2011;305:1–7. doi: 10.1016/j.canlet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satoh T, Izumi M, Inukai Y, Tsutsumi Y, Nakayama N, Kosaka K, Shimojo Y, Kitajima C, Itoh K, Yokoi T. Carnosic acid protects neuronal HT22 cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners. Neurosci Lett. 2008;434:260–265. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JJ, Syed DN, Suh Y, Heren CR, Saleem M, Siddiqui IA, Mukhtar H. Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: implications for chemoprevention. Cancer Prev Res (Phila) 2010;3:1112–1123. doi: 10.1158/1940-6207.CAPR-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poeckel D, Greiner C, Verhoff M, Rau O, Tausch L, Hornig C, Steinhilber D, Schubertzsilavecz M, Werz O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem Pharmacol. 2008;76:91–97. doi: 10.1016/j.bcp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Kim JS, Cho HS, Lee HJ, Kim SY, Kim S, Lee SY, Chun HS. Carnosol, a component of rosemary (Rosmarinus officinalis L. ) protects nigral dopaminergic neuronal cells. Neuroreport. 2006;17:1729–1733. doi: 10.1097/01.wnr.0000239951.14954.10. [DOI] [PubMed] [Google Scholar]
- 6.Lin C, Zhang X, Su Z, Xiao J, Lv M, Cao Y, Chen Y. Carnosol improved lifespan and healthspan by promoting antioxidant capacity in caenorhabditis elegans. Oxid Med Cell Longev. 2019;2019:1–13. doi: 10.1155/2019/5958043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samarghandian S, Aziminezhad M, Borji A, Samini M, Farkhondeh T. Protective effects of carnosol against oxidative stress induced brain damage by chronic stress in rats. BMC Complement Altern Med. 2017;17:249. doi: 10.1186/s12906-017-1753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CR, Chang HC, Cheng YD, Lan WC, Yang SE, Ching H. Aqueous extract of davallia mariesii attenuates 6-hydroxydopamine-induced oxidative damage and apoptosis in B35 cells through inhibition of caspase cascade and activation of PI3K/AKT/GSK-3β pathway. Nutrients. 2018;10 doi: 10.3390/nu10101449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos AL, Sinha S, Lindner AB. The good, the bad, and the ugly of ROS: new insights on aging and aging-related diseases from eukaryotic and prokaryotic model organisms. Oxid Med Cell Longev. 2018;2018:1–23. doi: 10.1155/2018/1941285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heng XP, Yang LQ, Li L. The new idea about early intervention for type 2 diabetes based on gan disease transferring to Pi in metabolic diseases. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35:746–751. [PubMed] [Google Scholar]
- 11.Katsuoka F, Motohashi H, Ishii T, Aburatani H, Engel JD, Yamamoto M. Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol Cell Biol. 2005;25:8044–8051. doi: 10.1128/MCB.25.18.8044-8051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 13.Qi D, Ouyang C, Wang Y, Zhang S, Ma X, Song YJ, Yu HL, Tang J, Fu W, Sheng L. HO-1 attenuates hippocampal neurons injury via the activation of BDNF-TrkB-PI3K/Akt signaling pathway in stroke. Brain Res. 2014;1577:69–76. doi: 10.1016/j.brainres.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Zhang Q, Hou N, Li J, Liu M, Peng S, Zhang Y, Luo Y, Zhao B, Wang S. Carnosol as a Nrf2 activator improves endothelial barrier function through antioxidative mechanisms. Int J Mol Sci. 2019;20:880. doi: 10.3390/ijms20040880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Tong X, Sui X, Wang Z, Qi B, Li Y, Jiang L. Antioxidant activity and protective effects of Alcalase-hydrolyzed soybean hydrolysate in human intestinal epithelial Caco-2 cells. Food Res Int. 2018;111:256–264. doi: 10.1016/j.foodres.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 16.T MM, Anand T, Khanum F. Attenuation of cytotoxicity induced by tBHP in H9C2 cells by Bacopa monniera and Bacoside A. Pathophysiology. 2018;25:143–149. doi: 10.1016/j.pathophys.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Noda S, Yamada A, Tanabe R, Nakaoka K, Hosoi T, Gosekisone M. Menaquinone-4 (vitamin K2) up-regulates expression of human intestinal alkaline phosphatase in Caco-2 cells. Nutr Res. 2016;36:1269–1276. doi: 10.1016/j.nutres.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Gao H, Song Q, Yang J, Yu S, Zhao J, Yu G. Carnosol inhibits Hedgehog signaling pathway in both LNCaP and DU145 prostate cancer cell lines. Cell Mol Biol (Noisy-le-grand) 2017;63:104–108. doi: 10.14715/cmb/2017.63.8.22. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Bundeesomchok K, Rakotomanomana N, Fabianotixier A, Bott R, Wang Y, Chemat F. Towards a zero-waste biorefinery using edible oils as solvents for the green extraction of volatile and non-volatile bioactive compounds from rosemary. Antioxidants. 2019;8:140. doi: 10.3390/antiox8050140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park KW, Kundu J, Chae I, Kim D, Yu M, Kundu JK, Chun K. Carnosol induces apoptosis through generation of ROS and inactivation of STAT3 signaling in human colon cancer HCT116 cells. Int J Oncol. 2014;44:1309–1315. doi: 10.3892/ijo.2014.2281. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Horke S, Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237:208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Ding Y, Chen M, Wang M, Li Y, Wen A. Posttreatment with 11-Keto-β-Boswellic acid ameliorates cerebral ischemia-reperfusion injury: Nrf2/HO-1 pathway as a potential mechanism. Mol Neurobiol. 2015;52:1430–1439. doi: 10.1007/s12035-014-8929-9. [DOI] [PubMed] [Google Scholar]
- 23.Campbell NK, Fitzgerald HK, Fletcher JM, Dunne A. Plant-derived polyphenols modulate human dendritic cell metabolism and immune function via AMPK-dependent induction of Heme Oxygenase-1. Front Immunol. 2019;10:345. doi: 10.3389/fimmu.2019.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamura T, Momozane T, Sanosaka M, Sugimura K, Iida O, Fuchino H. Carnosol is a potent lung protective agent: experimental study on mice. Transplant Proc. 2015;47:1657–1661. doi: 10.1016/j.transproceed.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Selimoglu-Buet D, Badaoui B, Benayoun E, Toma A, Fenaux P, Quesnel B. Accumulation of classical monocytes defines a subgroup of MDS that frequently evolves into CMML. Blood. 2017;130:832–835. doi: 10.1182/blood-2017-04-779579. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZH, Xie YX, Zhang JW, Qiu XH, Hou YB. Carnosol protects against spinal cord injury through Nrf-2 upregulation. J Recept Signal Transduct Res. 2015;36:72. doi: 10.3109/10799893.2015.1049358. [DOI] [PubMed] [Google Scholar]
- 27.Diehl N, Schaal H. Make yourself at home: viral hijacking of the PI3K/Akt signaling pathway. Viruses. 2013;5:3192. doi: 10.3390/v5123192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Revathidevi S, Munirajan AK. Akt in cancer: mediator and more. Semin Cancer Biol. 2019;59:80–91. doi: 10.1016/j.semcancer.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi: 10.1016/j.gene.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 30.Song M, Bode AM, Dong Z, Lee M. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 31.Jin X, Xu Z, Cao J, Yan R, Li Y. HO-1/EBP interaction alleviates cholesterol-induced hypoxia through the activation of the AKT and Nrf2/mTOR pathways and inhibition of carbohydrate metabolism in cardiomyocytes. Int J Mol Med. 2017;39:1409–1420. doi: 10.3892/ijmm.2017.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Huang W, Wang J, Song H, Cen J, Ji B. Anthraquinone derivative exerted hormetic effect on the apoptosis in oxygen-glucose deprivation-induced PC12 cells via ERK and Akt activated Nrf2/HO-1 signaling pathway. Chem Biol Interact. 2017;262:1–11. doi: 10.1016/j.cbi.2016.12.001. [DOI] [PubMed] [Google Scholar]