Abstract
Pretreatment inflammatory indexes including neutrophil-lymphocyte ratio (NLR), lymphocyte-monocyte ratio (LMR), platelet-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) are associated with poor outcomes in various malignant tumors, but their prognostic value in patients with osteosarcoma is poorly known. This was a retrospective study of patients with osteosarcoma treated between 01/2010 and 12/2013 at Chongqing University Cancer Hospital. Follow-up was calculated from the date of initial histological diagnosis to December 2018 or death or loss of follow-up. Receiver operating characteristic (ROC) analysis was used to determine the NLR, LMR, PLR, and SII cut-off values (low (L) vs. high (H)). The Kaplan-Meier method was used for survival analysis. Univariable and multivariable Cox analyses were performed to determine the independent prognostic factors. Patients with LNLR had better survival than those with HNLR (median, 38.0 vs. 13.0, P<0.001). Patients with LSII had better survival (26.0 vs. 10.0 months, P=0.001) than those with HSII. The areas under the curves for NLR, LMR, PLR, SII, and ALP were 0.761 (P<0.001), 0.683 (P=0.012), 0.697 (P=0.002), 0.653 (P=0.031), and 0.515 (P=0.837), respectively. In the univariable analyses, Enneking’s stage, systemic chemotherapy, surgery, NLR, PLR, LMR, and SII were associated with overall survival (OS). The multivariable analysis showed that HNLR (HR=2.507; 95% CI=1.364-4.606; P=0.003) was independent unfavorable prognostic factors. This preliminary study suggests that NLR is associated with poor prognosis in osteosarcoma. NLR could be a potential prognostic marker of osteosarcoma.
Keywords: Osteosarcoma, systemic immune-inflammation index, neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, lymphocyte-monocyte ratio, prognosis
Introduction
Osteosarcoma is a rare cancer of mesenchymal origin characterized by the production of osteoid (or immature bone) by the malignant cells [1,2]. It is the most common bone sarcoma in children and adolescent, causing just under two thirds of the malignant bone cancer cases in young children [1-4]. Among children aged 0-19 years, the incidence of osteosarcoma is 5.5 per million boys and 4.5 per million girls [5]. Osteosarcoma is rare in adults, with only 400-450 new cases each year in the United States, with an incidence of 8 per million people-year in adults aged 15-19 years, 1.5 per million people-year among adults aged 35-64 years, and 2.5 per million people-year among adults aged 80-84 years [6].
Recent advances in effective chemotherapy have improved the 5-year survival in osteosarcoma patients to up to 60%-70%, but there is a lack of novel therapeutic strategies to further improve survival [7]. Osteosarcoma can appear and progress rapidly, leading to poor prognosis and high mortality. A primary reason for the poor prognosis of osteosarcoma is the lack of reliable biomarkers, making it difficult to identify the early stages of the disease that are treatable. Traditional approaches such as imaging often have limited uses as prognostic tools [8]. Further exploration of the underlying biology of osteosarcoma is thus warranted in order to identify novel biomarkers useful for the clinical staging of the disease.
Inflammation is known to be an important hallmark of cancer, contributing to tumor cell proliferation and genomic instability [9]. Inflammation activates a number of oncogenic processes that ultimately contribute to tumor progression and metastasis, including increased angiogenesis, chemotherapy resistance, and immunosuppression [10]. Owing to the key role of inflammation in cancer progression, hematological inflammatory indexes such as neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), and systemic immune-inflammation index (SII) have been suggested for the assessment of different types of malignant tumors [5,11-14].
Nevertheless, whether these inflammatory indexes are relevant in osteosarcoma is poorly known. Therefore, the aim of the present retrospective study was to examine the prognostic value of NLR, LMR, PLR, and SII in patients with osteosarcoma. The results could provide new insights for the staging of the disease and to improve management.
Materials and methods
Study design and patients
This was a retrospective study of patients with osteosarcoma treated between January 2010 and December 2013 at the Chongqing University Cancer Hospital. The study was approved by the ethics committee of Chongqing University Cancer Hospital. The inclusion criteria were: 1) histologically confirmed osteosarcoma; 2) no previous anti-cancer treatment; 3) complete medical records available; and 4) available follow-up. The exclusion criteria were: 1) presence of a pre-existing hematological disease; 2) infection, fever, or other inflammatory diseases prior to treatment; 3) incomplete clinical data; or 4) previously treated with non-steroid anti-inflammatory drugs, as these might impact blood tests.
Data collection
Two authors worked independently to extract the clinical data of interest. Relevant clinicopathological data such as sex, age, Enneking’s stage, tumor location, chemotherapy, surgery, and pathological fracture were collected from the medical records. Routine laboratory data including absolute lymphocyte count (ALC), absolute neutrophil count (ANC), absolute monocyte count (AMC), platelets, and alkaline phosphatase (ALP) were obtained from the records from the diagnosis period, 7 days before initiation of any treatment. The pretreatment baseline NLR, LMR, and PLR were calculated using the following formulae: NLR = ANC/ALC, LMR = ALC/AMC, PLR = platelet count/ALC [15], and SII = platelet count × ANC/ALC [16].
Follow-up
The guidelines issued by the National Comprehensive Cancer Network [2] were routinely used for the follow-up of all patients. Overall survival (OS) was the primary outcome. Follow-up was conducted once every three months for the first 3 years, every six months for years 4-5, and yearly thereafter. Physical examinations, surgical site X-ray, chest CT scans, and laboratory tests were conducted routinely during follow-up. In addition, bone scans were conducted every 6 months. For the present study, the duration of follow-up was calculated from the date of initial histological diagnosis to the date of the latest follow-up of this study (December 2018) or death or loss of follow-up.
Statistical analysis
SPSS 22.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Categorical variables were presented as frequencies and were compared using the chi-square test. The Kaplan-Meier method was used to construct survival curves, with comparisons carried out using the log-rank test. Cox univariable and multivariable tests were used to determine the independent prognostic factors. The NLR, PLR, LMR, and SII cut-off values were established using receiver operating characteristic (ROC) curves, with 5-year OS as the outcome and the maximum Youden index point being used to guide cut-off selection [17]. Those values were then used to classify patients into two groups based on whether they were above or below the specified cut-off value. ROC areas under the curve (AUCs) were compared to determine how effective each prognostic variable was. P<0.05 was considered statistically significant.
Results
Clinicopathological characteristics
From 96 patients treated during the study period, 77 patients were included in the present study. Table 1 summarizes the clinical characteristics of the 77 osteosarcoma patients (43 males and 34 females). The median patient age at diagnosis was 19 (range 7-66) years. Thirty-three patients (42.9%) were <18 years of age at diagnosis, while 44 patients (57.1%) were >18 years of age. The majority of the tumors (n=63, 81.8%) were found in the extremities. Among the 77 patients, 68 were Enneking’s stage I-II (88.3%), while nine were stage III (11.7%). For histological subtypes, there were 70 (90.9%), 1 (1.3%), 1 (1.3%), and 5 (6.5%) patients diagnosed with conventional, telangiectatic, intramedullary, and periosteal osteosarcoma, respectively. Pathological fractures were found in 29 patients (37.7%). During follow-up, 41 (53.2%) patients received systemic chemotherapy and 52 (67.5%) underwent operation.
Table 1.
Association of the patients’ clinicopathological features with inflammatory indexes
| Clinical para | Total n=77 | NLR | P | PLR | P | LMR | P | SII | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| LNLR n=37 (48.1%) | HNLR n=40 (51.9%) | LPLR n=31 (40.3%) | HPLR n=46 (59.7%) | LLMR n=26 (32.5%) | HLMR n=51 (67.5%) | LSII n=52 (67.5%) | HSII n=25 (32.5%) | ||||||
| Age (years) | 0.147 | 0.421 | 0.060 | 0.182 | |||||||||
| ≤18 | 33 (42.9%) | 19 | 14 | 15 | 18 | 15 | 18 | 25 | 8 | ||||
| >18 | 44 (57.1%) | 18 | 26 | 16 | 28 | 11 | 33 | 27 | 17 | ||||
| Sex | 0.539 | 0.539 | 0.088 | 0.985 | |||||||||
| Female | 34 (44.2%) | 15 | 19 | 15 | 19 | 15 | 19 | 23 | 11 | ||||
| Male | 43 (55.8%) | 22 | 21 | 16 | 27 | 11 | 32 | 29 | 14 | ||||
| Tumor location | 0.667 | 0.411 | 0.865 | 0.774 | |||||||||
| Extremities | 63 (81.8%) | 31 | 32 | 24 | 39 | 21 | 42 | 43 | 20 | ||||
| Non-extremities | 14 (18.2%) | 6 | 8 | 7 | 7 | 5 | 9 | 9 | 5 | ||||
| Enneking’s stage | 0.484 | 0.785 | 0.248 | 0.953 | |||||||||
| I-II | 68 (88.3%) | 34 | 34 | 27 | 41 | 25 | 43 | 46 | 22 | ||||
| III | 9 (11.7%) | 3 | 6 | 4 | 5 | 1 | 8 | 6 | 3 | ||||
| Pathological fracture | 0.976 | 0.199 | 0.373 | 0.426 | |||||||||
| No | 48 (62.3%) | 23 | 25 | 22 | 26 | 18 | 30 | 34 | 14 | ||||
| Yes | 29 (37.7%) | 14 | 15 | 9 | 20 | 8 | 21 | 18 | 11 | ||||
| Chemotherapy | 0.049 | 0.011 | 0.577 | 0.010 | |||||||||
| No | 36 (46.8%) | 13 | 23 | 9 | 27 | 11 | 25 | 19 | 17 | ||||
| Yes | 41 (53.2%) | 24 | 17 | 22 | 19 | 15 | 26 | 33 | 8 | ||||
| Surgery | 0.142 | 0.579 | 0.188 | 0.044 | |||||||||
| No | 25 (32.5%) | 9 | 16 | 9 | 16 | 11 | 14 | 13 | 12 | ||||
| Yes | 52 (67.5%) | 28 | 24 | 22 | 30 | 15 | 37 | 39 | 13 | ||||
| ALP | 0.490 | 0.022 | 0.820 | 0.118 | |||||||||
| Normal | 28 (36.4%) | 12 | 16 | 16 | 12 | 9 | 19 | 22 | 6 | ||||
| High | 49 (63.6%) | 25 | 24 | 15 | 34 | 17 | 32 | 30 | 19 | ||||
NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; LMR, lymphocyte-monocyte ratio; SII, systemic immune-inflammation index; ALP, alkaline phosphatase; L: low; H: high.
Determination of inflammatory indexes and ALP cut-off values
ROC analyses were performed to establish the best cut-off for each inflammatory biomarker, based on the maximum Youden index. For NLR, LMR, PLR, ALP, and SSI, the cut-off values were 2.65 (Youden index of 0.47), 5.16 (Youden index of 0.37), 125.0 (Youden index of 0.33), 198.42 (Youden index of 0.15), and 728.24 (Youden index of 0.31), respectively (Figure 1). These cut-off values were used to divide the patients based on whether they were above or below these specified values. Of the 77 patients, 37 (48.1%) were in the low NLR (LNLR) group, while 40 (51.9%) were in high NLR (HNLR) group. The numbers of patients in the low PLR (LPLR) and high LMR (HLMR) were 31 (40.3%) and 46 (59.7%), respectively. The numbers of patients in the low LMR (LLMR) and high LMR (HLMR) were 25 (32.5%) and 52 (67.5%), respectively. The patient numbers with low ALP (LALP) and high ALP (HALP) were 28 (36.4%) and 49 (63.6%), respectively. Finally, 52 (67.5%) patients were in the low SII (LSII) group, while 25 (32.5%) were in the high SII (HSII) group (Table 1).
Figure 1.
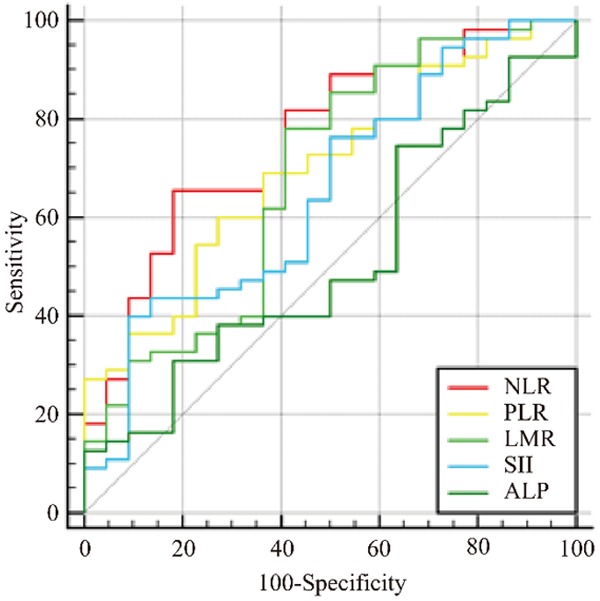
ROC curves of blood neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), systemic immune-inflammation index (SII), and alkaline phosphatase (ALP) for predicting overall survival. The areas under the curves (AUCs) for NLR, PLR, LMR, SII, and ALP were 0.761, 0.697, 0.683, 0.653, and 0.515, respectively.
ROC curves for inflammatory indexes and ALP
The AUC was 0.761 (95% CI=0.650-0.851; P<0.001) for NLR (sensitivity of 65.5%, specificity of 81.8%) (Table 2). The LMR AUC was 0.683 (95% CI=0.567-0.784; P=0.012; sensitivity of 78.2%, specificity of 59.1%). The PLR AUC was 0.697 (95% CI=0.581-0.796; P=0.002; sensitivity of 69.1%, specificity of 63.6%). The SII AUC was 0.653 (95% CI=0.536-0.758; P=0.031; sensitivity of 40.0%, specificity of 90.9%). The ALP AUC was 0.515 (95% CI=0.398-0.630; P=0.837; sensitivity of 49.1%, specificity of 36.4%). Due to its limited AUC (P>0.05), ALP was not further analyzed (Table 2 and Figure 1).
Table 2.
Cut-off value and AUC for prognostic factors
| Prognostic factors | Cut-off value | AUC | Sensitivity (%) | Specificity (%) | P |
|---|---|---|---|---|---|
| NLR | 2.65 | 0.761 | 65.45 | 81.82 | <0.001 |
| PLR | 125.0 | 0.697 | 69.09 | 63.64 | 0.002 |
| LMR | 5.16 | 0.683 | 78.18 | 59.09 | 0.012 |
| SII | 728.24 | 0.653 | 40.00 | 90.91 | 0.031 |
| ALP | 198.42 | 0.515 | 49.09 | 36.36 | 0.837 |
NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; LMR, lymphocyte-monocyte ratio; SII, systemic immune-inflammation index; ALP, alkaline phosphatase.
Clinicopathological significance of inflammatory indexes
To investigate the relationship between clinicopathological features of the patients with osteosarcoma and NLR, PLR, LMR, and SII, comparisons between different groups were made. Chemotherapy was significantly associated with the NLR (P=0.049), PLR (P=0.011), and SII groups (P=0.010). ALP and surgery were associated with the high PLR (P=0.022) and SII (P=0.044) groups, respectively (Table 1). No significant associations were observed between LMR and the other clinicopathological factors.
Survival analysis
Patients in the LNLR group had a median survival of 38.0 months, while those in the HNLR group had a median survival of 13.0 months (P<0.001) (Figure 2A). Those in the HPLR group and poorer survival than those in the LPLR group (median, 13.0 vs. 32.0 months, P=0.009) (Figure 2B). For LMR, the LLMR group had poorer survival than the HLMR group (median, 21.0 vs. 26.0 months, P=0.016) (Figure 2C). Compared with the patients in the LSII group, patients in the HSII group had shorter OS (median, 26.0 vs. 10.0 months, P=0.001) (Figure 2D). Patients with chemotherapy and surgery had better survival (median, 30.0 vs. 11.0 months, P<0.001 and median, 24 vs. 11 months, P=0.023, respectively) than patients without chemotherapy and surgery (Figure 2E, 2F). Favorable OS (median, 24.0 vs. 7.0 months, P=0.013) was found in patients with Enneking’s stage I-II compared with stage III (Figure 2G).
Figure 2.
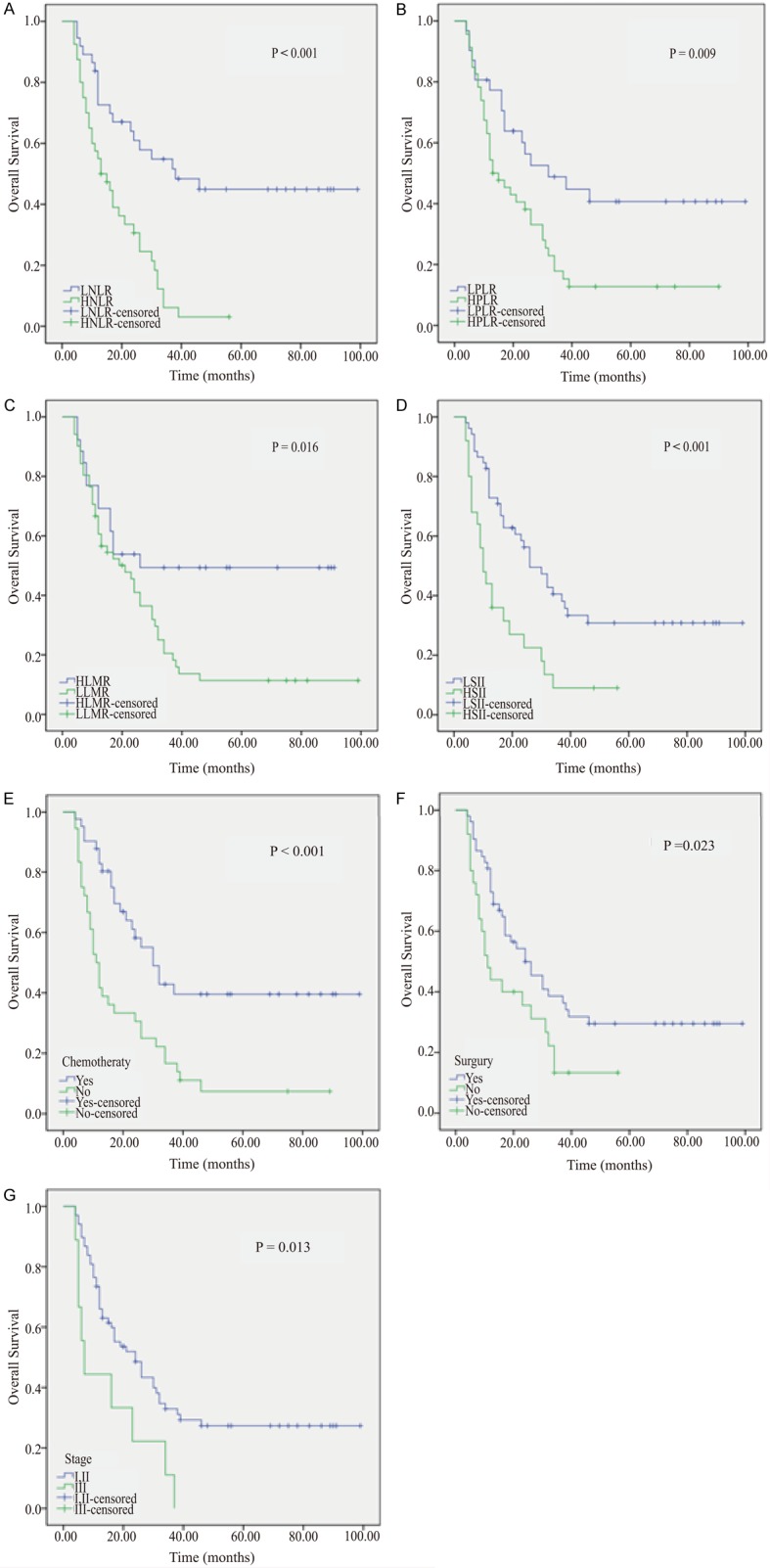
Overall survival Kaplan-Meier curves from 77 patients with osteosarcoma based on (A) neutrophil-lymphocyte ratio (NLR), (B) platelet-lymphocyte ratio (PLR), (C) lymphocyte-monocyte ratio (LMR), (D) systemic immune-inflammation index (SII), (E) chemotherapy, (F) surgery, and (G) Enneking’s stage.
Univariable and multivariable analysis for survival
The univariable analyses showed that the Enneking’s surgical staging (P=0.018), systemic chemotherapy (P=0.001), surgery (P=0.028), NLR (P<0.001), PLR (P=0.012), LMR (P=0.011), and SII (P=0.001) were associated with survival (Table 3). A multivariable analysis of these factors showed that HNLR (HR=2.507; 95% CI=1.364-4.606; P=0.003) and no systemic chemotherapy (HR=2.045; 95% CI=1.161-3.602; P=0.013) were independent unfavorable prognostic factors (Table 3).
Table 3.
Univariable and multivariable analyses of overall survival using the Cox proportional hazard model
| Variable | Median OS (95% CI) (months) | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | 0.767 | ||||
| ≤18 | 17 (9.185-24.815) | Reference | |||
| >18 | 24 (9.086-38.914) | 1.084 (0.635-1.850) | |||
| Sex | 0.662 | ||||
| Female | 21 (8.633-33.367) | Reference | |||
| Male | 23 (13.396-32.604) | 1.126 (0.662-1.916) | |||
| Tumor location | 0.791 | ||||
| Extremities | 21 (13.622-28.378) | Reference | |||
| Non-extremities | 32 (0.221-63.779) | 1.101 (0.538-2.254) | |||
| Enneking’s stage | 0.018 | 0.054 | |||
| I-II | 24 (14.731-33.267) | Reference | Reference | ||
| III | 7 (4.078-9.922) | 2.392 (1.163-4.916) | 2.048 (0.988-4.248) | ||
| PF | 0.383 | ||||
| No | 24 (13.495-34.505) | Reference | |||
| Yes | 19 (2.344-35.656) | 1.273 (0.741-2.188) | |||
| Chemotherapy | 11 (8.480-13.520) | 0.001 | 0.013 | ||
| No | 30 (21.972-38.028) | Reference | Reference | ||
| Yes | 2.622 (1.523-4.514) | 2.045 (1.161-3.602) | |||
| Surgery | 0.028 | ||||
| No | 11 (7.328-14.672) | Reference | |||
| Yes | 24 (14.125-33.875) | 1.855 (1.071-3.213) | |||
| NLR | <0.001 | 0.003 | |||
| LNLR | 38 (12.010-63.990) | Reference | Reference | ||
| HNLR | 13 (7.051-18.949) | 3.212 (1.797-5.741) | 2.507 (1.364-4.606) | ||
| PLR | 0.012 | ||||
| LPLR | 32 (9.804-54.196) | Reference | |||
| HPLR | 13 (7.246-18.754) | 2.094 (1.774-3.737) | |||
| LMR | 0.011 | ||||
| LLMR | 21 (13.881-28.119) | Reference | |||
| HLMR | 26 (11.109-30.981) | 2.309 (1.212-4.400) | |||
| SII | 0.001 | ||||
| LSII | 26 (17.646-34.354) | Reference | |||
| HSII | 10 (6.736-13.264) | 2.478 (1.432-4.286) | |||
| ALP | 0.650 | ||||
| Normal | 30 (17.151-42.849) | Reference | |||
| High | 17 (7.932-26.068) | 1.135 (0.658-1.958) | |||
HR, hazard ratio; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; LMR, lymphocyte-monocyte ratio; SII, systemic immune-inflammation index; ALP, alkaline phosphatase; L: low; H: high.
Discussion
There are numerous studies demonstrating the relationship between cancer and inflammatory biomarkers [18-21]. NLR, LMR, PLR, and SII are associated with poor outcomes in various malignant tumors [5,11-14], but their prognostic value in patients with osteosarcoma is poorly known. Therefore, this study aimed to examine the prognostic value of NLR, LMR, PLR, and SII in patients with osteosarcoma. Using multivariable analysis, the present preliminary study suggests that NLR is associated with poor prognosis in osteosarcoma and that it could be a potential prognostic marker of osteosarcoma.
Cancer-associated inflammation is a crucial indicator of cancer, and systemic inflammation has a well-documented association with carcinogenesis [22]. For instance, neutrophils can release and respond to several chemokines and cytokines [23,24], which in turn can participate in angiogenesis, tumor progression, and metastatic spread [25-27]. Several studies confirmed that monocyte cell lineage stimulates the migration of neoplastic cells, enhances angiogenesis, and inhibits antitumor immunity [28,29]. While lymphocytes are important for antitumor immunity [30,31], platelets are also involved in the growth and development of tumors [32,33]. Based on these results, several inflammation-based predictive biomarkers, including NLR, LMR, PLR, and SII have been suggested to be risk factors for malignancy, independent of one another [12,14,19,20]. In the present study, we found that chemotherapy was associated with the NLR, PLR, and SII groups. ALP and surgery were closely associated with PLR and SII, respectively. The univariable analyses showed that Enneking’s stage I-II, no chemotherapy, no surgery, HNLR, LLMR, HPLR, and HSII were significantly associated with poor prognosis in patients with osteosarcoma. The results were similar to previous studies on various types of tumors [16,30,31]. In osteosarcoma, Liu et al. [34] showed that pre-operative LLMR was associated with poor survival among patients with osteosarcoma, but they did not analyze NLR and PLR. Xia et al. [15] showed that advanced stage and metastasis at diagnosis were associated with HNLR and HPLR, and that the OS is independently associated with NLR. Liu et al. [35] showed that HNLR, HPLR, and LLMR are associated with poor prognosis of patients with osteosarcoma. Huang et al. [36] demonstrated that HSII was closely associated with poor prognosis of patients with osteosarcoma, but they did not analyze the relationship between survival to osteosarcoma and NLR, LMR, and PLR. Interestingly, the multivariable analysis demonstrated that only NLR was an independent predictor of prognosis, as supported by two previous studies [15,35]. A ROC analysis was performed to compare the utility of NLR, other inflammatory biomarkers, and ALP in predicting patient prognosis. The AUC for NLR was significantly larger than that for PLR, LMR, SII, and ALP, consistent with the multivariable analysis. These results are supported by previous studies [20,37]. In osteosarcoma, Xia et al. [15] and Liu et al. [35] also showed that NLR is more predictive of OS than PLR, but they did not analyze LMR. Nevertheless, the cut-off values vary among the present and previous studies [15,34,35] and much work remains to be done before standard reference values can be obtained.
The SII is based on neutrophil, platelet, and lymphocyte counts and is increasingly being recognized as a useful index for the prediction of survival in patients with various types of cancer, including pancreas cancer [38], non-small cell lung cancer [39], small cell lung cancer [16], hepatocellular carcinoma [40], esophageal cancer [41], cervical cancer [42], and gastric cancer [43]. On the other hand, conflicting results were obtained [44,45], and its usefulness in cancer prognosis is thus uncertain. A recent meta-analysis of 22 papers and 7657 patients suggested that the SII is a potential prognosis marker and is associated with poor patient outcomes [46]. More specifically, this meta-analysis showed that the SII is associated with OS, time to recurrence, PFS, cancer-specific survival, relapse-free survival, and disease-free survival. Differences could exist among cancer types. Indeed, the meta-analysis by Yang et al. [46] showed that even if the SII were associated with OS to hepatocellular carcinoma, gastric cancer, esophageal squamous cell carcinoma, urinary system cancer, small cell lung cancer, non-small cell lung cancer, and acral melanoma, the strongest association was observed for hepatocellular carcinoma. Of course, a publication bias could be observed here since negative results are less likely to be published and data regarding SII being not associated with cancer prognosis are more difficult to be found. In the present study, patients in the HSII group had significantly shorter OS (median, 26.0 vs. 10.0 months), but the multivariable analysis did not identify SII as being independently associated with survival of patients with osteosarcoma. This is in contrast to the study by Huang et al. [36] conducted with 126 patients, which showed that SII was independently associated with OS. As the literature regarding the value of SII in osteosarcoma, additional studies are necessary to determine its exact value in those patients.
This study has limitations. First, this was only a retrospective single-center study, with a small population size. Second, since the patients were mainly from poorer areas of southwest China, not all patients received standard chemotherapy, which could have biased the OS rate. In addition, there was treatment heterogeneity among these patients, further biasing the experimental findings. Finally, only Chinese patients were assessed in the present study. Caution is warranted in interpreting the results of the present study for other ethnic groups.
In conclusion, this study strongly suggests that the pretreatment inflammatory indexes NLR, LMR, PLR, and SII were correlated with OS in patients with osteosarcoma. NLR is a more robust predictor of OS to osteosarcoma than LMR, PLR, and SII. Therefore, a more aggressive chemotherapeutic regimen may be necessary if patients present with one or multiple of these risk factors. More careful long-term follow-up may also be warranted. Nevertheless, multicenter prospective studies are needed to confirm these results.
Acknowledgements
This work was partly supported by Science and Health Joint Medical Research Project of Ch-ongqing (2018QNXM019) and the Basic Science and Frontier Technology Project of Chongqing (cstc2018jcyjAX0777) to SY.
Disclosure of conflict of interest
None.
References
- 1.Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res. 2014;162:65–92. doi: 10.1007/978-3-319-07323-1_4. [DOI] [PubMed] [Google Scholar]
- 2.NCCN CLinical Practice Guidelines in Oncology (NCCN Guidelines) Version 1.2019. Fort Washington: National Comprehensive Cancer Network; 2018. Bone Cancer. [Google Scholar]
- 3.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 4.ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii113–23. doi: 10.1093/annonc/mdu256. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Wang C, Xu M, Kong C, Qu A, Zhang M, Zheng Z, Zhang G. Preoperative NLR for predicting survival rate after radical resection combined with adjuvant immunotherapy with CIK and postoperative chemotherapy in gastric cancer. J Cancer Res Clin Oncol. 2017;143:861–871. doi: 10.1007/s00432-016-2330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duong LM, Richardson LC. Descriptive epidemiology of malignant primary osteosarcoma using population-based registries, United States, 1999-2008. J Registry Manag. 2013;40:59–64. [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47:283–292. doi: 10.1016/j.ocl.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Li YJ, Dai YL, Cheng YS, Zhang WB, Tu CQ. Positron emission tomography (18)F-fluorodeoxyglucose uptake and prognosis in patients with bone and soft tissue sarcoma: a meta-analysis. Eur J Surg Oncol. 2016;42:1103–1114. doi: 10.1016/j.ejso.2016.04.056. [DOI] [PubMed] [Google Scholar]
- 9.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(Suppl 1):S79–84. doi: 10.1007/s10875-012-9847-0. [DOI] [PubMed] [Google Scholar]
- 10.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 11.Koh CH, Bhoo-Pathy N, Ng KL, Jabir RS, Tan GH, See MH, Jamaris S, Taib NA. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113:150–158. doi: 10.1038/bjc.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu X, Sun S, Gao XS, Xiong W, Qin S, Qi X, Ma M, Li X, Zhou D, Wang W, Yu H. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 patients. Sci Rep. 2016;6:23893. doi: 10.1038/srep23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan D, Fu Y, Su Q, Wang H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3837. doi: 10.1097/MD.0000000000003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie QK, Chen P, Hu WM, Sun P, He WZ, Jiang C, Kong PF, Liu SS, Chen HT, Yang YZ, Wang D, Yang L, Xia LP. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med. 2018;16:273. doi: 10.1186/s12967-018-1638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia WK, Liu ZL, Shen D, Lin QF, Su J, Mao WD. Prognostic performance of pre-treatment NLR and PLR in patients suffering from osteosarcoma. World J Surg Oncol. 2016;14:127. doi: 10.1186/s12957-016-0889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236:297–304. doi: 10.1620/tjem.236.297. [DOI] [PubMed] [Google Scholar]
- 17.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, Sun H, Chen J, Wang F, Gao T, Zhang L, Wang S. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13:66. doi: 10.1186/s12967-015-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stotz M, Pichler M, Absenger G, Szkandera J, Arminger F, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110:435–440. doi: 10.1038/bjc.2013.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Teng F, Kong L, Yu J. Pretreatment neutrophil-to-lymphocyte ratio as a survival predictor for small-cell lung cancer. Onco Targets Ther. 2016;9:5761–5770. doi: 10.2147/OTT.S106296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Murri AM, Wilson C, Lannigan A, Doughty JC, Angerson WJ, McArdle CS, McMillan DC. Evaluation of the relationship between the systemic inflammatory response and cancer-specific survival in patients with primary operable breast cancer. Br J Cancer. 2007;96:891–895. doi: 10.1038/sj.bjc.6603682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Bausch D, Pausch T, Krauss T, Hopt UT, Fernandez-del-Castillo C, Warshaw AL, Thayer SP, Keck T. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis. 2011;14:235–243. doi: 10.1007/s10456-011-9207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179:1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold S, Mira E, Muneer S, Korpanty G, Beck AW, Holloway SE, Mañes S, Brekken RA. Forced expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC. Exp Biol Med (Maywood) 2008;233:860–873. doi: 10.3181/0801-RM-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swierczak A, Mouchemore KA, Hamilton JA, Anderson RL. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015;34:735–751. doi: 10.1007/s10555-015-9594-9. [DOI] [PubMed] [Google Scholar]
- 28.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer. 2011;11:64. doi: 10.1186/1471-2407-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 33.Takagi S, Takemoto A, Takami M, Oh-Hara T, Fujita N. Platelets promote osteosarcoma cell growth through activation of the platelet-derived growth factor receptor-Akt signaling axis. Cancer Sci. 2014;105:983–988. doi: 10.1111/cas.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T, Fang XC, Ding Z, Sun ZG, Sun LM, Wang YL. Pre-operative lymphocyte-to-monocyte ratio as a predictor of overall survival in patients suffering from osteosarcoma. FEBS Open Bio. 2015;5:682–7. doi: 10.1016/j.fob.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu B, Huang Y, Sun Y, Zhang J, Yao Y, Shen Z, Xiang D, He A. Prognostic value of inflammation-based scores in patients with osteosarcoma. Sci Rep. 2016;6:39862. doi: 10.1038/srep39862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X, Hu H, Zhang W, Shao Z. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J Cell Physiol. 2019;234:18408–18414. doi: 10.1002/jcp.28476. [DOI] [PubMed] [Google Scholar]
- 37.Zhou B, Zhan C, Wu J, Liu J, Zhou J, Zheng S. Prognostic significance of preoperative neutrophil-to-lymphocyte ratio in surgically resectable pancreatic neuroendocrine tumors. Med Sci Monit. 2017;23:5574–5588. doi: 10.12659/MSM.907182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aziz MH, Sideras K, Aziz NA, Mauff K, Haen R, Roos D, Saida L, Suker M, van der Harst E, Mieog JS, Bonsing BA, Klaver Y, Koerkamp BG, van Eijck CH. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study. Ann Surg. 2019;270:139–146. doi: 10.1097/SLA.0000000000002660. [DOI] [PubMed] [Google Scholar]
- 39.Tomita M, Ayabe T, Maeda R, Nakamura K. Systemic immune-inflammation index predicts survival of patients after curative resection for non-small cell lung cancer. In Vivo. 2018;32:663–667. doi: 10.21873/invivo.112291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 41.Geng Y, Shao Y, Zhu D, Zheng X, Zhou Q, Zhou W, Ni X, Wu C, Jiang J. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis. Sci Rep. 2016;6:39482. doi: 10.1038/srep39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, Liu L. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9:3284. doi: 10.1038/s41598-019-39150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Yan Y, Zhu L, Cong X, Li S, Song S, Song H, Xue Y. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. 2017;9:849–867. doi: 10.2147/CMAR.S151026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao XH, Tian L, Wu J, Ma XL, Zhang CY, Zhou Y, Sun YF, Hu B, Qiu SJ, Zhou J, Fan J, Guo W, Yang XR. Circulating CD14(+) HLA-DR(-/low) myeloid-derived suppressor cells predicted early recurrence of hepatocellular carcinoma after surgery. Hepatol Res. 2017;47:1061–1071. doi: 10.1111/hepr.12831. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Sun X, Liu J, Kong P, Chen S, Zhan Y, Xu D. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8:339–45. doi: 10.1016/j.tranon.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9:3295–3302. doi: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]


