Abstract
Background: In the world, there are approximately 160,000 cases of laryngeal cancer newly diagnosed every year and 95% of the cases are squamous cell carcinoma (LSCC). We conduct this study to investigate the influencing factors in LSCC. Method: We used cohort of LSCC cases form the Surveillance, Epidemiology, and End Results (SEER) database (1973-2014) to investigate the relationship between gender and survival. We conducted 1:1 propensity matching to mimic randomized controlled trials. Using the matched group, we investigate the effect of gender on cancer-specific survival (CSS) and overall survival (OS). Result: In total, 47881 patients were brought into an unmatched cohort and 17985 cases were brought into a matched cohort. Using the matched group, we conducted a survival analysis. The 1-year, 3-year, and 5-year CSS and OS rates were better in female patients and the subgroup analysis showed the same trend. Cox regression analysis showed gender was an independent prognostic indicator for LSCC patients. Conclusion: Gender is an independent prognostic indicator for LSCC patients. Male patients are a high-risk population.
Keywords: Laryngeal neoplasms, gender, propensity score matching, SEER
Introduction
In the world, there are approximately 160,000 cases of laryngeal cancer newly diagnosed every year [1]. Among them, 95% of the cases are laryngeal squamous cell carcinoma (LSCC) [2]. Although the treatment methods have developed over the past 30 years, the survival rates of patients with LSCC have not significantly improved [3]. In order to make the therapy more efficient and improve LSCC patient prognosis and long-term quality of life, understanding the potential influencing factors of LSCC is important.
It has been reported that in Europe, the United States, and Korea, females have an advantage over males in surviving a diagnosis of cancer [4]. Endogenous sex hormones may lead to the difference in survival rates [5]. Another possibility is that women generally have healthier attitudes and living habits [6,7]. However, few studies have included gender-associated differences in the survival rates of patients with LSCC.
In our study, we obtained data on patients with a diagnosis of LSCC in the United States between 1973 and 2014 from the Surveillance, Epidemiology, and End Results (SEER) database. We used the propensity score matching method creating well-matched cohort to investigate the effects of gender on clinical outcomes of LSCC patients.
Materials and methods
Data extraction and management
We used a cohort of LSCC cases form the SEER database (1973-2014) for analysis. Using the topography codes (C32.0-C32.3 and C32.8-C32.9) and historical type code (8070/3) of the International Classification of Diseases for Oncology, third edition (ICD-O-3), we retrieved the LSCC patients’ data. We excluded patients using the following criteria: (1) age at diagnosis < 18 years; (2) LSCC was not the first tumor; (3) lack of histologic confirmation; (4) missing essential information. The patient demographics, clinical characteristics, follow-up, and vital status were acquired using SEER*Stat software (version 8.3.4; National Cancer Institute, Bethesda, MD, USA). We set cancer-specific survival (CSS) and overall survival (OS) as the endpoints.
Statistical analysis
For baseline characteristics, continuous variables were described as the means and standard deviations, and compared by t-test. Categorical variables were shown using frequencies and percentages, and compared using the Chi-square test or Fisher’s exact test. The survival period was calculated from the date of LSCC diagnosis until the time of death or the last follow-up. Survival analysis was conducted using Kaplan-Meier method with log-rank test. We also conducted univariate and multivariate Cox regression method to ascertain the prognostic value of gender in LSCC.
We used a propensity score matching (1-to-1) method to mimic randomized controlled trials and reduce the selection bias. Nearest-neighbor matching was performed with a stringent caliper of 0.05 [8], and all the baseline variables were selected into the logistic regression model. We conducted all the analyses and generated matched datasets using SPSS, version 24.0 (SPSS Inc., Chicago, IL). Two-sided P < 0.05 was considered significant.
Results
Demographics
61880 patients diagnosed with LSCC between 1973 and 2014 from the SEER database were extracted. After excluding the cases according to the selection criteria, 47881 patients were brought into unmatched cohort (Figure 1). In this group, 38887 cases were male and 8994 cases were female, and the baseline characteristics showed significant differences (Table 1).
Figure 1.
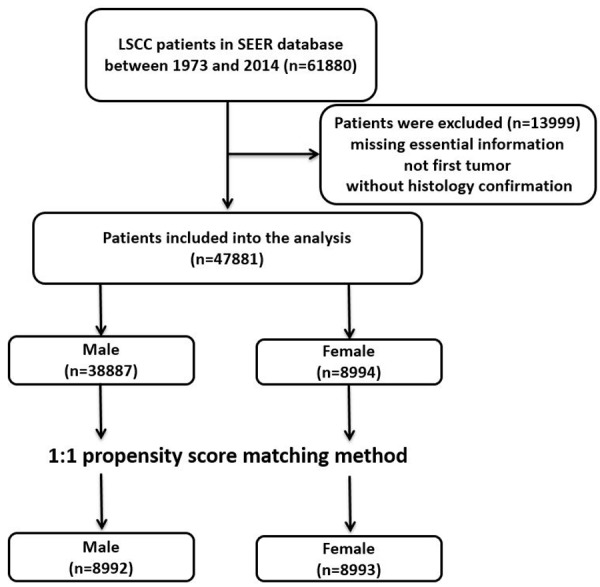
Flow chart for this study.
Table 1.
Baseline characteristics of the male and female patients with LSCC in the original/matched cohort
| Characteris | Original cohort (n = 47881) | Matched cohort (n = 17985) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Female | Male | P-value | Female | Male | P-value | |
| Year of diagnosis | < 0.001 | 0.875 | ||||
| 1973-1982 | 1215 | 6258 | 1215 | 1184 | ||
| 1983-1992 | 1479 | 6501 | 1479 | 1487 | ||
| 1993-2002 | 2319 | 9380 | 2318 | 2300 | ||
| 2003-2014 | 3981 | 16748 | 3981 | 4021 | ||
| Age at diagnosis | < 0.001 | 0.700 | ||||
| ≤ 60 years | 3837 | 15428 | 3836 | 3810 | ||
| > 60 years | 5157 | 23459 | 5157 | 5182 | ||
| Race | 0.010 | 0.559 | ||||
| White | 7338 | 32029 | 7338 | 7309 | ||
| Black | 1312 | 5399 | 1312 | 1304 | ||
| Others | 293 | 1319 | 293 | 328 | ||
| Unknown | 51 | 140 | 50 | 51 | ||
| Marital status | < 0.001 | 0.940 | ||||
| Married | 4882 | 22122 | 4882 | 4891 | ||
| Unmarried | 3671 | 15034 | 3671 | 3671 | ||
| Unknown | 441 | 1731 | 440 | 430 | ||
| Site | < 0.001 | 0.999 | ||||
| Supraglottis | 4786 | 11787 | 4785 | 4783 | ||
| Glottis | 3155 | 22354 | 3155 | 3157 | ||
| Subglottis | 119 | 497 | 119 | 121 | ||
| Others | 934 | 4249 | 934 | 931 | ||
| Grade | < 0.001 | 0.054 | ||||
| Well differentiated | 1454 | 6145 | 1454 | 1472 | ||
| Moderately differentiated | 4144 | 16851 | 4144 | 4142 | ||
| Poorly differentiated | 1574 | 7136 | 1574 | 1587 | ||
| Undifferentiated | 40 | 226 | 40 | 30 | ||
| Unknown | 1782 | 8529 | 1781 | 1761 | ||
After we conducted 1-to-1 propensity score matching, there were 17985 cases (8992 men and 8993 women) brought into analysis. All the baseline characteristics were well-matched between male and female patient groups.
Effect of gender in CSS and OS
As shown in Table 2, the 1-year, 3-year, and 5-year CSS rates were 79%, 70%, and 65% for female patients, and 75%, 64%, and 59% for male patients. Median survival months were 181.4 and 135.2, for female and male patients. The 1-year, 3-year and 5-year OS rates were 72%, 59% and 50% for female patients, and 68%, 53% and 44% for male patients. Median survival months were 73.2 and 56.5 for female and male patients. The Kaplan-Meier analysis showed that, in both original and matched groups, female patients had better prognosis than male patients (Figure 2). As shown in Table 3, in univariate analysis for CSS, all baseline characteristics were identified as significantly predictive factors, except for patients diagnosed in 1983-1992 (P=0.156), as well as blacks (P=0.65) and other races (P=0.144), and location of the tumor in the subglottis (P=0.726). The multivariate analysis results showed that, most variables were still independent prognostic indicators, except race, marital status, and pathologic grade (aside from the grade for moderately differentiated). The univariate analysis for OS showed similar results as for CSS. Black race, other races, subglottic location, and most pathologic grades (moderately differentiated, poorly differentiated and undifferentiated) were not independent prognostic indicators. As for the multivariate analysis results, they were basically the same as the results of the previously obtained multivariate analysis for OS, except that all pathologic grades were not associated with patient outcome.
Table 2.
Univariate and multivariate analysis of the effect of gender on survival outcome in LSCC
| Cancer-specific Survival S | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
|
|
|
|
|
|||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender | ||||||||
| Female | Reference | Reference | Reference | Reference | ||||
| Male | 1.19 (1.14-1.25) | < 0.001 | 1.20 (1.16-1.25) | < 0.001 | 1.15 (1.11-1.19) | < 0.001 | 1.16 (1.12-1.20) | < 0.001 |
| Year of diagnosis | ||||||||
| 1973-1982 | Reference | Reference | Reference | Reference | ||||
| 1983-1992 | 1.04 (0.99-1.09) | 0.156 | 1.05 (1.00-1.10) | 0.051 | 1.10 (1.04-1.16) | 0.001 | 1.02 (0.97-1.08) | 0.440 |
| 1993-2002 | 1.14 (1.09-1.19) | < 0.001 | 1.15 (1.10-1.20) | < 0.001 | 1.18 (1.12-1.25) | < 0.001 | 1.13 (1.07-1.19) | < 0.001 |
| 2003-2014 | 1.18 (1.13-1.24) | < 0.001 | 1.16 (1.11-1.22) | < 0.001 | 1.19 (1.13-1.26) | < 0.001 | 1.12 (1.06-1.19) | < 0.001 |
| Age at diagnosis | ||||||||
| ≤ 60 years | Reference | Reference | Reference | Reference | ||||
| > 60 years | 1.31 (1.27-1.35) | < 0.001 | 1.46 (1.42-1.51) | < 0.001 | 1.79 (1.73-1.86) | < 0.001 | 1.91 (1.84-1.99) | < 0.001 |
| Race | ||||||||
| White | Reference | Reference | Reference | Reference | ||||
| Black | 1.04 (1.00-1.09) | 0.65 | 0.97 (0.93-1.02) | 0.213 | 1.03 (0.98-1.09) | 0.190 | 0.97 (0.92-1.02) | 0.195 |
| Others | 1.06 (0.98-1.16) | 0.144 | 1.02 (0.94-1.11) | 0.680 | 1.00 (0.91-1.10) | 0.961 | 0.98 (0.89-1.08) | 0.643 |
| Unknown | 1.31 (1.05-1.63) | 0.016 | 1.08 (0.87-1.35) | 0.491 | 1.26 (1.01-1.58) | 0.044 | 0.98 (0.84-1.32) | 0.63 |
| Marital status | ||||||||
| Married | Reference | Reference | Reference | Reference | ||||
| Unmarried | 1.14 (1.10-1.17) | < 0.001 | 1.02 (0.99-1.05) | 0.190 | 1.09 (1.05-1.13) | < 0.001 | 1.02 (0.99-1.06) | 0.243 |
| Unknown | 1.16 (1.08-1.25) | < 0.001 | 1.04 (0.97-1.11) | 0.307 | 1.11 (1.03-1.21) | 0.010 | 1.05 (0.97-1.14) | 0.214 |
| Site | ||||||||
| Supraglottis | Reference | Reference | Reference | Reference | ||||
| Glottis | 0.37 (0.46-0.38) | < 0.001 | 0.35 (0.34-0.36) | < 0.001 | 0.52 (0.50-0.55) | < 0.001 | 0.49 (0.47-0.51) | < 0.001 |
| Subglottis | 1.02 (0.91-1.15) | 0.726 | 0.97 (0.86-1.10) | 0.645 | 1.00 (0.87-1.17) | 0.954 | 0.93 (0.79-1.08) | 0.322 |
| Others | 1.17 (1.12-1.22) | < 0.001 | 1.21 (1.15-1.27) | < 0.001 | 1.13 (1.07-1.19) | < 0.001 | 1.15 (1.08-1.22) | < 0.001 |
| Grade | ||||||||
| Well differentiated | Reference | Reference | Reference | Reference | ||||
| Moderately differentiated | 0.95 (0.91-0.99) | 0.022 | 1.07 (1.02-1.11) | 0.006 | 0.99 (0.94-1.04) | 0.681 | 1.01 (0.96-1.06) | 0.698 |
| Poorly differentiated | 0.90 (0.85-0.94) | < 0.001 | 1.01 (0.96-1.07) | 0.642 | 0.95 (0.89-1.00) | 0.070 | 1.00 (0.94-1.06) | 0.860 |
| Undifferentiated | 0.61 (0.49-0.77) | < 0.001 | 0.80 (0.63-1.00) | 0.051 | 0.82 (0.62-1.08) | 0.162 | 0.82 (0.62-1.08) | 0.148 |
| Unknown | 0.87 (0.83-0.91) | < 0.001 | 0.96 (0.91-1.01) | 0.084 | 0.93 (0.88-0.99) | 0.013 | 0.96 (0.91-1.02) | 0.202 |
Figure 2.
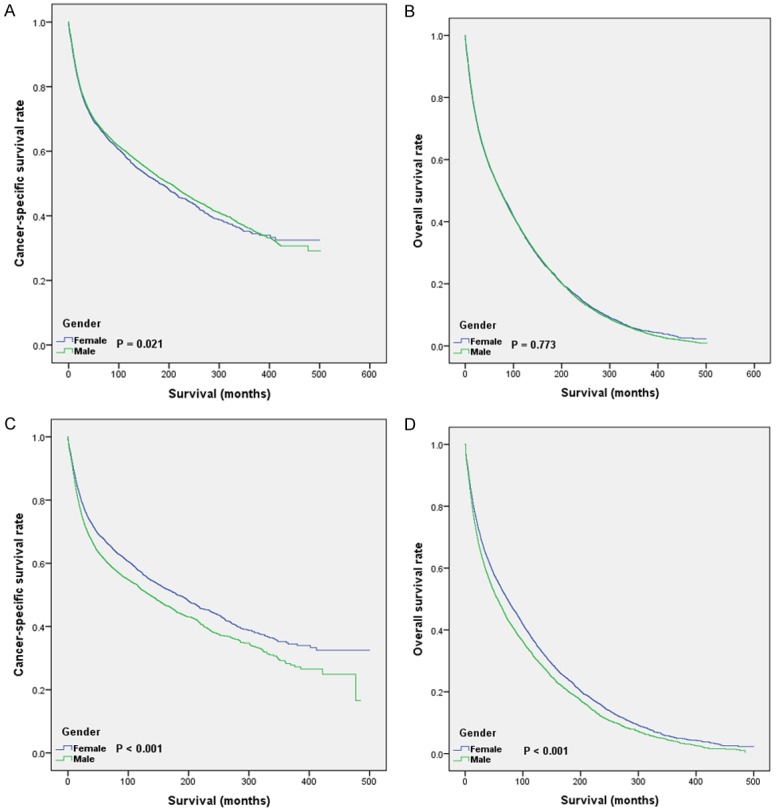
Kaplan-Meier curves for LSCC patients in original and matched groups. A. CSS of LSCC patients in original group; B. OS of LSCC patients in original group; C. CSS of LSCC patients in matched group; D. OS of LSCC patients in matched group.
Table 3.
Subgroup analysis of the effect of gender on survival outcome in LSCC
| Subgroup | Cancer-specific Survival | Overall Survival | ||
|---|---|---|---|---|
|
|
|
|||
| aHR | P-value | aHR | P-value | |
| Year of diagnosis | ||||
| 1973-1982 | 1.25 (1.11-1.41) | < 0.001 | 1.23 (1.13-1.33) | < 0.001 |
| 1983-1992 | 1.14 (1.02-1.26) | 0.017 | 1.12 (1.04-1.21) | 0.003 |
| 1993-2002 | 1.17 (1.08-1.28) | < 0.001 | 1.09 (1.03-1.17) | 0.006 |
| 2003-2014 | 1.21 (1.12-1.31) | < 0.001 | 1.18 (1.11-1.26) | < 0.001 |
| Age at diagnosis | ||||
| ≤ 60 years | 1.32 (1.23-1.43) | < 0.001 | 1.27 (1.20-1.35) | < 0.001 |
| > 60 years | 1.11 (1.04-1.18) | 0.001 | 1.07 (1.03-1.12) | 0.001 |
| Race | ||||
| White | 1.21 (1.15-1.27) | < 0.001 | 1.15 (1.11-1.20) | < 0.001 |
| Black | 1.11 (0.98-1.25) | 0.094 | 1.12 (1.03-1.23) | 0.012 |
| Others | 1.25 (0.97-1.60) | 0.084 | 1.18 (0.98-1.43) | 0.085 |
| Unknown | 1.06 (0.61-1.85) | 0.829 | 1.21 (0.77-1.90) | 0.416 |
| Marital status | ||||
| Married | 1.18 (1.11-1.26) | < 0.001 | 1.14 (1.09-1.20) | < 0.001 |
| Unmarried | 1.20 (1.12-1.29) | < 0.001 | 1.17 (1.11-1.24) | < 0.001 |
| Unknown | 1.23 (1.01-1.52) | 0.045 | 1.07 (0.92-1.25) | 0.383 |
| Site | ||||
| Supraglottis | 1.25 (1.18-1.33) | < 0.001 | 1.20 (1.14-1.26) | < 0.001 |
| Glottis | 1.11 (1.01-1.23) | 0.040 | 1.12 (1.05-1.19) | < 0.001 |
| Subglottis | 0.86 (0.60-1.25) | 0.432 | 0.85 (0.63-1.14) | 0.278 |
| Others | 1.26 (1.11-1.42) | < 0.001 | 1.17 (1.06-1.30) | 0.002 |
| Grade | ||||
| Well differentiated | 1.18 (1.05-1.32) | 0.004 | 1.17 (1.08-1.27) | < 0.001 |
| Moderately differentiated | 1.23 (1.15-1.32) | < 0.001 | 1.19 (1.13-1.25) | < 0.001 |
| Poorly differentiated | 1.15 (1.03-1.29) | 0.013 | 1.11 (1.02-1.20) | 0.017 |
| Undifferentiated | 2.04 (0.88-4.74) | 0.098 | 2.02 (1.15-3.57) | 0.015 |
| Unknown | 1.16 (1.04-1.28) | 0.006 | 1.09 (1.01-1.18) | 0.023 |
aHR, adjusted hazard ratio; CI, confidence interval.
Subgroup analysis for different genders
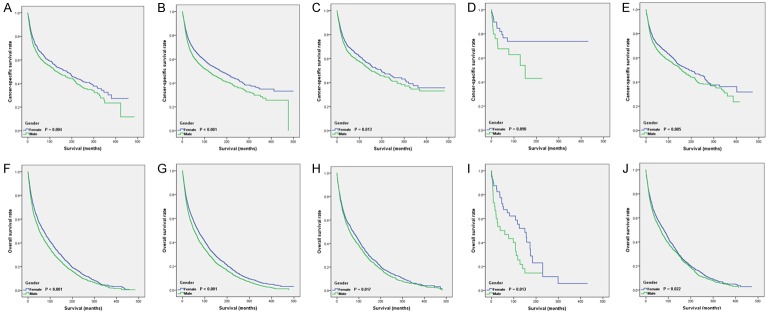
Because of the distribution difference of pathologic grade between the two groups in the matched cohort, we conducted subgroup analysis according to gender. The Kaplan-Meier survival results for CSS (Figure 3A-E) and OS (Figure 3F-J) showed that female patients had a better prognosis at almost all pathologic grades (except for patients with pathologic grade of undifferentiated). As shown in Table 4, we also performed a subgroup analysis grouped by year of diagnosis, age at diagnosis, race, marital status, and tumor site. Female gender was also a protective effect in those subgroups, except with other races, unknown races, and subglottic location. However, in the black race, unknown marital status, and undifferentiated pathological grades, the results of the subgroup analysis were inconsistent in OS/CSS.
Figure 3.
Kaplan-Meier curves for LSCC patients with different pathology grades. Survival curves for CSS (A-E) and OS (F-J) were stratified by gender. (A, F) grade I; (B, G) grade II; (C, H) grade III; (D, I) grade IV; (E, G) grade unknown.
Table 4.
Survival status stratified by gender
| Characteristics | n | Survival rate (%) | Median Survival (month) | ||
|---|---|---|---|---|---|
|
| |||||
| 1-year | 3-year | 5-year | |||
| CSS | |||||
| Female | 8993 | 79 | 70 | 65 | 181.44 |
| Male | 8992 | 75 | 64 | 59 | 135.24 |
| OS | |||||
| Female | 8993 | 72 | 59 | 50 | 73.27 |
| Male | 8992 | 68 | 53 | 44 | 56.55 |
Discussion
In the past few years, radiation and chemotherapy or surgery strategies based on prognostic classifiers have slightly improved the survival rate of laryngeal squamous cell carcinoma (LSCC) [9]. It is important to know the interactions of multiple factors affecting the LSCC survival. Clinical factors and demographic data have been studied as prognostic factors for cancers, including LSCC. From the present studies, tumor characteristics such as primary tumor location and TNM stage are important factors for LSCC outcome by both univariate and multivariate analysis [10]. There are only a few old studies on the relationship between demographic characteristics such as sex and clinical outcomes in LSCC patients [11-14], and this is controversial. Hence, it is important to use a database to focus on this issue.
In our study, all the data in the SEER database were collected directly by clinical staff. The data were then extracted according to our research requirements. The only inclusion standard was adult patients with a primary diagnosis of LSCC. Data storage and evaluation were performed by different teams. As the data had already existed in the SEER before we performed the plan, our subjective awareness did not interfere in patient selection and treatment, which ensures that our data are real and our results are believable. However, it is hard to avoid selection bias and subjective interference in some previous retrospective studies, and this may affect research results. Also, the number of patients in our study was much larger than in any other former studies, and our study duration was much longer. Therefore, several confounding factors between the two groups of males and females are more balanced.
Several studies have found the relationship between sex and incidence and outcome in patients with cancer diseases. Women have better outcome than men in some cancer types. Studies have shown that females have a significant survival advantage for most cancers, including salivary gland cancer, head and neck cancer, esophageal cancer, gastric cancer, colon and rectal cancer, pancreatic cancer, lung cancer, pleural cancer, bone cancer, kidney cancer, and brain cancer [15]. Only in very few cancers do women have a higher incidence than men, such as thyroid cancer. There are several views relating to reasons for different outcome in female and male cancer patients.
First, behavioral and occupational factors are widely acknowledged as potential determinants. Men have more frequent drinking occasions and smoking behavior. Smoking is a strong risk factor for LSCC in Eastern and Central Europe [16]. Current smokers have a 15-fold increased risk of laryngeal cancer and former smokers have a five-fold increase. With alcohol drinking, the risk of laryngeal cancer increases approximately 1.5 to 2.0 times. Furthermore, the researchers observed that the effect of alcohol and smoking on the risk of laryngeal cancer is greater than the multiplicative effect [17]. However, when the risk factors have been adjusted, women still have a better outcome than men in most cancers [18,19]. Thus, there must be other causes for the cancer incidence and survival difference in men and women. One cause may be the cellular/molecular mechanism for differences in cancer susceptibility between males and females, with a focus on the complicated effects of sex chromosomes and sex hormones. The X chromosome is rich in immune related genes [20], and some X-linked microRNAs may promote sex-specific modulation of immune responses by targeting related immune genes [21,22]. Whatever the detailed mechanisms are, women are indeed more susceptible to autoimmune diseases and may also have enhanced immune surveillance for many tumor types.
Some sex hormones, such as growth hormone (GH), can get through the membrane of specific cells and combine directly with receptors that can influence the expression of specific genes [23]. The action of these hormone signaling can lead to different DNA methylation levels and chromatin conformation [24,25]. It has been reported that GH may affect cancer in these areas, such as liver, breast, skin, and brain [26]. The three major sex hormone receptors in our body, ER α, ER β and AR, play an important role in cell renewal, the microenvironment of tumor, the immune system, and glucose metabolism [27]. These reasons may partly explain our results.
However, there are still some disadvantages for our study: (1) SEER database didn’t record the margin status, chemotherapy and radiotherapy information which could be important in survival prediction. (2) We only used one database data for analysis; more multi-center studies need to be conduct for further research. (3) Information about recurrence and comorbidities was not available.
Thus gender is an independent prognostic indicator for LSCC patients, and male patients have worse short-term and long-term survival.
Disclosure of conflict of interest
None.
Abbreviations
- LSCC
laryngeal squamous cell carcinomas
- SEER
Surveillance, Epidemiology, and End Results database
- CSS
cancer specific survival
- OS
overall survival
References
- 1.Gao L, Cao H, Cheng X. A positive feedback regulation between long noncoding RNA SNHG1 and YAP1 modulates growth and metastasis in laryngeal squamous cell carcinoma. Am J Cancer Res. 2018;8:1712–1724. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Gao P, Gong L, Wang X. Induction chemotherapy in patients with resectable laryngeal cancer: a meta-analysis. Mol Clin Oncol. 2018;9:155–162. doi: 10.3892/mco.2018.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erkul E, Yilmaz I, Narli G, Babayigit MA, Gungor A, Demirel D. The presence and prognostic significance of human papillomavirus in squamous cell carcinoma of the larynx. Eur Arch Otorhinolaryngol. 2017;274:2921–2926. doi: 10.1007/s00405-017-4573-0. [DOI] [PubMed] [Google Scholar]
- 4.Ellison LF. Differences in cancer survival in Canada by sex. Health Rep. 2016;27:19–27. [PubMed] [Google Scholar]
- 5.Majek O, Gondos A, Jansen L, Emrich K, Holleczek B, Katalinic A, Nennecke A, Eberle A, Brenner H GEKID Cancer Survival Working Group. Sex differences in colorectal cancer survival: population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS One. 2013;8:e68077. doi: 10.1371/journal.pone.0068077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20:1629–1637. doi: 10.1158/1055-9965.EPI-11-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papaleontiou M, Haymart MR. New insights in risk stratification of differentiated thyroid cancer. Curr Opin Oncol. 2014;26:1–7. doi: 10.1097/CCO.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sturmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59:437–447. doi: 10.1016/j.jclinepi.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirisola V, Mora R, Esposito AI, Guastini L, Tabacchiera F, Paleari L, Amaro A, Angelini G, Dellepiane M, Pfeffer U, Salami A. A prognostic multigene classifier for squamous cell carcinomas of the larynx. Cancer Lett. 2011;307:37–46. doi: 10.1016/j.canlet.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Vlachtsis K, Nikolaou A, Markou K, Fountzilas G, Daniilidis I. Clinical and molecular prognostic factors in operable laryngeal cancer. Eur Arch Otorhinolaryngol. 2005;262:890–898. doi: 10.1007/s00405-005-0916-3. [DOI] [PubMed] [Google Scholar]
- 11.Boffetta P, Merletti F, Faggiano F, Migliaretti G, Ferro G, Zanetti R, Terracini B. Prognostic factors and survival of laryngeal cancer patients from Turin, Italy. A population-based study. Am J Epidemiol. 1997;145:1100–1105. doi: 10.1093/oxfordjournals.aje.a009072. [DOI] [PubMed] [Google Scholar]
- 12.Eiband JD, Elias EG, Suter CM, Gray WC, Didolkar MS. Prognostic factors in squamous cell carcinoma of the larynx. Am J Surg. 1989;158:314–317. doi: 10.1016/0002-9610(89)90123-2. [DOI] [PubMed] [Google Scholar]
- 13.Jin YT, Kayser S, Kemp BL, Ordonez NG, Tucker SL, Clayman GL, Goepfert H, Luna MA, Batsakis JG, El-Naggar AK. The prognostic significance of the biomarkers p21WAF1/CIP1, p53, and bcl-2 in laryngeal squamous cell carcinoma. Cancer. 1998;82:2159–2165. [PubMed] [Google Scholar]
- 14.Lassaletta L, Garcia-Pallares M, Morera E, Bernaldez R, Gavilan J. T3 glottic cancer: oncologic results and prognostic factors. Otolaryngol Head Neck Surg. 2001;124:556–560. doi: 10.1067/mhn.2001.115498. [DOI] [PubMed] [Google Scholar]
- 15.Harshman LC. Mind the gap: what is driving the survival disparity between the sexes in bladder cancer? Cancer. 2016;122:1966–1970. doi: 10.1002/cncr.30027. [DOI] [PubMed] [Google Scholar]
- 16.Wilsnack RW, Vogeltanz ND, Wilsnack SC, Harris TR, Ahlstrom S, Bondy S, Csemy L, Ferrence R, Ferris J, Fleming J, Graham K, Greenfield T, Guyon L, Haavio-Mannila E, Kellner F, Knibbe R, Kubicka L, Loukomskaia M, Mustonen H, Nadeau L, Narusk A, Neve R, Rahav G, Spak F, Teichman M, Trocki K, Webster I, Weiss S. Gender differences in alcohol consumption and adverse drinking consequences: cross-cultural patterns. Addiction. 2000;95:251–265. doi: 10.1046/j.1360-0443.2000.95225112.x. [DOI] [PubMed] [Google Scholar]
- 17.Hashibe M, Boffetta P, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, Fabianova E, Rudnai P, Brennan P. Contribution of tobacco and alcohol to the high rates of squamous cell carcinoma of the supraglottis and glottis in Central Europe. Am J Epidemiol. 2007;165:814–820. doi: 10.1093/aje/kwk066. [DOI] [PubMed] [Google Scholar]
- 18.Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J. Clin. Oncol. 2007;25:1705–1712. doi: 10.1200/JCO.2006.08.1455. [DOI] [PubMed] [Google Scholar]
- 19.OuYang PY, Zhang LN, Lan XW, Xie C, Zhang WW, Wang QX, Su Z, Tang J, Xie FY. The significant survival advantage of female sex in nasopharyngeal carcinoma: a propensity-matched analysis. Br J Cancer. 2015;112:1554–1561. doi: 10.1038/bjc.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai R, Ahmed SA. Sexual dimorphism of miRNA expression: a new perspective in understanding the sex bias of autoimmune diseases. Ther Clin Risk Manag. 2014;10:151–163. doi: 10.2147/TCRM.S33517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianchi I, Lleo A, Gershwin ME, Invernizzi P. The X chromosome and immune associated genes. J Autoimmun. 2012;38:J187–192. doi: 10.1016/j.jaut.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto T, Sakari M, Okada M, Yokoyama A, Takahashi S, Kouzmenko A, Kato S. The androgen receptor in health and disease. Annu Rev Physiol. 2013;75:201–224. doi: 10.1146/annurev-physiol-030212-183656. [DOI] [PubMed] [Google Scholar]
- 24.Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, Orlov YL, Velkov S, Ho A, Mei PH, Chew EG, Huang PY, Welboren WJ, Han Y, Ooi HS, Ariyaratne PN, Vega VB, Luo Y, Tan PY, Choy PY, Wansa KD, Zhao B, Lim KS, Leow SC, Yow JS, Joseph R, Li H, Desai KV, Thomsen JS, Lee YK, Karuturi RK, Herve T, Bourque G, Stunnenberg HG, Ruan X, Cacheux-Rataboul V, Sung WK, Liu ET, Wei CL, Cheung E, Ruan Y. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462:58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lea RW, Dawson T, Martinez-Moreno CG, El-Abry N, Harvey S. Growth hormone and cancer: GH production and action in glioma? Gen Comp Endocrinol. 2015;220:119–123. doi: 10.1016/j.ygcen.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Clocchiatti A, Cora E, Zhang Y, Dotto GP. Sexual dimorphism in cancer. Nat Rev Cancer. 2016;16:330–339. doi: 10.1038/nrc.2016.30. [DOI] [PubMed] [Google Scholar]