Abstract
We aimed to investigate the effect of Keap1/Nrf2 pathway on the biologic function of trophoblast cells in the oxidative stress model at the cellular level, and analyze the expression levels and clinical significance of Keap1/Nrf2 related antioxidant factors in placental tissues of preeclampsia (PE) patients at clinical level. In this study, we found that under hypoxia/reoxygenation conditions, the activities of oxidative stress-related enzymes (CAT, GSH-Px, SOD) in HTR8/SVneo cells were significantly lower than those before treatment (P<0.01). The activities of CAT, GSH-Px and SOD in HTR8/SVneo cells in siRNA+H/R group decreased significantly (P<0.01), which indicated the important defensive effect of Keap1/Nrf2 pathway in oxidative stress. Compared with Nrf2 siRNA+H/R group, Si-NC+H/R group had CAT, GSH-Px and SOD activities decreasing, which were similar to those in the H/R group. Moreover, the activities of oxidative stress-related active enzymes in patients with preeclampsia were further confirmed by detecting and comparing the activities of CAT, GSH-Px and SOD in placental tissues. The results showed that the activities of SOD (P<0.001), GSH-Px (P<0.01) and CAT (P<0.01) in placental tissues of patients with PE were significant different from those of normal placental tissues. The expression level of Keap1 in placenta of patients with PE was slightly lower than that of normal placenta, while the expression of Nrf2 and HO-1 in placenta of patients with PE were significantly higher than those of normal placenta, which implicated the importance of Keap-1/Nrf2 pathway in PE.
Keywords: Keap-1/Nrf2, oxidative stress, preeclampsia
Introduction
Preeclampsia (PE) is one of the leading causes of maternal mortality and morbidity and a life-threatening pregnancy complication for both mother and embryos. It is characterized by new-onset hypertension and proteinuria after 20 weeks of gestation, and its incidence is about 2-9.4% [1-3]. Various factors have been proposed as risk factors associated with preeclampsia including age, obesity, chronic hypertension, renal disease and diabetes mellitus [4-6]. The development process of PE can be divided into the following two stages: the first stage is the invasion of the embryonic trophoblast into the myometrium of the mother, resulting in insufficient remodeling of the uterine spiral artery. In the second stage, the endothelial dysfunction caused by the systemic inflammatory response and the increase in blood pressure caused by decreased renal function are the main features, eventually leading to clinical syndrome characterized by maternal hypertension and proteinuria. Despite the involvement of multiple risk factors in preeclampsia, the pathogenesis of PE development remains unclear [7,8]. The only effective way to relieve maternal symptoms is delivery of the placenta and fetus [9-11].
One pathophysiologic mechanism which was significantly associated with PE is oxidative stress (OS). OS is defined as a homeostatic imbalance within the reduction-oxidation (redox) environment that involves a dysregulation between oxidants and antioxidants [12-14]. Oxidative stress arises during pregnancy including either from an excessive production by the placental metabolism of reactive oxygen species (ROS) or from an inadequate supply of antioxidant substances [15-18]. Endogenous ROS play a critical role in maintaining biologic homeostasis and have been identified as signaling molecules in physiologic and pathophysiologic processes. Specifically, ROS can activate redox-sensitive transcription factors to regulate inflammatory cytokines or activate protein kinases to promote cell proliferation.
Transcription factor NF-E2 p45-related factor 2 (Nrf2, gene name NFE2L2) regulates the expression of a large network of genes encoding inducible cytoprotective proteins that allow mammalian cells and organisms to adapt and survive under various conditions including oxidative stress. Together with its principal negative regulator, Kelch-like ECH-associated protein 1 (Keap1), Nrf2 forms a molecular effector and sensor system that robustly responds to perturbations of the cellular redox balance and orchestrates a comprehensive defense program, which in turn restores homeostasis [19,20]. The Nrf2 status affects the production of ROS by the two major ROS producing systems, mitochondria and NADPH oxidase. Among the Nrf2 transcriptional targets are proteins with critical roles in the generation and utilization of reducing equivalents such as NADPH and reduced glutathione (GSH), as well as thioredoxin, thioredoxin reductase, peroxiredoxin, and sulfiredoxin, which collectively provide compartmentalized redox sensing of ROS to maintain redox balance under homeostatic conditions [21,22].
In present study, we investigated the effect of the Keap1/Nrf2 pathway on the biological function of trophoblast cells in the oxidative stress model at the cellular level, and analyzed the expression level and clinical significance of Keap1/Nrf2 related antioxidant factors in placental tissues of PE patients at the clinical level. The aim of this study is to clarify the role of Keap1/Nrf2 pathway in the regulation of oxidative stress in PE patients, and provide a theoretical basis for follow-up research and drug development.
Materials and methods
Patients
Participants were recruited after written informed consent under a protocol approved by the Committee on Human Research at the Shandong Provincial Qianfoshan Hospital, The First Hospital Affiliated with Shandong First Medical University. All samples in our study were from placenta of single pregnancy. Placental tissues from normal pregnancy (n=20), and preeclampsia patients (n=20) were obtained from individuals at Shandong Provincial Qianfoshan Hospital. Several aliquots of tissue were collected randomly from the maternal side of the placenta. The tissues were snap frozen in liquid nitrogen and stored at -80°C before use.
Cell culture HTR8/SVneo cell hypoxia reoxygenation model
The trophoblastic oxidative stress model uses a human chorionic trophoblast cell line HTR8/SVneo in early pregnancy. Cell cultured in a 37°C, 5% CO2 incubator, Gibco RPMI 1640 medium, 10% fetal bovine serum, 1% penicillin/streptomycin double antibody. HTR8/SVneo cells were cultured in the above cell culture manner, and the cells were cultured in a three-gas incubator (O2, CO2, N2) to detect oxidative stress related indicators. HTR8/SVneo cell hypoxia/reoxygenation (H/R) is a PE cell model, which is the most commonly used culture with mixed gas. In this study, HTR8/SVneo cells were cultured in 2% CO2 for 8 hours as hypoxia, and then cultured in 20% O2 for 16 hours to reoxygenate.
Construction of Nrf2 siRNA interferes with lentiviral vector and transfects HTR8/SVneo cells
Due to the lack of a specific Keap1/Nrf2 signaling pathway inhibitor, viral transfection induces Nrf2 gene silencing in HTR8/SVneo cells to inhibit Nrf2 expression: selection of small interfering RNA (siRNA) interfering with Nrf2. The viral vector was then transfected into HTR8/SVneo cells.
Preparation of dichlorofluorescin (DCFH)
DCFDA was hydrolyzed to dichlorofluorescin (DCFH) according to a reported method. Briefly, 0.5 mL DCFDA in water (1 mmol L-1) was added to 2 mL of 0.01 N NaOH and allowed to stand at room temperature for 30 min. The hydrolysate was neutralized with 10 mL PBS (25 mmol L-1), pH 7.2, and was always freshly prepared prior to use.
Oxidation of DCFH
100-μL aliquot of DCFH (40 μmol L-1) was added to 100 μL PBS containing Cu2+ or Cu+ and fluorescence development was monitored in an automated plate reader at 37°C (Wallac). Excitation wavelength was set at 485 nm and emission was recorded at 535 nm. At the indicated time points, 20 μL of the respective copper chelator or PBS (for control) was added to give the final concentrations.
Antioxidant status in placental tissues
Intracellular antioxidant enzyme concentrations were measured as a proxy for oxidative stress in fetal membranes using a Milliplex Human Oxidative Stress Magnetic Bead Panel (Catalog no. HOXSTMAG-18K, EMD Millipore, Merck, Barmstadt, Germany). Catalase (CAT), Glutathione peroxidase (GSH-Px) and superoxide dismutases (SOD) were measured in tissue homogenates. Samples were run in duplicate, and the manufacturer’s instructions were followed for conducting this assay after reconstituting tissue lysates and diluting them in the MAP Assay Buffer. The plates were analyzed using the Luminex system. Data were normalized to total protein concentration prior to analysis and reported as median fluorescence intensity (MFI).
RNA extraction and real-time reverse transcriptase-PCR
Total RNA of tissue samples were isolated using TRIzol (Life Technologies, Inc., Rockville, MD) according to the manufacturer’s instructions. cDNA was generated from 1 μg of each RNA sample and a reverse transcribed using a transcription kit (Takara, Kyoto, Japan). Real-time quantitative reverse transcriptase-PCR (RT-PCR) was done in the 7300 Real Time PCR System (Applied Biosystems).
Western blotting analysis
Denatured protein samples were resolved on SDS-PAGE and transferred to PVDF membrane (Millipore, Billerica, MA, USA). After blocking with non-fat milk, membrane was incubated overnight at 4°C with antibodies including Keap-1, Nrf2, HO-1 and β-actin (Abcam, USA) followed by incubation with the antirabbit HRP-conjugated secondary antibodies (Santa Cruz, Billerica, MA, USA). Chemiluminescence detection was performed using ECL advance western blotting detection reagents (GE healthcare, Little Chalfont, Buckinghamshire, UK). The relative expression was quantified by image software.
Statistical analysis
The χ2 test or Fisher’s exact test were used to compare qualitative variables, while continuous variables were compared using Student’s t-test or Mann-Whitney test for variables with an abnormal distribution. Receiver operating characteristic curve analysis was used to determine the optimal cut-offs of continuous variables. All statistical evaluations were carried out using SPSS software (Statistical Package for the Social Science, version 15.0, SPSS Inc.) and GraphPad Prism 5 (Version 5.01, GraphPad Software, Inc.). A value of P<0.05 was considered significant in all analyses.
Results
Identification of Nrf2 transfection efficiency in HTR8/SVneo cells
The lentiviral vector was transfected into HTR8/SVneo cells, and the optimal infection value of lentivirus with a titer of 1×107 TU/ml was 40; the transfection efficiency was 90% after stable transfection. The expression of Nrf2 mRNA after transfection was detected by qRT-PCR. The expression of Nrf2 mRNA in Si-Nrf2 group was significantly lower than that in Si-NC group (P<0.001, Figure 1A, 1B). By western blotting, the expression of Nrf2 in Si-Nrf2 group was significantly lower than that in Si-NC group (P<0.001, Figure 1C). We applied DCFH that were commonly used to detect reactive oxygen species (ROS) levels. The ROS levels in hypoxia/reoxygenation(H/R) group of HTR8/SVneo cells were significantly higher than that in Non-treated group (P<0.01, Figure 1D), indicating that hypoxia/reoxygenation had a significant effect on intracellular oxidative stress levels; ROS levels in Nrf2 SiRNA+H/R group were significantly higher than those in H/R group (P<0.01), indicating that Nrf2 participated in and regulated the oxidative stress levels in HTR8/SVneo cells.
Figure 1.
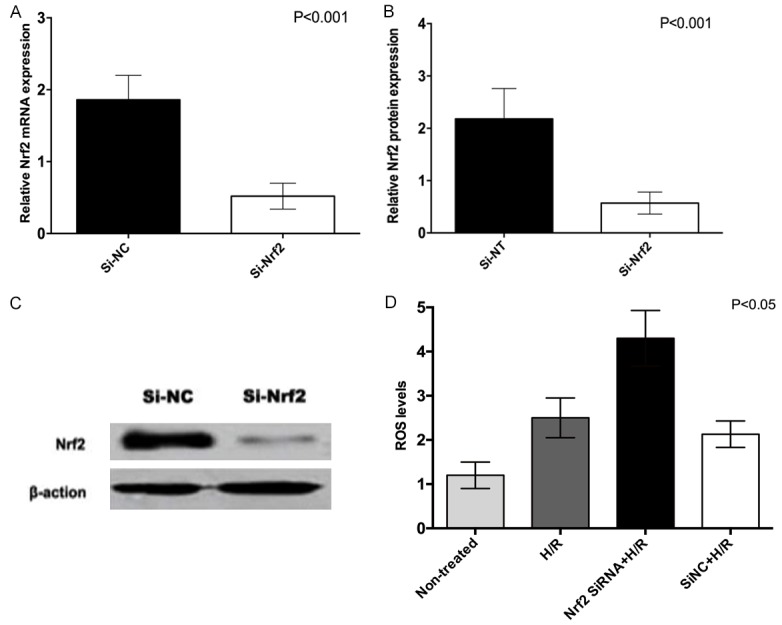
Nrf2 interference efficiency in HTR8/SVneo cells (normal group value is set to 1).
Detection and comparison of CAT, GSH-Px, and SOD activities in different groups of HTR8/SVneo cells and the effect of Nrf2 on oxidative stress-related active enzymes
Under hypoxia/reoxygenation conditions, the activities of oxidative stress-related enzymes (CAT, GSH-Px, SOD) in HTR8/SVneo cells were significantly lower than before treatment (P<0.01). The activities of CAT, GSH-Px, and SOD in HTR8/SVneo cells in the SiRNA+H/R group decreased significantly (P<0.01), which indicated the important defensive effect of Keap1/Nrf2 pathway in oxidative stress. Compared with Nrf2 SiRNA+H/R group, Si-NC+H/R group had decreasing CAT, GSH-Px and SOD activities, which were similar to those in the H/R group (P>0.05, Figure 2).
Figure 2.
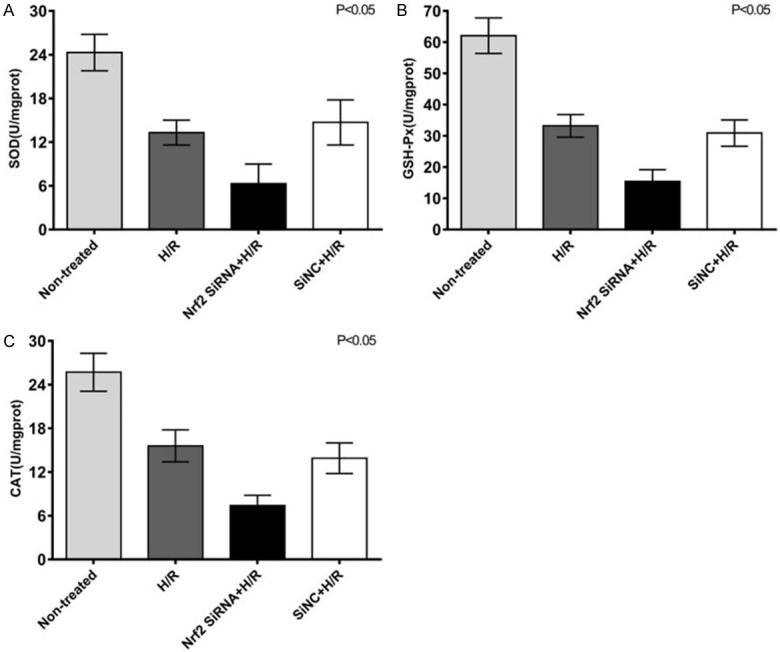
Comparison of SOD (A), GSH-Px (B) and CAT (C) enzyme activities in different groups of HTR8/SVneo cells.
Detection and comparison of expression levels of anti-oxidation related genes Keap1, Nrf2, and HO-1 in HTR8/SVneo cells under hypoxia-reoxygenation
The results showed that the expression of Keap1 mRNA in HTR8/SVneo cells was slightly lower than that of normal controls after hypoxia-reoxygenation (P<0.05, Figure 3A). The expression of Nrf2 mRNA in HTR8/SVneo cells was significantly higher than that in the normal control group (P<0.001, Figure 3B); HO-1 mRNA expression levels were significantly higher in HTR8/SVneo cells after hypoxia-reoxygenation than in normal cells (P<0.001, Figure 3C). The expression of Keap1 protein in HTR8/SVneo cells was significantly lower than that in the normal control group (Figure 3D, P<0.05). The expression of Nrf2 protein in HTR8/SVneo cells was significantly higher than that in normal cells after hypoxia-reoxygenation (P<0.001, Figure 3D); HO-1 protein expression level was also significantly higher than normal cells after hypoxia-reoxygenation (P<0.001, Figure 3D).
Figure 3.
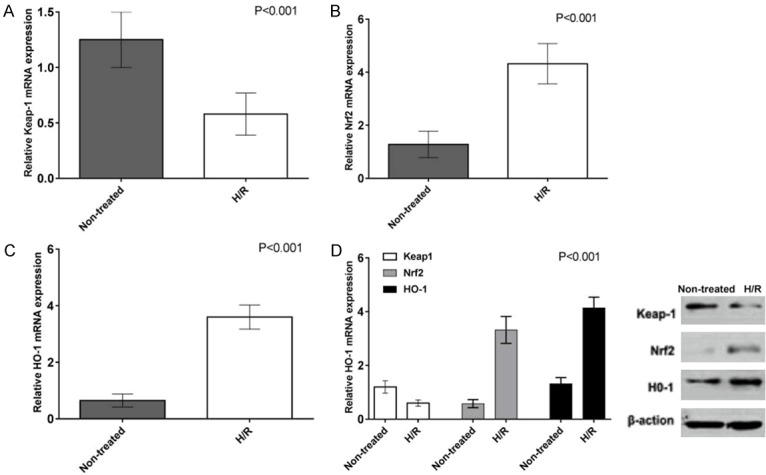
Detection and comparison of mRNA and protein expression levels of anti-oxidation genes Keap1, Nrf2, and HO-1 in HTR8/SVneo cells by hypoxia-reoxygenation.
Comparison of clinical characteristics between normal group and preeclampsia group
There were no significant differences in age, BMI, and previous smoking history between the two groups of patients (P>0.05). There were significant differences in the gestational age, systolic blood pressure, diastolic blood pressure and neonatal weight between the normal group and preeclampsia group (Table 1).
Table 1.
Demographics and clinical characteristics of all patients
| variables | Normal group | Preeclampsia group | P value |
|---|---|---|---|
| Case (n) | 20 | 20 | |
| Maternal age (years) | 30.2±3.8 | 29.3±4.6 | >0.05 |
| Gestational age (weeks, days) | 38.6±1.6 | 35.3±3.1 | <0.05 |
| BMI (kg/m2) | 25.4±3.2 | 27.1±2.1 | >0.05 |
| Smoking (N) | 2 | 5 | >0.05 |
| Systolic pressure (mmHg) | 129.7±7.5 | 158.6±10.6 | <0.01 |
| Diastolic pressure (mmHg) | 79.3±8.2 | 120.7±9.2 | <0.01 |
| Birthweight (g) | 3500.2±2.8 | 2601.7±8.5 | <0.01 |
Abbreviations: BMI, body mass index.
Detection and comparison of enzymes related to oxidative stress in placental tissues of patients in normal and preeclampsia group
The activities of oxidative stress-related active enzymes in patients with preeclampsia were further confirmed by detecting and comparing the activities of CAT, GSH-Px and SOD in placental tissues. The results showed that the activities of SOD (P<0.001, Figure 4A), GSH-Px (P<0.01, Figure 4B), and CAT (P<0.01, Figure 4) in placental tissues of patients with preeclampsia were significant different from those of normal placental tissues.
Figure 4.
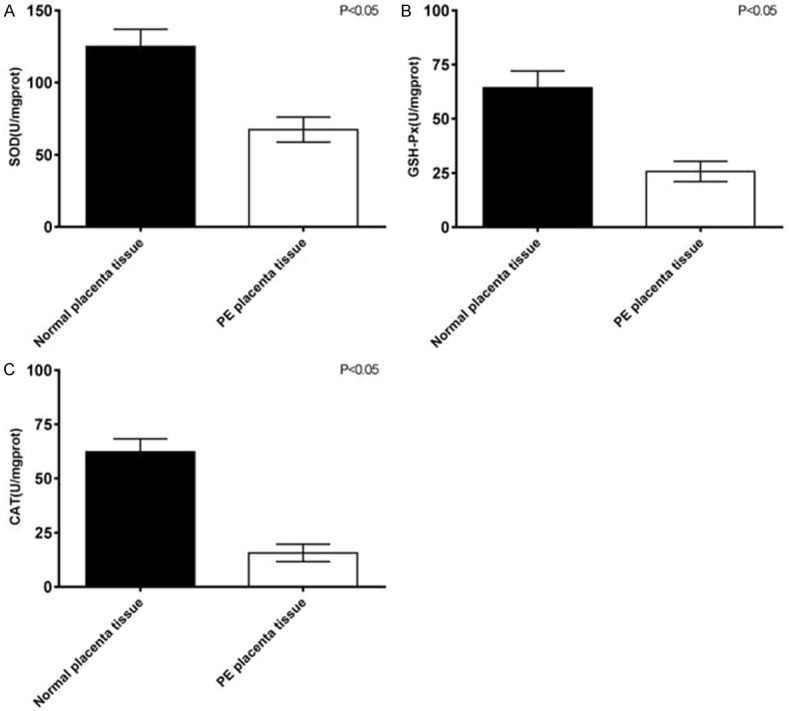
Comparison of SOD (A), GSH-Px (B) and CAT (C) enzyme activities between normal placental tissue and placental tissue in preeclampsia patients.
Detection and comparison of mRNA expression levels of anti-oxidation related genes Keap1, Nrf2, and HO-1 in placental tissues of patients in the normal and preeclampsia group
The mRNA expression levels of Keap1, Nrf2 and HO-1 in the placenta tissues of the two groups were detected by qRT-PCR. The results showed that the expression level of Keap1 mRNA in placenta of patients with preeclampsia was slightly lower than that of normal placenta (P<0.05, Figure 5A). The expression of Nrf2 mRNA in placenta of patients with preeclampsia was significantly higher than that of normal placenta (P<0.001, Figure 5B). HO-1 mRNA expression in placenta of patients with preeclampsia was significantly higher than that of normal placenta (P<0.001, Figure 5C).
Figure 5.
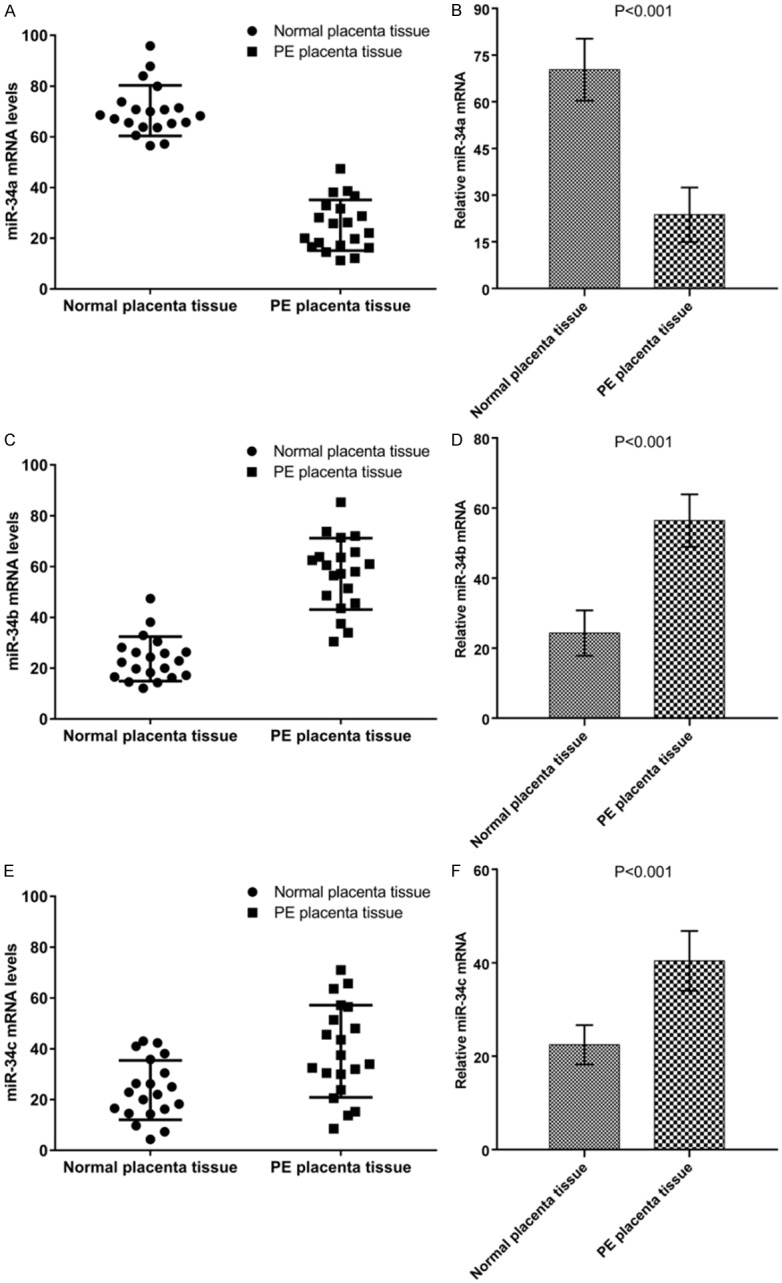
Detection and comparison of mRNA expression levels of anti-oxidation related genes Keap1 (A, B), Nrf2 (C, D), and HO-1 (E, F) in placental tissue of normal patients with those of preeclampsia patients.
Detection and comparison of protein expression levels of anti-oxidation related genes Keap1, Nrf2, and HO-1 in placental tissues of patients in normal and preeclampsia group
The results showed that the expression level of Keap1 in placenta of patients with preeclampsia was slightly lower than that of normal placenta (P<0.05, Figure 6). The expression of Nrf2 and HO-1 in placenta of patients with preeclampsia were significantly higher than that of normal placenta. Tissue (P<0.001, Figure 6).
Figure 6.
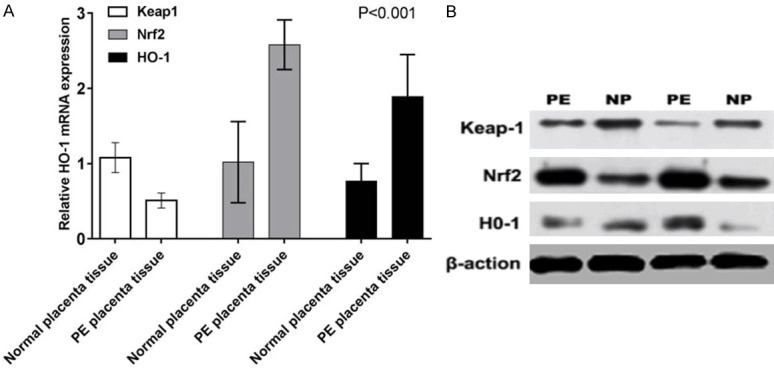
Detection and comparison of protein expression levels of anti-oxidation related genes Keap1, Nrf2, and HO-1 in placental tissue of normal patients with those of preeclampsia patients.
Discussion
Classically, PE is defined by de novo maternal hypertension (>140/90 mmHg systolic/diastolic blood pressure) and proteinuria (>300 mg/24 h) [23]. In severe cases, the mother may develop comorbidities such as hepatic alterations (HELLP syndrome), edema, disseminated vascular coagulation (DIC), and eclampsia, particularly targeting the brain (cerebral edema) [24,25]. Over the last decade, substantial progress had been made in understanding the pathophysiology of PE [26-28]. Oxidative stress damage in the placenta had been reported to lead to inflammation, apoptosis, and the release of cellular debris into maternal circulation, along with several anti-angiogenic factors, such as soluble fms-like tyrosine kinase-1 (sFlt1) and soluble Endoglin (sEng), cytokines, and oxidants [29,30]. These placental-derived factors played on the maternal vascular endothelium, inducing oxidative stress and stimulating the production and secretion of pro-inflammatory cytokines, as well as vasoactive compounds. Therefore, oxidative stress appeared to be the central component of both placental and endothelial dysfunction, the causative etiology of PE [31].
During the first stage of oxidative stress, Nrf2 is activated by the disassociation of Nrf2 from its repressor protein in the cytoplasm. Keap-1 contains cysteine residues and reacts with oxidative and electrophilic radicals leading to conformational changes and the release of Nrf2 [32,33]. Subsequently, the translocation of Nrf2 to the nucleus takes place and it binds to Antioxidant Response Element (ARE), resulting in the transcription of defensive genes [34]. The activation of the transcription involves Nrf2 recognizing its own promoter and establishing an effective interaction with it and the newly formed and accumulated Nrf2 in the nucleus binds to promoters of other specific genes [35].
In the present study, we found that under hypoxia/reoxygenation conditions, the activity of oxidative stress-related enzymes (CAT, GSH-Px, SOD) in HTR8/SVneo cells was significantly lower than that before treatment (P<0.01). The activities of CAT, GSH-Px, and SOD in HTR8/SVneo cells in SiRNA+H/R group decreased significantly (P<0.01), which indicated the important defensive effect of Keap1/Nrf2 pathway in oxidative stress. Compared with the Nrf2 SiRNA+H/R group, Si-NC+H/R group had CAT, GSH-Px and SOD activities decreasing, which were similar to those in the H/R group. Moreover, the activities of oxidative stress-related active enzymes in patients with preeclampsia were further confirmed by detecting and comparing the activities of CAT, GSH-Px and SOD in placental tissues. The results showed that the activity of SOD (P<0.001), GSH-Px (P<0.01), and CAT (P<0.01) in placental tissues of patients with preeclampsia were significantly different from those of normal placental tissues. The expression level of Keap1 in placenta of patients with preeclampsia was slightly lower than that of normal placenta, while the expression of Nrf2 in placenta of patients with preeclampsia was significantly higher than that of normal placenta. HO-1 expression in placenta of patients with preeclampsia was significantly higher than that of normal placenta.
In summary, we found that decreased antioxidant enzymes were observed under hypoxia/reoxygenation conditions in HTR8/SVneo cells. The levels of anti-oxidation related genes Keap1, Nrf2, and HO-1 in HTR8/SVneo cells also were significantly different. Moreover, the activities of oxidative stress-related active enzymes in placental tissues of patients with preeclampsia were significantly lower than those in the normal placental tissues. These results implicate the importance of Keap-1/Nrf2 pathway in PE.
Acknowledgements
This work was supported by the Health Department of Shandong Provincial Foundation of China (grant number 2017WS452).
Disclosure of conflict of interest
None.
References
- 1.Kaduma J, Seni J, Chuma C, Kirita R, Mujuni F, Mushi MF, van der Meer F, Mshana SE. Urinary tract infections and preeclampsia among pregnant women attending two hospitals in mwanza city, tanzania: a 1:2 matched case-control study. Biomed Res Int. 2019;2019:3937812. doi: 10.1155/2019/3937812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kristensen JH, Basit S, Wohlfahrt J, Damholt MB, Boyd HA. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. 2019;365:l1516. doi: 10.1136/bmj.l1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masoumi Z, Maes GE, Herten K, Cortés-Calabuig Á, Alattar AG, Hanson E, Erlandsson L, Mezey E, Magnusson M, Vermeesch JR, Familari M, Hansson SR. Preeclampsia is associated with sex-specific transcriptional and proteomic changes in fetal erythroid cells. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20082038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosimann B, Pfiffner C, Amylidi-Mohr S, Risch L, Surbek D, Raio L. First trimester combined screening for preeclampsia and small for gestational age - a single centre experience and validation of the FMF screening algorithm. Swiss Med Wkly. 2017;147:w14498. doi: 10.4414/smw.2017.14498. [DOI] [PubMed] [Google Scholar]
- 5.O’Gorman N, Wright D, Poon LC, Rolnik DL, Syngelaki A, de Alvarado M, Carbone IF, Dutemeyer V, Fiolna M, Frick A, Karagiotis N, Mastrodima S, de Paco Matallana C, Papaioannou G, Pazos A, Plasencia W, Nicolaides KH. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation: comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol. 2017;49:756–760. doi: 10.1002/uog.17455. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, Lopez-Lopez C, Felix C. Obesity and preeclampsia: common pathophysiological mechanisms. Front Physiol. 2018;9:1838. doi: 10.3389/fphys.2018.01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masoura S, Makedou K, Theodoridis T, Kourtis A, Zepiridis L, Athanasiadis A. The involvement of uric acid in the pathogenesis of preeclampsia. Curr Hypertens Rev. 2015;11:110–5. doi: 10.2174/1573402111666150529130703. [DOI] [PubMed] [Google Scholar]
- 8.Williamson RD, O’Keeffe GW, Kenny LC. Activin signalling and pre-eclampsia: from genetic risk to pre-symptomatic biomarker. Cytokine. 2015;71:360–5. doi: 10.1016/j.cyto.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Farina A, Rapacchia G, Freni Sterrantino A, Pula G, Morano D, Rizzo N. Prospective evaluation of ultrasound and biochemical-based multivariable models for the prediction of late pre-eclampsia. Prenat Diagn. 2011;31:1147–52. doi: 10.1002/pd.2849. [DOI] [PubMed] [Google Scholar]
- 10.Chasan-Taber L. Physical activity and dietary behaviors associated with weight gain and impaired glucose tolerance among pregnant Latinas. Adv Nutr. 2012;3:108–18. doi: 10.3945/an.111.001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa PJ, Palmeiro Y, Soto MJ, Ugarte C, Illanes SE. Etiopathogenesis, prediction, and prevention of preeclampsia. Hypertens Pregnancy. 2016;35:280–94. doi: 10.1080/10641955.2016.1181180. [DOI] [PubMed] [Google Scholar]
- 12.Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol. 2014;5:567. doi: 10.3389/fimmu.2014.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta EH, Behnia F, Boldogh I, Saade GR, Taylor BD, Kacerovský M, Menon R. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol Hum Reprod. 2016;22:143–57. doi: 10.1093/molehr/gav074. [DOI] [PubMed] [Google Scholar]
- 14.Martin LF, Moço NP, de Lima MD, Polettini J, Miot HA, Corrêa CR, Menon R, da Silva MG. Histologic chorioamnionitis does not modulate the oxidative stress and antioxidant status in pregnancies complicated by spontaneous preterm delivery. BMC Pregnancy Childbirth. 2017;17:376. doi: 10.1186/s12884-017-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Micle O, Muresan M, Antal L, Bodog F, Bodog A. The influence of homocysteine and oxidative stress on pregnancy outcome. J Med Life. 2012;5:68–73. [PMC free article] [PubMed] [Google Scholar]
- 16.Tang C, Liang J, Qian J, Jin L, Du M, Li M, Li D. Opposing role of JNK-p38 kinase and ERK1/2 in hydrogen peroxide-induced oxidative damage of human trophoblast-like JEG-3 cells. Int J Clin Exp Pathol. 2014;7:959–68. [PMC free article] [PubMed] [Google Scholar]
- 17.Wu F, Tian FJ, Lin Y. Oxidative stress in placenta: health and diseases. Biomed Res Int. 2015;2015:293271. doi: 10.1155/2015/293271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jauniaux E, Burton GJ. The role of oxidative stress in placental-related diseases of pregnancy. J Gynecol Obstet Biol Reprod (Paris) 2016;45:775–785. doi: 10.1016/j.jgyn.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Muller SG, Jardim NS, Quines CB, Nogueira CW. Diphenyl diselenide regulates Nrf2/Keap-1 signaling pathway and counteracts hepatic oxidative stress induced by bisphenol A in male mice. Environ Res. 2018;164:280–287. doi: 10.1016/j.envres.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Jiang C, Zhang K, Lan X, Chen X, Zang W, Wang Z, Guan F, Zhu C, Yang X, Lu H, Wang J. Melatonin receptor activation provides cerebral protection after traumatic brain injury by mitigating oxidative stress and inflammation via the Nrf2 signaling pathway. Free Radic Biol Med. 2019;131:345–355. doi: 10.1016/j.freeradbiomed.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Ci X, Lv H, Wang L, Wang X, Peng L, Qin FX, Cheng G. The antioxidative potential of farrerol occurs via the activation of Nrf2 mediated HO-1 signaling in RAW 264.7 cells. Chem Biol Interact. 2015;239:192–9. doi: 10.1016/j.cbi.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Zhu C, Dong Y, Liu H, Ren H, Cui Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed Pharmacother. 2017;88:124–133. doi: 10.1016/j.biopha.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 23.Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens. 2015;24:131–8. doi: 10.1097/MNH.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 24.Farajian-Mashhadi F, Eskandari F, Rezaei M, Eskandari F, Najafi D, Teimoori B, Moradi-Sharbabak M, Salimi S. The possible role of maternal and placental vitamin D receptor polymorphisms and haplotypes in pathogenesis of preeclampsia. Clin Exp Hypertens. 2020;42:171–176. doi: 10.1080/10641963.2019.1601203. [DOI] [PubMed] [Google Scholar]
- 25.Lv X, Li X, Dai X, Liu M, Wu C, Song W, Wang J, Ren X, Cai Y. Investigation heme oxygenase-1 polymorphism with the pathogenesis of preeclampsia. Clin Exp Hypertens. 2020;42:167–170. doi: 10.1080/10641963.2019.1601202. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhary P, Malhotra SS, Babu GS, Sobti RC, Gupta SK. HGF promotes HTR-8/SVneo cell migration through activation of MAPK/PKA signaling leading to up-regulation of WNT ligands and integrins that target beta-catenin. Mol Cell Biochem. 2019;453:11–32. doi: 10.1007/s11010-018-3428-3. [DOI] [PubMed] [Google Scholar]
- 27.Chen SSM, Leeton L, Castro JM, Dennis AT. Myocardial tissue characterisation and detection of myocardial oedema by cardiovascular magnetic resonance in women with pre-eclampsia: a pilot study. Int J Obstet Anesth. 2018;36:56–65. doi: 10.1016/j.ijoa.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Than NG, Romero R, Tarca AL, Kekesi KA, Xu Y, Xu Z, Juhasz K, Bhatti G, Leavitt RJ, Gelencser Z, Palhalmi J, Chung TH, Gyorffy BA, Orosz L, Demeter A, Szecsi A, Hunyadi-Gulyas E, Darula Z, Simor A, Eder K, Szabo S, Topping V, El-Azzamy H, LaJeunesse C, Balogh A, Szalai G, Land S, Torok O, Dong Z, Kovalszky I, Falus A, Meiri H, Draghici S, Hassan SS, Chaiworapongsa T, Krispin M, Knöfler M, Erez O, Burton GJ, Kim CJ, Juhasz G, Papp Z. Integrated systems biology approach identifies novel maternal and placental pathways of preeclampsia. Front Immunol. 2018;9:1661. doi: 10.3389/fimmu.2018.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solevåg AL, Schmölzer GM, Cheung PY. Novel interventions to reduce oxidative-stress related brain injury in neonatal asphyxia. Free Radic Biol Med. 2019;142:113–122. doi: 10.1016/j.freeradbiomed.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Roumeliotis S, Eleftheriadis T, Liakopoulos V. Is oxidative stress an issue in peritoneal dialysis? Semin Dial. 2019;32:463–466. doi: 10.1111/sdi.12818. [DOI] [PubMed] [Google Scholar]
- 31.Goulopoulou S, Davidge ST. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol Med. 2015;21:88–97. doi: 10.1016/j.molmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Zhang J, Ji Q, Yu K, Wang P, Song M, Cao Z, Zhang X, Li Y. Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotoxicol Environ Saf. 2019;173:131–141. doi: 10.1016/j.ecoenv.2019.01.095. [DOI] [PubMed] [Google Scholar]
- 33.Zhang F, Munoz FM, Sun L, Zhang S, Lau SS, Monks TJ. Cell-specific regulation of Nrf2 during ROS-Dependent cell death caused by 2,3,5-tris(glutathion-S-yl)hydroquinone (TGHQ) Chem Biol Interact. 2019;302:1–10. doi: 10.1016/j.cbi.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Lu MC, Ji JA, Jiang ZY, You QD. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med Res Rev. 2016;36:924–63. doi: 10.1002/med.21396. [DOI] [PubMed] [Google Scholar]
- 35.Fan J, Lv H, Li J, Che Y, Xu B, Tao Z, Jiang W. Roles of Nrf2/HO-1 and HIF-1alpha/VEGF in lung tissue injury and repair following cerebral ischemia/reperfusion injury. J Cell Physiol. 2019;234:7695–7707. doi: 10.1002/jcp.27767. [DOI] [PubMed] [Google Scholar]


