Abstract
Recent studies have indicated that ANXA7 promotes progression and metastasis of hepatocellular carcinoma (HCC). In this study we found a significant negative correlation between the levels of miR-124-3p and ANXA7 protein in HCC. Level of miR-124-3p in tumor tissues was negatively correlated, while ANXA7 protein was positively correlated, with TNM stage and tumor metastasis. Furthermore, we confirmed ANXA7 was a target gene of miR-124-3p by a dual luciferase reporter assay. In vitro, up-regulation of miR-124-3p promotes apoptosis and inhibits migration and invasion of Hca-F. Bcl-2 correlates X protein (Bax) protein level was up-regulated, while ANXA7, B-cell lymphoma-2 (Bcl-2), Matrix metalloproteinase (MMP-9) and C-X-C motif chemokine 12 (CXCL12) protein levels were suppressed relative to miR-124-3p over-expression. In vivo, up-regulation of miR-124-3p suppresses lymph node metastasis (LNM) and tumorigenicity of Hca-F cells. The expression of ANXA7, MMP-9, and CXCL12 protein in transplanted tumors was suppressed relative to miR-124-3p overexpression. In addition, we found the levels of Bcl-2, MMP-9, and CXCL12 in Hca-F cells decreased significantly after transfection of shRNA-Anxa7 in vitro. In conclusion, our study revealed miR-124-3p inhibits tumor growth, invasion, and lymphatic metastasis in HCC by down-regulation of ANXA7 gene, thereby reducing the expression of Bcl-2, MMP-9, and CXCL12.
Keywords: MiR-124-3p, ANXA7, hepatocellular carcinoma, invasion, lymphatic metastasis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in clinic. It has the characteristics of high invasion, early metastasis, high recurrence and mortality. Tumor metastasis is an important cause of death in patients with HCC [1]. Lymphatic metastasis is an important way of HCC metastasis. The degree and extent of lymphatic metastasis is an important factor determining the prognosis of patients [2]. Studying the mechanism of lymphatic metastasis is of great value for understanding the biological characteristics of HCC and guiding clinical treatment.
Annexin A7 (ANXA7) is an important member of the Annexin family in vertebrate cells [3]. It plays an important role in cytoskeleton activity, membrane phospholipidization, membrane receptor regulation, and mitosis [4,5]. Recent studies have found that ANXA7 plays an important role in tumorigenesis, development, invasion and metastasis [6,7]. In the past, our team has found ANXA7 gene is one of the important differentially expressed genes that affect the lymphatic metastasis potential of tumors which differs between Hca-F cells (lymph node metastasis rate > 70%) and Hca-P cells (lymph node metastasis rate < 30%) by gene chip, protein spectrum, and suppression subtraction [8,9]. Up-regulation of ANXA7 gene expression enhanced the proliferation and migration of Hca-F cells, and down-regulation of ANXA7 gene expression reduced the proliferation and migration of Hca-F cells, suggesting that ANXA7 plays a role in promoting lymphatic metastasis of HCC [10,11].
MicroRNAs (miRNAs) are 18-24 nucleotide endogenous non-coding small RNAs [12]. Previous studies have shown that miRNAs have significant effects on signal transduction pathways, cell apoptosis and metabolism, myogenesis, cancer, viral infection, and body development [13-15]. miRNAs may interact with ANXA7 and play an important role in tumorigenesis, development, metastasis, and invasion. However, the interaction between ANXA7 gene and microRNAs has not yet been described.
Therefore, we aimed to explore the effects of miR-124-3p and ANXA7 in HCC, in the hope of providing possible indicators for clinical diagnosis of hepatocellular carcinoma and a potential molecular target for HCC treatment.
Materials and methods
Patients and specimens
60 tumor tissues were obtained from patients with HCC. The patients with HCC underwent surgical resection at the First Affiliated Hospital of Dalian Medical University from August 2017 to July 2019. The specimens were collected with the consent of the patients. These patients did not have history of radiotherapy or chemotherapy before collecting specimens. The experiment was approved by the Ethics Committee of First Affiliated Hospital of Dalian Medical University.
Cell culture
The Hca-F cells (Mouse hepatocellular carcinoma cells with high lymphatic metastasis potential) were established in the Department of Pathology, Dalian Medical University. NCTC1469 cell and Human embryonic kidney cell line HEK-293T was obtained from American Type Culture Collection (ATCC). Cells were cultured in 90% RPMI 1640 (Gibco, Grand Island, NY, USA) which was supplemented with streptomycin/penicillin (Gibco, USA) and 10% fetal bovine serum (Gibco, USA). The cells were cultured in a humidified incubator with 5% CO2 at 37°C.
MiRNA prediction
ANXA7 was input into NCBI GENE (https://www.ncbi.nlm.nih.gov/gene/) as a search term to query the Ensembl sequence of ANXA7 (species: mice). The miRNAs that could regulate ANXA7 were retrieved from TargetScan (http://www.targetscan.org/mmu_72/) (species: mice), and a number of miRNAs were predicted by the database. According to the basic principles of bioinformatics prediction of target genes: high degree of conservativeness of target gene “seed sequence” in humans, mice, squirrels, rabbits and other species, low binding free energy between microRNAs and target genes, and closely relation to cell proliferation, candidate microRNAs were selected.
Cell transfection and grouping
MiRNA vectors, miR-124-3p mimics, precursor miR-124-3p scrambled control clones (N-control), and miR-124-3p inhibitors were purchased from GenePharma (Hangzhou, China). The lentiviral packaging kit (Open Biosystems, Huntsville, AL, USA) was used to package the lentiviruses that carried the compounds mentioned above, in accordance with the manual. The shRNA-ANXA7 (hairpin sequence: 5’-GTCAGAATTGAGTGG-GAATT-3’) and negative control shRNA (hairpin sequence: 5’-GTTCTCCGAACGTGTCACGT-3’) were transfected the un-manipulated Hca-F cells.
Dual-luciferase reporter gene assay
The miRNAs that could regulate ANXA7 were retrieved from TargetScan (http://www. targetscan.org/). “ANXA7” served as the keyword, and a number of miRNAs were predicted in the database. Wild-type pGL3-ANXA7-3’UTR (WT-ANXA7-3’UTR) and mutant pGL3-ANXA7-3’UTR (MUT-ANXA7-3’UTR) plasmids were obtained from Shanghai Genechem Co. Ltd. (Shanghai, China). After 48 h transfection, Passive Lysis Buffer (Promega, USA) was used to collect the lysates of HEK-293t cells from the treatment groups with its protocols. Dual-Luciferase Reporter Assay System (Promega, USA) was used to analyze the relative luciferase activity relative to the Renilla luciferase activity in the same sample.
Flow cytometry
The transfected cells were centrifuged and washed by cold PBS for 2 times. 10 μL Annexin V-FITC and 10 μL PI (Invitrogen; Thermo Fisher Scientific, Inc.) were employed to incubate the harvested cells (3 × 105) at room temperature for 15 min in dark room. A flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) was used to measure the apoptotic cells.
Transwell assay
The transwell assay detected the cell motility capacities through the Corning transwell chambers with the 8-μm pore size (Corning Co, Corning, USA) with/without matrigel. 4 × 103 cells in serum-free media were put in the upper chamber after 48 h of transfection. The cells in upper membrane were wiped off 48 h after the incubation. The migrated cells were stained with 0.1% crystal violet. A microscope was used to count the cells. A total of three independent experiments were conducted.
Mouse xenograft mode
Fifteen 8-week-old male 615 mice were obtained from Institute of Genome Engineered Animal Models for Human Disease of Dalian Medical University (China). The mice were randomly divided into NC group, miR-124 mimics group, miR-124 inhibitor group, five mice in each. Transfected Hca-F cell suspension 0.05 ml (containing 2 × 106 cells) was injected into each mouse’s right foot pad. Tumor growth was monitored and measured every 4 days, and the tumor volume was calculated through the formula [(length (mm) × width (mm)) 2]/2. After 28 days, the mice were euthanized by cervical dislocation under CO2 anesthesia and the tumor was dissected and weighed. The regional lymph nodes (inguinal and axillary) were dissected to analyze the lymph node metastatic rate. The transplanted tumors were washed with sterile PBS, and dried with filter paper for western blot. Part of lymph node metastases of transplanted tumors was fixed with 10% formalin for immunohistochemical stains. All of the procedures were performed by the Animal Ethics Committee of the Dalian Medical University.
Immunohistochemical analysis
The tumor tissues were sliced into sections of 4 µm, and then they underwent deparaffination and rehydration. The anti-ANXA7 (1:200, Santa Cruz Biotechnology Inc.) and anti-MMP-9 (1:200, Santa Cruz Biotechnology Inc.) was used as the primary antibody at 4°C overnight. The following day, peroxidase conjugated secondary antibody (ZSGB-BIO, Beijing, China) was used to incubate the slides for 30 min. Signal development was done with DAB solution, a peroxidase-labeled polymer, for 5 min. After counterstaining the sections with hematoxylin, dehydrating and mounting were carried out. Immunohistochemistry stain was analyzed by Image-Pro-Plus (IPP) 6.0 software.
RNA extraction and real-time quantitative PCR (q-PCR) analysis
The isolation of total RNA from cells was realized through TRIzol reagent (Invitrogen) in accordance with the protocol of the manufacturer. The primers of genes were as below: miR-124-3p forward, 5’-CGGGTAGCAGGCTTCTGAGT-3’ and reverse, 5’-AAACCCCTCTCTGTCGGTAGCT-3’; U6 forward, 5’-CTTTGCCGCACGGCACA-3’ and reverse, 5’-AAGCAACTTYCACGYCGT-3’. ANXA7 forward, 5’-AAUGACCUGCAUGCTTGACT-3’ and reverse, AGCUAACCGAGAAAGAGCTT; β-actin, forward, 5’-AGCGCCATCCGCAACAAGTT-3’; and reverse, 5’-GGCACGGGCTCAAGATCATT-3’. qRT-PCR was analyzed through Bio-Rad CFX Manager 2.1 software. All of the experiments were performed in triplicate. The 2ΔΔCt was used as the analytic formula.
Western blot analysis
For western blot, the proteins were transferred electrophoretically to PVDF membranes (Merck KGaA, Darmstadt, Germany), which were blotted with antibody overnight through anti-ANXA7 (1:500; ab-63842; Abcam, Cambridge, MA), anti-Bax (1:500, sc-6106, Santa Cruz Biotechnology Inc, CA.), anti-Bcl-2 (1:500, sc-58012, Santa Cruz Biotechnology Inc.), anti-MMP-9 (1:500, SC-6840, Santa Cruz Biotechnology Inc.), anti-CXCL12 (1:500, SC-6372, Santa Cruz Biotechnology Inc.), GAPDH (1:1000, 21872-1-AP, Wuhan Sanying Biotechnology, Wuhan, China) or β-tubulin (1:500, sc-63410, Santa Cruz Biotechnology Inc.) at 4°C. The membranes were washed in TBST and incubated with anti-mouse horseradish peroxidase-conjugated secondary antibody (1:1000, Biosynthesis Biotechnology, China) at room temperature for 2 h. Chemiluminescence (GE, USA) was used to visualize the immunocomplexes according to the manufacturer’s protocol.
Statistical analysis
The mean ± standard deviation (SD) expressed the continuous variables. GraphPad Prism software, version 5.0 (GraphPad, La Jolla, CA, USA) was used to perform one-way ANOVA for multiple comparisons. A significant difference was indicated by P-values < 0.05 (*P < 0.05). All data are representative of at least three different experiments.
Results
miR-124-3p and ANXA7 are involved in HCC
A total of 5 ANXA7-related miRNAs, namely, miR-19-3p, miR-124-3p, miR-135-5p, miR-190-5p, miR-323-3p were retrieved from TargetScan. It was found that the position 155-162 of ANXA7 3’UTR segment was consequentially pairing to target region of miR-124-3p. Sequence alignment showed that the conserved sequence of ANXA7 in human, mouse, squirrel, rabbit and other species was strictly conserved, while the free energy was low.
miR-124-3p was down-regulated while ANXA7 was up-regulated in Hca-F cells and HCC
We tested tumor and related adjacent tissues from 60 HCC patients by qPCR and immunohistochemical analysis. The results revealed that miR-124-3p was down-regulated while ANXA7 protein was up-regulated in tumor tissues (Figure 1A-C). Pearson analysis showed that the expression level of ANXA7 protein decreased significantly with the increase of the expression of miR-124-3p. There was a significant negative correlation (r = -0.7573, P < 0.05, Figure 1D). Using q-PCR, we detected the miR-124-3p was down-regulated in Hca-F cells, compared with a normal hepatic cell line NCTC1469 (P < 0.05) (Figure 1E). Using western blot, we detected the ANXA7 protein expression was higher in Hca-F cells, compared with NCTC1469 (P < 0.05) (Figure 1F and 1G).
Figure 1.
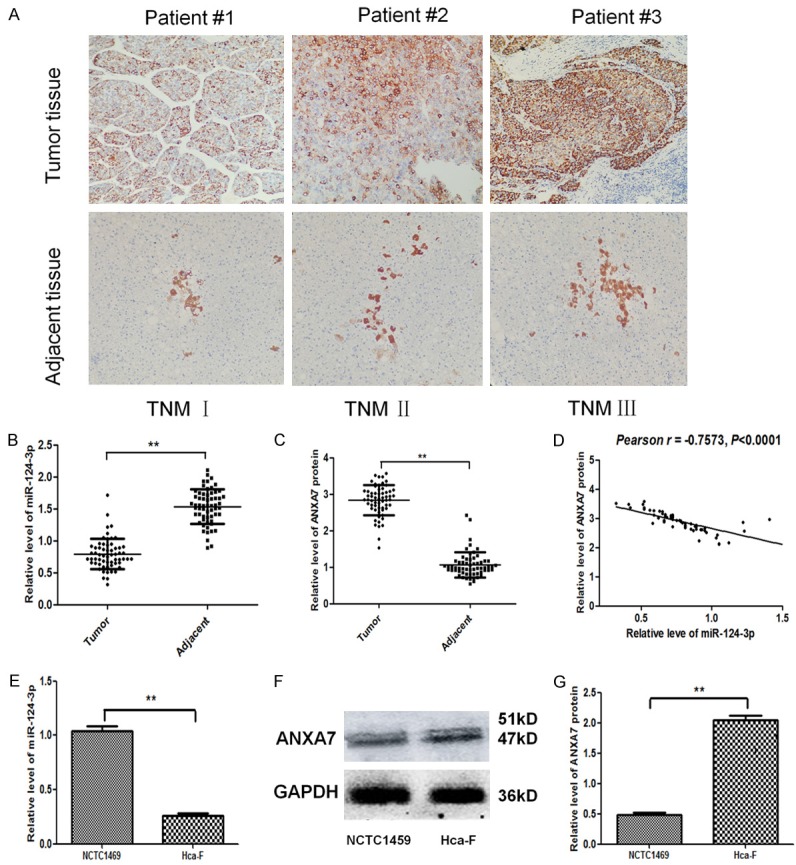
miR-124-3p was down-regulated while ANXA7 was up-regulated in Hca-F cells and HCC. A. Protein expression of ANXA7 in tumor tissues and related adjacent tissues from HCC patients by immunohistochemical stain revealed that miR-124-3p was down-regulated while ANXA7 protein was up-regulated in tumor tissue. B. miR-124-3p level in tumor tissues and related adjacent tissues from HCC patients by q-PCR. C. Protein expression of ANXA7 in tumor tissues and related adjacent tissues from HCC patients. D. Pearson analysis between level of ANXA7 protein and level of miR-124-3p. There was a significant negative correlation. E. miR-124-3p level in Hca-F cells, compared with a normal hepatic cell line NCTC1469 by q-PCR. F, G. Protein expression of ANXA7 in Hca-F cells, compared with a normal hepatic cell line NCTC1469 by western blot. The error bar represents the SD (n = 3). **P < 0.01.
Level of miR-124-3p and ANXA7 was associated with HCC progression
We collected clinicopathological data and conducted an analysis. The results showed that the level of miR-124-3p in tumor tissues was negatively correlated, while ANXA7 protein was positively correlated with TNM stage and tumor metastasis (P < 0.05). There was no significant difference in the expression of miR-124-3p and ANXA7 between different age, gender, AFP level, alcohol consumption, and HBV/HCV infection groups (Table 1).
Table 1.
Clinicopathologic characteristics of HCC patients according to miR-124-3p expression and ANXA7 protein expression
| Characteristic | n | miR-124-3p expression | P value | ANXA7 protein expression | P value |
|---|---|---|---|---|---|
| Age (years) | 0.8171 | 0.8954 | |||
| ≥ 60 | 42 | 0.78±0.12 | 2.89±0.38 | ||
| < 60 | 18 | 0.80±0.07 | 2.87±0.32 | ||
| Gender | 0.9721 | 0.9921 | |||
| male | 38 | 0.79±0.11 | 2.88±0.36 | ||
| female | 22 | 0.79±0.09 | 2.88±0.33 | ||
| TNM stage | 0.0021* | 0.0010* | |||
| I-II | 46 | 0.85±0.13 | 2.77±0.41 | ||
| III | 14 | 0.62±0.07 | 3.27±0.30 | ||
| AFP level | 0.1026 | 0.1973 | |||
| ≥ 400 | 45 | 0.77±0.13 | 2.91±0.35 | ||
| < 400 | 15 | 0.85±0.10 | 2.82±0.27 | ||
| Alcohol consumption | 0.6842 | 0.8362 | |||
| Yes | 25 | 0.77±0.09 | 2.90±0.33 | ||
| No | 35 | 0.80±0.12 | 2.88±0.35 | ||
| HBV/HCV infection | 0.9183 | 0.9072 | |||
| Yes | 39 | 0.79±0.12 | 2.89±0.38 | ||
| No | 21 | 0.80±0.08 | 2.88±0.34 | ||
| Tumor metastasis | 0.0013* | 0.0001* | |||
| Yes | 12 | 0.60±0.07 | 3.35±0.28 | ||
| No | 48 | 0.84±0.14 | 2.77±0.41 |
p < 0.05 indicates a significant association among the variables.
ANXA7 is a target gene of miR-124-3p
The luciferase activity of WTANXA7-3’UTR was inhibited by miR-124-3p (P < 0.05), while that of MUT-ANXA7-3’UTR did not exhibit the same effect (P > 0.05) (Figure 2B). ANXA7 protein expression level was down-regulated relative to miR-124-3p over-expression, However, miR-124-3p down-regulation had the opposite effect (P < 0.05) (Figure 2C and 2D). These findings indicated that miR-124-3p may specifically bind to ANXA7-3’UTR and down-regulate ANXA7 gene expression at a post-transcriptional level. The aforementioned results suggest that ANXA7 is a target gene of miR-124-3p.
Figure 2.
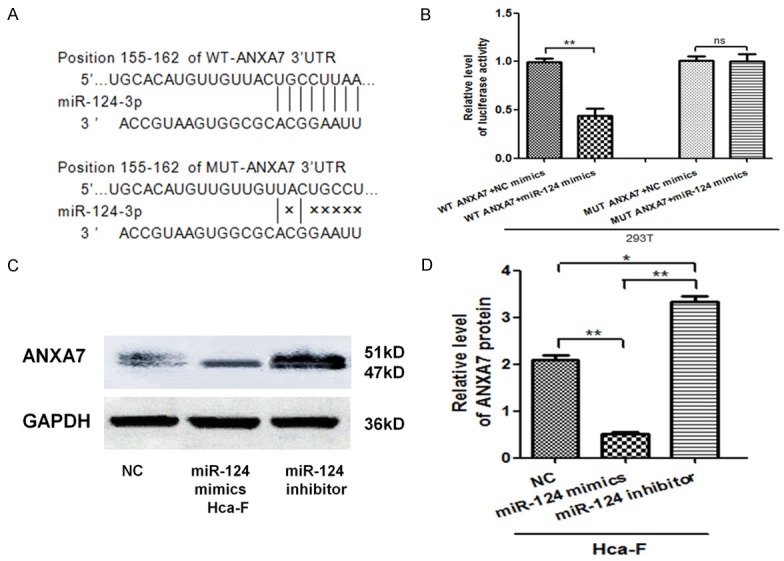
ANXA7 is a target gene of miR-124-3p. A. Sequencing results of WT ANXA7 3’-UTR gene and MUT ANXA7 3’-UTR gene. The sequences were consistent with miRanda, Pubmed, and Targetscans databases and there were binding sites with miR-124-3p in the 155-162 bp sites, while the MUT ANXA7’-UTR 155-162 sites had no miR-124-3p binding sites. B. miR-124-3p binding site in WT ANXA7 3’-UTR and MUT ANXA7 3’-UTR mutation site. C. Dual-luciferase reporter gene assay. D. Comparison of ANXA7 protein levels in Hca-F cells after transfection of miR-124-3p mimics, and miR-124-3p inhibitor. The error bar represents the SD (n = 3). *P < 0.05, **P < 0.01.
Up-regulation of miR-124-3p promotes Hca-F cell apoptosis
In order to evaluate whether miR-124-3p regulated HCC cell apoptosis, apoptosis assay was conducted by flow cytometry. There was an increased apoptotic population after miR-124-3p mimic transfection in Hca-F cells, but the apoptosis rate of Hca-F cells decreased as miR-124-3p expression was inhibited (Figure 3A). Furthermore, we detected the protein expression of ANXA7, Bax, and Bcl-2 in Hca-F cells. As the data show (Figure 3B and 3C), Bax protein expression was up-regulated relative to miR-124-3p over-expression, while ANXA7 and Bcl-2 protein expression were suppressed. However, miR-124-3p down-regulation had an opposite effect.
Figure 3.
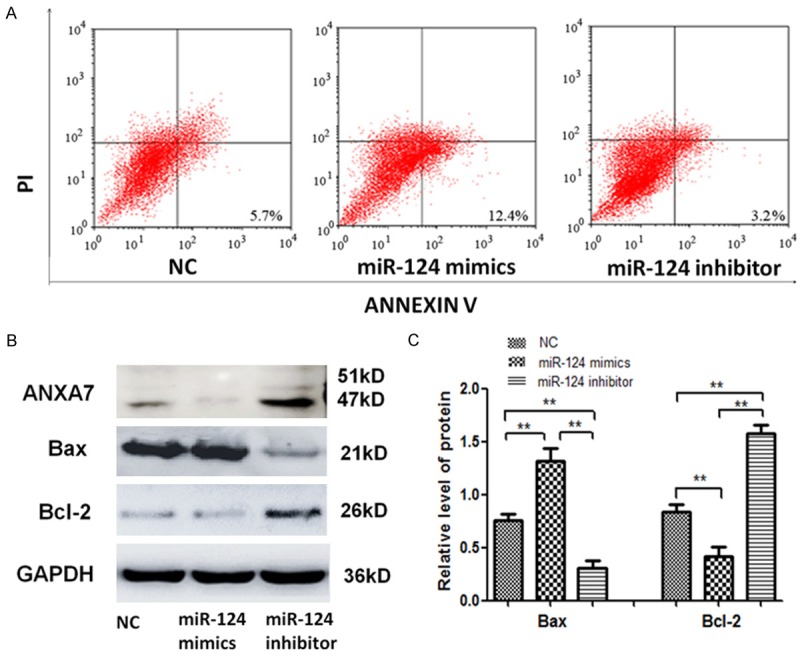
Upregulation of miR-124-3p promotes Hca-F cell apoptosis. A. Annexin V-FITC/PI flow cytometry was used to detect the apoptosis of Hca-F cells, miR-124-3p mimic cells, and miR-124-3p inhibitor cells. B, C. The levels of ANAX7, Bax, and Bcl-2 proteins in Hca-F cells, miR-124-3p mimic cells, and miR-124-3p inhibitor cells were detected by western blot. The error bar represents the SD (n = 3). **P < 0.01.
Up-regulation of miR-124-3p inhibits Hca-F cell migration and invasion
To detect the correlation of miR-124-3p level with the migration and invasion of HCC progression, Hca-F cells were infected with a miR-124-3p mimic or inhibitors. Fewer migrated miR-124-3p over-expressing cells were seen on the insert membrane than control cells. In contrast, silencing miR-124-3p in HCC cells significantly increased cell invasion and metastasis compared to the control group (Figure 4A and 4B). To further assess the contribution of miR-124-3p on cell invasion, we investigated the protein expression of ANXA7, MMP-9, and CXCL12 in Hca-F cells by western blot. As the results showed, the expression of MMP-9 and CXCL12 in HCC cells with stable over-expression of miR-124-3p inhibitors was higher than in the other control cells. However, miR-124-3p down-regulation had an opposite effect (Figure 4C and 4D).
Figure 4.
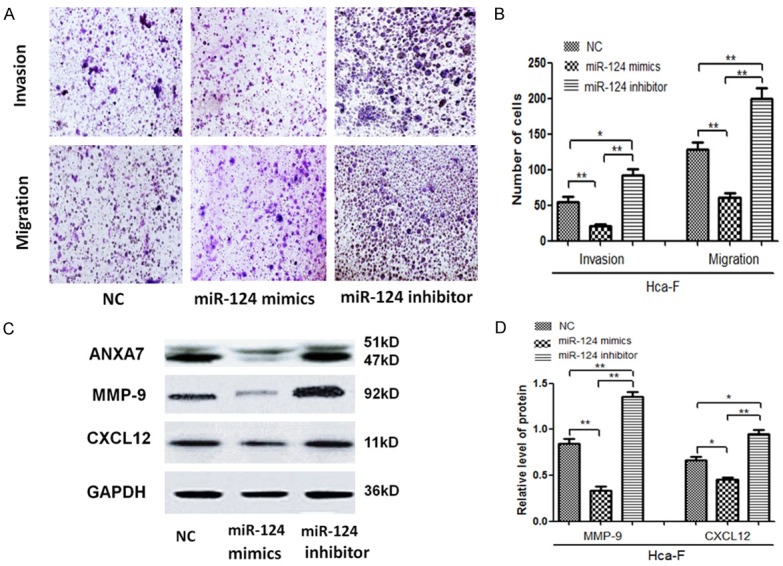
Upregulation of miR-124-3p promotes Hca-F cell apoptosis. A. Transwell migration and invasion experiment in Hca-F cells, miR-124-3p mimic cells, and miR-124-3p inhibitor cells. B. The number of cells on the insert membrane after transwell migration and invasion experiments in Hca-F cells, miR-124-3p mimic cells, and miR-124-3p inhibitor cells. C, D. The levels of ANXA7, MMP-9, and CXCL12 protein in Hca-F cells, miR-124-3p mimic cells, and miR-124-3p inhibitor cells were detected by western blot. The error bar represents the SD (n = 3). *P < 0.05, **P < 0.01.
Up-regulation of miR-124-3p suppresses LNM and tumorigenicity in 615 mice with Hca-F cells
Hca-F cells were chosen as the LNM model for in vivo study. As the figure shows (Figure 5A), in comparison with the NC group, growth was significantly increased in the miR-124-3p inhibitor group. The tumor volume and weight from miR-124-3p mimics cells decreased at day 28 days post-injection (Figure 5B and 5D). Moreover, we observed the LNM rate in different groups. The result showed that there were more visible metastases in the axillary and inguinal lymph nodes (Figure 5C and Table 2). The LNM rate in the miR-124-3p mimics group was lower compared with the NC group and the miR-124-3p inhibitor group (30% vs. 50.0%, 30% vs. 80.0%). The axillary and inguinal lymph node volume from miR-124-3p mimic cells decreased at day 28 days post injection (Figure 5E). The expression levels of ANXA7, MMP-9, and CXCL12 protein in transplanted tumors in the miR-124 mimics group were significantly lower than those in the NC group, while the expressions of ANXA7, MMP-9 and CXCL12 protein in the miR-124 inhibitor group were significantly higher than those in the NC group (P < 0.05) (Figure 5F and 5G). The expressions of ANXA7, MMP-9, and CXCL12 were detected by immunohistochemical assay. Figure 6A and 6B show the levels of ANXA7, MMP-9, and CXCL12 protein in lymph node metastases of the miR-124-3p mimics group were significantly lower than those of the NC group, and the levels of ANXA7, MMP-9, and CXCL12 protein in lymph node metastases of the miR-124-3p inhibitor group were significantly higher than those of the NC group (P < 0.05).
Figure 5.
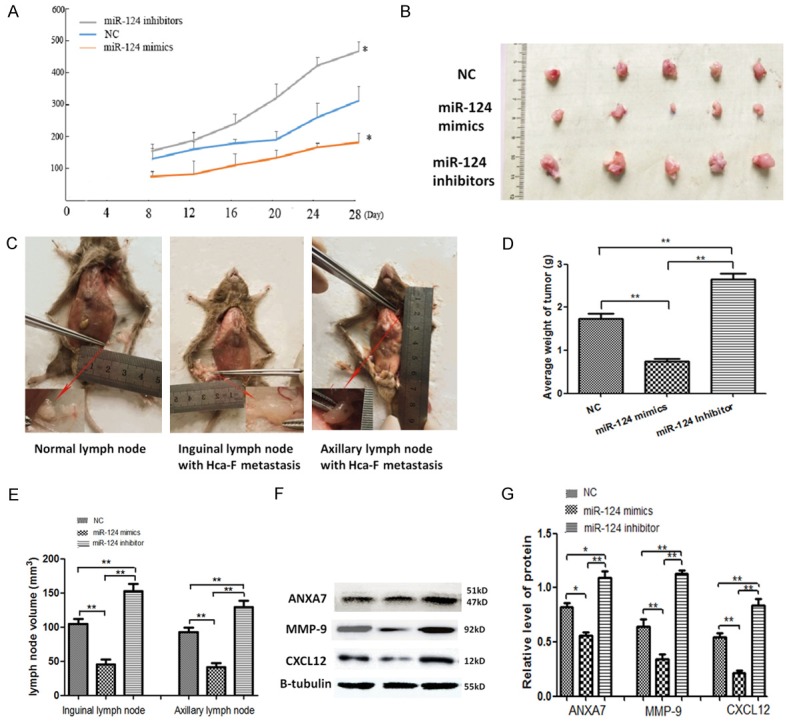
Upregulation of miR-124-3p suppresses LNM and tumorigenicity in 615 mice with Hca-F cells. A. The volume of transplanted tumors on the footpad of mice in each group at different time points. B. The transplanted tumors at the footpad of mice in each group at 28 days. C. The volume of normal lymph nodes smaller than the nodes infected with Hca-F cells. D. The average weight of transplanted tumors on the footpad of mice in each group at 28 days. E. The axillary and inguinal lymph node volume in each group on 28 days. F, G. The expression levels of ANXA7, MMP-9, and CXCL12 proteins in transplanted tumors in each group. The error bar represents the SD (n = 3). *P < 0.05, **P < 0.01.
Table 2.
Lymph node metastaticsis of LNM model in different groups
| Group | LN | Lymph node metastatic rate level | ||
|---|---|---|---|---|
|
| ||||
| LNM rate | % LNM rate | P value | ||
| NC | Inguinal | 3/5 | 50.00 | |
| axillary | 2/5 | |||
| miR-124 mimics | Inguinal | 2/5 | 30.00 | < 0.05* |
| axillary | 1/5 | |||
| miR-124 inhibitors | Inguinal | 5/5 | 80.00 | |
| axillary | 3/5 | |||
p < 0.05 indicates a significant association among the variables.
Figure 6.
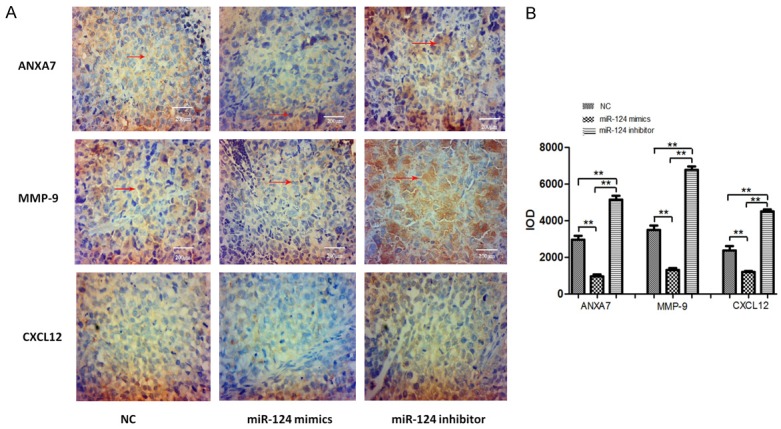
The expression of ANXA7, MMP-9, and CXCL12 detected by immunohistochemical assay. (A) Immunostaining results of ANXA7, MMP-9, and CXCL12 in transplanted tumors of mice in each group, using a magnification of 40 × 10, (B) IOD values of positive results in each group. The error bar represents the SD (n = 3). **P < 0.01.
Silencing ANXA7 affects miR-124-3p related protein in Hca-F cells
The expression vector ANXA7 and its unrelated sequence were transfected into Hca-F cells by Lipofectamine 2000. The green fluorescent protein was observed under fluorescence microscope after incubation at 37°C for 24-48 hours (Figure 7A). qPCR showed no significant difference in the level of ANXA7 gene between transfected unrelated sequence and Hca-F cells (P > 0.05). The level of ANXA7 gene in transfected expression vector was significantly lower than that in Hca-F cells and transfected unrelated sequence (P < 0.05) (Figure 7B). Western blot showed that there was no significant difference between the level of ANXA7 protein in transfected unrelated sequence and Hca-F cells (P > 0.05). The level of ANXA7 protein in transfected expression vector was significantly lower than that in Hca-F cells and transfected unrelated sequence (P < 0.05) (Figure 7C and 7D). The levels of Bax, Bcl-2, MMP-9, and CXCL12 in Hca-F cells did not change significantly after transfection of unrelated sequence (P > 0.05), but the levels of Bcl-2, MMP-9, and CXCL12 in Hca-F cells decreased significantly after transfection of shRNA-Anxa7 (P < 0.05), while the levels of Bax and protein did not change significantly (P > 0.05) (Figure 7E-G).
Figure 7.
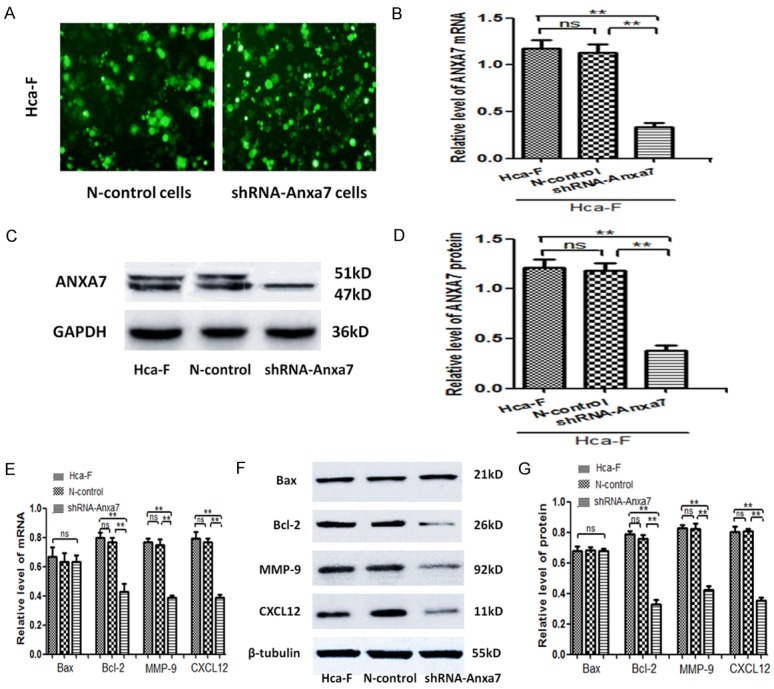
Silencing ANXA7 affects miR-124-3p related protein in Hca-F cells. A. The expression vector ANXA7 and its unrelated sequence were transfected into Hca-F cells by Lipofectamine 2000. The green fluorescent protein was observed under fluorescence microscope after incubation at 37°C for 24-48 hours. B. The levels of ANXA7 gene in Hca-F group, N-control group, and shRNA-Anxa7 group were detected by qPCR. C, D. Western blot was used to detect the levels of ANXA7 protein in Hca-F group, N-control group, and shRNA-Anxa7 group. E. q-PCR was used to detect the levels of Bax, Bcl-2, MMP-9, and CXCL12 gene in the Hca-F group, N-control group, and shRNA-Anxa7 group. F, G. Western blot was used to detect the levels of Bax, Bcl-2, MMP-9, and CXCL12 protein in the Hca-F group, N-control group, and shRNA-Anxa7 group. The error bar represents the SD (n = 3). *P < 0.05, **P < 0.01, ns P > 0.05.
Discussion
ANXA7 protein is one of the Annexin family in vertebrate cells which plays an important role in cytoskeleton activity, membrane phospholipidization, membrane receptor regulation, and mitosis [16,17]. In the past, our team has found that ANXA7 plays a role in promoting lymphatic metastasis of HCC. Up-regulation of ANXA7 gene expression enhanced the proliferation and migration of Hca-F cells, and down-regulation of ANXA7 gene expression reduced the proliferation and migration of Hca-F cells [18,19].
In this study, miR-124-3p was selected as a candidate according to the basic principles of bioinformatics prediction. miR-124-3p is one of the important members of the miR-124 family that was first extracted from mouse brain by Mishima in 2007. miR-124 can inhibit the occurrence of colon cancer [20]. Tian et al. found that, miR-124 can inhibit the invasion and metastasis of cholangiocarcinoma [21]. Hu et al. found that, there is an abnormal decrease of miR-124 in nasopharyngeal carcinoma [22]. In this study we demonstrated miR-124-3p was down-regulated while ANXA7 protein was up-regulated in HCC. There was a significant negative correlation between level of ANXA7 protein and miR-124-3p. Furthermore, we collected clinicopathologic data and conducted an analysis. The results showed that levels of miR-124-3p in tumor tissues was negatively correlated, while ANXA7 protein was positively correlated with TNM stage and tumor metastasis (Table 1). These results suggest that miR-124-3p and ANXA7 play an important role in occurrence and development of HCC, and are closely related to the progression and metastasis of HCC.
The luciferase reporter assay demonstrated that there was a binding site of miR-124-3p in the 3’-UTR of ANXA7, and miR-124-3p decreased the activity of ANXA7 3’-UTR, while no change was seen in the mutant group (Figure 2). Then we chose miR-124-3p mimics and inhibitor for further mechanism investigation. We demonstrated the over-expression of miR-124-3p can induce the apoptosis rate in Hca-F cells partly though the mitochondrial apoptotic pathway. miR-124-3p enhanced the Bax level and inhibited the expression of Bcl-2 and ANXA7 (Figure 3). Bax and Bcl-2 are two important proteins involved in apoptosis that belong to the Bcl-2 family [23,24]. Studies have shown that Bcl-2 protein can stabilize the mitochondrial membrane and inhibit the opening of mitochondrial membrane channels, thereby inhibiting the release of apoptosis-related proteins in mitochondria [25]. Bcl-2 can also inhibit the formation of apoptotic bodies by inhibiting the release of cytochrome C and the binding of caspase-9 to cytochrome C [26]. Bax protein is the first confirmed apoptotic protein in the Bcl-2 family [27]. The results in this study showed that miR-124-3p could promote the apoptosis of Hca-F cells by stimulating the expression of Bax and inhibiting the expression of Bcl-2.
Next, the functional studies were performed to reveal the correlation of miR-124-3p level with the migration and invasion of HCC progression. HCC progression was inhibited by up-regulating miR-124-3p through suppression migration and invasion in vitro, whereas the activities were facilitated by miR-124-3p knockdown. As the results showed, the expression of MMP-9 and CXCL12 in HCC cells with stable over-expression of miR-124-3p inhibitors was higher than that of the other control cells (Figure 4). MMP-9, also known as gelatinase B, is an important member of the matrix metalloproteinase family [28]. Samad et al. reported that MMP-9 plays an important role in promoting metastasis of hepatocellular carcinoma Hep G2 cells [29]. Frenoux et al. found that serum MMP-9 levels were significantly increased in patients with metastasis after radiofrequency ablation of HCC [30]. CXCL12, also known as matrix-derived factor 1, is an important member of the chemokine CXC family. Recent studies have found that CXCL12 is also highly expressed in lung, cervical, esophageal, and hepatocellular carcinomas, and plays an important role in directional migration of cancer cells [31,33]. Semaan reported that CXCL12 promotes the adhesion of hepatocellular carcinoma cells by binding to its receptor CXCR4 through autocrine or paracrine mechanisms, thus promoting the invasion and metastasis of tumors [34]. The results showed that miR-124-3p could inhibit the migration and invasion of Hca-F cells by inhibiting the expression of MMP-9 and CXCL12.
In vitro, overexpression of miR-124-3p de-creased the tumor volume significantly and resulted in fewer lymph node metastases in mice. The opposite effects were yielded by miR-124-3p knockdown (Figure 5). In this study the expression of ANXA7, MMP-9, and CXCL12 was detected by immunohistochemical assay. As the data showed, the levels of ANXA7, MMP-9, and CXCL12 protein in lymph node metastases of the miR-124-3p mimics group were significantly lower than those of the NC group (Figure 6). It was further confirmed that the expression of MMP-9 and CXCL12 could be regulated by miR-124-3p and ANXA7 to inhibit lymphatic metastasis of HCC. The expression vector ANXA7 and its unrelated sequence were transfected into Hca-F cells by Lipofectamine 2000. The data demonstrated that silencing ANXA7 affects miR-124-3p related protein on Hca-F cells, such as Bcl-2, MMP-9, and CXCL12 in Hca-F cells (Figure 7). As predicted, we believe that miR-124-3p can regulate the expression of Bcl-2, MMP-9, and CXCL12 by targeting the ANXA7 gene, promoting the apoptosis of Hca-F cells, and inhibiting the migration, invasion, and lymphatic metastasis of Hca-F cells.
In summary, miR-124-3p was downregulated while ANXA7 protein was upregulated in HCC, and both are closely related to the progression and metastasis of HCC. Our results revealed miR-124-3p inhibits tumor growth, invasion, and lymphatic metastasis in HCC by down-regulating the ANXA7 gene, thereby reducing the expression of Bcl-2, MMP-9, and CXCL12. The positive expression of miR-124-3p and ANXA7 in primary and metastatic tumors also provides a possible marker for the clinical diagnosis of the occurrence and development of HCC, and might be a potential therapeutic target for HCC.
Acknowledgements
This work was supported by Liaoning Provincial Program for Top Discipline of Basic Medical Sciences, the grants from the National Natural Science Foundation of China (No. 81071725) and the Financial Department of Liaoning Province, PRC (No. 20121203).
Disclosure of conflict of interest
None.
References
- 1.Sonder SL, Boye TL, Toelle R, Dengjel J, Maeda K, Jaattela M, Simonsen AC, Jaiswal JK, Nylandsted J. Annexin A7 is required for ESCRT III-mediated plasma membrane repair. Sci Rep. 2019;9:6726. doi: 10.1038/s41598-019-43143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Ye W, Li Y, Fan L, Zhao Q, Yuan H, Tan B, Zhang Z. Effect of annexin A7 suppression on the apoptosis of gastric cancer cells. Mol Cell Biochem. 2017;429:33–43. doi: 10.1007/s11010-016-2934-4. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Sun M, Li R, Liu S, Mao J, Huang Y, Wang B, Hou L, Ibrahim MM, Tang J. Ech1 is a potent suppressor of lymphatic metastasis in hepatocarcinoma. Biomed Pharmacother. 2013;67:557–560. doi: 10.1016/j.biopha.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Guo C, Liu S, Greenaway F, Sun MZ. Potential role of annexin A7 in cancers. Clin Chim Acta. 2013;423:83–89. doi: 10.1016/j.cca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Hago AM, Gamallat Y, Mahmoud SA, Huang Y, Zhang J, Mahmoud YK, Wang J, Wei Y, Wang L, Zhou S, Awsh MA, Yabasin IB, Tang J. Ezrin expression is altered in mice lymphatic metastatic hepatocellular carcinoma and subcellular fractions upon Annexin 7 modulation in-vitro. Biomed Pharmacother. 2017;85:209–217. doi: 10.1016/j.biopha.2016.10.071. [DOI] [PubMed] [Google Scholar]
- 7.Bai L, Guo Y, Du Y, Wang H, Zhao Z, Huang Y, Tang J. 47 kDa isoform of Annexin A7 affecting the apoptosis of mouse hepatocarcinoma cells line. Biomed Pharmacother. 2016;83:1127–1131. doi: 10.1016/j.biopha.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Du Y, Huang Y, Gao Y, Song B, Mao J, Chen L, Bai L, Tang J. Annexin A7 modulates BAG4 and BAG4-binding proteins in mitochondrial apoptosis. Biomed Pharmacother. 2015;74:30–34. doi: 10.1016/j.biopha.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Song L, Mao J, Zhang J, Ibrahim MM, Li LH, Tang JW. Annexin A7 and its binding protein galectin-3 influence mouse hepatocellular carcinoma cell line in vitro. Biomed Pharmacother. 2014;68:377–384. doi: 10.1016/j.biopha.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Torosyan Y, Simakova O, Naga S, Mezhevaya K, Leighton X, Diaz J, Huang W, Pollard H, Srivastava M. Annexin-A7 protects normal prostate cells and induces distinct patterns of RB-associated cytotoxicity in androgen-sensitive and -resistant prostate cancer cells. Int J Cancer. 2009;125:2528–2539. doi: 10.1002/ijc.24592. [DOI] [PubMed] [Google Scholar]
- 11.Riedel R, Perez-Perez A, Carmona-Fernandez A, Jaime M, Casale R, Luis Duenas J, Guadix P, Sanchez-Margalet V, Varone CL, Maymo JL. Human amniotic membrane conditioned medium inhibits proliferation and modulates related microRNAs expression in hepatocarcinoma cells. Sci Rep. 2019;9:14193. doi: 10.1038/s41598-019-50648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng J, Zhuo H, Xu M, Wang L, Xu H, Peng J, Hou J, Lin L, Cai J. Regulatory network of circRNA-miRNA-mRNA contributes to the histological classification and disease progression in gastric cancer. J Transl Med. 2018;16:216. doi: 10.1186/s12967-018-1582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Huang Y, Zhang J, Xing B, Xuan W, Wang H, Huang H, Yang J, Tang J. High co-expression of the SDF1/CXCR4 axis in hepatocarcinoma cells is regulated by AnnexinA7 in vitro and in vivo. Cell Commun Signal. 2018;16:22. doi: 10.1186/s12964-018-0234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Ye W, Li Y, Fan L, Zhao Q, Yuan H, Tan B, Zhang Z. Annexin A7 expression is downregulated in late-stage gastric cancer and is negatively correlated with the differentiation grade and apoptosis rate. Oncol Lett. 2018;15:9836–9844. doi: 10.3892/ol.2018.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bera A, Leighton XM, Pollard H, Srivastava M. Cyclin E and FGF8 are downstream cell growth regulators in distinct tumor suppressor effects of ANXA7 in hormone-resistant cancer cells of breast versus prostate origin. Trends Cancer Res. 2018;13:55–62. [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, Du Y, Zhang X, Bai L, Mibrahim M, Zhang J, Wei Y, Li C, Fan S, Wang H, Zhao Z, Tang J. Down-regulated expression of Annexin A7 induces apoptosis in mouse hepatocarcinoma cell line by the intrinsic mitochondrial pathway. Biomed Pharmacother. 2015;70:146–150. doi: 10.1016/j.biopha.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim MM, Sun MZ, Huang Y, Jun M, Jin Y, Yue D, Wang J, Zhang J, Qazi AS, Sagoe K, Tang J. Down-regulation of ANXA7 decreases metastatic potential of human hepatocellular carcinoma cells in vitro. Biomed Pharmacother. 2013;67:285–291. doi: 10.1016/j.biopha.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T, Takizawa T. RT-PCR-based analysis of microRNA (miR-1 and-124) expression in mouse CNS. Brain Res. 2007;1131:37–43. doi: 10.1016/j.brainres.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Sadaf N, Kumar N, Ali M, Ali V, Bimal S, Haque R. Arsenic trioxide induces apoptosis and inhibits the growth of human liver cancer cells. Life Sci. 2018;205:9–17. doi: 10.1016/j.lfs.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Huang C, Li R, Zhang Y, Gong J. Amarogentin induces apoptosis of liver cancer cells via upregulation of p53 and downregulation of human telomerase reverse transcriptase in mice. Technol Cancer Res Treat. 2017;16:546–558. doi: 10.1177/1533034616657976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian F, Chen J, Zheng S, Li D, Zhao X, Jiang P, Li J, Wang S. miR-124 targets GATA6 to suppress cholangiocarcinoma cell invasion and metastasis. BMC Cancer. 2017;17:175. doi: 10.1186/s12885-017-3166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enomoto A, Yamada J, Morita A, Miyagawa K. Bisdemethoxycurcumin enhances X-ray-induced apoptosis possibly through p53/Bcl-2 pathway. Mutat Res. 2017;815:1–5. doi: 10.1016/j.mrgentox.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi K, Sugito N, Kumazaki M, Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama K, Akao Y. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett. 2015;363:17–27. doi: 10.1016/j.canlet.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Frenoux C, Rebischung C, Quesada JL, Mendosa C, Letoublon C, Trocme C. How to predict the relapse after surgery or radiofrequency of liver metastases of colorectal cancer? Interest of the serum kinetic variation of a matrix metalloproteinase cluster. Bull Cancer. 2018;105:884–895. doi: 10.1016/j.bulcan.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Samad NA, Abdul AB, Rahman HS, Rasedee A, Tengku Ibrahim TA, Keon YS. Zerumbone suppresses angiogenesis in HepG2 cells through inhibition of matrix metalloproteinase-9, vascular endothelial growth factor, and vascular endothelial growth factor receptor expressions. Pharmacogn Mag. 2018;13(Suppl 4):S731–S736. doi: 10.4103/pm.pm_18_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyna DE, Garner TP, Lopez A, Kopp F, Choudhary GS, Sridharan A, Narayanagari SR, Mitchell K, Dong B, Bartholdy BA, Walensky LD, Verma A, Steidl U, Gavathiotis E. Direct activation of BAX by BTSA1 overcomes apoptosis resistance in acute myeloid leukemia. Cancer Cell. 2017;32:490–505. e10. doi: 10.1016/j.ccell.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenk RL, Strasser A, Dewson G. BCL-2: long and winding path from discovery to therapeutic target. Biochem Biophys Res Commun. 2017;482:459–469. doi: 10.1016/j.bbrc.2016.10.100. [DOI] [PubMed] [Google Scholar]
- 30.Kozlowska J, Mikula T, Suchacz M, Jablonska J, Stanczak W, Cianciara J, Wiercinska-Drapalo A. Pigment epithelium-derived factor and matrix metalloproteinase-9 in liver cirrhosis. Saudi J Gastroenterol. 2016;22:375–379. doi: 10.4103/1319-3767.191143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsura M, Shoji F, Okamoto T, Shimamatsu S, Hirai F, Toyokawa G, Morodomi Y, Tagawa T, Oda Y, Maehara Y. Correlation between CXCR4/CXCR7/CXCL12 chemokine axis expression and prognosis in lymph-node-positive lung cancer patients. Cancer Sci. 2018;109:154–165. doi: 10.1111/cas.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semaan A, Dietrich D, Bergheim D, Dietrich J, Kalff JC, Branchi V, Matthaei H, Kristiansen G, Fischer HP, Goltz D. CXCL12 expression and PD-L1 expression serve as prognostic biomarkers in HCC and are induced by hypoxia. Virchows Archiv. 2017;470:185–196. doi: 10.1007/s00428-016-2051-5. [DOI] [PubMed] [Google Scholar]
- 33.Uchi Y, Takeuchi H, Matsuda S, Saikawa Y, Kawakubo H, Wada N, Takahashi T, Nakamura R, Fukuda K, Omori T, Kitagawa Y. CXCL12 expression promotes esophageal squamous cell carcinoma proliferation and worsens the prognosis. BMC Cancer. 2016;16:514. doi: 10.1186/s12885-016-2555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah AD, Bouchard MJ, Shieh AC. Interstitial fluid flow increases hepatocellular carcinoma cell invasion through CXCR4/CXCL12 and MEK/ERK signaling. PLoS One. 2015;10:e0142337. doi: 10.1371/journal.pone.0142337. [DOI] [PMC free article] [PubMed] [Google Scholar]


