Abstract
Idiopathic pulmonary fibrosis (IPF) is a devastating disease, which is characterized by the progressive deterioration in lung function. In the pathogenesis of IPF, insulin-like growth factor-1 (IGF-1) has been found to be heavily involved. Metformin, a commonly used oral antidiabetic agent, is known to inhibit IGF-1 by the reversal of hyperinsulinemia. In this study, we evaluated the effects of metformin in pulmonary fibrosis in C57/BL6J mice, and further understand the role of IGF-1 signaling pathway involving in this process. Pulmonary fibrosis was induced experimentally in these mice by the intratracheal injection of bleomycin (BLM). Metformin was given orally the day before or 14 days after bleomycin injection, while pirfenidone was used as the positive control. Our study showed that intratracheal injection of bleomycin induced pulmonary fibrosis in mice, with observed elevation in collagen, fibronectin and α-SMA level, characterized by the enhanced IGF-1 and PI3K expression. Metformin was able to inhibit these effects significantly, and its antifibrotic effect had no marked difference with pirfenidone. Our results show that metformin attenuates bleomycin-induced pulmonary fibrosis via IGF-1 pathway.
Keywords: Pulmonary fibrosis, metformin, IGF-1, bleomycin, murine model
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive fibrosing interstitial pneumonia, which is characterized by the worsening dyspnea and the irreversible loss of lung function [1]. IPF is the most common and one of the most life-threatening interstitial lung diseases (ILD) among the over 150 recognized types. The median survival of IPF is only 3 to 5 years, and its incidence rate is gradually increasing globally [2]. Currently, the etiology and pathogenesis of IPF is undetermined. Therefore, the available therapeutic methods and options are very limited. At the present, the only known efficient drugs for the treatment of IPF are pirfenidone and nintedanib [3,4]. However, these drugs only delay the worsening of lung function in the patient. Innovative treatment strategies to target against this lethal disease is needed.
The prevailing hypothesis for the pathogenesis of IPF involves abnormal wound healing in the lung [5]. Ongoing alveolar epithelial injury in the multiple, microscopic sites are observed. This is characterized by severe fibroblast-myofibroblast migration and proliferation, and decreased myofibroblast apoptosis, with increased activity and responses to fibrogenic cytokines.
Insulin-like growth factor-1 (IGF-1) is an important factor in controlling the metabolism and the growth of the body. IGF-1 is also a potent survival factor, acting by inhibiting the apoptosis and inducing the proliferation in various cells [6-8]. Among a variety of profibrotic cytokines, IGF-1 has been proved to promote the proliferation, migration and collagen production of fibroblasts [9]. Moreover, the high expression of IGF-1 is found in bronchoalveolar lavage fluid (BALF) of IPF patients [10]. The blocking of IGF-1 pathway by a monoclonal antibody against the IGF-I receptor (IGF-1R), has been shown to attenuate bleomycin-induced lung injury in animal models [11]. Among the pathways which are activated by IGF-1R mediated signaling, the phosphatidylinositol-3-kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR) pathway is one of the fundamental pathways for cell proliferation and survival [12]. Hence, the pharmacological suppression of the IGF-1 signaling and its downstream pathways might exhibit crucial therapeutic potentials in the treatment of IPF.
Metformin is one of the most commonly prescribed biguanide oral agents, that is used to lower blood glucose in type 2 diabetes patients. Metformin decreases the inflammatory cytokine secretion from the primary cystic fibrosis airway cells. It also reduces the radiological and histological signs of fibrosis, inflammatory infiltration and lower the alterations to the alveolar structures in radiation-induced pulmonary injury murine model [13,14]. Recent papers have demonstrated that metformin inhibits myofibroblast differentiation and attenuates lung fibrosis through modulating NADPH oxidase 4 (NOX4) signaling [15] or via AMP-activated protein kinase (AMPK)-dependent activation of autophagy [16,17]. Based on the pharmacological effects, metformin inhibits IGF-1 by the reversal of hyperinsulinemia, resulting in the inhibition of the negative feedback of insulin on IGF-binding protein-1 (IGFBP-1) [18].
We suggest that metformin could treat pulmonary fibrosis by suppressing IGF-1 pathway. We investigated the anti-fibrotic effect of metformin to illustrate its underlying mechanism. The efficacy of metformin and pirfenidone was compared in bleomycin (BLM)-induced pulmonary fibrosis mouse model.
Materials and methods
Mouse model
C57BL/6J mice (Vital River Laboratory Animal Technology Co., Beijing, China) were obtained (n = 10 per group, male, 8~9 weeks old, average weight 23~25 g). They were kept at a controlled temperature of 24±1°C with a 12:12 h light-dark cycle and being fed with a standard diet. A dose of 3.5 mg/kg (for evaluating the preventive effect of metformin) or 1.5 mg/kg (for evaluating the reversal effect of metformin) bleomycin (Nippon Kayaku Co., Tokyo, Japan) was intratracheally administered to these mice. The oral dose of metformin (200 mg/kg/day) was given once a day from the day before bleomycin injection to day 20 (part 1 of the experiment) and from day 14 to day 27 (part 2 of the experiment). Pirfenidone was administered orally twice a day at a dose of 300 mg/kg/day, also from day 14 to day 27. On the 21st day (part 1 of the experiment) and the 28th day (part 2 of the experiment) the lungs were removed and prepared for histological staining and extraction of protein. Meanwhile, the plasm and BALF of each mouse was obtained for the ELISA detection.
CT scanning
High-resolution computed tomography (HRCT) was performed on day 28 by Skyscan 1176 system (Bruker, Germany) with the following parameters: 90 kV, 150 mA, 80 mm FOV, 1.00-mm slice thickness over 3.5-cm scan region (entire lung).
Histologic analysis
The preparation of mouse lungs for histology analysis was performed as previously described [19]. The lungs were dehydrated, paraffin-embedded, and cut into 4-μm sections, then stained with H&E and Masson’s trichrome stain. For immunohistochemistry staining, the sections were deparaffinized and rehydrated as described above, then heated in a microwave oven for 20 min in ethylenediaminetetraacetic acid (EDTA) buffer. The endogenous peroxidase activity was blocked by 0.3% hydrogen peroxide in methanol for 15 min in dark. After blocking in 5% goat serum for 20 min, the sections were incubated with anti-collagen I antibody (ab2413, Abcam), anti-fibronectin antibody (ab2413, Abcam) and anti-α-SMA antibody (ab124964, Abcam), then stored overnight at 4°C.
Hydroxyproline assay
The level of total lung collagen fiber was determined by the analysis of hydroxyproline on day 28 after bleomycin injectionn [20]. Briefly, the right lung was removed and homogenized. Lung homogenates in 1.5 ml HCl (2 mol/l) were incubated at 110°C overnight. Chloramine-T solution (56 mmol/l chloramine T and 10% 1-propanol in citrate/acetate buffer) was added < in each sample > at room temperature for 20 min. The mixture was then incubated with Ehrlich’s solution at 65°C for 20 min. After cooling to room temperature, the absorbance of the mixture was measured at 550 nm by a spectrophotometer and L-hydroxyproline content was determined using a standard curve method.
Protein extraction and western blot
Protein from lung sections was separated on a SDS-PAGE gel and then transferred onto polyvinylidene difluoride membranes (Millipore, USA). After blocking, the membranes were incubated overnight at 4°C with primary antibodies of anti-mouse fibronectin (ab2413, Abcam), α-SMA (ab124964, Abcam), and β-actin (ab6267, Abcam). The membranes were then treated with IRDye™800 (green) or IRDye™700 (red) conjugated affinity purified anti-rabbit or anti-mouse IgG (LI-COR, NE, USA) respectively. The positive western bands were visualized using a LI-COR Odyssey infrared double-fluorescence imaging system (LI-COR).
ELISA
The levels of IGF-1 in plasma, BALF and lung tissues were measured by ELISA kit (R&D Systems, Abingdon, UK). The level of PI3K in lung tissues was also measured by ELISA kit (BLUE GENE, Shanghai, China).
Statistics analysis
SPSS 16.0 (SPSS, Chicago, IL, USA) software was used for the analyses. Statistical analyses were carried out using the unpaired, two-tailed Student’s t test. The comparison of the difference among the multiple groups was analyzed using two-way analysis of variance (ANOVA) test. P value <0.05 was considered statistically significant. Kaplan-Meier survival analysis with log-rank test was used to compare survival rates. The data was reported as mean ± standard error of the mean (SEM). Statistical tests and graphs were done with GraphPad Prism 5.0 (GraphPad Inc., San Diego, USA). IHC staining statistics was analysed by Image-Pro Plus 6.0 (Media Cybernetics Inc., Rockville, USA).
Patient and public involvement statement
Patients and the public were not involved in the design, conduct and reporting of this research.
Results
Part one: metformin attenuated bleomycin-induced mouse pulmonary fibrosis
We monitored the survival condition of mice during the course of 21 days. When metformin was administered to the mice the day before the injection of bleomycin, the survival rate of the bleomycin-treated mice at day 21 was significantly lower than that of the control mice and the metformin-treated mice (Figure 1A). The median survival time of bleomycin-treated mice was 11 days. For the metformin-treated mice, the median survival time had increased to 20.5 days. Therefore, the administration of metformin was determined to improve the declined trend of the survival rate of bleomycin-induced pulmonary fibrosis mice.
Figure 1.
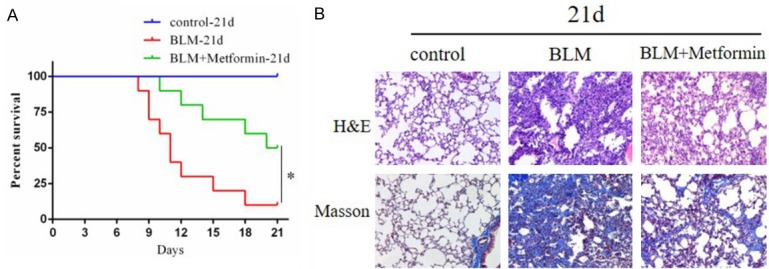
A. Kaplan-Meier curve shows the survival condition of mice during the course of 21 days. 50% of the metformin treated mice still survived at day 21, but only 10% of the bleomycin treated mice survived for the same period. *P<0.05. B. Photomicrographs (original magnification 200×) of H&E staining (upper panel) and Masson’s trichrome staining (lower panel) of mouse lungs at day 21. The first column is normal control group which shows normal alveoli with intact lung tissue architecture and small amount of collagen fibers (stained blue) in the bronchiolar wall. The second column is bleomycin-treated group which shows alveolar damage with thickened alveolar septum, fibroblast proliferation and replacement by fibrosis tissue, like severe collagen deposition. The third column is metformin-treated group which shows marked reduction in alveolar damage, fibrosis and the content of collagen fiber.
As indicated by H&E staining of lung sections, the intratracheal injection of bleomycin led to the destruction of normal pulmonary architecture, the prominently thickening of alveolar septum and the mass filling of fibrous tissue. As illustrated by Masson’s trichrome staining, the deposition of collagen fibers was largely increased in bleomycin-induced lung injury. Apparently, metformin could remarkably alleviate these pathological changes and decrease the production of collagen. Under the Ashcroft scoring system for the degree of fibrosis, the mice in the metformin-treated group showed a lower score than the mice in the bleomycin-treated group (Figure 1B).
Part two: Metformin reversed bleomycin-induced mouse lung fibrosis by the inhibition of IGF-1
Metformin attenuated bleomycin damage expressed in bodyweight, imaging and pathology
To determine whether metformin has a positive effect on pulmonary fibrosis, we gave metformin, pirfenidone and these two drugs’ combination to mice 14 days after bleomycin administered, and continued intervening until 28 days. As illustrated in the weight curve, the bodyweight of bleomycin treated mice showed a significant trend of decline compared with the control mice. Metformin remarkably inhibited this trend, which had the same effect as pirfenidone and combination therapy (Figure 2A). Chest CT photographing and H&E staining of lung sections were performed in each group of mice at day 28. The results of chest CT indicated that bleomycin reduced lung volume and transmittance. Concurrently, severe fibrotic changes had taken place in the lung after bleomycin injury. Impressively, these imaging and pathological damages prominently improved after metformin, pirfenidone or combination therapy (Figure 2B).
Figure 2.
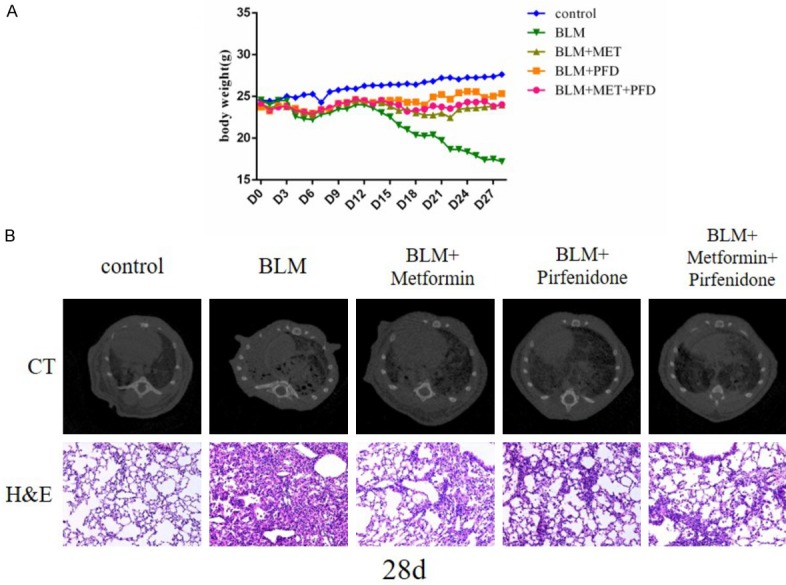
A. The average body weight of the control group, BLM group, BLM + metformin group, BLM + pirfenidone group, BLM + metformin + pirfenidone group from day 0 to day 28. There is little difference between BLM group and BLM + drugs groups from day 0 to day 7. After metformin and pirfenidone applied, the loss of body weight in BLM + metformin group, BLM + pirfenidone group and BLM + metformin + pirfenidone group is significantly reduced. B. Computed Tomography of Chest scan (upper panel) and photomicrographs (original magnification 200×) of H&E staining (lower panel) of lung sections of mice at day 28. The five columns are the control group, BLM group, BLM + metformin group, BLM + pirfenidone group, BLM + metformin + pirfenidone group, respectively.
Metformin inhibited the production of type I collagen and fibronectin in mouse lungs
As demonstrated by Masson trichrome staining and the IHC staining of type I collagen, the deposition of collagen fiber was substantially increased in bleomycin-induced lung fibrosis at day 28. The administration of metformin, pirfenidone or the two in combination significantly reduced the deposition of collagen fiber (Figure 3A, 3C).
Figure 3.
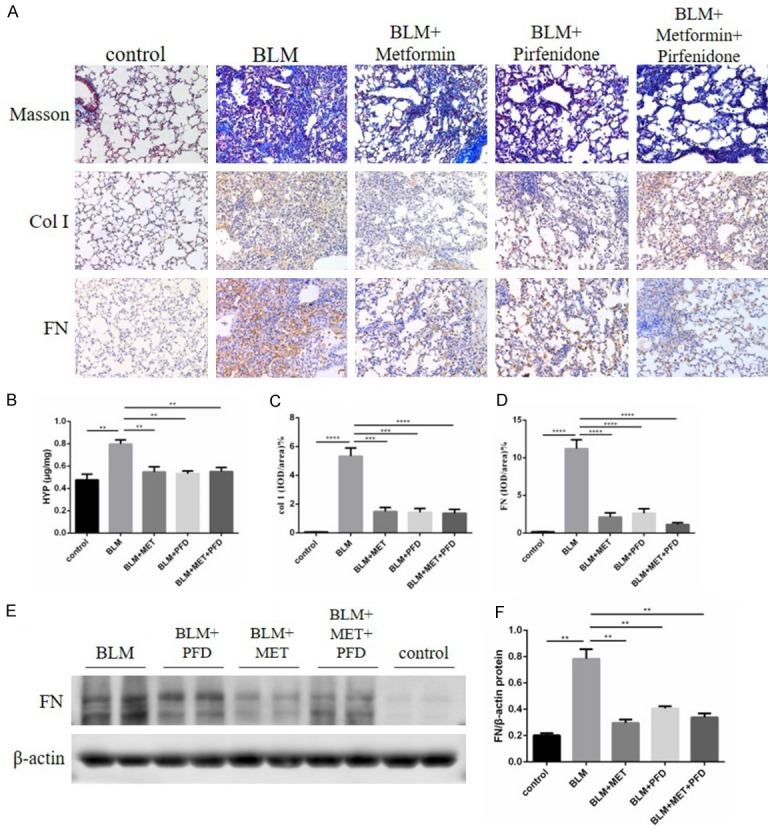
A. Photomicrographs (original magnification 200×) of Masson’s trichrome staining (upper panel) and Masson’s trichrome staining (lower panel) of mouse lungs at day 21. Photomicrographs (original magnification 200×) of Masson’s trichrome staining (upper panel) and immunohistochemical staining of type I collagen (middle panel) and fibronectin (lower panel) in mouse lungs at day 28. The five columns are the normal control group, BLM group, BLM + metformin group, BLM + pirfenidone group, BLM + metformin + pirfenidone group, respectively. B. Hydroxyproline content in mouse lung tissues of each group on day 28, results are expressed as mean ± SEM, n = 4-6 mice per group, **P<0.01. C, D. Statistical results of immunohistochemistry staining of type I collagen and fibronectin were shown as positive area ratio total area, and results are expressed as mean ± SEM, n = 4-6 mice per group, ***p<0.001 ****P<0.0001. E, F. Whole lung tissues western blotting of fibronectin at day 28 and the statistical chart of it, results are expressed as mean ± SEM, n = 4-6 mice per group, **P<0.01.
We also measured the amount of hydroxyproline (Hyp) in lung tissues. Hyp is a specific type of collagen protein marker, which can represent the content of collagen fiber. Both metformin, pirfenidone and the combination therapy reduced the raised level of Hyp induced by bleomycin (Figure 3B). Fibronectin is another pro-fibrotic marker and was detectable by IHC staining and western blotting. The results showed that the content of fibronectin was increased after bleomycin injury. Markedly, this phenomenon was also improved after metformin, pirfenidone, or the combination therapy (Figure 3A, 3D-F).
The anti-fibrotic effect of metformin associated with suppressing IGF-1 pathway
To explore the participation of IGF-1 signaling pathway in the bleomycin-induced pulmonary fibrosis in mice and in amelioration of fibrosis by metformin, the level of IGF-1 and its main downstream cytokine PI3K were measured by ELISA. Compared with the control group, bleomycin administered significantly increased the level of IGF-1 in both plasma and BALF as well as that of PI3K in lung samples. Meanwhile, metformin treatment clearly and significantly suppressed IGF-1 expression level in BALF and PI3K expression level in lung tissues contrast with bleomycin group. However, we did not see the same effect of metformin acting on the level of IGF-1 in mouse plasma. There was no statistical difference in the IGF-1 expression level in lung tissues among the metformin-treated group, the pirfenidone-treated group, or the combination therapy group (Figure 4A, 4B).
Figure 4.
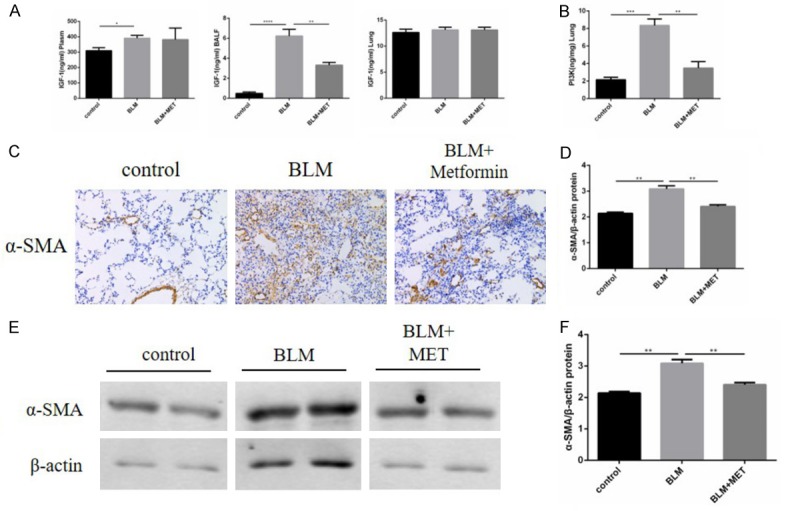
A, B. The level of IGF-1 in mouse plasma, BALF and lung tissues as well as its main downstream cytokine PI3K content in mouse lungs were measured by ELISA, results are expressed as mean ± SEM, n = 4-6 mice per group, *P<0.05 **P<0.01 ***P<0.001 ****P<0.0001. C. Immunohistochemical staining of α-SMA in mouse lungs at day 28, original magnification 200×. The three columns are the control group, BLM group, BLM + metformin group, respectively. D. Statistical results of α-SMA staining were shown as positive area ratio total area, and results are expressed as mean ± SEM, n = 4-6 mice per group, **P<0.01. E, F. Whole lung tissues western blotting of α-SMA at day 28 and the statistical chart of it, results are expressed as mean ± SEM, n = 4-6 mice per group, **P<0.01.
We then measured the pulmonary α-SMA content of mice by IHC staining and western blotting to observe the level of myofibroblasts. α-SMA is a major marker of myofibroblasts. Through these two detection methods, we could see that bleomycin induced high levels of α-SMA, while metformin decreased α-SMA level significantly. Therefore, metformin inhibited the process of fibroblasts differentiated into myofibroblasts (Figure 4C-F).
Discussion
In the present study, we established a mouse model of pulmonary fibrosis by intratracheal injection of bleomycin, and demonstrated that the preventive administration of metformin the day before bleomycin injection had improved the survival rate and median survival time of mice.
The recommendations for pharmacotherapy in IPF patients was in clinical practice guidelines 2015, and pirfenidone were recommended conditionally for treatment as antifibrotic therapy [3]. The evidence that supported the use of pirfenidone was based on prospective, randomized clinical trials. Pirfenidone reduced the disease progression, as reflected by lung function, exercise tolerance, and progression-free survival, in patients with idiopathic pulmonary fibrosis [21]. In order to further explore the therapeutic effect of metformin, we administered metformin, pirfenidone or both of the two drugs from 14 days after the induction of bleomycin. Lung fibrosis is generally considered to have already formed stable after acute inflammatory reaction on day 14. The results showed that after two weeks of drugs intervention, metformin significantly ameliorated the pulmonary fibrosis of mice in terms of body weight, CT patterns, pathomorphological changes, collagen deposition and fibronectin production. Furthermore, there was no marked difference in the effect of metformin and pirfenidone or the combination therapy.
It is generally believed that IPF originates from the abnormal repair of alveolar epithelial injury [5]. Repeated and unexplained stimulations result in the sustained damages to the alveolar epithelial cells and the release of massive cytokines, which enable the fibroblast to proliferate and differentiate into myofibroblasts, promoting the formation of the fibroblast foci [22,23]. Myofibroblasts produce extracellular matrix (ECM) such as collagen, and the deposition of a large amount of ECM leads to the thickening of alveolar septum, which eventually causes the loss of normal structure and function of the lung tissue [5]. A large number of studies have shown that IGF-1, as a cytokine regulating cell proliferation and apoptosis and tissue damage repair, is closely related to the occurrence and the development of IPF. It has been implicated as a mediator that can promote the proliferation, migration and differentiation of fibroblasts, enhance the fibroblasts’ ability of synthesizing fibronectin, α-SMA, type I and type III collagen, consequently increase the deposition of ECM [9,24-27]. In this study, we found that metformin significantly decreased the level of IGF-1 in BALF and α-SMA expression in lung tissues. Based on the pathogenesis of IPF, α-SMA produced by myofibroblasts which was differentiated by fibroblasts, in turn, the content of α-SMA reflects the level of activated fibroblasts [28]. In vivo, IGF-1 functions by binding to insulin receptor (IR) and/or IGF-1R and then activates multiple downstream signaling pathways, and the PI3K pathway is the most important one among them [12,29]. It has been reported that metformin inhibits the proliferation of tumor cells by reducing the expression of IGF-1R, and various factors in the PI3K signaling pathway in some cancer models [30]. We demonstrated that metformin significantly reduced the content of PI3K in lung tissues, as well as the level of activated fibroblasts, suggesting that metformin may directly or indirectly block PI3K pathway by suppressing IGF-1, and then inhibit the activation of fibroblasts, and eventually achieve the effect of attenuating pulmonary fibrosis.
Pirfenidone has pleiotropic antifibrotic, anti-inflammatory, and antioxidant effects in animal and cell-based models [31-33]. The mechanism of pirfenidone has been shown to decreased the production of profibrotic cytokines, chemokines, and growth factors, including TGF-β, basic fibroblast growth factor, tumor necrosis factor-a, IL-1β, IL-6, CXCL12, and CCL2, as well as markers of oxidative stress [34-37]. In studies in vitro, pirfenidone has been demonstrated to inhibit multiple profibrotic behaviors of fibroblasts, including their abilities of proliferation, differentiation to myofibroblasts, and synthesis of collagen [32,34,37-39]. In this study, the combination treatment with metformin and pirfenidone did not show any more benefit than the single treatment, or reduction in efficacy, suggesting that the mechanism of metformin in pulmonary fibrosis may be similar to that of pirfenidone.
Common side effects of pirfenidone include nausea and gastrointestinal upset as well as skin rash and photosensitivity [21], which affects the patient’s compliance with medication to a certain extent. Metformin, as a classical drug for lowering blood glucose in clinic, has fewer adverse effects, which is of great significance in the future application as a potential anti-fibrotic drug.
Acknowledgements
This study is supported by grants from National Natural Science Foundation of China [No. 81430001 and 81870056].
Disclosure of conflict of interest
None.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TJ, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, Johkoh T, Martinez FJ, Myers J, Protzko SL, Richeldi L, Rind D, Selman M, Theodore A, Wells AU, Hoogsteden H, Schunemann HJ. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G. Pharmacotherapy for idiopathic pulmonary fibrosis: current landscape and future potential. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0071-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 6.LeRoith D, Roberts CJ. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 7.Kurmasheva RT, Houghton PJ. IGF-I mediated survival pathways in normal and malignant cells. Biochim Biophys Acta. 2006;1766:1–22. doi: 10.1016/j.bbcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Krein PM, Winston BW. Roles for insulin-like growth factor I and transforming growth factor-beta in fibrotic lung disease. Chest. 2002;122:289S–293S. doi: 10.1378/chest.122.6_suppl.289s. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Geng J, Xu X, Huang X, Leng D, Jiang D, Liang J, Wang C, Jiang D, Dai H. miR-130b-3p modulates epithelial-mesenchymal crosstalk in lung fibrosis by targeting IGF-1. PLoS One. 2016;11:e150418. doi: 10.1371/journal.pone.0150418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pala L, Giannini S, Rosi E, Cresci B, Scano G, Mohan S, Duranti R, Rotella CM. Direct measurement of IGF-I and IGFBP-3 in bronchoalveolar lavage fluid from idiopathic pulmonary fibrosis. J Endocrinol Invest. 2001;24:856–864. doi: 10.1007/BF03343942. [DOI] [PubMed] [Google Scholar]
- 11.Choi J, Lee S, Sunde DA, Huizar I, Haugk KL, Thannickal VJ, Vittal R, Plymate SR, Schnapp LM. Insulin-like growth factor-I receptor blockade improves outcome in mouse model of lung injury. Am J Resp Crit Care. 2009;179:212–219. doi: 10.1164/rccm.200802-228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klement RJ, Fink MK. Dietary and pharmacological modification of the insulin/IGF-1 system: exploiting the full repertoire against cancer. Oncogenesis. 2016;5:e193. doi: 10.1038/oncsis.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myerburg MM, King JJ, Oyster NM, Fitch AC, Magill A, Baty CJ, Watkins SC, Kolls JK, Pilewski JM, Hallows KR. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2010;42:676–684. doi: 10.1165/rcmb.2009-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Wang Y, Han J, Mei H, Yu D, Ding Q, Zhang T, Wu G, Peng G, Lin Z. Metformin attenuates radiation-induced pulmonary fibrosis in a murine model. Radiat Res. 2017;188:105–113. doi: 10.1667/RR14708.1. [DOI] [PubMed] [Google Scholar]
- 15.Sato N, Takasaka N, Yoshida M, Tsubouchi K, Minagawa S, Araya J, Saito N, Fujita Y, Kurita Y, Kobayashi K, Ito S, Hara H, Kadota T, Yanagisawa H, Hashimoto M, Utsumi H, Wakui H, Kojima J, Numata T, Kaneko Y, Odaka M, Morikawa T, Nakayama K, Kohrogi H, Kuwano K. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res. 2016;17:107. doi: 10.1186/s12931-016-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB, Abraham E, Darley-Usmar V, Thannickal VJ, Zmijewski JW. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 2018;24:1121–1127. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamad N, Malik S, Suchal K, Vasisht S, Tomar A, Arava S, Arya DS, Bhatia J. Metformin alleviates bleomycin-induced pulmonary fibrosis in rats: pharmacological effects and molecular mechanisms. Biomed Pharmacother. 2018;97:1544–1553. doi: 10.1016/j.biopha.2017.11.101. [DOI] [PubMed] [Google Scholar]
- 18.Aljada A, Mousa SA. Metformin and neoplasia: implications and indications. Pharmacol Therapeut. 2012;133:108–115. doi: 10.1016/j.pharmthera.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Minagawa S, Lou J, Seed RI, Cormier A, Wu S, Cheng Y, Murray L, Tsui P, Connor J, Herbst R, Govaerts C, Barker T, Cambier S, Yanagisawa H, Goodsell A, Hashimoto M, Brand OJ, Cheng R, Ma R, McKnelly KJ, Wen W, Hill A, Jablons D, Wolters P, Kitamura H, Araya J, Barczak AJ, Erle DJ, Reichardt LF, Marks JD, Baron JL, Nishimura SL. Selective targeting of TGF-beta activation to treat fibroinflammatory airway disease. Sci Transl Med. 2014;6:241r–279r. doi: 10.1126/scitranslmed.3008074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B, Huang X, Liu Z, Xu X, Xiao H, Zhang X, Dai H, Wang C. Ouabain ameliorates bleomycin induced pulmonary fibrosis by inhibiting proliferation and promoting apoptosis of lung fibroblasts. Am J Transl Res. 2018;10:2967–2974. [PMC free article] [PubMed] [Google Scholar]
- 21.King TE, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 22.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krein PM, Winston BW. Roles for insulin-like growth factor I and transforming growth factor-beta in fibrotic lung disease. Chest. 2002;122:289S–293S. doi: 10.1378/chest.122.6_suppl.289s. [DOI] [PubMed] [Google Scholar]
- 25.Hung CF, Rohani MG, Lee SS, Chen P, Schnapp LM. Role of IGF-1 pathway in lung fibroblast activation. Respir Res. 2013;14:102. doi: 10.1186/1465-9921-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hetzel M, Bachem M, Anders D, Trischler G, Faehling M. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183:225–237. doi: 10.1007/s00408-004-2534-z. [DOI] [PubMed] [Google Scholar]
- 27.Chetty A, Cao GJ, Nielsen HC. Insulin-like growth Factor-I signaling mechanisms, type I collagen and alpha smooth muscle actin in human fetal lung fibroblasts. Pediatr Res. 2006;60:389–394. doi: 10.1203/01.pdr.0000238257.15502.f4. [DOI] [PubMed] [Google Scholar]
- 28.Li M, Krishnaveni MS, Li C, Zhou B, Xing Y, Banfalvi A, Li A, Lombardi V, Akbari O, Borok Z, Minoo P. Epithelium-specific deletion of TGF-beta receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J Clin Invest. 2011;121:277–287. doi: 10.1172/JCI42090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 2015;5:390–401. doi: 10.1016/j.apsb.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang JC, An R, Jiang YQ, Yang J. Effects and mechanisms of metformin on the proliferation of esophageal cancer cells in vitro and in vivo. Cancer Res Treat. 2017;49:778–789. doi: 10.4143/crt.2015.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. aiming to rein in runaway wound-healing responses. Am J Resp Crit Care. 2014;190:867–878. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K. Antifibrotic activities of pirfenidone in animal models. Eur Respir Rev. 2011;20:85–97. doi: 10.1183/09059180.00001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maher TM. Pirfenidone in idiopathic pulmonary fibrosis. Drugs Today (Barc) 2010;46:473–482. doi: 10.1358/dot.2010.46.7.1488336. [DOI] [PubMed] [Google Scholar]
- 34.Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A, Matsushima S, Torii M, Arimura A. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol. 2008;590:400–408. doi: 10.1016/j.ejphar.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 35.Inomata M, Kamio K, Azuma A, Matsuda K, Kokuho N, Miura Y, Hayashi H, Nei T, Fujita K, Saito Y, Gemma A. Pirfenidone inhibits fibrocyte accumulation in the lungs in bleomycin-induced murine pulmonary fibrosis. Respir Res. 2014;15:16. doi: 10.1186/1465-9921-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;289:211–218. [PubMed] [Google Scholar]
- 37.Iyer SN, Wild JS, Schiedt MJ, Hyde DM, Margolin SB, Giri SN. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J Lab Clin Med. 1995;125:779–785. [PubMed] [Google Scholar]
- 38.Hirano A, Kanehiro A, Ono K, Ito W, Yoshida A, Okada C, Nakashima H, Tanimoto Y, Kataoka M, Gelfand EW, Tanimoto M. Pirfenidone modulates airway responsiveness, inflammation, and remodeling after repeated challenge. Am J Respir Cell Mol Biol. 2006;35:366–377. doi: 10.1165/rcmb.2005-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;291:367–373. [PubMed] [Google Scholar]


