Abstract
Objectives: Hepatitis C virus (HCV) infection is associated with abnormal immune responses. Since regulatory T (Tregs) and B (Bregs) cells modulate the progression of infectious diseases, this study aimed at examining how these cells are involved with the development of HCV infection. Methods: The frequencies of circulating Bregs and Tregs were characterized using flow cytometry. Both the association and dynamic changes of these cells with related clinical parameters were analyzed after Direct-Acting Antiviral (DAA) agent treatments. Additionally, both regulatory B and T and naïve B and T cells were sorted and stimulated with healthy or HCV sera in vitro. Results: Bregs frequency in HCV-infected patients increased significantly and were positively correlated with levels of sera HCV RNA load, Alanine aminotransferase (AST) and total bilirubin (TBILI). Additionally, the increased Bregs returned to normal levels after DAA treatment. However, Tregs increased markedly in patients with HCV-cirrhosis and were significantly associated with Aspartate aminotransferase to Platelet Ratio Index (APRI) and Fibrosis 4 (FIB-4) scores. Furthermore, HCV sera doesn’t expand either Tregs or Bregs, however, it does induce the IL-10 expression in B cells although it fails to induce FOXP3 expression in CD4+ T cells. Conclusions: Increased Bregs not only may be associated with poor viral eradication and liver injury but also may provide a predictive marker of HCV disease therapeutic efficacy following DAA-treatment. HCV sera may selectively induce Bregs. Tregs probably do not control disease status in the early stages but may contribute to the progression of liver fibrosis in the late stages of HCV infection.
Keywords: Regulatory B cells, HCV infection, DAA, regulatory T cells
Introduction
Hepatitis C virus (HCV) infection still remains a major global health problem. Persistent infection with HCV causes chronic hepatitis C (CHC) and many patients progressively and eventually develop liver cirrhosis, hepatocellular carcinoma (HCC) and end-stage liver disease [1].
During chronic viral infection, effector T cells become progressively exhausted and their protective antiviral immune activity is reduced [2]. A growing body of studies emphasized the role of regulatory T cells in chronic viral infectious diseases [3]. It is well known that Tregs are critical for the suppression of immune responses and maintain homeostasis of immune function [4-6]. Many studies have shown Tregs contribute to the impairment of virus-specific T cell responses involved in the pathogenesis of chronic HCV infection [7].
Similar to Tregs, regulatory B cells (Breg), a newly defined B cell subgroup with the capacity of inhibiting immune responses, play an important role in maintenance of peripheral tolerance [8,9]. Breg cells mainly produce regulatory cytokines, such as IL-10, and are comprised of many phenotypic subsets. CD1dhiCD5+ cells, have been identified to be responsible for IL-10 production by B cells [10], while in the CD24hiCD27+ B cell subset, IL-10 producing cells are not only highly concentrated but also have a higher proliferative capacity in response to mitogenic stimulation [11]. Moreover, in the human, a subset of Bregs that exhibits a CD19+CD24hiCD38hi phenotype [12], has been identified within immature transitional B cells and plays a crucial role in regulating T-cell responses by releasing IL-10 [11]. IL-10 serves as the most important functional molecule for Bregs, its regulation is controlled by many transcription factors, such as c-Maf, AhR [13,14]. To date, the role of Breg cells has been identified in the regulation of several immune-mediated processes of different disease types, such as autoimmune diseases [12], infectious diseases [15], and cancers [16].
It is now well-established that the antiviral immune response determines the outcome of virus infection. The direct-acting antiviral (DAA) regimen is one of the major safe and effective antiviral therapeutic procedures [17]. DAA-induced sustained virologic responses (SVR) reduce the risk of cirrhosis and hepatocellular carcinoma as well as many other extra-hepatic manifestations of HCV infection [18].
In the present study, we directed a major investigative effort at not only identifying regulatory T and B cells in peripheral blood of HCV-infected patients but also correlating the frequencies and dynamic changes of these immune cell subpopulations prior to and after DAA drug treatment. To our knowledge, this is the first study that investigates the frequency and correlation of CD19+CD24hiCD38hi B regulatory cells in HCV-infected patients.
Materials and methods
Human subjects
A total of 109 patients with chronic HCV infection and 49 healthy control subjects from the Third Affiliated Hospital of Sun Yat-Sen University and Guangzhou blood center were enrolled in the study (Table 1). The diagnosis of chronic HCV infection was based on the detection of serum HCV RNA in the sera for more than 6 months. Subjects infected with the hepatitis A, B, D, or E viruses, those co-infected with human immunodeficiency virus (HIV), and those with other liver diseases were excluded. Peripheral blood samples were obtained from all control subjects, and patients with HCV infection prior to, during and after DAA drug treatment. All human studies were approved by the Research Ethical Committee from The Third Affiliated Hospital of Sun Yat-Sen University and signed the informed consent.
Table 1.
Baseline characteristics of HCV-infected patients and healthy controls
| Characteristic | Patients (109) (mean ± SEM) | HC§ (49) (mean ± SEM) |
|---|---|---|
| Age (years) | 46.99 ± 1.405 | 41.93 ± 2.163 |
| Gender (M/F) | 62/47 | 27/20 |
| HCV genotype (1/no 1) | 51/58 | NA§ |
| Baseline HCV RNA (log10 U/L) | 6.07 (3.44-8.05) | NA§ |
| ALT§ level (U/L) | 74.7 (14-344) | ≤40 |
| AST§ level (U/L) | 64.6 (17-277) | ≤40 |
Abbreviations: HC§: healthy control; ALT§: alanine aminotransferase; AST§: aspartate aminotransferase; NA§: not applicable. Date are shown as medium (range).
Clinical index assay
The clinical data of individual subjects enrolled in the study were collected from hospital records. Moreover, the APRI and Fibrosis-4 indices were non-invasive scoring systems that are usually used to estimate the degree of liver fibrosis based on several laboratory tests. The APRI values were calculated using the following formula: APRI = (AST[U/L]/platelets [109/L]) × 100. An APRI index ≤0.5 signified the absence of significant fibrosis; APRI index ≤1.0 signified the absence of cirrhosis; APRI >1.5 signified the presence of significant fibrosis; and APRI >2 signified the presence of cirrhosis. APRI scores between 1.0 and 1.5 signified the progressive stages of fibrosis [19,20]. FIB-4 values were determined using the following formula: age (years) × AST [U/L]/(Platelets [109/L] × (ALT [U/L])1/2). A Fibrosis 4 score (FIB-4) <1.45 and >3.25 enables the correct identification of patients who have moderate or significant fibrosis [20,21].
Flow cytometry analysis
Peripheral venous blood samples (5 ml) were collected from all subjects and analyzed immediately. Freshly peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation using Ficoll (Axis-Shield) and stained using the following anti-human monoclonal antibodies: anti-CD4, anti-CD25, anti-CD127, anti-CD226, anti-TIGIT, anti-CD19, anti-CD38 and anti-CD24. These samples were incubated at 4°C for 30 min and protected from light. The cells were analyzed with BD LSR Fortessa flow cytometry (BD Biosciences) after washed once with staining buffer. The collected data were analyzed with FlowJo software (version V10).
Cells isolation and culture
All CD19+CD24hiCD38hi regulatory B cells, CD19+CD24intCD38int naïve B cells, CD4+CD25+CD127low regulatory T cells, CD4+CD45RA+ naïve T cells were sorted with flow cytometry sorter (purify >95%). Regulatory or naïve B cells were stimulated with Lipopolysaccharides (LPS) 1 ug/ml and CD40L 1 ug/ml in the presence of HCV patients or healthy control sera. Placenta blue count at day 0, day 3, and day 6. IL-10 expression was detected by flow cytometry. Regulatory T cells were stimulated with anti-CD3/CD38 dynabeads (beads: cells = 1:3) and rhIL-2 (300 U/ml), rhTGF-β (5 ng/ml) under HCV or healthy sera, Placenta blue count at day0, day3 and day6. Naïve T cells were stimulated with anti-CD3/CD38 dynabeads (beads: cells = 1:5) and rhIL-2 (100 U/ml), rhTGF-β (5 ng/ml) during different serum stimulation for 3 days. FOXP3 expression was tested Flow cytometry. For intracellular staining of cytokine, cells were stimulated with PMA (0.25 mg/ml), ionomycin (0.25 mg/ml) and Brefeldin A (5 mg/ml) at the last five hours. Then cells fixed, permeabilized and stained (Invitrogen) with anti-IL-10 antibody.
Real-time polymerase chain reaction (RT-PCR)
Total RNA was isolated from naïve B cells stimulated with different sera using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions; 1 μg RNA was used to synthesize the first strand of cDNA using the PrimeScript RT reagent Kit (Takara). PCR was performed on a 7500 fast (ABI) RT-PCR machine using SYBR Premix Ex Taq (Takara). Primers sequences for the following mRNA were used: IL-10 (Forward: GGCGCTGTCATCGATTTCTTC Reverse: GCCACCCTGATGTCTCAGTT), c-Maf (Forward: AGCAAGTCGACCACCTCAAG Reverse: CTGGAATCGCGTGTCAGACT), GATA3 (Forward: GCCCCTCATTAAGCCCAAG Reverse: TTGTGGTGGTCTGACAGTTCG), TGF-β (Forward: CGACTCGCCAGAGTGGTTAT Reverse: AGTGAACCCGTTGATGTCCA), AhR (Forward: ACATCACCTACGCCAGTCG Reverse: CGCTTGAAGGATTTGACTTGA). The amplified conditions included a three-step schedule as follows: 95°C for 10 min, 95°C for 15 s, and 60°C for 60 s for 40 cycles. The expression levels of the mRNAs were reported as fold change vs healthy control serum.
Statistical analysis
All data comparisons and the correlated figures were made by GraphPad Prism version 7.0 software (GraphPad Software). All data were presented as mean values ± the standard error of the mean (SEM). The Student’s t test was used for two-group comparisons. A one-way ANOVA test was used for assessment of the multiple groups. Correlation analyses were carried out using linear regression analysis. P values less than 0.05 were considered statistically significant.
Results
Characteristics of HCV-infected patients
A total 109 of HCV patients (including chronic HCV patients 89 and HCV patients with cirrhosis 20) and 49 healthy controls were recruited. As expected, all patients had disease duration more than 6 months and were consistent with their chronic state. In comparison with that in the HC, abnormally higher levels of sera ALT, AST, positive HCV RNA load were identified (Table 1).
High frequency of CD19+CD24hiCD38hi regulatory B cells but not CD4+CD25+CD127low regulatory T cells in the peripheral blood of HCV-infected patients compared to healthy controls
We first characterized the frequency of CD19+CD24hiCD38hi Bregs and CD4+CD25+CD127low Tregs in these subjects (Figure 1A-C). The proportion of CD19+CD24hiCD38hi Bregs in peripheral blood CD19+ B cells of HCV-infected patients was significantly greater than HC (P<0.001) (Figure 1B). In addition, Tregs are characterized by high expression of CD4, CD25 and low expression of CD127 (IL-7 receptor α) in the peripheral blood [22]. However, there were no significant differences in the percentage of CD25+CD127low cells within CD4+ T cells among these two groups of subjects (P>0.05) (Figure 1D). Bregs may play a pivotal role in the persistent infection of HCV virus.
Figure 1.
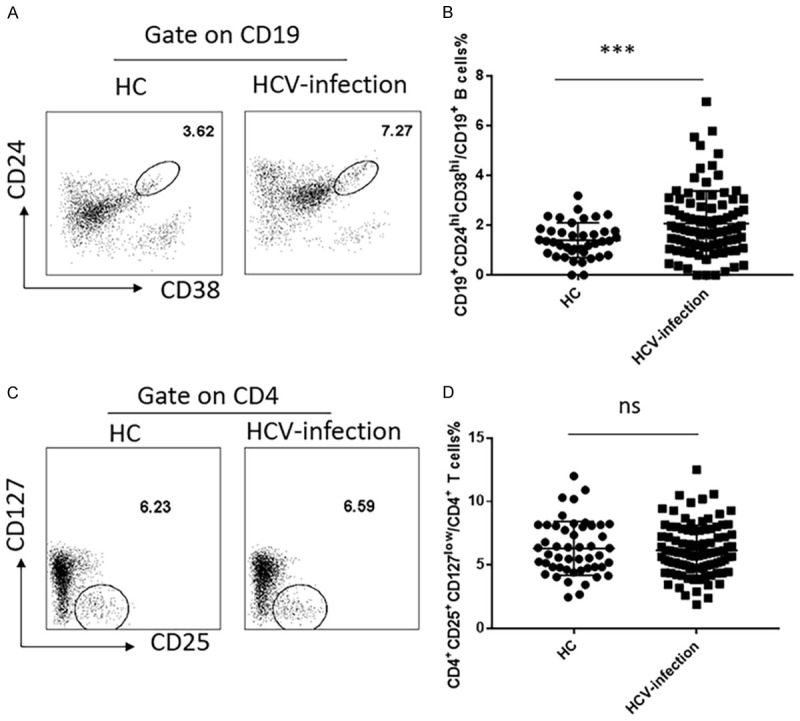
Increased frequency of CD19+CD24hiCD38hi regulatory B cells but not CD4+CD25+CD127low regulatory T cells in HCV-infected patients and healthy controls. A. CD19+CD24hiCD38hi regulatory B cells were detected by flow cytometry. B. The proportion of CD19+CD24hiCD38hi regulatory B cells was compared between HCV-infected patients (2.069 ± 0.1344) (N = 95) and Healthy controls (1.401 ± 0.1115) (N = 39). C. Gating strategy of CD4+CD25+CD127low regulatory T cells on flow cytometry. D. The proportion of CD4+CD25+CD127low regulatory T cells was compared between HCV-infected patients (6.15 ± 0.1975) (N = 99) and Healthy controls (6.308 ± 0.3033) (N = 49). Values were Mean ± SEM. Statistical analyses were performed using t test. ***P<0.001, ns, not significant.
Increased numbers of Bregs are positively correlated with the values of clinical measures in the HCV-infected patients
To further understand the significance of increased numbers of Breg cells in the pathogenesis of HCV infection, we analyzed the potential relationship between the numbers of Bregs and the values of clinical parameters. As shown in Figure 2A, the proportion of circulating Breg cells was correlated positively with the levels of sera HCV RNA loads, AST and total bilirubin levels, though the correlation was not very strong. Moreover, the percentage of Bregs was significantly higher in ALT abnormal patients than that in patients with normal sera ALT levels (Supplementary Figure 1A). Nonetheless, the genotype seems to not affect the proportion of peripheral Bregs (Supplementary Figure 1B). As for Treg cells, no significant correlation was found between the proportion and HCV RNA loads, total bilirubin levels, but low positively correlated with sera AST levels (Figure 2B). Therefore, increased numbers of Breg but not Treg cells are associated with the virus replication and liver injury in HCV patients in this population.
Figure 2.
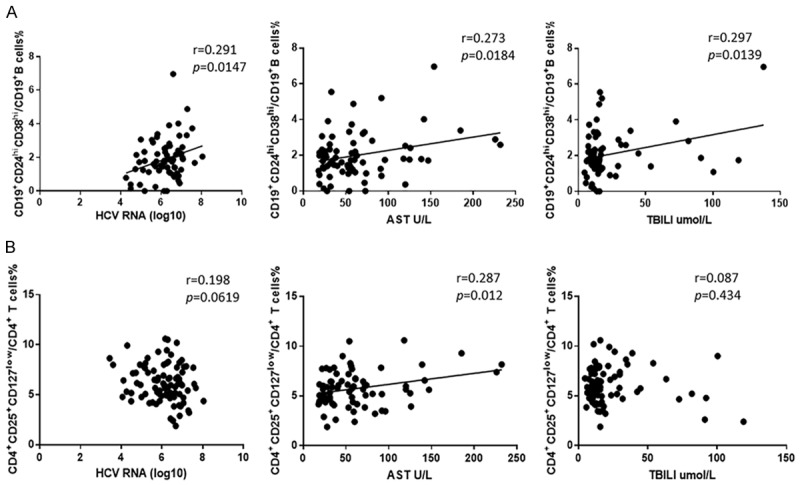
Correlation analysis between CD19+CD24hiCD38hi regulatory B cells or CD4+CD25+CD127low regulatory T cells and clinical parameters. A. The proportion of CD19+CD24hiCD38hi regulatory B cells was positively correlated with HCV RNA loads (r = 0.291, P = 0.0147, N = 83) (left) and sera AST levels (r = 0.273, P = 0.0184, N = 87) (middle) and sera total bilirubin levels (r = 0.297, P = 0.0139, N = 70) (right). B. The proportion of CD4+CD25+CD127low regulatory T cells was not associated with HCV RNA loads (r = 0.198, P = 0.0619, N = 90) (left) and sera total bilirubin levels (r = 0.087, P = 0.434, N = 83) (right), but positively correlated with sera AST levels (r = 0.287, P = 0.0012, N = 91) (middle). Statistical analyses were performed using linear regression analysis.
CD4+CD25+CD127low regulatory T cells increase in patients with hepatitis C cirrhosis and have the positive correlation with APRI and FIB-4 scores than CD19+CD24hiCD38hi regulatory B cells
Since previous studies reported that Tregs can activate fibrogenesis in hepatic stellate cells, and promote the progression the chronic HCV induced liver fibrosis [23], we next investigated whether the frequency of peripheral circulating Tregs and Bregs could relate to liver fibrosis and be associated with disease involvement. As shown in Figure 3A, chronic hepatitis C patients (CHC) and patients with hepatitis C cirrhosis (HCV-LC) had a higher percentage in Bregs. However, there were no statistical differences between the two groups. Unlike Bregs, the patients with hepatitis C cirrhosis had a significantly higher percentage of Tregs compared with HC and CHC (Figure 3C). In addition, since APRI index and Fibrosis-4 score were usually used for estimating the degree of liver fibrosis [20]. We studied the correlation and observed that Tregs were significantly positively associated with the APRI index and Fibrosis-4 score (Figure 3D). Moreover, patients with higher APRI or FIB-4 indexes had significantly higher percentages of Tregs than those patients with lower APRI or FIB-4 indexes (P<0.05) (Supplementary Figure 2A, 2B). These results suggest that the circulating Tregs but not Bregs play a more important role in the progression of cirrhosis.
Figure 3.
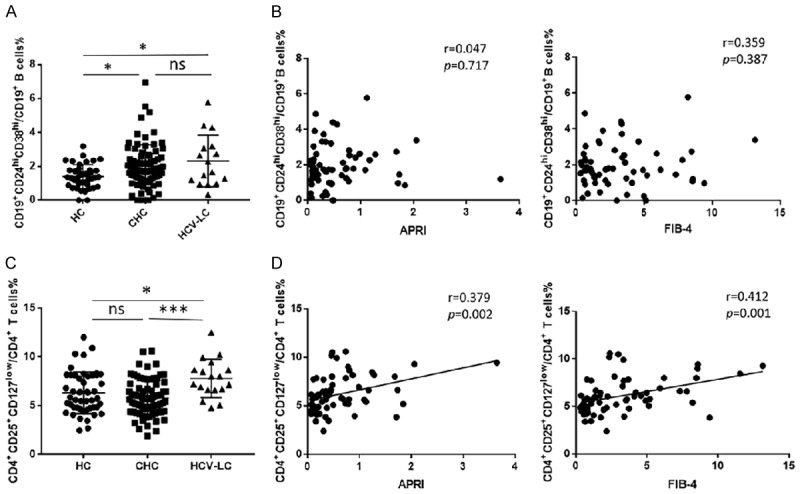
CD4+CD25+CD127low regulatory T cells increase in patients with hepatitis C cirrhosis and have the positive correlation with APRI index and Fibrosis-4 scores than CD19+CD24hiCD38hi regulatory B cells. A. The proportion of CD19+CD24hiCD38hi regulatory B cells were compared between chronic HCV patients (N = 89) and HCV liver cirrhosis patients (N = 16) and healthy controls (N = 47). C. CD4+CD25+CD127low regulatory T cells were compared between chronic HCV patients (N = 89) and HCV liver cirrhosis patients (N = 18) and healthy controls (N = 49). Values were Mean ± SEM. Statistical analyses were performed using one-way ANOVA. (*: P<0.05, **: P<0.01, ***: P<0.001). B. The correlation between CD19+CD24hiCD38hi regulatory B cells and APRI index (r = 0.047, P = 0.717, N = 64) (left) and Fibrosis-4 score (r = 0.359, P = 0.387, N = 60) (right). D. The correlation between CD4+CD25+CD127low regulatory T cells and the APRI index (r = 0.379, P = 0.002, N = 67) (left), Fibrosis-4 score (r = 0.412, P = 0.001, N = 62) (right). Statistical analyses were performed using linear regression analysis.
Previous study revealed that the expression of CD226 and TIGIT on human Tregs might be involved in the stability and immunosuppressive function of Treg [24]. In our data, the expression of TIGIT on Treg in HCV-infected patients was significantly elevated, primarily in chronic hepatitis C patients (Supplementary Figure 3C). Data indicated that the expression of TIGIT on Tregs was positively correlated with the percentages of Tregs in the peripheral blood and sera AST levels (Supplementary Figure 4A, 4B). TIGIT+ Tregs may be the crucial subpopulation of Tregs that contributes to the development of HCV infection.
The dynamic changes of the CD19+CD24hiCD38hi regulatory B cells and CD4+CD25+CD127low regulatory T cells in HCV-infected patients with DAA treatment
In recent years, although the development of DAA treatment has dramatically improved HCV treatment options [25]. A statistically significant decrease in the proportion of Bregs was observed during and after DAA treatment compared to prior treatment. Usually patients take DAA for 12 weeks, 0-12 weeks were named during DAA treatment and 12 weeks was named after DAA treatment, when patients achieve a sustained virologic response. Additionally, there were no significant differences of Bregs among the during after treatment and healthy controls (Figure 4B). Interestingly, the frequency of Tregs did not show significant changes after antiviral treatment (Figure 4D). It is likely that the important indicator of successful therapy in HCV patients is the viral load; Bregs but not Tregs had a positive correlation with HCV RNA load. After treatment, the amount of virus in patients was significantly reduced and diminished eventually; Bregs then gradually returned to normal levels.
Figure 4.
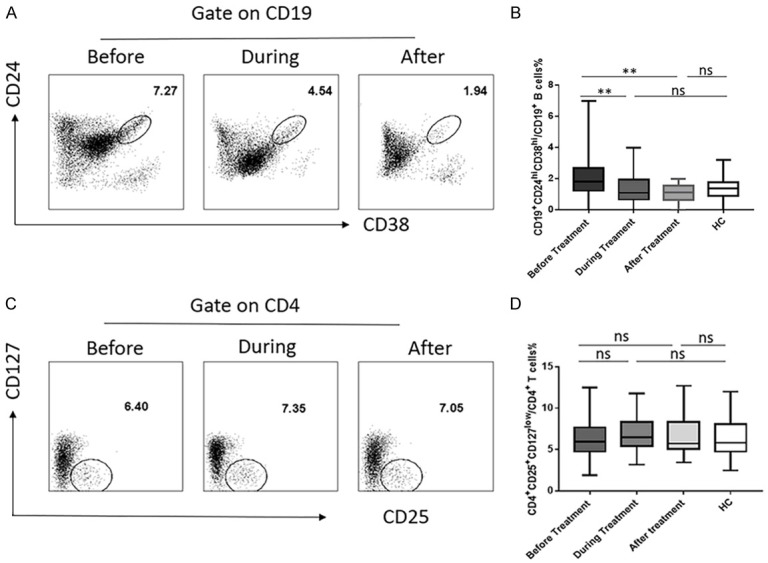
The dynamic changes of the CD19+CD24hiCD38hi regulatory B cells and CD4+CD25+CD127low regulatory cells in HCV-infected patients with DAA treatment. (A) Change of the CD19+CD24hiCD38hi regulatory B cells before (N = 97), during (N = 34), and after DAA (N = 16) treatment and (B) statistical chart. (C) Change of the CD4+CD25+CD127low regulatory cells before (N = 99), during (N = 67) and after DAA (N = 22) treatment and (D) statistical chart. Values were Mean ± SEM. Statistical analyses were performed using one-way ANOVA. *: P<0.05, **: P<0.01, ***: P<0.001, ns, not significant.
The sera from HCV-infected patients could induce more IL-10 production in B cells but not more FOXP3 expression in T cells
To further understand why the numbers of regulatory B cells increased in HCV-infected patients, we conducted a series of experiments in vitro to determine whether the increase of regulatory B cells was due to the amplification of Breg cells or the induction from non-Breg cells. First, we observed that the healthy PBMC stimulated by the sera from HCV-infected patients produced a significant higher proportion of the CD19+CD24hiCD38hi regulatory B cells when compared with healthy control sera (Figure 5A). Then, we sorted the CD19+CD24hiCD38hi regulatory B cells for the amplification or induction of Breg cells from CD19+CD24intCD38int naïve B cells that had been stimulated with LPS and CD40L. Previous study has demonstrated this protocol can induce IL-10+ B cells [11].
Figure 5.
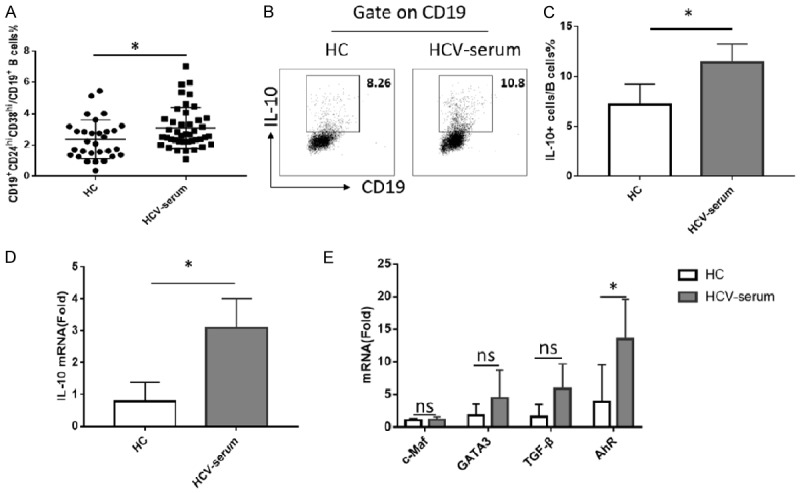
The sera from HCV-infected patients could induce more IL-10 produce in B cells but not more FOXP3 expression in T cells. (A) Healthy PBMC stimulated with LPS (1 ug/ml) for 3 days under HCV-sera (N = 42), produced high percentages of CD19+CD24hiCD38hi regulatory B cells compared with healthy control (N = 30). (B) Sorted CD19+CD24intCD38int naïve B cells from healthy control stimulated with LPS (1 ug/ml) and CD40L (1 ug/ml) under different sera for 3 days. HCV sera induced B cell produce more IL-10, including mRNA (D) and protein level (C). (E) The mRNA levels of relative transcription factors were detected in B cells stimulated with different serum conditions. Values were Mean ± SEM of three independent experiments. Statistical analyses were performed using t test. *: P<0.05, ns, not significant.
Interestingly, there was no significant difference in the total numbers of Breg amplified during six days culture under different conditions (Supplementary Figure 5). Since IL-10 is the most important functional molecule for Bregs, we selected this cytokine as the differentiation indicator. As we expected, we noted that sera from HCV patients indeed induced naïve B cells to produce more IL-10 including mRNA and protein levels (Figure 5B-D). We also did a series of similar experiments on CD4+CD25+CD127low regulatory T cells, but no significant differences were found (Supplementary Figure 6A-E), suggesting HCV-sera neither expands nor induces Treg cells. Given many transcription factors, such as c-Maf, GATA3, AhR, play a crucial role in regulating the secretion of IL-10 by regulatory B cells, and AhR could directly bind to and regulate the expression of IL-10 in Bregs [13,14], we also detected the expression of those transcription factors and found that, compared with c-Maf and GATA3, AhR increased significantly after HCV serum stimulation (Figure 5E). It is possible that the increasing of IL-10 secreting in B cells under HCV-infected patients is mediated by the HCV-sera-AhR signaling.
Discussion
Approximately 70% of HCV infected individuals may develop chronic infection [16,20]. A complex and mutual interaction between innate and adaptive immune responses plays a major role in the clearance of HCV infection [26]. Many patients develop immunodeficiency with impaired T cells responses and dysfunctional B cell activation [27]. Meanwhile, regulatory cells take part in the viral evasion and the persistence of HCV infection [28].
Among immune suppressors including T, B, DC, macrophage and other subsets, Tregs play a dominate role in immune tolerance [28-31]. Treg cells comprise a collection of innate or natural Treg subsets (tTreg), induced in the periphery (pTreg) and differentiated ex vivo (iTreg) [32,33]. Treg cells regulate immune response through direct cell to cell contact or through the elaboration of cytokines [34,35]. In addition, B regulatory (Breg) cells, which mainly secret IL-10 suppressive cytokines, have been rigorously studied in the last decade [10,36]. A subset of Bregs in human peripheral blood exhibit a CD19+CD24hiCD38hi phenotype and are the IL-10 producer which expresses both CD1dhi and immunoglobulin D [35,37]. These cells represent about 1% of peripheral blood mononuclear cells and decrease in patients with systemic lupus erythematosus (SLE), fail to suppress tumor necrosis factor (TNF) and IFN production by Th1 cells [12,37]. Many studies have begun to focus on the relationship between this group of Bregs and immune-related disorders [12,38] and hold a great promise for their clinical application.
In the present study, we found that the frequencies of circulating CD19+CD24hiCD38hi regulatory B cells were significantly increased in HCV patients. This finding could implicate that Bregs may have been activated after HCV antigenic stimulation in helping the virus escape from attacking via their immunosuppressive role. In addition, we found significant percentages of circulating Bregs were positively correlated with the levels of sera AST, TBILI and HCV virus load which reflect to liver damage [39]. Other similar studies have reported that circulating regulatory B cells may have an impact on the pathogenesis of various diseases such as chronic hepatitis B virus [40], rheumatoid arthritis [37], even in cancers [38].
We previously reported that Treg cells are effective in preventing liver damage [41,42]. Our results were also different from previous studies reporting data that CD4+CD25+Foxp3+ Tregs were increased in patients with chronic hepatitis C and may mediate the sustained suppression of HCV-specific T-cell responses [3,43-45]. Those differences may derive from phenotypic differences in patients recruited, disease duration, and methods used for Treg cells evaluation. Additionally, race, disease activity and treatment also have been shown to have an impact on the frequencies of Treg [4,42].
Nonetheless, major findings of the present study are that Tregs but not Bregs were significantly positively associated with the APRI index and Fibrosis-4 scores. These results indicate that Tregs may have a pivotal role in the disease progression stage and promote the occurrence and development of liver cirrhosis in the late stage, which is consistent with other studies [23,46]. The liver fibrosis process is mainly caused by the imbalance of synthesis and degradation of extracellular matrix produced by activated hepatitis stellate cells [46,47], they could promote the activation of hepatic stellate through the secretion of cytokines, such as TGF-β [48]. It is known that TGF-β promotes the liver fibrosis [49]. Thus, Treg cells have multiple roles in the pathogenesis of liver inflammation, damage and fibrosis. In the early stage, Treg can suppress inflammation and damage, nonetheless, Treg cells may promote liver fibrosis through TGF-β. TGF-β is crucial for Treg development and differentiation, forming a feedback circulation of Treg, TGF-β, Treg and fibrosis.
With regard to the treatment of HCV, sustained virologic response is the primary purpose because the clinical remission is associated closely with viral clearance. The successful effectiveness and tolerance of direct-acting antivirals (DAAs) in eradicating HCV virus is confirmedly established and become the greatest triumphs of medical therapeutics in the last 20 years [17,50]. There was a significant decrease of Bregs during and after treatment compared to baseline levels. As this immune mediator was activated by antigenic stimulation at baseline, along with the dramatic viral decline and the reduced liver inflammation, effective therapy aiming at HCV virus plays a strong role in immune balance recovery. After treatment, the amounts of virus in the patients were significantly reduced and diminished eventually, Bregs then gradually return to normal levels, which suggest that the higher frequencies of Bregs in patients with chronic HCV infection may play a relevant role in DAA-induced antiviral responses and can be an important predictor of the achievement of virologic response following DAA drugs treatment in HCV-infected patients. Nevertheless, Tregs may modify multiple immune responses and thus determine the further course of liver disease after HCV clearance, and play a profound impact on the pathogenesis of HCV associated liver cirrhosis.
We also demonstrated that increased circulating Bregs may be due to the induced differentiation rather than amplification. It is likely that HCV-sera induces the differentiation of Breg cells and its function through mediating an AhR signaling. Indeed, AhR directly binds and regulates the expression of IL-10 in Bregs [13]. Nonetheless, HCV-serum does not significantly affect Treg cells. It is under so far why HCV-sera has a different role in Treg and Breg cells. Additionally, underlying mechanisms thereby HCV-sera promotes Breg cells warrant a further study in the future.
Acknowledgements
We also thank the study volunteers for their participation and the staff of the infection department for their assistance in the execution of this study. This work was supported by the grant of the Guangdong Innovative and Entrepreneurial Research Team Program [2016ZT06S252 to X.Z]; Science and technology of Guangdong Province: Special Funds for Public Welfare Research and Capacity Building in Guangdong Province [2014B020212025 to X.Z]. Q.F, J.W and S.G.Z is in part supported by the grants from NIH R01 AR059103, NIH STAR Award and NIH R61 AR073409.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Wang L, Qiu J, Yu L, Hu X, Zhao P, Jiang Y. Increased numbers of CD5+CD19+CD1dhighIL-10+ Bregs, CD4+Foxp3+ Tregs, CD4+ CXCR5+Foxp3+ follicular regulatory T (TFR) cells in CHB or CHC. J Transl Med. 2014;12:251. doi: 10.1186/s12967-014-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeidi A, Zandi K, Cheok YY, Saeidi H, Wong WF, Lee CYQ, Cheong HC, Yong YK, Larsson M, Shankar EM. T-cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front Immunol. 2018;9:2569. doi: 10.3389/fimmu.2018.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhai N, Chi X, Li T, Song H, Li H, Jin X, Crispe IN, Su L, Niu J, Tu Z. Hepatitis C virus core protein triggers expansion and activation of CD4(+)CD25(+) regulatory T cells in chronic hepatitis C patients. Cell Mol Immunol. 2015;12:743–749. doi: 10.1038/cmi.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Liu ZM, Wang KP, Ma J, Guo Zheng S. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell Mol Immunol. 2015;12:553–557. doi: 10.1038/cmi.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu G, Liu Z, Zheng C, Zheng SG. Antigen-non-specific regulation centered on CD25+Foxp3+ Treg cells. Cell Mol Immunol. 2010;7:414–418. doi: 10.1038/cmi.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langhans B, Braunschweiger I, Arndt S, Schulte W, Satoguina J, Layland LE, Vidovic N, Hoerauf A, Oldenburg J, Sauerbruch T, Spengler U. Core-specific adaptive regulatory T-cells in different outcomes of hepatitis C. Clin Sci (Lond) 2010;119:97–109. doi: 10.1042/CS20090661. [DOI] [PubMed] [Google Scholar]
- 8.Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Wang P, Zheng SG. Regulatory T cells and B cells implication on autoimmune diseases. Int J Clin Exp Pathol. 2013;6:2668–2674. [PMC free article] [PubMed] [Google Scholar]
- 10.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Piper CJM, Rosser EC, Oleinika K, Nistala K, Krausgruber T, Rendeiro AF, Banos A, Drozdov I, Villa M, Thomson S, Xanthou G, Bock C, Stockinger B, Mauri C. Aryl hydrocarbon receptor contributes to the transcriptional program of IL-10-producing regulatory B cells. Cell Rep. 2019;29:1878–1892. e1877. doi: 10.1016/j.celrep.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu M, Zhao X, Ma Y, Zhou Y, Deng M, Ma Y. Transcription factor c-Maf is essential for IL-10 gene expression in B cells. Scand J Immunol. 2018;88:e12701. doi: 10.1111/sji.12701. [DOI] [PubMed] [Google Scholar]
- 15.Majlessi L, Lo-Man R, Leclerc C. Regulatory B and T cells in infections. Microbes Infect. 2008;10:1030–1035. doi: 10.1016/j.micinf.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Biragyn A, Lee-Chang C. A new paradigm for an old story: the role of regulatory B cells in cancer. Front Immunol. 2012;3:206. doi: 10.3389/fimmu.2012.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Au JS, Pockros PJ. Novel therapeutic approaches for hepatitis C. Clin Pharmacol Ther. 2014;95:78–88. doi: 10.1038/clpt.2013.206. [DOI] [PubMed] [Google Scholar]
- 18.Singer AW, Reddy KR, Telep LE, Osinusi AO, Brainard DM, Buti M, Chokkalingam AP. Direct-acting antiviral treatment for hepatitis C virus infection and risk of incident liver cancer: a retrospective cohort study. Aliment Pharmacol Ther. 2018;47:1278–1287. doi: 10.1111/apt.14593. [DOI] [PubMed] [Google Scholar]
- 19.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Zhang L, Li H, Chen Z, Luo A, Liu B, Chen M, Peng M, Ren H, Hu P. Circulating T follicular helper cells are associated with rapid virological response in chronic hepatitis C patients undergoing peginterferon therapy. Int Immunopharmacol. 2016;34:235–243. doi: 10.1016/j.intimp.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 22.Khalili A, Ebrahimpour S, Maleki I, Abediankenari S, Afrouzi MM. CD4+CD25+ CD127low FoxP3+ regulatory T cells in Crohn’s disease. Rom J Intern Med. 2018;56:158–166. doi: 10.2478/rjim-2018-0006. [DOI] [PubMed] [Google Scholar]
- 23.Langhans B, Kramer B, Louis M, Nischalke HD, Huneburg R, Staratschek-Jox A, Odenthal M, Manekeller S, Schepke M, Kalff J, Fischer HP, Schultze JL, Spengler U. Intrahepatic IL-8 producing Foxp3(+)CD4(+) regulatory T cells and fibrogenesis in chronic hepatitis C. J Hepatol. 2013;59:229–235. doi: 10.1016/j.jhep.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Elhai M, Chiocchia G, Marchiol C, Lager F, Renault G, Colonna M, Bernhardt G, Allanore Y, Avouac J. Targeting CD226/DNAX accessory molecule-1 (DNAM-1) in collagen-induced arthritis mouse models. J Inflamm (Lond) 2015;12:9. doi: 10.1186/s12950-015-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langhans B, Nischalke HD, Krämer B, Hausen A, Dold L, van Heteren P, Hüneburg R, Nattermann J, Strassburg CP, Spengler U. Increased peripheral CD4+ regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J Hepatol. 2017;66:888–896. doi: 10.1016/j.jhep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Burton JR Jr, Klarquist J, Im K, Smyk-Pearson S, Golden-Mason L, Castelblanco N, Terrault N, Rosen HR Virahep-C Study Group. Prospective analysis of effector and regulatory CD4+ T cells in chronic HCV patients undergoing combination antiviral therapy. J Hepatol. 2008;49:329–338. doi: 10.1016/j.jhep.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 28.Eiza N, Toubi E, Vadasz Z. Increased T and B regulatory cell function contributes to the persistence of HCV and other viral infections. Isr Med Assoc J. 2016;18:159–61. [PubMed] [Google Scholar]
- 29.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz DA, Zheng SG, Gray JD, Wang JH, Ohtsuka K, Yamagiwa S. Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease. Semin Immunol. 2004;16:135–143. doi: 10.1016/j.smim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Lan Q, Fan H, Quesniaux V, Ryffel B, Liu Z, Zheng SG. Induced Foxp3(+) regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol. 2012;4:22–28. doi: 10.1093/jmcb/mjr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002;169:4183–9. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 33.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DA, Ziegler SF. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 34.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–21. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 35.Zhong H, Liu Y, Xu Z, Liang P, Yang H, Zhang X, Zhao J, Chen J, Fu S, Tang Y, Lv J, Wang J, Olsen N, Xu A, Zheng SG. TGF-β-induced CD8+CD103+ regulatory T cells show potent therapeutic effect on chronic graft-versus-Host disease lupus by suppressing B cells. Front Immunol. 2018;9:35. doi: 10.3389/fimmu.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 37.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5:173ra123. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 38.Gheybi MK, Farrokhi S, Ravanbod MR, Ostovar A, Mehrzad V, Nematollahi P. The correlation of CD19+CD24+CD38+ B cells and other clinicopathological variables with the proportion of circulating tregs in breast cancer patients. Breast Cancer. 2017;24:756–764. doi: 10.1007/s12282-017-0775-y. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Gu J, Liu R, Zhou H, Lu L, Dai X, Qian X. IL-2/IL-6 ratio correlates with liver function and recovery in acute liver injury patients. APMIS. 2019;127:468–474. doi: 10.1111/apm.12944. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Cheng LS, Wu SD, Wang SQ, Li L, She WM, Li J, Wang JY, Jiang W. IL-10-producing regulatory B cells suppressed effector T but enhanced regulatory T cells in chronic HBV infection. Clin Sci (Lond) 2016;130:907–919. doi: 10.1042/CS20160069. [DOI] [PubMed] [Google Scholar]
- 41.Billerbeck E, Bottler T, Thimme R. Regulatory T cells in viral hepatitis. World J Gastroenterol. 2007;13:4858–4864. doi: 10.3748/wjg.v13.i36.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Feng M, Gu J, Xia Z, Zhang H, Zheng S, Duan Z, Hu R, Wang J, Shi W, Ji C, Shen Y, Chen G, Zheng SG, Han YP. Restoration of intrahepatic regulatory T cells through MMP-9/13-dependent activation of TGF-beta is critical for immune homeostasis following acute liver injury. J Mol Cell Biol. 2013;5:369–379. doi: 10.1093/jmcb/mjt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsacker F, Thimme R. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860–7867. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–1071. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 45.Ayers CL, Firan M, Pillai V, Lee WM, Karandikar NJ. Viral interactions with B-cells contribute to increased regulatory T-cells during chronic HCV infection. Viral Immunol. 2011;24:119–129. doi: 10.1089/vim.2010.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward SM, Fox BC, Brown PJ, Worthington J, Fox SB, Chapman RW, Fleming KA, Banham AH, Klenerman P. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol. 2007;47:316–324. doi: 10.1016/j.jhep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Ouyang X, Xu Z, Chen J, Huang Q, Liu Y, Xu T, Wang J, Olsen N, Xu A, Zheng SG. CD8+CD103+ iTregs inhibit chronic graft-versus-host disease with lupus nephritis by the increased expression of CD39. Mol Ther. 2019;27:1963–1973. doi: 10.1016/j.ymthe.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu L, Ma J, Wang X, Wang J, Zhang F, Yu J, He G, Xu B, Brand DD, Horwitz DA, Shi W, Zheng SG. Synergistic effect of TGF-beta superfamily members on the induction of Foxp3+ Treg. Eur J Immunol. 2010;40:142–152. doi: 10.1002/eji.200939618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu X, Zhang Y, Zheng X, Deng J, Li H, Kang Z, Cao Z, Huang Z, Ding Z, Dong L, Chen J, Zang Y, Zhang J. TGF-beta-induced hepatocyte lincRNA-p21 contributes to liver fibrosis in mice. Sci Rep. 2017;7:2957. doi: 10.1038/s41598-017-03175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ioannou GN, Feld JJ. What are the benefits of a sustained virologic response to direct-acting antiviral therapy for hepatitis C virus infection? Gastroenterology. 2019;156:446–460. e442. doi: 10.1053/j.gastro.2018.10.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


