Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors with a high mortality rate and low survival rate. This study was designed to explore a novel molecular with high sensitivity and specificity, which can be applied in early diagnosis and therapeutic evaluation of HCC. The current study aims to investigate the effect and important role of Axin1 on cell proliferation, invasion, migration and epithelial-mesenchymal transition (EMT) in hepatocellular carcinoma. qRT-PCR results showed lower Axin1 expression level and higher miR-650 expression level in HCC. Luciferase reporter assay was carried out to verify the negative correlation between Axin1 and miR-650 mRNA levels. CCK-8 assay results showed that the cell proliferation ability was significantly suppressed by Axin1 overexpression in SK-HEP-1 cells. The results in wound healing assay uncovered that cell migration ability was markedly suppressed by Axin1 overexpression. The results in trans-well invasion assay showed that Axin1 overexpression caused decreased invasive ability in SK-HEP-1 cells. The WB results showed that the protein level of E-cad was significantly increased and the protein levels of N-cad, vimentin and snail were obviously reduced following Axin1 overexpression. Whereas, the suppressive effects on cell proliferation, migration, invasion and EMT caused by Axin1 overexpression were abolished by miR-650 mimic. All the results in the current study confirmed the truth that Axin1 overexpression could suppress cell proliferation, migration, invasion and EMT by downregulating miR-650 expression.
Keywords: Axin1, MiR-650, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in digestive system [1]. According to statistics, HCC is the sixth most common malignant tumor in the world with the third highest mortality rate, which seriously endangers human health [2]. At present, surgery is still the only method to cure HCC. However, due to the hidden nature of onset of HCC, most patients are already at the intermediate or advanced stage when diagnosis and are not candidates for curative surgical treatment [3]. Besides, the effects of other treatments such as intervention, molecular targeted therapy and chemotherapy are still unsatisfactory. Progression-free survival and median survival time are still very poor [4]. Thus, new molecular probes for target recognition with high sensitivity and specificity need to be found out and applied to early diagnosis, early warning, metastasis and therapeutic evaluation of liver cancer.
The early detection and timely treatment are the key to improving prognosis and reducing mortality of patients with HCC [5]. In recent years, molecular targeted therapy has become a hot topic in the treatment of advanced HCC [6]. MicroRNAs (miRNAs) are a class of short nucleotide sequences with the length of only 20-24 nt, which are widely expressed in multicellular eukaryotes. MiRNAs regulate the target genes in post-transcriptional level by pairing with complimentary mRNAs [7]. Recently, more and more studies have found that miRNAs play important roles in the occurrence and development of various diseases and have great potential in the diagnosis, treatment and prognosis of certain diseases, which make miRNAs potential as disease biomarkers and novel therapeutic targets [8].
Evidence has proved that miRNAs are involved in a variety of life processes in liver cells, especially in liver cancer [9]. Yun et al. found that the expression of miR-548b in HCC tissues and cell lines was weak. MiR-548b overexpression significantly promoted HCC cell proliferation, colony formation, and metastasis and induced apoptosis in vitro [10]. Recently, miR-650 has been proved to play important roles in many kinds of cancers [11,12]. Besides, a research showed that miR-650 could promote the migration, invasion and Epithelial-mesenchymal transition (EMT) of HCC cells [13]. Therefore, miR-650 may serve as a novel biomarker for HCC patients.
Axin (Axis inhibition), the product of the mouse Fused (Fu) gene found in 1997, is an important scaffold protein of the Wnt signaling pathway. It transmits cellular signaling to downstream effective molecule in order to regulate the signaling transduction among different signaling pathways [14]. It had been proved that Axin was associated with carcinoma tumorigenesis and progression [15]. EMT is a key factor in promoting the invasion and metastasis tumor cells. Wu et al. demonstrate that Axin could induce a functional EMT program and drive metastatic activity rather than function as a tumor suppressor [16]. Moreover, revelant research showed that miRNA could play biologic role by binding to AXIN, which was closely associated with the genesis and development of HCC [17,18]. However, whether AXIN1 could affect HCC by targeting miR-650 is unknown.
Considering the important role of AXIN1 and miR-650 in HCC development, the present study aimed to test the expression of AXIN1 and miR-650 in HCC cell lines, to verify the relativity between them, and to examine the effect of AXIN1 on the biological function in hepatocellular carcinoma. In short, findings in our current study suggested that Axin1 could inhibit cell proliferation, invasion, migration and EMT in hepatocellular carcinoma by targeting miR-650. Therefore, in-depth study on the molecular mechanisms may make AXINI as a potential agent hepatocellular carcinoma and provide a new strategy for the treatment.
Materials and methods
Cell culture and treatment
LO2, SK-HEP-1, HUH-7, LM6 and Li-7 cell lines were purchased from American Type Culture Collection (ATCC). Cell lines were cultured with Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA) containing 10% heat-inactivated FBS (Invitrogen), 100 IU/ml penicillin (Sigma Aldrich, St. Louis, MO) and 100 μg/ml streptomycin (Sigma-Aldrich) at 37°C in a humidified atmosphere of 5% CO2.
Cell transfection
LO2 and SK-HEP-1 cell lines were transfected with miR-650 mimic/NC and pcDNA-Axin1/pcDNA-NC. According to the manufacturer’s instructions, the cells were placed in a 6-well plate and then transfected with plasmids upon reaching 75% to 90% confluence using Lipofectamine 2000 (Invitrogen, USA). After 24-48 h, transfected cells were used for analyses.
Cell viability assay
The proliferation of SK-HEP-1 cells was evaluated using cell counting kit-8 (CCK-8) assay. Briefly, after LPS (1, 2, 4 or 8 μg/ml) treatment, 5×103 SK-HEP-1 cells were seeded into the 96-well plate and incubated in a 37°C incubator with 5% CO2 for 24 h. Immediately following, 10 μl of CCK-8 solution was added into each well and sustained for another 2 h. Subsequently, the absorbance at 450 nm was recorded with Micro-plate Reader (Bio-Rad, Hercules, CA, USA). Cell viability (%) was calculated and analyzed accordingly.
Quantitative real-time PCR
Briefly, total RNA from hepatocellular carcinoma cell lines was extracted for reverse transcription using TRIzol reagent (Invitrogen, USA). After dissolving RNA with diethyl pyrocarbonate (DEPC) treated water, the absorbance at 260 nm and 280 nm was measured to determine the RNA purity and concentration. The cDNA was synthesized using a commercial PrimeScript™ II 1st strand cDNA synthesis kit (TaKaRa, Japan). RT-PCR was performed using SYBR Premix kit (TaKaRa Biotechnology) with an ABI Prism 7500 Sequence Detection system (Thermo Fisher Scientific, USA). Reaction conditions were as follows: Initial denaturation at 95°C for 2 min, followed by 35 cycles of denaturation at 95°C for 30 sec and annealing at 60°C for 30 sec. β-actin was used as an internal control. The 2-ΔΔCq method was used to calculate the gene expression levels [19]. All experiments were conducted three times.
Western blot
After washing with pre-cooled phosphate buffer saline (PBS) buffer for 3 times, the cells were lysed by RIPA Buffer (Beyotime, Shanghai, CN). Then, the supernatants were collected by 12000 rpm centrifugation at 4°C for 10 min. The proteins were quantified using the BCA™ Protein Assay Kit (Beyotime) and stored at -80°C. Protein samples were separated by SDS-PAGE and then transferred onto polyvinylidene fluoride (PVDF) membranes. After blocking with 5% skim milk for 1 h, the membranes incubated with primary antibodies against Axin1 (Abcam, 1:2000, ab233652), MMP9 (Abcam, 1:1000, ab38898), MMP12 (Abcam, 1:3000, ab137444), E-cad (Abcam, 1:500, ab15148), N-cad (Abcam, 1:2000, ab98952), Vimentin (Abcam, 1:1000, ab8978), Snail (Abcam, 1:1000, ab53519) and GAPDH (Abcam, 1:20000, ab181602) at 4°C overnight. After washing with 1×TBST buffer, the membranes were incubated with the corresponding secondary antibody at room temperature for 1-1.5 h. After washing, the proteins were detected by enhanced ECL. All Western blotting bands were analyzed by Image J software (NIH, USA).
Colony formation assay
Briefly, the transfected cells were seeded in fresh 6-well plates in triplicate at a density of 1×103 per well and maintained in medium containing 10% fetal bovine serum (FBS) for 2 weeks. Cells were washed with PBS, and fixed in 20% methanol for 15 min at room temperature. Then, cells were stained with 0.5% crystal violet for 15 min. The colonies were counted using a microscope (Leica, Germany).
Wound healing assay
Transfected SK-HEP-1 cells (2×106/well) were seeded in a 6-well plate and incubated until reaching at least 90% confluence. Then a 20-μl plastic pipette tip was used to produce a scratch. After washing with PBS, the cells were incubated in culture medium without serum for 72 h. The wound closure was photographed under a microscope at 0 h and 72 h. The relative migrated areas were analyzed using Image J software (NIH, USA).
Cell invasion assay
The treated cells (1×105 cells/ml) were suspended in serum-free medium, and 200 μl cell suspension was added to the upper chamber of the trans-well plate. The upper chambers were coated with Matrigel. Then, medium containing 10% FBS was added into the lower chambers. The cells were incubated in a humidified incubator for 72 h. Then, the cells on the top side were removed with cotton swabs, and the cells that passed through the membrane were fixed with 0.1% crystal violet for 15 min. The invasive cells were counted under a light microscope (Leica, Germany).
Luciferase reporter assay
The Axin1 wild-type (WT) and mutant (MUT) 3’UTR firefly luciferase construct were generated. In brief, cells were seeded in 24-well plates and cultured for 24 h before luciferase reporter assay. Then, SK-HEP-1 cells (80% confluence) were cotransfected with Axin1-WT-3’UTR or Axin1-MUT-3’UTR luciferase reporter and Renilla luciferase reporter (pRL), and miR-NC or miR-650-mimic by using Lipofectamine 2000 reagent (Invitrogen, USA). Luciferase activity was detected 48 h post-transfection using the Dual Luciferase Reporter Assay System (Promega, USA). The relative fluorescence intensity was calculated and normalized to the internal control for transfection efficiency.
Statistical analysis
All statistical analysis in the present study were performed using GraphPad Prism 7.0 and each data are expressed as the mean + standard deviation. Differences among multiple groups were analyzed using one-way analysis of variance (ANOVA) and statistical analysis between two groups was performed using Student’s t-test. P<0.05 was considered as statistical differences.
Results
Expression levels of Axin1 and miR-650 in hepatocellular carcinoma
To examine Axin1 and miR-650 expressions in hepatocellular carcinoma, we measured the Axin1 expression level and miR-650 expression level in normal liver epithelial LO2 cells and liver cancer cells including SK-HEP-1, HUH-7, LM6, Li-7 cells. Compared to the normal liver epithelial LO2 cells, lower Axin1 expression level was found in liver cancer cell lines in qRT-PCR results (Figure 1A). Then, we further detected the Axin1 expression level in LO2 cells and four GC cells by western blot assay. The data also demonstrated that the Axin1 expression level in HCC cells was significantly reduced (Figure 1B). In addition, the miR-650 expression level in HCC cells was also measured by qRT-PCR and the data demonstrated higher miR-650 expression level in HCC cells than that in the normal liver epithelial LO2 cells (Figure 1C). Furthermore, we analyzed the correlation between BMP2 and miR-378 in GC tissues. As expected, the data revealed that, in GC tissues, miR-378 expressions were negatively correlated to the BMP2 expressions.
Figure 1.
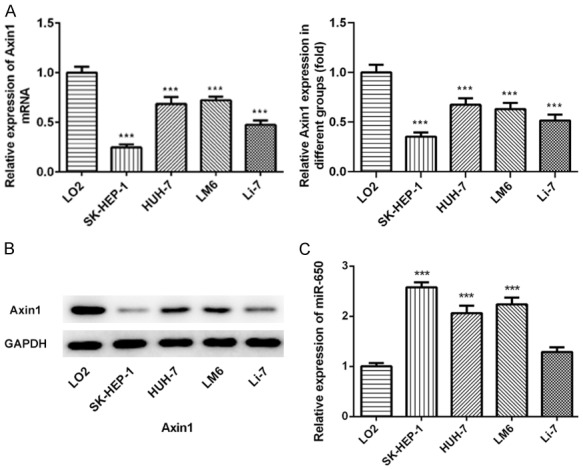
Expression levels of Axin1 and miR-650 in hepatocellular carcinoma. A. qRT-PCR result of Axin1 expression in normal liver epithelial LO2 cells and liver cancer cells including SK-HEP-1, HUH-7, LM6, Li-7 cells. B. WB result of Axin1 expression in normal liver epithelial LO2 cells and liver cancer cells including SK-HEP-1, HUH-7, LM6, Li-7 cells. C. qRT-PCR result of miR-650 expression in normal liver epithelial LO2 cells and liver cancer cells including SK-HEP-1, HUH-7, LM6, Li-7 cells (***P<0.001 vs. LO2 cells group).
MiR-650 is a direct target of Axin1
Genes targeted by Axin1 were screened using prediction software. Among all the genes common to databases, we chose miR-650, a key tumor promoter, as a target for the further research due to its vital role in cancer migration, invasion and EMT. Luciferase reporter assay was carried out to explore whether Axin1 targets and regulates miR-650 expression (Figure 2A). Meanwhile, we examined miR-650 expression after transfection with miR-650 mimic in SK-HEP-1 cells (Figure 2B). The result of luciferase reporter assay uncovered that miR-650 overexpression inhibited the luciferase activity of Axin1-WT but had no obvious effects on Axin1-MUT (Figure 2C). Axin1 expression was detected after transfection with Axin1 overexpression vector (Figure 2D). Meanwhile, the data of qRT-PCR demonstrated that miR-650 expression was significantly downregulated by Axin1 overexpression (Figure 2E). These data above all demonstrated that Axin1 acts as a molecular sponge for miR-650 and negatively regulate its expression.
Figure 2.
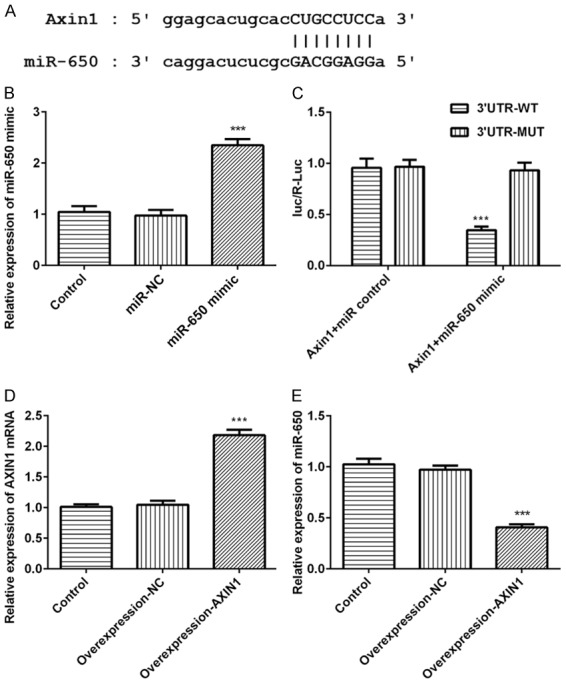
MiR-650 is a direct target of Axin1. A. The predictive binding sites between Axin1 and miR-650. B. MiR-650 expression level detected by RT-qPCR assay. C. The luciferase activity detected by luciferase reporter assay. D. Axin1 expression level detected by RT-qPCR assay. E. MiR-650 expression level detected by RT-qPCR assay (***P<0.001 vs. control group).
Axin1 overexpression suppressed cell proliferation by downregulating miR-650 expression
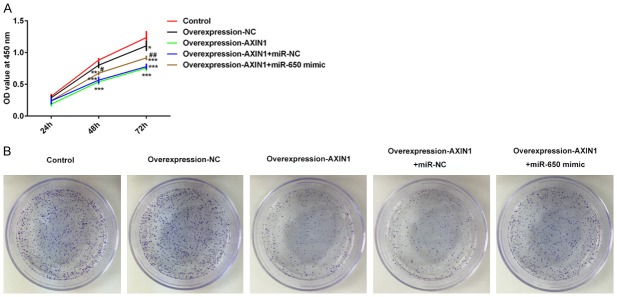
The negative correlation between Axin1 and miR-650 mRNA levels indicated a regulatory association of Axin1 and miR-650. As shown in Figure 3A, CCK-8 assay results showed that the cell proliferation ability was significantly suppressed by Axin1 overexpression in SK-HEP-1 cells, which was abolished by miR-650 treatment. Clone formation assay (Figure 3B) also demonstrated that Axin1 overexpression suppressed cell proliferation by downregulating miR-650 expression.
Figure 3.
Axin1 overexpression suppressed cell proliferation by downregulating miR-650 expression. A. Cell viability determined by CCK8 assay. B. The cell clone number determined by clone formation assay (*P<0.05, **P<0.01, ***P<0.001 vs. control group; #P<0.05, ##P<0.01 vs. Axin1 group).
Axin1 overexpression suppressed cell migration and invasion by downregulating miR-650 expression
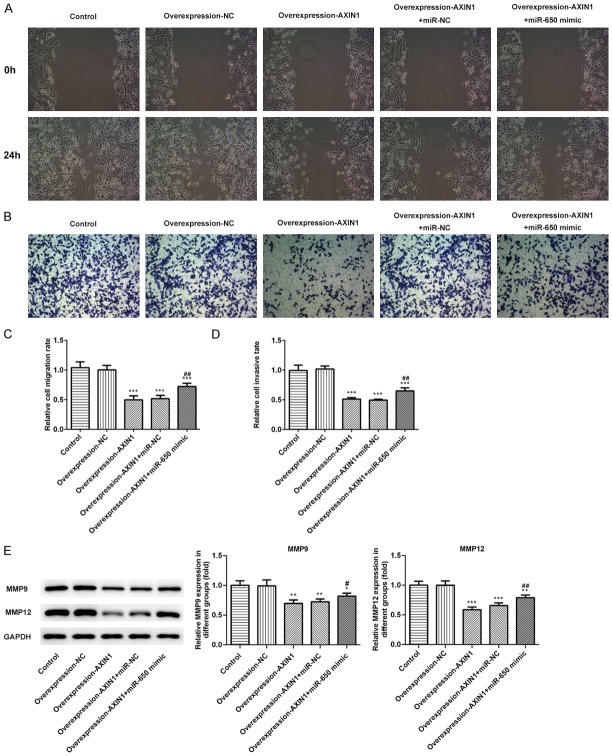
Three different approaches were performed to explore the critical role of β-arrestin1 on cell migration and invasion in HCC. As shown in Figure 4A, wound healing assay was applied to evaluate the migration ability of SK-HEP-1 cells. The results in wound healing assay uncovered that relative ratios of wound closures were markedly suppressed by Axin1 overexpression, which was reversed by miR-650 treatment (Figure 4C). Then, trans-well invasion assay was performed to investigate the influence of Axin1 on cell invasion in HCC. The results in trans-well invasion assay showed that Axin1 overexpression caused a decreased invasive ability in SK-HEP-1 cells, while miR-650 overexpression extremely abolished the effects and promoted the invasion of SK-HEP-1 cells (Figure 4B and 4D). Meanwhile, the expression levels of MMP9 and MMP12 detected by western blot were significantly downregulated by Axin1 overexpression compared to the control group, which were also abolished by miR-650 treatment (Figure 4E). All the data above indicated that Axin1 overexpression could suppress cell migration and invasion by downregulating miR-650 expression.
Figure 4.
Axin1 overexpression suppressed cell migration and invasion by downregulating miR-650 expression. A. Wound healing assays were performed to determine cell migration. B. Trans-well assays were performed to determine cell invasion. C. Quantitative results regarding the cell migration. D. Quantitative results regarding the cell invasion. E. The protein levels of MMP9 and MMP12 were measured by western blot assay (*P<0.05, **P<0.01, ***P<0.001 vs. control group; #P<0.05, ##P<0.01 vs. Axin1 group).
Axin1 overexpression suppressed EMT by downregulating miR-650 expression
Since EMT is a crucial mechanism in tumor progress, we speculated whether the Axin1/miR-650 axis is involved in EMT and then detected the protein levels of several EMT markers including E-cad, N-cad, vimentin and snail. The results showed that the protein level of E-cad was significantly increased and the protein levels of N-cad, vimentin and snail were obviously reduced in K-HEP-1 cells following Axin1 overexpression (Figure 5), Whereas, miR-650 mimic abolished the suppressive effects on EMT caused by Axin1 overexpression. Together, these results implicated that Axin1 overexpression may suppress EMT by downregulating miR-650 expression.
Figure 5.
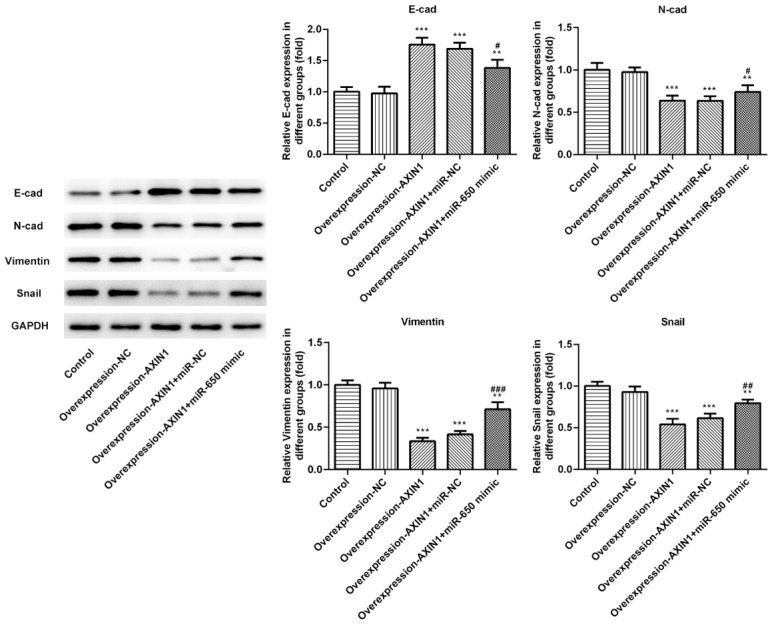
Axin1 overexpression suppressed EMT by downregulating miR-650 expression. The protein levels of EMT markers were measured by western blot assay (**P<0.01, ***P<0.001 vs. control group; #P<0.05, ##P<0.01, ###P<0.001 vs. Axin1 group).
Discussion
In recent years, the incidence of HCC has gradually increased, which has been concerned worldwide [2]. It is hard for patients with HCC to discover the disease, causing difficulty in surgery and prognosis [20]. What’s more, neither the sensitivity nor the specificity of traditional diagnostic biomarkers of liver cancer is desirable, which is inconducive to the diagnosis and treatment of HCC [21]. Thus, our study aimed to search for new diagnostic and therapeutic targets on HCC.
As a negative regulator of Wnt signaling pathway, AXIN1 has also been considered as a tumor suppressor [22]. The present study revealed that the protein level of AXIN1 was strikingly lower in HCC cell lines than in hepatic epithelial cells. MiRNAs are short non-coding RNAs that modulate gene expression at the post transcriptional level. They have a variety of biological functions, playing important roles in tumor progression, metastasis, drug resistance and immune regulation [23]. Recently, research proved that AXIN1 could affect cancers by binding to miRNAs [17,24]. Our research showed that the expression of miR-650 in hepatic epithelial cells was weak and its level in HCC cell lines was enhanced. Besides, we proved that AXIN1 could target miR-650 in HCC cell lines.
Accumulating studies have shown that AXIN1 and miR-650 can potentially be used as biomarkers for early diagnosis, treatment and prognosis [25,26]. Similarly, our results revealed that overexpression of Axin1 could significantly inhibit the proliferation and cloning formation of HCC cells. While this effect was reversed by transfecting miR-650 mimic. Recent research indicated that miR-650 could markedly promoted the migration and invasion of cancer cells [27]. As expected, our study suggested that overexpression of AXIN1 could inhibit migration and invasion of HCC cell lines. However, miR-650 could rescue the effects of AXIN1 on HCC cell migration and invasion. It is known that EMT takes part in tumor invasion and metastasis. MiRNAs could participate in the regulation of EMT in HCC cells through various pathways, thus affecting the occurrence and development of HCC [28,29]. Our result showed that AXIN1 had the significant action of inhibiting or reversing the EMT in HCC cells by targeting inhibition of miR-650.
Conclusion
The present study not only evaluated the functions of AXIN1 in HCC progression, but also provided a solid basis for us to explore the functional mechanism of AXIN1 and develop therapeutic strategies for HCC.
Acknowledgements
Suzhou medical science program (KJXW2018028); Suzhou key clinic disease program (LCZX 201711); Suzhou health key discipline construction (SZXK201808).
Disclosure of conflict of interest
None.
References
- 1.Jiang JW, Chen XH, Ren Z, Zheng SS. Gut microbial dysbiosis associates hepatocellular carcinoma via the gut-liver axis. Hepatobiliary Pancreat Dis Int. 2019;18:19–27. doi: 10.1016/j.hbpd.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Herszényi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249–58. [PubMed] [Google Scholar]
- 3.Wang L, Wang FS. Clinical immunology and immunotherapy for hepatocellular carcinoma: current progress and challenges. Hepatol Int. 2019;13:521–533. doi: 10.1007/s12072-019-09967-y. [DOI] [PubMed] [Google Scholar]
- 4.Lee DW, Jang MJ, Lee KH, Cho EJ, Lee JH, Yu SJ, Kim YJ, Yoon JH, Kim TY, Han SW, Oh DY, Im SA, Kim TY. TTP as a surrogate endpoint in advanced hepatocellular carcinoma treated with molecular targeted therapy: meta-analysis of randomised controlled trials. Br J Cancer. 2016;115:1201–1205. doi: 10.1038/bjc.2016.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddique O, Yoo ER, Perumpail RB, Perumpail BJ, Liu A, Cholankeril G, Ahmed A. The importance of a multidisciplinary approach to hepatocellular carcinoma. J Multidiscip Healthc. 2017;10:95–100. doi: 10.2147/JMDH.S128629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu MJ, He XX, Bi NR, Wang MM, Xiong ZF, Yang SL. Effects of liver-targeted drugs on expression of immune-related proteins in hepatocellular carcinoma cells. Clin Chim Acta. 2018;485:103–105. doi: 10.1016/j.cca.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Callegari E, Gramantieri L, Domenicali M, D’Abundo L, Sabbioni S, Negrini M. MicroRNAs in liver cancer: a model for investigating pathogenesis and novel therapeutic approaches. Cell Death Differ. 2015;22:46–57. doi: 10.1038/cdd.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timis TL, Orasan RI. Understanding psoriasis: role of miRNAs. Biomed Rep. 2018;9:367–374. doi: 10.3892/br.2018.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jun L, Yang G, Zhisu L. The utility of serum exosomal microRNAs in hepatocellular carcinoma. Biomed Pharmacother. 2019;111:1221–1227. doi: 10.1016/j.biopha.2018.12.131. [DOI] [PubMed] [Google Scholar]
- 10.Yun Z, Meng F, Jiang P, Yue M, Li S. MicroRNA-548b suppresses aggressive phenotypes of hepatocellular carcinoma by directly targeting high-mobility group box 1 mRNA. Cancer Manag Res. 2019;11:5821–5834. doi: 10.2147/CMAR.S198615. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.You Q, Li H, Liu Y, Xu Y, Miao S, Yao G, Xue Y, Geng J, Jin X, Meng H. MicroRNA-650 targets inhibitor of growth 4 to promote colorectal cancer progression via mitogen activated protein kinase signaling. Oncol Lett. 2018;16:2326–2334. doi: 10.3892/ol.2018.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orlandella FM, Mariniello RM, Iervolino PLC, Imperlini E, Mandola A, Verde A, De Stefano AE, Pane K, Franzese M, Esposito S, Basolo F, Orrù S, Salvatore G. MiR-650 promotes motility of anaplastic thyroid cancer cells by targeting PPP2CA. Endocrine. 2019;65:582–594. doi: 10.1007/s12020-019-01910-3. [DOI] [PubMed] [Google Scholar]
- 13.Han LL, Yin XR, Zhang SQ. MiR-650 promotes the metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by directly inhibiting LATS2 expression. Cell Physiol Biochem. 2018;51:1179–1192. doi: 10.1159/000495495. [DOI] [PubMed] [Google Scholar]
- 14.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–92. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 15.Mazzoni SM, Fearon ER. AXIN1 and AXIN2 variants in gastrointestinal cancers. Cancer Lett. 2014;355:1–8. doi: 10.1016/j.canlet.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu ZQ, Brabletz T, Fearon E, Willis AL, Hu CY, Li XY, Weiss SJ. Canonical Wnt suppressor, Axin2, promotes colon carcinoma oncogenic activity. Proc Natl Acad Sci U S A. 2012;109:11312–7. doi: 10.1073/pnas.1203015109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L, Yang L, Li YJ, Mei R, Yu HL, Gong Y, Du MY, Wang F. MicroRNA-128 protects dopamine neurons from apoptosis and upregulates the expression of excitatory amino acid transporter 4 in parkinson’s disease by binding to AXIN1. Cell Physiol Biochem. 2018;51:2275–2289. doi: 10.1159/000495872. [DOI] [PubMed] [Google Scholar]
- 18.Yang YF, Zhang MF, Tian QH, Zhang CZ. TRIM65 triggers β-catenin signaling via ubiquitylation of Axin1 to promote hepatocellular carcinoma. J Cell Sci. 2017;130:3108–3115. doi: 10.1242/jcs.206623. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Tighe SP, Iqbal U, Fernandes CT, Ahmed A. Treatment of inoperable hepatocellular carcinoma with immunotherapy. BMJ Case Rep. 2019;12 doi: 10.1136/bcr-2019-229744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai XR, Wang LH, Ren JQ, Bai XW, Zeng LW, Shen AG, Hu JM. Accurate clinical diagnosis of liver cancer based on simultaneous detection of ternary specific antigens by magnetic induced mixing surface-enhanced raman scattering emissions. Anal Chem. 2019;91:2955–2963. doi: 10.1021/acs.analchem.8b05153. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Zhang P, Wang Y, Zhang Y, Li K, Song Y, Su M, Zhou B, Zhang L. Association between AXIN1 gene polymorphisms and bladder cancer in Chinese han population. Dis Markers. 2019;2019:3949343. doi: 10.1155/2019/3949343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anfossi S, Fu X, Nagvekar R, Calin GA. MicroRNAs, regulatory messengers inside and outside cancer cells. Adv Exp Med Biol. 2018;1056:87–108. doi: 10.1007/978-3-319-74470-4_6. [DOI] [PubMed] [Google Scholar]
- 24.Prossomariti A, Piazzi G, D’Angelo L, Miccoli S, Turchetti D, Alquati C, Montagna C, Bazzoli F, Ricciardiello L. MiR-155 is downregulated in familial adenomatous polyposis and modulates WNT signaling by targeting AXIN1 and TCF4. Mol Cancer Res. 2018;16:1965–1976. doi: 10.1158/1541-7786.MCR-18-0115. [DOI] [PubMed] [Google Scholar]
- 25.You Q, Li H, Liu Y, Xu Y, Miao S, Yao G, Xue Y, Geng J, Jin X, Meng H. MicroRNA-650 targets inhibitor of growth 4 to promote colorectal cancer progression via mitogen activated protein kinase signaling. Oncol Lett. 2018;16:2326–2334. doi: 10.3892/ol.2018.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen J, Yu Z, Li N. The E3 ubiquitin ligase RNF146 promotes colorectal cancer by activating the wnt/β-catenin pathway via ubiquitination of Axin1. Biochem Biophys Res Commun. 2018;503:991–997. doi: 10.1016/j.bbrc.2018.06.107. [DOI] [PubMed] [Google Scholar]
- 27.Ningning S, Libo S, Chuanbin W, Haijiang S, Qing Z. MiR-650 regulates the proliferation, migration and invasion of human oral cancer by targeting growth factor independent 1 (Gfi1) Biochimie. 2019;156:69–78. doi: 10.1016/j.biochi.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Lei B, Huang Q. SOX18 affects cell viability, migration, invasiveness, and apoptosis in hepatocellular carcinoma (HCC) cells by participating in epithelial-to-mesenchymal transition (EMT) progression and adenosine monophosphate activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) Med Sci Monit. 2019;25:6244–6254. doi: 10.12659/MSM.915729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Y, Lv B, Xie J, Zhang Y, Lin Y, Wang S, Zhong J, Chen Y, Peng Y, Ma J. Plumbagin promotes human hepatoma SMMC-7721 cell apoptosis via caspase-3/vimentin signal-mediated EMT. Drug Des Devel Ther. 2019;13:2343–2355. doi: 10.2147/DDDT.S204787. [DOI] [PMC free article] [PubMed] [Google Scholar]