Abstract
Dialysis disequilibrium syndrome (DDS) is a rare syndrome characterised by neurological symptoms related to cerebral oedema. New patients who are started on haemodialysis are at the greatest risk for developing dialysis disequilibrium syndrome. Classical DDS develops during or immediately after haemodialysis. It is a generally self-limiting condition and settles with supportive management. Our case report describes DDS in a patient on chronic haemodialysis. She developed a tonic-clonic seizure shortly after completing 4 h of haemodialysis. This occurred in the context of having missed one session of dialysis, but with no new changes made to her usual dialysis regime. She was managed supportively in the intensive care unit and made a full recovery.
Keywords: Cerebral oedema, dialysis disequilibrium, haemodialysis
Background
Dialysis disequilibrium syndrome (DDS) has become a disappearing entity owing to strategies and protocols put in place in dialysis centres to reduce its incidence. Its occurrence over the last decade has been vastly scarce. When it does occur, patients having their first haemodialysis (HD) session are mostly susceptible. This case report describes an unusual case of DDS occurring in a patient on chronic HD for three years. There was no change in her usual HD regime, but she developed DDS having missed one session of HD.
Case presentation
A 72-year-old female presented to the emergency department with confusion and bradycardia. She had a background of end-stage renal failure secondary to hypertension and was on haemodialysis (HD) three times per week for many years. Her left brachial fistula through which she had HD had been partially thrombosed, and attempts at HD two days prior to presenting had been unsuccessful.
On examination, she had a Glasgow Coma Scale (GCS) of 14 (E4V4M6). Her bloods showed a metabolic acidosis, hyperkalaemia of 9 mmol/L, a urea of 62 mmol/L and creatinine of 1876 µmol/L. Her electrocardiography showed widespread features of hyperkalaemia.
She was transferred to the renal unit, where HD was initiated via her newly inserted femoral vascath. She had HD for a total of 4 h; pump speed at 300 mL/min, with a total of 1 L of fluid removed.
Immediately on completion of HD, the patient had a witnessed tonic-clonic seizure which lasted 30 s and self-terminated. She subsequently had a respiratory arrest secondary to this seizure with a corresponding GCS of 3. Her post-seizure venous blood gas was as follows: pH – 6.82; pCO2 – 10; pO2 – 9.7; K – 3.43; Na – 138; HCO3 – 13.3; BE: – 20.5; lactate – 15.
She was intubated and ventilated by the intensive care team and transferred to CT.
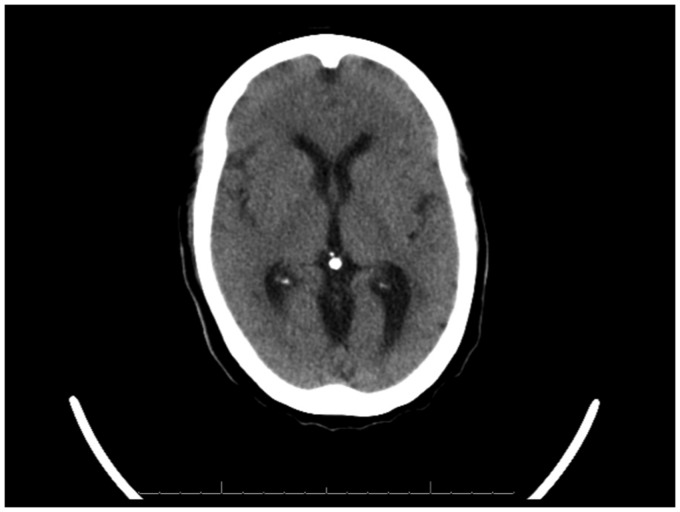
Her CT-head showed features of cerebral oedema (Figure 1). Her blood glucose, electrolytes (including sodium, potassium, calcium, magnesium and chloride) and renal function were all within normal levels. On this basis, a diagnosis of DDS was made.
Figure 1.
Non-contrasted CT-head showing features of cerebral oedema post-HD.
She was transferred to the intensive care unit for supportive management. She was managed with controlled short periods of continuous venovenous hemodiafiltration (CVVHDF) via a right femoral vascath and with vasopressors. Her blood tests returned to their baseline levels.
Following 24 h of intermittent CVVHDF, her sedation was weaned and she was successfully extubated. Her GCS upon discharge from intensive care was 15.
Differential diagnosis
DDS remains a clinical diagnosis, which relies on cerebral imaging to rule out other potential diagnoses. Therefore, it remains a diagnosis of exclusion. Diagnoses such as uraemia itself, subdural haematoma, cerebral infarction, intracerebral haemorrhage, meningitis, metabolic disturbances (hypo/hypernatraemia, hypo/hypercalcaemia and hypoglycaemia) and drug-induced encephalopathy should also be considered.
Outcome and follow-up
The patient continued to have her usual regime of HD via her vascath. In the interim, she was referred to the vascular team to salvage her fistula for future HD which was accomplished.
Discussion
DDS is a rare syndrome characterized by neurological symptoms of varying severity that affect dialysis patients.1,2 It is believed to be due to cerebral oedema. Although the incidence of DDS is poorly defined, most reports state that there is a decline in its incidence.2 Some reports suggest, however, that due to the broad spectrum of clinical manifestations, the syndrome is underreported.3
Many risk factors exist for DDS. New patients who are just being started on intermittent HD are at greatest risk for DDS, particularly if the blood urea is markedly elevated (>60 mmol/L). Other predisposing factors include severe metabolic acidosis, older age, chronic kidney disease, paediatric patients, pre-existing neurologic conditions (such as head trauma, stroke or seizure disorder), conditions that are associated with cerebral oedema (such as hyponatraemia, hepatic encephalopathy and malignant hypertension), or any condition that increases permeability of the blood–brain barrier (such as sepsis, vasculitis, thrombotic thrombocytopenic purpura/haemolytic uraemic syndrome, encephalitis or meningitis).1–5 In our patient, we see that she had many risk factors for the development of DDS – a raised blood urea, metabolic acidosis, older age and chronic kidney disease.
The symptoms of DDS are caused by water movement into the brain, leading to cerebral oedema.4 Two theories of pathogenesis exist, with the former being substantiated more so than the latter:
Reverse osmotic shift: Haemodialysis rapidly removes small solutes such as urea. The reduction in blood urea lowers the plasma osmolality, thereby creating a transient osmotic gradient that promotes water movement into cells. In the brain, this water shift produces cerebral oedema. This has been demonstrated by experiments in uraemic rats.6–8 Furthermore, animal studies have suggested that in the context of uraemia, there may be a decrease in urea transporters and an increase in water channels which can potentiate this osmotic gradient.9
Intracerebral acidosis and idiogenic osmoles: Because urea is a highly permeable solute, some investigators have suggested an alternative hypothesis for the development of cerebral oedema. They remark that urea movement out of the brain is sufficiently rapid, and therefore a large osmotic gradient between the brain and extracellular fluid does not exist to produce cerebral oedema.1 Instead, they propose that a decrease in cerebral pH by an increase in the production of organic acids (via an unclear mechanism) is the primary aetiological feature.1,8 A decrease in intracerebral pH results in the displacement of bound sodium and potassium ions by the excess hydrogen ions. This promotes an increase in intracellular osmolality and results in water movement into the brain.10
Classic DDS develops during or immediately after HD11 as seen in our case. Our patient developed tonic-clonic seizure just moments after completing 4 h of HD. The early findings of DDS include headache, nausea, disorientation, restlessness, blurred vision and asterixis. More severely affected patients progress to confusion, seizures, coma and even death. Milder signs and symptoms include muscle cramps, anorexia and dizziness. These milder symptoms can explain why the syndrome may be underreported.
Measures to prevent DDS should be used among patients at high risk. The most important preventive measure is to limit the reduction in urea per treatment so that there is a gradual reduction that is distributed over several days. Slow urea removal can be achieved by the following methods:
Using a relatively low initial blood flow rate, e.g. 150 to 250 mL/min with a small surface area dialyser (0.9 to 1.2 m2) for 1–2 h. This regimen can be repeated daily for a few days. If the patient shows no signs of DDS, the blood flow rate can be increased (in 50 mL/min increments) and duration of HD can be increased (in 30-min increments).12
Patients who also have marked fluid overload can be treated with ultrafiltration (which removes less urea per unit time), followed by a short period of HD.12 In our patient, short controlled periods of CVVHDF allowed for less urea movement per unit time.
In patients with extremely elevated urea or neurological symptoms, HD should be initiated as an inpatient.
Because our patient had at least four risk factors for developing DDS, she can be classed as being at high risk for developing DDS. It is likely that the faster rate of urea reduction with flow rates of 300 mL/min was responsible for her developing cerebral oedema, according to the reverse osmotic shift theory. Her seizure self-terminated which is typical of the symptoms of DDS – most symptoms of DDS are self-limiting and usually dissipate within several hours owing to the highly permeable nature of urea.10
When a patient develops features of DDS, the treatment depends upon the severity of symptoms13:
Patients with mild, non-specific symptoms, e.g. nausea, vomiting, are treated symptomatically. If these also are acutely uraemic, then the blood flow rate should be reduced or the HD sessions stopped.
In patients who develop seizures or coma, HD should be stopped immediately, and the patency of the airway should be maintained. In this setting, causes of these symptoms other than severe DDS should be considered.
Hypertonic saline or mannitol is not recommended for the treatment of DDS.4
From the intensivist point of view, it is generally a self-limiting condition and would settle with supportive management.
Learning points/take home messages
DDS can present as a spectrum of neurological symptoms related to cerebral oedema. New patients being started on HD are at the greatest risk for developing DDS.
DDS is rare, as measures have been initiated in dialysis centres to reduce its incidence; lower blood flow rates are used and smaller surface area dialysers.
The pathogenesis of DDS is probably related to a rapid reduction in urea by HD. This lowers the plasma osmolality of the blood. Water therefore moves into cerebral cells, which results in cerebral oedema.
DDS is a diagnosis of exclusion. Diagnoses such as uraemia itself, subdural haematoma, cerebral infarction, intracerebral haemorrhage, meningitis, metabolic disturbances (hypo/hypernatraemia, hypo/hypercalcaemia and hypoglycaemia) and drug-induced encephalopathy should also be considered.
When DDS is suspected, dialysis should be stopped. It is managed supportively with a key focus on airway patency. Neither hypertonic saline nor mannitol should be used in its management.
Consent
Published with the written consent of the patient.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Arieff AI. Dialysis disequilibrium syndrome: current concepts on pathogenesis and prevention. Kidney Int 1994; 45: 629. [DOI] [PubMed] [Google Scholar]
- 2.Patel N, Dalal P, Panesar M. Dialysis disequilibrium syndrome: a narrative review. Semin Dial 2008; 21: 493. [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw SM, Peets AD, Hameed M, et al. Dialysis disequilibrium syndrome: brain death following hemodialysis for metabolic acidosis and acute renal failure – a case report. BMC Nephrol 2004; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zepeda-Orozco D, Quigley R. Dialysis disequilibrium syndrome. Pediatr Nephrol 2012; 27: 2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall MR, Golper TA. Low-efficiency acute renal replacement therapy: role in acute kidney injury. Semin Dial 2011; 24: 142. [DOI] [PubMed] [Google Scholar]
- 6.Silver SM, DeSimone JA, Jr, Smith DA, et al. Dialysis disequilibrium syndrome (DDS) in the rat: role of the “reverse urea effect”. Kidney Int 1992; 42: 161. [DOI] [PubMed] [Google Scholar]
- 7.Silver SM, Sterns RH, Halperin ML. Brain swelling after dialysis: old urea or new osmoles? Am J Kidney Dis 1996; 28: 1. [DOI] [PubMed] [Google Scholar]
- 8.Galons JP, Trouard T, Gmitro AF, et al. Hemodialysis increases apparent diffusion coefficient of brain water in nephrectomized rats measured by isotropic diffusion-weighted magnetic resonance imaging. J Clin Invest 1996; 98: 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinh-Trang-Tan MM, Cartron JP, Bankir L. Molecular basis for the dialysis disequilibrium syndrome: altered aquaporin and urea transporter expression in the brain. Nephrol Dial Transplant 2005; 20: 1984. [DOI] [PubMed] [Google Scholar]
- 10.Ali II and Pirzada NA. Neurologic complications associated with dialysis and chronic renal insufficiency. In: Henrich WL (ed.) Principles and practice of dialysis. Philadelphia: Lippincott, Williams & Wilkins, 2004, p.507.
- 11.Bellomo R, Cass A, et al. RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009; 361: 1627. [DOI] [PubMed] [Google Scholar]
- 12.Silver SM. Cerebral edema after rapid dialysis is not caused by an increase in brain organic osmolytes. J Am Soc Nephrol 1995; 6: 1600. [DOI] [PubMed] [Google Scholar]
- 13.Bergman H, Daugirdas JT and Ing TS. Complications during hemodialysis. In: Daugirdas JG, Blake PG and Ing TS (eds) Handbook of dialysis. Topic 1851 Version 13.0. Philadelphia: Lippincott, Williams & Wilkins, 2001, p.158.