Abstract
Liver cancer is the second most lethal cancer in the world with limited treatment options. Hepatocellular carcinoma (HCC), which accounts for more than 80% of all liver cancers, has had increasing global incidence over the past few years. There is an urgent need for novel and better therapeutic intervention for HCC patients. The JAK/STAT signaling pathway plays a multitude of important biological functions in both normal and malignant cells. In a subset of HCC, JAK/STAT signaling is aberrantly activated, leading to dysregulation of downstream target genes that controls survival, angiogenesis, stemness, immune surveillance, invasion and metastasis. In this review, we will focus on the role of JAK/STAT signaling in HCC and discuss the current clinical status of several JAK/STAT inhibitors.
Keywords: : hepatocarcinogenesis, hepatocellular carcinoma, JAK/STAT signaling, liver cancer, targeted therapy
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the second most common cause of cancer-related deaths worldwide [1]. The global incidence of HCC has been increasing, with an estimated range of 600,000–800,000 new cases occurring annually [2–4]. HCC is a highly heterogenous disease with multiple risk factors and etiologies, including chronic hepatitis B or hepatitis C virus (HBV/HCV) infections, excessive alcohol consumption, aflatoxin exposure and diabetes or obesity-related metabolic syndromes, that vary depending on the geographic distribution [5]. Generally, the dominant risk factors for HCC in high incidence rate countries such as those in Asia and Africa are HBV infections and aflatoxin B exposure, whereas HVC virus infections, alcohol consumption and metabolic syndromes are more important risk factors in low incidence regions, which include countries in Europe, North and South America and the Middle East [6,7]. Nevertheless, about 70–80% of HCC cases develop from a background of liver cirrhosis, with a median time to development of 10 years [8–10]. The underlying diseased/cirrhotic liver contributes to the poor prognosis/high mortality of many HCC patients, along with the difficulty of early diagnosis and a lack of effective late-stage treatment options.
Currently, the types of interventions available for treatment of HCC vary depending on the stage of the disease. Early-stage HCC patients are amenable for potentially curative treatments, such as surgical resection and liver transplantation, while patients with intermediate stage HCC are often given locoregional therapies, which include radiofrequency ablation, transarterial chemoembolization and radioembolization [11]. Unfortunately, many HCC cases often present at advanced and unresectable stages and systemic therapy is usually the only viable option for such patients [12,13]. Currently, the clinical standard of care systemic treatment for advanced HCC is the small molecule inhibitor sorafenib, a multi-kinase inhibitor targeting RAF, VEGFR 2, VEGFR 3, PDGFRβ, c-KIT, FLT-3 and RET [14,15]. Since its approval by the US FDA in 2007, sorafenib has been the sole systemic drug for HCC for more than a decade. However, in recent years, several other drugs have also been approved for advanced HCC, including lenvatinib (an alternative first-line treatment), regorafenib, cabozantinib and ramucirumab (second-line treatments for sorafenib-refractory patients) [16–19]. Lenvatinib, regorafenib and cabozantinib are multi-kinase inhibitors, like sorafenib, whereas ramucirumab is a monoclonal antibody against VEGFR2. Important targets of lenvatinib include VEGFR 1-3, FGFR 1-4, PDGFRα, RET and c-KIT; for regorafenib, VEGFR 1-3, PDGFRβ, FGFR 1, c-KIT, RET and B-RAF; and for cabozantinib, VEGFR 1-3, MET and AXL.
Nevertheless, while these drugs have shown clinical benefits, the improvements in patient survival and outcome remain marginal [5]. The effectiveness of these treatments has also been hampered by the development of drug resistance and underlying liver dysfunction [12,20]. Even for curative treatments, disease recurrence represents a major drawback, with a 5-year incidence rate of over 70% [21]. These factors explain why HCC remains a highly difficult cancer to treat. Hence, there is an urgent need to develop better therapeutic strategies, especially for advanced HCC patients. In this regard, given the multitude of molecular signaling pathways that contribute to HCC development, targeted therapy based on identification and understanding of these molecular mechanisms provides a promising alternative/approach for treatment of HCC.
JAK/STAT signaling pathway
Aberrant activation of various intracellular signaling pathways involved in cell growth, differentiation, apoptosis and survival have been found to contribute to HCC development and progression [22,23]. These include known oncogenic signaling pathways such as Wnt/β-catenin pathway, PI3K/Akt/mTOR pathway, Ras/Raf/MAPK pathway and JAK/STAT pathway [23].
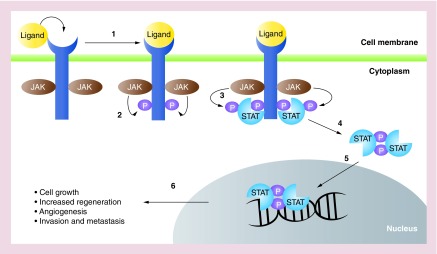
The JAK/STAT signaling pathway plays important roles in many cellular functions, including cell proliferation, stem cell maintenance and differentiation as well as modulation of the immune/inflammatory response [24]. JAK/STAT signaling has also been reported to regulate liver regeneration and gluconeogenesis [25]. The JAK/STAT pathway can be activated by various cytokines and growth factors, such as interleukins, interferons and EGF family members, which bind to their respective transmembrane receptors. The cytoplasmic tails of some of these receptors are associated with Janus kinases (JAKs) that become activated upon ligand-induced conformational change of the receptors. These activated JAKs then phosphorylate tyrosine residues on the cytoplasmic tail of the receptor, creating docking sites for a family of signal transducers and activators of transcription, known as STATs. Upon binding to the receptor, these STATs are phosphorylated by JAKs, become activated and form dimers, which then translocate to the cell nucleus. Subsequently, the STAT dimers recognize and bind to specific promoter sequences to activate transcription of their target genes, for example, CCND1, BIRC5 and Mcl-1 (Figure 1) [26].
Figure 1. . JAK/STAT signaling pathway overview.
(1) Ligands such as cytokines and growth factors bind to transmembrane receptors, activating receptor-associated JAKs. (2) JAKs phosphorylate cytoplasmic tails of receptors, (3) recruiting STATs to the receptor and become phosphorylated by JAKs. (4) Activated STATs dimerize and (5) translocate into the nucleus where they bind to DNA and (6) activate transcription of target genes such as those involved in regulating cell growth.
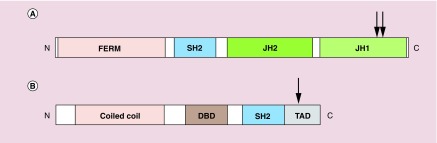
In humans, there are four members in the JAK family – JAK1, JAK2, JAK3 and TYK2. The JAK proteins contain two adjacent kinase domains that serve different functions (Figure 2A). JH1 domain performs the typical phosphorylation of STATs and receptors, while the JH2 domain regulates JH1 [24]. Additionally, JAKs also contain a FERM domain (4.1 protein, ezrin, radixin and moesin) that is responsible for interacting with receptors and a SH2 (Src homology 2) domain that binds to phosphorylated tyrosine residues [27,28].
Figure 2. . Schematic structures of JAK and STAT proteins.
(A) JAK proteins contain a FERM domain that associates with receptors, a SH2 domain that binds phosphorylated tyrosine residues and two kinase domains JH1 and JH2. Arrowheads indicate phosphorylation sites (tyrosine residues) required for JAK activation. (B) STAT proteins contain a coiled coil domain for dimerization, a DBD, a SH2 domain and a TAD for transcriptional activation of target genes. Arrowheads indicate the conserved tyrosine residue that needs to be phosphorylated for STAT activation. N and C represents the amino- and carboxy-terminal ends respectively.
DBD: DNA-binding domain; TAD: Transactivation domain.
The human STAT protein family comprises of seven members: STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6. These proteins share several functional domains, including the SH2 domain, which recognizes phosphorylated tyrosine residues on the receptors, and activated STAT proteins, and a coiled-coil domain, which enables dimerization of activated STATs as well as interaction with other proteins (Figure 2B) [27]. In addition to binding to DNA, the DNA-binding domain is also involved in nuclear translocation of STAT dimers. STATs also contain a C-terminal transactivation domain necessary for activation of transcription.
Homeostatic regulation of JAK/STAT signaling is mediated by negative regulators that work at multiple levels of the pathway. These include phosphatases that remove phosphate groups from JAKs and STATs, some SOCS proteins that can competitively bind to receptor binding sites of STATs and can target JAK/STATs for proteasomal degradation, as well as protein inhibitors of activated STAT (PIAS), which prevents DNA binding and nuclear translocation of STATs [24,29]. As transcription of SOCS genes are regulated by STATs, this negative feedback loop provides an additional level of control over the pathway and ensures that activation of JAK/STAT signaling is transient.
While the JAK/STAT pathway appears relatively simple compared with other intracellular signaling pathways, the diversity of ligands and receptors that can activate the pathway as well as the relationship between different JAKs and STATs contribute to its complexity and the range of cellular responses. For example, STAT3 and STAT5A/B have been found to promote cancer progression while STAT1 has tumor suppressive effects [24,30].
Many studies have shown that the JAK/STAT pathway is often deregulated in cancer, including HCC. In fact, STAT3 was reported to be constitutively active in up to 60% of the HCC cases [31]. An increase in inflammatory signaling, growth factor stimulation, oxidative stress and epigenetic silencing of SOCS genes were some of the contributing factors for the upregulated JAK/STAT signaling [31]. Furthermore, 9% of HBV-related HCC cases contained missense mutations in JAK1, which were found to increase phosphorylation of JAK1 and STAT3, allowing cytokine-independent growth [32].
The role of STAT3 in HCC
STAT3 is generally accepted as a bona fide oncogene in promoting HCC development. Activation of STAT3 as a transcription factor leads to the expression of several genes which contribute to the various hallmarks of cancer, highlighting the essential role of STAT3 in HCC (Figure 3).
Figure 3. . The role of STAT3 in hepatocellular carcinoma.
The regulation of target genes and proteins by STAT3 promotes the progression of hepatocellular carcinoma by contributing to key hallmarks of tumorigenesis. Shown in green are genes and proteins which are upregulated while genes in red are inhibited by STAT3 activation.
STAT3 in survival & proliferation
The oncogenic and proliferative potential of STAT3 was first reported in 1999 [33]. Cells with constitutively activated STAT3 express higher levels of CCND1, which drives cell cycle progression from the G1 to S phase [33]. The pharmacological inhibition of the JAK2/STAT3 pathway has shown a marked downregulation of CCND1 and growth arrest at the G0/G1 phase in HCC cell lines [34]. The proliferative properties of STAT3 were also observed in vivo. Nude mice injected with cells harboring STAT3 clones grew tumors at the site of injection as opposed to STAT3-negative cells [33]. Similarly, the introduction of STAT3-specific short-hairpin RNA in diethylnitrosamine-induced HCC mice models failed to induce tumor development, supporting the oncogenic role of STAT3 in HCC [35]. Conversely, the inhibition of STAT3 activity via antisense oligonucleotides (ASOs) successfully mitigated tumor growth, leading to a reduction in tumor volume and doubling of survival time in orthotopically-implanted HCCLM3 mice models of HCC [36].
In addition, STAT3 is known to drive the expression of anti-apoptotic genes such as BCL2, BCL2L1, BIRC5 and MCL1 [37–40]. Simultaneously, STAT3 inhibits the expression of pro-apoptotic proteins such as TP53, BAX and CHOP [41,42]. Inhibition of STAT3 by JAK2 inhibitor, AG490, further induced apoptosis in HCC cell line, Hep3B, by downregulating the expression of anti-apoptotic proteins Bcl-xL and survivin [34]. Additionally, STAT3 has also been implicated in the development of sorafenib resistance in HCC cell line Huh7 by the regulation of anti-apoptotic protein, Mcl-1 [43]. The overexpression of JAK1/2 and constitutive phosphorylation of STAT3 (Tyr705) results in the nuclear localization of STAT3 and expression of Mcl-1 [43]. Knockdown of STAT3 led to a downregulation of Mcl-1 expression in vitro, rendering the cells sensitive to sorafenib-induced cell death. Collectively, these findings demonstrate that STAT3 promotes cell survival and drug resistance while allowing HCC cells to evade apoptosis and continue proliferating.
STAT3 in angiogenesis
STAT3 can promote angiogenesis by regulating the expression of several pro-angiogenic modulators in the tumor microenvironment. These are traditionally attributed to the bFGF, VEGF and HIF-1 axis [28,44,45]. STAT3 activation was shown to regulate the expression of Akt, a key regulator of Hif-1 expression [45]. Hif-1, together with STAT3, function as transcriptional activators of VEGF by binding to the VEGF promoter [45]. VEGF secreted by the cells can then bind to receptors on endothelial cells and stimulate the formation of new blood vessels. Conversely, inhibition of STAT3 has also been shown to downregulate the activation of the PI3K/Akt pathway and subsequent Hif-1 and VEGF expression [44,45]. The use of anti-STAT3 ASOs can also lead to a reduction in tumor microvessel density as a result of decreased circulating VEGF and bFGF levels in vivo [36]. Therefore, elevated STAT3 levels facilitate tumor development by upregulating pro-angiogenic factors, thereby providing tumors with greater perfusion and promoting tumor growth.
STAT3 in immunity & inflammation
STAT3 plays a key role in regulating the inflammatory and immune environment of the tumor. STAT3 activation in tumor cells aids in evasion of immune surveillance during hepatocarcinogenesis through several mechanisms, one of which is by maintaining an activation loop with the immune cells present in the tumor microenvironment. Activation of STAT3 in tumor cells by IL-6 triggers a downstream inflammatory response, inducing the expression and secretion of STAT3-activating cytokines and chemokines such as IL-6 and IL-1b [46]. Consequently, these secreted factors activate STAT3 signaling in the surrounding stromal cells, which also synthesize and secrete the same cytokines, resulting in a paracrine tumor-stroma positive feedback or activation loop [47].
This continuous activation of STAT3 modulates the tumor immune microenvironment, ensuring that it is conducive for the tumor cell. The activation of STAT3 in regulatory dendritic cells derived from carcinoma-associated fibroblasts leads to increased secretion of IDO in vitro [48]. IDO impairs T-cell proliferation and response, promoting the survival of the tumor cells. Additionally, STAT3 induces the differentiation of monocytes into myeloid-derived suppressor cells in vitro, further impeding T-cell function and suppressing the antitumor immune response [49].
Apart from IL-6, immune cells may also respond to other interleukin and inflammatory molecules present in the microenvironment, leading to STAT3 activation. For instance, the presence of IL-4 leads to the activation of STAT3 in macrophages, inducing a polarization from the antitumoral M1 phenotype into the pro-tumorigenic M2 phenotype both in vitro and in vivo [50]. The presence of M2 macrophages in the microenvironment promotes proliferation, invasion and migration of HCC cells [50].
The tumor-promoting effects of STAT3 signaling is further established when tumor progression is inhibited upon blockade of the IL-6/STAT3 pathway, resulting in an alteration of the cytokines present in the microenvironment. Specifically, TGF-β and IL-10 levels were reduced while type I interferon expression was elevated, which reactivates natural killer cells and recovers the antitumor immune response [51].
In summary, STAT3 activation is essential in modulating the immune cells and cytokines present in the tumor microenvironment to ensure that the tumor cells can evade apoptosis and survive. Targeting STAT3 therefore offers a potential immunotherapy for HCC by suppressing the tumor immune microenvironment.
STAT3 in cancer stem cells
The activation of STAT3 has been demonstrated to correlate with cancer stem cell markers that confer stem cell-like properties to tumor cells. STAT3 activation has been shown to correlate with the self-renewing side population/CD44-positive (SP/CD44+) cells in HCC [52]. CD44 is traditionally reported to maintain cell populations with cancer stem cell-like properties in HCC [53]. Inhibition of STAT3 via small molecule inhibitors significantly reduced the SP/CD44+ cells in vitro and diminished the tumor formation capacity in vivo. This demonstrates the potential of targeting STAT3 in controlling the population of cells containing stem cell-like properties in HCC [52]. Additionally, STAT3 phosphorylation and activation have also been reported to regulate the expression of other cancer stem cell markers in HCC, namely CD133 and NANOG [54,55]. Inhibition of STAT3 led to a reduction in the population of cancer stem-like cells in vitro and impeded the tumor-initiating capacity of HCC cells in vivo. STAT3 has also been implicated in the maintenance of the stem cell-like population of cells in HCC via the upregulation of the Notch signaling pathway [56]. Attenuation of the IL-6/STAT3 pathway led to a deactivation of the Notch pathway, hindering the growth and invasion of HCC cells [56]. Collectively, the evidence demonstrates the role of STAT3 in maintaining the population of cells with stem cell-like and tumor initiating capacities in HCC. Thus, targeting STAT3 offers a promising strategy to reduce the population of cancer stem cells with self-renewal capabilities in HCC tumors.
STAT3 in HCC metabolism
STAT3 is implicated in the adaptation of metabolic processes in cancer cells to allow efficient generation of energy biomolecules like ATP [57]. Bi et al. showed that STAT3 and PKM2 can be activated and enhance the Warburg effect in HCC [58]. The Warburg effect occurs when cancerous cells transform significant amounts of glucose into lactate regardless of oxygen availability. PKM2 is a key enzyme that regulates this process, which allows tumor cells to meet the energetic demands for expansive proliferation. Bi et al. observed that in transformed hepatic progenitor cells, the consumption of glucose, production of lactate and ATP levels were all decreased following the use of a small molecule inhibitor of STAT3 (Stattic) in vitro [58]. Additionally, this inhibition of STAT3 also decreased phospho-PKM2 expression [58]. This suggests that STAT3 regulates the expression of PKM2 and, in turn, plays a key role in altering cancer cell metabolism to meet the energetic demands of the disease. Targeting STAT3 can thus help to mitigate this aspect of HCC and reduce tumor growth.
STAT3 in invasion & metastasis
STAT3 plays a role in promoting the invasive capacities of HCC cells by regulating the expression of MMPs, such as MMP-2 and MMP-9 [36,59]. Secreted MMPs cleave the extracellular matrix in the tumor microenvironment, removing the physical barrier for cancer cells to invade into the surrounding tissue. Li et al. showed that anti-STAT3 ASOs reduced MMP-2 and MMP-9 expression levels in vitro [36]. Consequently, this inhibited lung metastasis formation and significantly prolonged survival time [36]. Zhao et al. also demonstrated that inhibition of STAT3 phosphorylation by CTS successfully impeded the invasion of HCC cells which was induced by peri-tumor fibroblasts [60]. Besides, STAT3 can also induce the invasiveness of HCC tumors by upregulating the expression of epithelial–mesenchymal transition proteins such as Slug and Twist [61,62]. In fact, targeting STAT3 led to a reduction in these proteins, while simultaneously increasing the expression of adhesion protein, E-cadherin, to reduce the metastatic potential of the cells both in vitro and in vivo [63].
Furthermore, STAT3 may promote the invasiveness of HCC by regulating alternative oncogenic pathways involved in metastasis. One such pathway is the PI3K/Akt2 signaling pathway, which modulates cell adhesion and invasion both in vitro and in vivo [64,65]. Zhang and colleagues demonstrated that STAT3 performs this function by regulating the expression of AKT2 [65]. HCC cells transfected with STAT3-specific small interfering RNAs (siRNAs) induced a downregulation of AKT2 expression along with its target genes, leading to a significant reduction in the invasive properties of the cells in vitro [65]. The direct crosstalk between the JAK/STAT and PI3K/Akt2 pathway further supports the role of STAT3 in promoting invasion and metastasis in HCC.
Overall, these studies demonstrate that STAT3 is a key regulator in the progression and development of HCC through its involvement in various aspects of tumorigenesis and metastasis. Thus, STAT3 offers a viable therapeutic target for treatment of HCC patients, allowing for early intervention by clinicians before the disease worsens.
The role of other STATs in HCC
Previous studies have shown that STAT1 and STAT2 exhibit antiproliferative effects in HCC both in vitro and in vivo [66]. The suppression of STAT1 activity was shown to correlate with the progression of HCC and prognosis in a set of HCC patient samples [67]. Additionally, suppression of STAT1 activity correlated with VEGF levels in HCC patients, indicating that STAT1 may exert its antitumorigenic properties by inhibiting angiogenesis [67]. Notably, the antitumorigenic properties of STAT1 correlate with the activity of STAT1 instead of the expression levels of the protein itself. In a recent study, the authors found that elevated expression of STAT1 without activation (i.e., unphosphorylated STAT1) was observed in HCC patients [68]. The presence of unphosphorylated STAT1 was able to sustain growth in HCC cell lines in vitro [68]. This is an important finding in guiding the development of drug targets against STAT1 as a therapeutic strategy. Specifically, it demonstrates that inducing STAT1 expression is not sufficient in treating HCC and might worsen the disease. Instead, specific activation of STAT1 serves as a more viable therapeutic strategy for HCC.
Similar to STAT1, STAT2 exhibits anticancer properties in HCC. STAT2 can exert its antitumorigenic properties in HCC by functioning as a transcription regulator of oncogenes. Testoni et al. showed that, in response to IFN-α induction, phosphorylated STAT2 directly binds to the the P2p73 promoter of oncogene DNp73 [69]. As a result, STAT2 recruits Ezh2 to the promoter to induce histone 3 lysine 27 methylation and hence the transcriptional repression of the oncogene [69]. The detailed antitumorigenic roles of STAT1 and STAT2 in HCC, however, still remain relatively unclear.
While the detailed role and function of STAT4 in HCC is not well established, several publications have demonstrated that STAT4 likely functions as a tumor suppressor in HCC. In these studies, the authors reported a significantly lower expression level of STAT4 in HCC tumors as compared with the normal tissues [70,71]. Furthermore, the knockdown of STAT4 via siRNAs in these two independent studies led to enhanced proliferation of HCC cell lines in vitro. In addition, several studies investigating polymorphisms in HCC patients have identified a specific STAT4 polymorphism (rs7574865) which increases the risk of HBV-related HCC in several cohorts, including Chinese, Thai, Korean, Vietnamese and Caucasian. This specific rs7574865 STAT4 variant also showed a corresponding reduction in STAT4 mRNA expression levels [72–76]. However, there are still no conclusive studies detailing the exact role of the variant.
The distinct functions of both isoforms of STAT5 (STAT5a and STAT5b) in HCC is still unclear and shows context specificity. Upregulation of STAT5 has been observed in HCC patients, suggesting that STAT5 exhibits pro-oncogenic properties [77]. Specifically, one group identified that STAT5b, and not STAT5a, was a driver of epithelial–mesenchymal transition in HBV-dependent HCC in vitro [78]. Several mouse models of HCC, however, have suggested that STAT5 exhibits hepatoprotective properties instead [66,79–81]. One group showed that STAT5 acts as a tumor suppressor in the context of hyperactive growth hormone signaling. In the study, the authors demonstrated that mice with hyperactive growth hormone signaling and a synthetic loss of STAT5 rapidly developed HCC as a result of increased compensatory STAT3 activation [79]. In a second study, Yu et al. demonstrated that in CCl4-induced HCC mouse models, STAT5 functions as a tumor suppressor by upregulating the expression of CDKN2B and CDKN1A [81]. Ablation of STAT5 in vivo led to a reduction in the p15INK4B protein levels, a compensatory activation of STAT3 and tumor progression [81]. Kaltenecker et al., however, did not witness a more aggressive HCC phenotype when STAT5 was lost in their diethylnitrosamine-induced mice models [82]. Taking the evidence together, they suggest that the function of STAT5 is extremely complex and context-dependent, thereby calling for deeper investigation into the role of STAT5 in HCC in humans.
Targeting the JAK/STAT pathway for HCC treatment
Different JAK/STAT inhibitors are already being studied for their clinical relevance in various cancers, including HCC. Two main classes of molecules that have been used are small molecule inhibitors and siRNAs (Table 1).
Table 1. . Clinical status of Jak/STAT inhibitors.
| Classification: small molecule inhibitors | Indication | Target | Clinical status | Findings/results | Ref. |
|---|---|---|---|---|---|
| WP1066 | Bladder cancer Malignant glioma Metastatic melanoma HCC |
JAK2 | Phase I (NCT01904123) (Pre-clinical for HCC) |
In vitro studies: in bladder and brain cancer cells, pre-clinical studies show promotion of apoptosis. Awaiting results for metastatic melanoma and malignant glioma in clinical trials (NCT01904123). In HCC: WP1066 was shown to inhibit MMPs and reduce migration and invasion of HCC cancer cells. |
[83–85] |
| Pacritinib | Malignant glioma Myelofibrosis HCC |
JAK2 | Phase III (NCT02055781) (Pre-clinical for HCC) |
In vitro studies: pacritinib decreased BTIC viability and sphere-forming potential. Improved response to TMZ in TMZ-resistant BTICs was also observed. In vivo studies: in orthotopically xenografted mice, pacritinib combined with TMZ showed penetration of the blood-brain barrier and led to overall median survival improvement. Phase III: for myelofibrosis and thrombocytopenia patients including those prior anti-Jak therapy, the twice-daily dosing of pacritinib was more effective than the best available treatment for reducing splenomegaly and symptoms. In HCC: in vivo studies showed fibrosis biomarker CK18 was effectively reduced by pacritinib. Fibrotic areas were also reduced in the mouse liver. |
[86–88] |
| CTS | Esophageal cancer HCC |
JAK2, STAT3 |
Pre-clinical |
In vitro and in vivo studies: migration and tumor growth of esophageal cancer cells was impeded with CTS. Inhibition of cell growth in mice xenografts was also observed without significant effect on body weight. In HCC: CTS was shown to promote apoptosis and immune response in vitro and in vivo. CTS also helped convert immune cells to the tumor suppressive M1 phenotype. |
[60,89,90] |
| Ruxolitinib (INCB018424) |
Leukemias HCC |
JAK1/2 | Approved Phase II (NCT00674479) (Pre-clinical for HCC) |
Approved for myelofibrosis, polycythemia vera, graft-vs-host disease. Phase II: modest antileukemia activity and an acceptable toxicity profile were seen in refractory leukemias. This includes post myeloproliferative neoplasm acute myeloid leukemia patients. In HCC (preclinical): Ruxolitinib was shown to inhibit colony-forming abilities and cell proliferation. |
[91,92] |
| Stattic | NPC HCC |
STAT3 | Pre-clinical |
In vitro studies: reduced growth and increased apoptosis of NPC was observed with Stattic. The drug also sensitized NPC to cisplatin and ionizing radiation. In HCC: Stattic-attenuated radiotherapy and reduced cancer functions such as invasiveness, survival and proliferation. |
[93,94] |
| OPB-111077 | Advanced HCC | STAT3 | Phase I (NCT01942083) |
The drug was compatible with advanced HCC patients that failed sorafenib therapy. Limited preliminary efficacy outcomes were shown. | [95] |
| OPB-31121 | Advanced cancer Solid tumor HCC |
STAT3 | Phase I (NCT00657176) PhaseI/II (NCT01406574) |
(NCT00657176): OPB-31121 was relatively well tolerated and has preliminary antitumor activity in solid tumors. In HCC (NCT01406574): limited survival benefits and insufficient antitumor activity were shown |
[96,97] |
| Napabucasin (BBI608) | Gastric cancer HCC |
STAT3 | + Paclitaxel: Phase I (JapicCTI-142420) Phase III (NCT02178956) + Sorafenib: Phase Ib/II (NCT02279719) |
(JapicCTI-142420): for Japanese patients with gastric cancer, the combination of napabucasin with paclitaxel was tolerated. No dose-limiting toxicities were observed and two patients reported partial response, stable disease and progressive disease each. Trial is ongoing for NCT02178956. In HCC (NCT02279719): recommended Phase II dose was determined for napabucasin and safely combined with sorafenib at full dose. Encouraging antitumor activity seen in HCC patients with no prior systemic chemotherapy. |
[98,99] |
| AZD9150 | Advanced cancers DLBCL Lymphoma HCC |
STAT3 | Phase I/II (NCT01563302) Phase I/Ib (NCT01839604)) |
(NCT01563302): in a subset of heavily pretreated DLBCL patients, AZD9150 was well tolerated and efficacious. In HCC (NCT01839604): maximum tolerated dose for AZD9150 was determined with preliminary activity and few serious adverse effects. |
[100,101] |
BTIC: Brain tumor-initiating cell; CTS: Cryptotanshinone; DLBCL: Diffuse large B-cell lymphoma; HCC: Hepatocellular carcinoma; NPC: Nasopharyngeal carcinoma; TMZ: Temozolomide.
Targeting JAKs
As JAKs function upstream of STATs along the signaling axis, it acts as a feasible target to inhibit the downstream effects of the JAK/STAT pathway. Currently, WP1066, pacritinib, cryptotanishinone and ruxolitinib are common JAK inhibitors being studied for their relevance in human diseases; however, these compounds are still in preclinical stages for HCC treatment.
Studies into brain and bladder cancers have demonstrated the efficacy of WP1066, which inhibits JAK2. In malignant glioma cells, WP1066 downregulated downstream targets of STAT3 such as Bcl-xL, Mcl-1 and c-myc. The drug also selectively activated BAX, induced apoptosis and downregulated several anti-apoptotic proteins. Thus, these findings indicate a strong correlation between WP1066 and programmed cell death. Currently, WP1066 is being investigated for brain metastasis in clinical trials (NCT01904123) [83,86]. Similarly, another study by Tsujita et al. showed that WP1066 demonstrated efficacy by promoting apoptosis of bladder cancer cells [84]. In HCC, WP1066 has been shown to inhibit MMPs and neutralize the activity of UCK2, which reduced the migration and invasion abilities of HCC cell lines [85]. Collectively, these insights demonstrate the potential of WP1066 as a treatment approach in HCC.
Another potent and selective JAK2 inhibitor is pacritinib, which was observed to have clinical efficacy for myelofibrosis patients when compared with the current best available treatment in a randomized Phase III trial (NCT02055781) [87]. Overall, the outcome showed improved total symptom score reduction and effective spleen volume reduction in pacritinib-treated patients compared with the best available treatment. Pacritinib was also well tolerated and adverse events were uncommon, with patients generally presenting mild gastrointestinal toxic effects. In another study, Jensen and colleagues used patient-derived brain tumor-initiating cells and demonstrated that pacritinib was able to reduce cell viability in these cells with satisfactory results [86]. Moreover, the team determined the compatibility of pacritinib in combination with temozolomide, the current standard of care chemotherapy for glioblastoma multiforme. This combination in mice xenografts led to an improvement in median survival, from 52 to 62.5 days, and reduced tumor growth. Thus, this study demonstrates the potential of pacritinib, as the JAK inhibitor could prolong survival periods in clinically relevant models. In HCC, pacritinib was found to reduce liver fibrosis in mouse models that mimic clinical HCC development and progression from hepatic steatosis [88]. High levels of CK-18, which predicts for increased fibrosis, was also effectively reduced in these mice upon treatment with pacritinib [88]. Given that liver fibrosis occurs in most patients with HCC, the antifibrotic effects of pacritinib observed in relevant disease models offer promising clinical applications for HCC treatment.
CTS is a plant-based quinone extracted from the root of Salvia miltiorrhiza Bunge that has inhibitory effects on the JAK/STAT pathway. In preclinical studies, CTS was shown to induce apoptosis, inhibit proliferation and reduce the migration of esophageal squamous cell carcinoma. In mice, tumor growth was effectively reduced with CTS treatment, with minimal effects on body weight, indicative of its low toxicity profile. Therefore, CTS has been shown to be a viable alternative for the treatment of esophageal squamous cell carcinoma [89]. In HCC, CTS treatment was found to inhibit the proliferation of mouse hepatoma cells by promoting cell apoptosis via JAK/STAT signaling. Furthermore, CTS was shown to promote immune response in vivo and aid in the conversion of macrophages to the M1 phenotype in vitro, allowing for increased proinflammatory and antitumor properties [90].
Ruxolitinib, a small molecule inhibitor of JAK1 and JAK2, was the first JAK inhibitor to be approved by the FDA (for primary myelofibrosis). Recently, a Phase II study of ruxolitinib in relapsed/refractory leukemia (NCT00674479) demonstrated satisfactory results, with limited grade 3 or higher toxicity [91]. Significant response was observed in 17% of postmyeloproliferative neoplasm acute myeloid leukemia patients, with complete remission in two patients. While ruxolitinib has been widely studied for the treatment of blood malignancies, studies in HCC are still preclinical. Ruxolitinib was shown to inhibit cell proliferation and colony-forming abilities in HCC cell lines [92]. Additionally, using HCC patient-derived xenograft models, tumors with JAK1 mutations (JAK1S703I) were observed to have increased sensitivity toward ruxolitinib as compared with other JAK1 mutant or wild-type tumors [102].
Targeting STAT3
Besides indirectly inhibiting STAT3 via JAKs, STAT3 can also be directly inhibited using small molecule compounds such as stattic, OPB-111077, OPB-31121, napabucasin or AZD9150, an siRNA.
Stattic has been shown to inhibit the activation, dimerization and translocation of STAT3 independent of its phosphorylation status [103]. In nasopharyngeal carcinoma (NPC) cell lines, Stattic promotes antitumor effects by decreasing the expression of STAT3-mediated CCND1 [93]. Furthermore, Stattic was shown to induce apoptosis and inhibit cell viability, effectively impeding cancer growth in vitro [93]. As a combination therapy, Stattic was shown to synergize well with other treatments such as cisplatin, as evidenced by the lowered IC50 values in NPC cells, demonstrating increased drug potency [93]. In HCC cell lines, Stattic promoted apoptosis induced by radiotherapy and reduced tumor cell survival and invasiveness in a dose-dependent manner [94].
In a Phase I study by Yoo et al., OPB-111077 was administered to sorafenib-refractory HCC patients to examine toxicity and safety profiles [95]. The drug was well tolerated overall, with limited patients experiencing dose-limiting toxicities and no treatment-related deaths reported. Unfortunately, preliminary outcomes showed limited efficacy, with zero cases of complete or partial response and a median progression-free survival of 1.4 months. Nevertheless, further investigations of OPB-111077 for combination therapy can be considered due to its acceptable safety profile.
Another STAT3 inhibitor is OPB-31121 which has been evaluated in a Phase I study for advanced solid tumors (NCT00657176) [96]. Common adverse events observed were gastrointestinal and included nausea, vomiting and diarrhea. Evaluable dose-limiting toxicities included grade 3 diarrhea and grade 3 vomiting. Overall, 800 mg/day was determined as the maximum tolerated dose. Stable disease was seen in eight patients, while disease progression was present in ten patients. Furthermore, OPB-31121 showed tumor shrinkage in one colon cancer and one rectal cancer patient. Hence, although the side effects are not ideal, the study demonstrates preliminary efficacy of OPB-31121 in advanced solid tumor patients. However, in a Phase I study by Okusaka et al. (NCT01406574), OPB-31121 showed poor antitumor efficacy in advanced HCC patients [97]. Despite six out of 25 patients having stable disease (≥8 weeks), the toxic side effects associated with the peripheral nervous system limited potential long-term usage of the drug.
Napabucasin is a STAT3 inhibitor that has been found to have preliminary clinical efficacy. In a clinical trial of napabucasin combined with paclitaxel (JapicCTI-142420), the two drugs were shown to be well tolerated in Japanese patients with gastric cancer [98]. Common adverse effects reported were generally mild and gastrointestinal, but concurrent administration with loperamide was able to control these negative effects. Napabucasin was found to have a satisfactory safety profile. Two patients in the study demonstrated preliminary signs of clinical activity in which partial response was achieved. In one of these patients, even after paclitaxel was discontinued at cycle 7, partial response was maintained until cycle 21 with napabucasin treatment alone. Collectively, this study demonstrates that drug combinations with napabucasin are a viable treatment approach with potential survival benefits and hence a Phase III trial is ongoing for this combination in gastric and gastroesophageal junction cancers (NCT02178956) [98]. In preclinical studies of HCC, napabucasin promoted apoptosis in vitro and suppressed tumor growth in orthotopic mouse models [104]. Using another orthotopic HCC resection mouse model, napabucasin treatment was also observed to decrease the incidence of recurrence after surgery (hepatectomy), which is likely mediated by inhibition of the IL-11/STAT3 signaling axis [104]. In addition, AFP and proliferating cell nuclear antigen levels were also reduced after treatment with napabucasin. Hence, there is evidence that targeting STAT3 signaling can reduce tumor growth and mitigate the risk of recurrence. Currently, napabucasin is being evaluated in a phase Ib/II clinical trial in combination with sorafenib for HCC (NCT02279719).
In contrast to small molecule inhibitors, AZD9150 is a siRNA that targets STAT3. In a Phase Ib study by Reilley et al., diffuse large B-cell lymphoma patients were treated with AZD9150 and the drug was well tolerated (NCT01563302) [100]. Drug-related side effects such as fatigue, transaminitis and thrombocytopenia were commonly observed; however, no patient withdrew treatment due to drug-related toxicities. Complete and partial responses were observed in two patients each with a median duration of response of 10.7 months for the complete response patients. Altogether, AZD9150 showed clinically meaningful antitumor activity and can be considered as a safe therapy for diffuse large B-cell lymphoma. In HCC, a Phase I/Ib study was performed with AZD9150 to evaluate its efficacy and safety profiles (NCT01839604) [101]. The study showed that AZD9150 was well tolerated with mild and a few serious adverse events. However, further studies are needed to elucidate its clinical efficacy.
Overall, these studies demonstrate the potential of targeting the JAK/STAT pathway in HCC. While clinical investigations for these inhibitors are still in early stages for HCC, the beneficial effects observed in other tumor types provide indications of possible clinical efficacy for HCC as well.
Future perspective
Activation of JAK/STAT signaling is a widely reported phenomenon in many cancers, including HCC. The multiple cellular effects of JAK/STAT signaling and its relationship with other signaling pathways have been shown to contribute to many key hallmarks of cancer development and progression. Thus, therapeutic targeting of activated JAK/STAT pathway is a rational approach in tumors with such aberrant signaling. In fact, the numerous clinical trials and studies on JAK/STAT inhibitors demonstrate the potential and efficacy of these compounds in mitigating various cancers. Nevertheless, in the case of HCC, clinical investigations into these compounds are still in the early stages, with limited benefits observed. As many of these studies in HCC were done using JAK/STAT inhibitors as monotherapy, perhaps these compounds could be applied in a combinatorial therapy setting. With its role in maintaining cancer stem-like cells, targeted inhibition of JAK/STAT could be investigated as an adjuvant therapy for HCC, which may help to suppress or eradicate the subpopulation of cells with tumor-propagating properties. Furthermore, as these cells often mediate chemoresistance in tumors, the use of JAK/STAT inhibitors could help mitigate this, thus reducing the risk of drug resistance and disease recurrence, two major setbacks in the current treatment landscape of HCC. Besides, given the highly heterogenous nature of HCC, it is likely that the response toward JAK/STAT inhibitors among patients would be varied as well, as seen in the case of increased ruxolitinib sensitivity in HCC patient-derived xenograft tumors with specific JAK1 mutations. Thus, it would be interesting to see if these mutations that correlate with treatment efficacy can be applied toward stratifying HCC patients in clinical trials to achieve better outcomes and survival. Overall, the JAK/STAT pathway is a promising therapeutic target for HCC, although further investigations are needed to fully understand the molecular mechanisms and side effects to improve clinical outcomes and possible personalized treatments.
Executive summary.
Hepatocellular carcinoma (HCC) is a highly challenging disease to treat, due to factors such as lack of effective treatments, high rate of recurrence, underlying liver dysfunction, drug resistance and heterogeneous tumor background.
Treatment options for HCC include surgical resection, liver transplantation, transarterial chemo- or radio-embolization and systemic drug therapy using small molecule inhibitors such as sorafenib, lenvatinib, regorafenib and cabozantinib.
Many intracellular signaling pathways contribute to hepatocarcinogenesis, including the JAK/STAT pathway, which has normal roles in regulating cell proliferation, survival and differentiation. However, deregulation of JAK/STAT signaling is observed in many cancers and contributes to various oncogenic effects.
In HCC, aberrant activation of JAK/STAT pathway promotes tumor growth, angiogenesis, invasion and metastasis. JAK/STAT signaling is also implicated in maintenance of cancer stem cells with tumor-propagating abilities in HCC as well as creation of an immunosuppressive microenvironment.
Due to the oncogenic role of JAK/STAT activation, especially in the context of STAT3 dysregulation, targeting this pathway represents an attractive/feasible approach for the treatment of HCC. In fact, various small molecule inhibitors and RNA therapies that target JAKs or STATs have been developed and tested for efficacy against tumor cells.
While many of the JAK/STAT-targeting compounds have shown clear antitumor effects on tumor growth and development in preclinical models of HCC, clinical investigations of these compounds for HCC are still limited. Nevertheless, the promising results of clinical trials in other cancer types highlight the potential of inhibiting the JAK/STAT pathway as an effective treatment for HCC.
Footnotes
Author contributions
JJH Tang, DKH Thng, JJ Lim and TB. Toh were major contributors in the writing of the manuscript. TB. Toh designed, revised and edited the manuscript. All authors read and approved the final manuscript.
Financial & competing interests disclosure
TB Toh gratefully acknowledges financial support from N.1 Translational Core. The other authors state no conflict of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Pan H, Fu X, Huang W. Molecular mechanism of liver cancer. Anticancer Agents Med. Chem. 11(6), 493–499 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class and effects on patient prognosis. Gastroenterology 152(4), 745–761 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 65(2), 87–108 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 15(10), 599–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin. Liver Dis. 19(2), 223–238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 16(10), 589–604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet JM, Zucman-Rossi J, Pikarsky E. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2, 16018 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 149(5), 1226–1239 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Sangiovanni A, Del Ninno E, Fasani P. et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 126(4), 1005–1014 (2004). [DOI] [PubMed] [Google Scholar]
- 11.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69(1), 182–236 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Liu CY, Chen KF, Chen PJ. Treatment of liver cancer. Cold Spring Harb. Perspect. Med. 5(9), a021535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrzesinski SH, Taddei TH, Strazzabosco M. Systemic therapy in hepatocellular carcinoma. Clin. Liver Dis. 15(2), 423–441 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llovet JM, Ricci S, Mazzaferro V. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359(4), 378–390 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Cheng AL, Kang YK, Chen Z. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10(1), 25–34 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Kudo M, Finn RS, Qin S. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase III non-inferiority trial. Lancet 391(10126), 1163–1173 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Qin S, Merle P. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, Phase III trial. Lancet 389(10064), 56–66 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Abou-Alfa GK, Meyer T, Cheng AL. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379(1), 54–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu AX, Kang Y-K, Yen C-J. et al. REACH-2: A randomized, double-blind, placebo-controlled Phase III study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafenib. J. Clin. Oncol. 36(Suppl. 15), 4003–4003 (2018). [Google Scholar]
- 20.Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin. Liver Dis. 25(2), 212–225 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin. Liver Dis. 25(2), 181–200 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Chen C, Wang G. Mechanisms of hepatocellular carcinoma and challenges and opportunities for molecular targeted therapy. World J. Hepatol. 7(15), 1964–1970 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alqahtani A, Khan Z, Alloghbi A, Said Ahmed TS, Ashraf M, Hammouda DM. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina 55(9), 526–547 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signaling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 113(3), 365–371 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svinka J, Mikulits W, Eferl R. STAT3 in hepatocellular carcinoma: new perspectives. Hepat. Oncol. 1(1), 107–120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosmorduc O, Desbois-Mouthon C. Targeting STAT3 in hepatocellular carcinoma: sorafenib again. J. Hepatol. 55(5), 957–959 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J. Immunol. 178(5), 2623–2629 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Subramaniam A, Shanmugam MK, Perumal E. et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim. Biophys. Acta 1835(1), 46–60 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Niu GJ, Xu JD, Yuan WJ. et al. Protein inhibitor of activated STAT (PIAS) negatively regulates the JAK/STAT pathway by inhibiting STAT phosphorylation and translocation. Front. Immunol. 9, 2392 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loh CY, Arya A, Naema AF, Wong WF, Sethi G, Looi CY. Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: functions and therapeutic implication. Front. Oncol. 9, 48 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He G, Karin M. NF-κB and STAT3 – key players in liver inflammation and cancer. Cell Res. 21(1), 159–168 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kan Z, Zheng H, Liu X. et al. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 23(9), 1422–1433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bromberg JF, Wrzeszczynska MH, Devgan G. et al. STAT3 as an oncogene. Cell 98(3), 295–303 (1999). [DOI] [PubMed] [Google Scholar]
- 34.Kusaba M, Nakao K, Goto T. et al. Abrogation of constitutive STAT3 activity sensitizes human hepatoma cells to TRAIL-mediated apoptosis. J. Hepatol. 47(4), 546–555 (2007). [DOI] [PubMed] [Google Scholar]
- 35.He G, Yu GY, Temkin V. et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell 17(3), 286–297 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li WC, Ye SL, Sun RX. et al. Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin. Cancer Res. 12(23), 7140–7148 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Alas S, Bonavida B. Rituximab inactivates signal transducer and activation of transcription 3 (STAT3) activity in B-non-Hodgkin's lymphoma through inhibition of the interleukin 10 autocrine/paracrine loop and results in down-regulation of Bcl-2 and sensitization to cytotoxic drugs. Cancer Res. 61(13), 5137–5144 (2001). [PubMed] [Google Scholar]
- 38.Kotha A, Sekharam M, Cilenti L. et al. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol. Cancer Ther. 5(3), 621–629 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Gritsko T, Williams A, Turkson J. et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin. Cancer Res. 12(1), 11–19 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Epling-Burnette PK, Liu JH, Catlett-Falcone R. et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J. Clin. Invest. 107(3), 351–362 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niu G, Wright KL, Ma Y. et al. Role of Stat3 in regulating p53 expression and function. Mol. Cell. Biol. 25(17), 7432–7440 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timofeeva OA, Tarasova NI, Zhang X. et al. STAT3 suppresses transcription of proapoptotic genes in cancer cells with the involvement of its N-terminal domain. Proc. Natl Acad. Sci. USA 110(4), 1267–1272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie L, Zeng Y, Dai Z. et al. Chemical and genetic inhibition of STAT3 sensitizes hepatocellular carcinoma cells to sorafenib induced cell death. Int. J. Biol. Sci. 14(5), 577–585 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carbajo-Pescador S, Ordonez R, Benet M. et al. Inhibition of VEGF expression through blockade of Hif1alpha and STAT3 signaling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br. J. Cancer 109(1), 83–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Q, Briggs J, Park S. et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 24(36), 5552–5560 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9(11), 798–809 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 7(1), 41–51 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Cheng JT, Deng YN, Yi HM. et al. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis 5, e198 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng Y, Cheng J, Fu B. et al. Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene 36(8), 1090–1101 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Yin Z, Ma T, Lin Y. et al. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J. Cell. Biochem. 119(11), 9419–9432 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Sun X, Sui Q, Zhang C, Tian Z, Zhang J. Targeting blockage of STAT3 in hepatocellular carcinoma cells augments NK cell functions via reverse hepatocellular carcinoma-induced immune suppression. Mol. Cancer Ther. 12(12), 2885–2896 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Toh TB, Lim JJ, Hooi L, Rashid M, Chow EK. Targeting Jak/Stat pathway as a therapeutic strategy against SP/CD44+ tumorigenic cells in Akt/β-catenin-driven hepatocellular carcinoma. J. Hepatol. 72(1), 104–118 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Asai R, Tsuchiya H, Amisaki M. et al. CD44 standard isoform is involved in maintenance of cancer stem cells of a hepatocellular carcinoma cell line. Cancer Med. 8(2), 773–782 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Won C, Kim BH, Yi EH. et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology 62(4), 1160–1173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell 9(1), 50–63 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Xiong S, Wang R, Chen Q. et al. Cancer-associated fibroblasts promote stem cell-like properties of hepatocellular carcinoma cells through IL-6/STAT3/Notch signaling. Am. J. Cancer Res. 8(2), 302–316 (2018). [PMC free article] [PubMed] [Google Scholar]
- 57.Huynh J, Chand A, Gough D, Ernst M. Therapeutically exploiting STAT3 activity in cancer – using tissue repair as a road map. Nat. Rev. Cancer 19(2), 82–96 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Bi YH, Han WQ, Li RF. et al. Signal transducer and activator of transcription 3 promotes the Warburg effect possibly by inducing pyruvate kinase M2 phosphorylation in liver precancerous lesions. World J. Gastroenterol. 25(16), 1936–1949 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang L, Jian Z, Gao Y. et al. RPN2 promotes metastasis of hepatocellular carcinoma cell and inhibits autophagy via STAT3 and NF-κB pathways. Aging (Albany NY) 11(17), 6674–6690 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Zhao Z, Xiong S, Wang R. et al. Peri-tumor fibroblasts promote tumorigenesis and metastasis of hepatocellular carcinoma via Interleukin6/STAT3 signaling pathway. Cancer. Manag. Res. 11, 2889–2901 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang FB, Wang L, Jia HC. et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 15, 45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang CH, Guo FL, Xu GL, Jia WD, Ge YS. STAT3 activation mediates epithelial-to-mesenchymal transition in human hepatocellular carcinoma cells. Hepatogastroenterology 61(132), 1082–1089 (2014). [PubMed] [Google Scholar]
- 63.Yang L, Zhang XY, Li K. et al. Protopanaxadiol inhibits epithelial-mesenchymal transition of hepatocellular carcinoma by targeting STAT3 pathway. Cell Death Dis. 10(9), 630 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arboleda MJ, Lyons JF, Kabbinavar FF. et al. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion and metastasis of human breast and ovarian cancer cells. Cancer Res. 63(1), 196–206 (2003). [PubMed] [Google Scholar]
- 65.Xie Y, Li J, Zhang C. STAT3 promotes the proliferation and migration of hepatocellular carcinoma cells by regulating AKT2. Oncol. Lett. 15(3), 3333–3338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verhoeven Y, Tilborghs S, Jacobs J. et al. The potential and controversy of targeting STAT family members in cancer. Semin. Cancer Biol. 60, 41– 55 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Hosui A, Klover P, Tatsumi T. et al. Suppression of signal transducers and activators of transcription 1 in hepatocellular carcinoma is associated with tumor progression. Int. J. Cancer 131(12), 2774–2784 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma B, Chen K, Liu P. et al. Dichotomal functions of phosphorylated and unphosphorylated STAT1 in hepatocellular carcinoma. J. Mol. Med. 97(1), 77–88 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Testoni B, Schinzari V, Guerrieri F, Gerbal-Chaloin S, Blandino G, Levrero M. p53-paralog DNp73 oncogene is repressed by IFNalpha/STAT2 through the recruitment of the Ezh2 polycomb group transcriptional repressor. Oncogene 30(23), 2670–2678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J, Liang L, Liu Y. et al. Clinicopathological significance of STAT4 in hepatocellular carcinoma and its effect on cell growth and apoptosis. Onco. Targets Ther. 9, 1721–1734 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang G, Chen JH, Qiang Y, Wang DZ, Chen Z. Decreased STAT4 indicates poor prognosis and enhanced cell proliferation in hepatocellular carcinoma. World J. Gastroenterol. 21(13), 3983–3993 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chanthra N, Payungporn S, Chuaypen N. et al. Single nucleotide polymorphisms in STAT3 and STAT4 and risk of hepatocellular carcinoma in Thai patients with chronic hepatitis B. Asian Pac. J. Cancer Prev. 16(18), 8405–8410 (2015). [DOI] [PubMed] [Google Scholar]
- 73.El Sharkawy R, Thabet K, Lampertico P. et al. A STAT4 variant increases liver fibrosis risk in Caucasian patients with chronic hepatitis B. Aliment. Pharmacol. Ther. 48(5), 564–573 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Jiang DK, Ma XP, Wu X. et al. Genetic variations in STAT4, C2, HLA-DRB1 and HLA-DQ associated with risk of hepatitis B virus-related liver cirrhosis. Sci. Rep. 5, 16278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang DK, Sun J, Cao G. et al. Genetic variants in STAT4 and HLA-DQ genes confer risk of hepatitis B virus-related hepatocellular carcinoma. Nat. Genet. 45(1), 72–75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi H, He H, Ojha SC. et al. Association of STAT3 and STAT4 polymorphisms with susceptibility to chronic hepatitis B virus infection and risk of hepatocellular carcinoma: a meta-analysis. Biosci. Rep. 39(6), 783–797 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calvisi DF, Ladu S, Gorden A. et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology 130(4), 1117–1128 (2006). [DOI] [PubMed] [Google Scholar]
- 78.Lee TK, Man K, Poon RT. et al. Signal transducers and activators of transcription 5b activation enhances hepatocellular carcinoma aggressiveness through induction of epithelial-mesenchymal transition. Cancer Res. 66(20), 9948–9956 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Friedbichler K, Themanns M, Mueller KM. et al. Growth-hormone-induced signal transducer and activator of transcription 5 signaling causes gigantism, inflammation and premature death but protects mice from aggressive liver cancer. Hepatology 55(3), 941–952 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Hosui A, Kimura A, Yamaji D, Zhu BM, Na R, Hennighausen L. Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-{beta} and STAT3 activation. J. Exp. Med. 206(4), 819–831 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu JH, Zhu BM, Wickre M. et al. The transcription factors signal transducer and activator of transcription 5A (STAT5A) and STAT5B negatively regulate cell proliferation through the activation of cyclin-dependent kinase inhibitor 2b (Cdkn2b) and Cdkn1a expression. Hepatology 52(5), 1808–1818 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaltenecker D, Themanns M, Mueller KM. et al. STAT5 deficiency in hepatocytes reduces diethylnitrosamine-induced liver tumorigenesis in mice. Cytokine 124, 154573 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Iwamaru A, Szymanski S, Iwado E. et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 26(17), 2435–2444 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Tsujita Y, Horiguchi A, Tasaki S. et al. STAT3 inhibition by WP1066 suppresses the growth and invasiveness of bladder cancer cells. Oncol. Rep. 38(4), 2197–2204 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Zhou Q, Jiang H, Zhang J. et al. Uridine-cytidine kinase 2 promotes metastasis of hepatocellular carcinoma cells via the Stat3 pathway. Cancer Manag. Res. 10, 6339–6355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jensen KV, Cseh O, Aman A, Weiss S, Luchman HA. The JAK2/STAT3 inhibitor pacritinib effectively inhibits patient-derived GBM brain tumor initiating cells in vitro and when used in combination with temozolomide increases survival in an orthotopic xenograft model. PLoS ONE 12(12), e0189670 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mascarenhas J, Hoffman R, Talpaz M. et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 4(5), 652–659 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Fayoumi S, Hashiguchi T, Shirakata Y, Mascarenhas J, Singer JW. Pilot study of the antifibrotic effects of the multikinase inhibitor pacritinib in a mouse model of liver fibrosis. J. Exp. Pharmacol. 10, 9–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji Y, Liu Y, Xue N. et al. Cryptotanshinone inhibits esophageal squamous-cell carcinoma in vitro and in vivo through the suppression of STAT3 activation. Onco. Targets Ther. 12, 883–896 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han Z, Liu S, Lin H. et al. Inhibition of murine hepatoma tumor growth by cryptotanshinone involves TLR7-dependent activation of macrophages and induction of adaptive antitumor immune defenses. Cancer Immunol. Immunother. 68(7), 1073–1085 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eghtedar A, Verstovsek S, Estrov Z. et al. Phase II study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood 119(20), 4614–4618 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson GS, Tian A, Hebbard L. et al. Tumoricidal effects of the JAK inhibitor Ruxolitinib (INC424) on hepatocellular carcinoma in vitro. Cancer Lett. 341(2), 224–230 (2013). [DOI] [PubMed] [Google Scholar]
- 93.Pan Y, Zhou F, Zhang R, Claret FX. Stat3 inhibitor Stattic exhibits potent antitumor activity and induces chemo- and radio-sensitivity in nasopharyngeal carcinoma. PLoS ONE 8(1), e54565 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu G, Zhu L, Wang Y, Shi Y, Gong A, Wu C. Stattic enhances radiosensitivity and reduces radio-induced migration and invasion in HCC cell lines through an apoptosis pathway. Biomed. Res. Int. 2017, 1832494 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoo C, Kang J, Lim HY. et al. Phase I dose-finding study of OPB-111077, a novel STAT3 inhibitor, in patients with avanced hepatocellular carcinoma. Cancer Res. Treat. 51(2), 510–518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oh DY, Lee SH, Han SW. et al. Phase I study of OPB-31121, an oral STAT3 inhibitor, in patients with advanced solid tumors. Cancer Res. Treat. 47(4), 607–615 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okusaka T, Ueno H, Ikeda M. et al. Phase I and pharmacological trial of OPB-31121, a signal transducer and activator of transcription-3 inhibitor, in patients with advanced hepatocellular carcinoma. Hepatol. Res. 45(13), 1283–1291 (2015). [DOI] [PubMed] [Google Scholar]
- 98.Shitara K, Yodo Y, Iino S. A Phase I study of napabucasin plus paclitaxel for japanese patients with advanced/recurrent gastric cancer. In Vivo 33(3), 933–937 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El-Rayes BF, Richards DA, Cohn AL. et al. BBI608-503-103HCC: a Phase Ib/II clinical study of napabucasin (BBI608) in combination with sorafenib or amcasertib (BBI503) in combination with sorafenib (Sor) in adult patients with hepatocellular carcinoma (HCC). J. Clin. Oncol. (Suppl. 15), 4077–4077 (2017). [Google Scholar]
- 100.Reilley MJ, McCoon P, Cook C. et al. STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a Phase Ib trial. J. Immunother. Cancer. 6(1), 119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee C, Cheung ST. STAT3: an emerging therapeutic target for hepatocellular carcinoma. Cancers 11(11), 1646–1665 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang S, Luo C, Gu Q. et al. Activating JAK1 mutation may predict the sensitivity of JAK-STAT inhibition in hepatocellular carcinoma. Oncotarget 7(5), 5461–5469 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 13(11), 1235–1242 (2006). [DOI] [PubMed] [Google Scholar]
- 104.Wang D, Zheng X, Fu B. et al. Hepatectomy promotes recurrence of liver cancer by enhancing IL-11-STAT3 signaling. EBioMedicine 46, 119–132 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]