Abstract
Background
Suxiao jiuxin wan is widely used in China for angina pectoris.
Objectives
The objective of this review is to determine the effects (benefits and harms) of suxiao jiuxin wan in the treatment of angina pectoris.
Search methods
We searched the Cochrane Central Register of Controlled Trials on The Cochrane Library (issue 4 2005), Medline (1995 to 2005), EMBASE (1995 to 2005), the Register of Chinese trials developed by the Chinese Cochrane Centre (to 2006), and the Chinese Biomedical Database (1995 to 2005), and handsearched 83 Chinese journals. We also searched reference lists, databases of ongoing trials and the Internet. Date of last search: November 2005.
Selection criteria
Randomised controlled trials of suxiao jiuxin wan compared to standard treatment in people with angina. Studies with a treatment duration > 4 weeks were included.
Data collection and analysis
Two reviewers independently applied the inclusion criteria, assessed trial quality and extracted the data.
Main results
Fifteen trials involving 1776 people were included. There was weak evidence that suxiao jiuxin wan compared with nitroglyerin (xiaoxintong) improved ECG measurements (RR 1.16, 95% CI 1.05 to 1.27), reduced symptoms (RR 1.09, 95% CI 1.04 to 1.13), reduced the frequency of acute attacks of angina (difference in means ‐0.70, 95% CI ‐0.90 to ‐0.50), reduced diastolic pressure (difference in means ‐3mmHg, 95% CI ‐5.73 to ‐0.27) and reduced the need for supplementary nitroglycerin (difference in means of ‐0.60, 95% CI ‐0.94 to ‐0.26). There was also weak evidence that suxiao jiuxin wan compared with Salvia miltiorrhiza (danshen) reduced symptoms (RR 1.21, 95% CI 1.11 to 1.31) and improved ECG measurements (RR 1.55, 95% CI 1.30 to 1.84). There was no significant difference when comparing suxiao jiuxin wan with isosorbide dinitrate (xiaosuanyishanlizhi) both for ECG improvement (RR 1.34, 95% CI 0.91 to 1.98) and for symptom improvement (RR 1.11, 95% CI 0.86 to 1.43).
Authors' conclusions
Suxiao jiuxin wan appears to be effective in the treatment of angina pectoris and no serious side effects were identified. However, the evidence remains weak due to poor methodological quality of including studies.
Keywords: Humans; Phytotherapy; Angina Pectoris; Angina Pectoris/drug therapy; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Isosorbide Dinitrate; Isosorbide Dinitrate/therapeutic use; Nitroglycerin; Nitroglycerin/therapeutic use; Randomized Controlled Trials as Topic; Vasodilator Agents; Vasodilator Agents/therapeutic use
Plain language summary
Chinese herbal medicine suxiao jiuxin wan for angina pectoris
Angina pectoris is pain or discomfort within the chest, typically provoked by exertion or anxiety. Angina is a sign that someone is at increased risk of heart attack, cardiac arrest or sudden cardiac death. The aim of treatment for angina is to control the symptoms and prevent a cardiovascular event such as a heart attack. In western medicine, treatment is usually with beta blockers, calcium channel blockers and nitrates (nitroglycerin). Suxiao jiuxin wan is widely used in China in conjunction with these western treatments. This review found weak evidence to suggest suxiao jiuxin wan alone or in combination with other anti‐anginal drugs reduces the symptoms of angina. However, because of the quality of the research, the role of suxiao jiuxin wan is uncertain and more high quality trials are required to assess the effects of suxiao jiuxin wan in the long term.
Background
Description of the condition Ischaemic heart disease (IHD) is the most common cause of death in western countries, where it accounts for almost 33% of overall mortality (Braunwald 1992). In recent years, there has been a tendency for the incidence of IHD to increase in less developed countries as their economies develop. For instance in Beijing, China, the mortality from IHD rose from 21.7 per 100,000 in 1970 to 62.0 per 100,000 in 1980 (Chen 2000). The first clinical sign in over half of IHD patients is angina pectoris (Gibbons 1999). Angina pectoris is generally defined as pain or discomfort within (or adjacent to) the chest, which is typically provoked by exertion or anxiety. The pain usually lasts for several minutes and is alleviated by rest, and does not result in myocardial necrosis. Although angina may happen during exercise, strong emotions or extreme temperatures may result in some people, such as those with a coronary artery spasm, experiencing angina when they are resting. Angina is a sign that someone is at increased risk of heart attack, cardiac arrest or sudden cardiac death. The reported annual incidence of angina is 213 per 100,000 people over 30 years old in USA (Gibbons 1999).
About 30‐40% of patients will have spontaneous remission of angina for 2 or more years (Cleland 1996). The most important determinants of prognosis are left ventricular systolic function, comorbid conditions, and the severity of coronary artery disease (Hilton 1991). In patients with good left ventricular function, despite severe coronary artery disease, the long‐term prognosis is similar with medical treatment or surgical revascularisation procedures (Alderman 1990; Asirvatham 1998; Yusuf 1994).
Description of the intervention Chinese herbal medicine is part of traditional Chinese medicine (TCM), which is a 3000‐year‐old holistic system of medicine combining the use of medicinal herbs, acupuncture, food therapy, massage, and therapeutic exercise for both treatment and prevention of disease (Fulder 1996). TCM has its unique theories for concepts of aetiology, systems of diagnosis and treatment that are vital to its practice. TCM drug treatment consists typically of complex prescriptions of a combination of several components. The combination, based on the Chinese diagnostic patterns (i.e. inspection, listening, smelling, inquiry, and palpation), follows a completely different rationale than many western drug treatments (Liu 2002).
In conventional medicine the purpose of treatment is to reduce angina attacks and to prevent cardiovascular events. Guidelines recommend that first‐line drugs for angina are beta blockers. If these are not effective then nitrates to improve symptoms only, together with beta blockers to improve prognosis, are recommended. Patients who remain resistant to treatment are then treated with calcium‐blockers, nitrates and beta‐blockers (ACC/AHA 1999). However, there is an increased risk of bradycardia, heart block and additive negative inotropic effects with calcium and beta‐blockers (Akhras 1991; Thadani 1999). Antianginal therapy with a single agent may be as effective as combination therapy with two or three agents (Jackson 2001). This has been demonstrated in two large randomized controlled trials (RCTs), TIBET (Fox 1996) and IMAGE (Savonitto 1996). Clinical judgement is therefore essential when selecting optimal treatment plans for individual patients.
Suxiao jiuxin wan is a new drug on the national essential drug list of China for the treatment of cardiocerebral vascular diseases (Feng 2000). It has been shown to cause remission of angina pectoris (Yuan 2002), improve anginal symptoms, and reduce the use of nitroglycerin ‐ a drug used to relieve quickly angina symptoms (Wei 1995). Suxiao jiuxin wan may be used on its own or in conjunction with conventional anti‐anginal treatments. In China, some people who suffer from angina pectoris take suxiao jiuxin wan to prevent and treat angina pectoris. However, suxiao jiuxin wan can lead to gastrointestinal reactions in some cases, but these can be relieved by taking it after a meal (Han 2000).
How the intervention might work In experimental studies, suxiao jiuxin wan was shown to significantly improve myocardial ischaemia and reduce the incidence of myocardial infarction by preventing hyperlipidaemia, improving microcirculation, increasing coronary arterial blood flow, dilating coronary vessels and improving myocardial blood supply (Liang 1999). The composition of suxiao jiuxin wan includes Ligusticum chuanxiong Hort. (also known as Radix chuanxiong) and Borneolum syntheticum (Zhuang 1999) (see Table 1 for further details of suxiao jiuxin wan composition). Radix chuanxiong dilates the coronary artery and increases the coronary flow. In aqueous solution or alcoholic infusion it can lower blood pressure. Its alkaloids, ferulic acid and cnidilide are antispasmodics. Borneolum syntheticum can increase the level of Radix chuanxiong in plasma (Liu 2003). Its solution, in low concentration, exerts anti‐inflammatory, astringent and antiseptic effects. The side effects of Borneolum syntheticum are eye, skin and respiratory irritation. It may be harmful through ingestion, inhalation or through skin contact (PTCL 2002).
1. Composition of suxiao jiuxin wan.
| Common name | Latin name | Alternative name |
| chuanxiong | Ligusticum chuanxiong Hort. | Radix chuanxiong |
| bingpian | Borneolum syntheticum | Borneol |
Why it is important to do this review The evidence on the effects of suxiao jiuxin wan has not been systematically assessed. The effects of this treatment need to be reviewed to inform clinical practice as well as highlight any areas for new research.
Objectives
The objective of this review is to determine the effects (benefits and harms) of suxiao jiuxin wan in the treatment of angina pectoris, in monotherapy or in combination with other antianginal therapy, as compared to placebo or other anti‐anginal drugs.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled studies were included.
Types of participants
Participants were male or female of any age or ethnic origin with chronic angina pectoris. Participants with acute myocardial infarction, heart failure, hepatic failure and renal failure were excluded.
Types of interventions
We included any studies in which suxiao jiuxin wan was used for treating angina pectoris. We included studies where suxiao jiuxin wan was used alone versus other anti‐anginal drugs (either western or traditional Chinese medicine) or placebo. We also accepted studies of suxiao jiuxin wan in combination therapy versus combination therapy without suxiao jiuxin wan. We excluded studies of less than 4 weeks treatment duration.
Types of outcome measures
Primary outcome measures 1. Mortality (sudden death from acute myocardial infarction) 2. Severity of angina pectoris 3. Frequency of acute attack angina 4. ECG improvement (an exercise ECG or a resting ECG) 5. Changes in dosage of nitroglycerin 6. Changes in symptoms (such as chest pain, breathlessness, etc)
Secondary outcome measures 1. Blood pressure 2. Levels of plasma endothelin level and nitric oxide 3. Health‐related quality of life (ideally, using a validated instrument) 4. Adverse effects
Search methods for identification of studies
A comprehensive and exhaustive search strategy was formulated in an attempt to identify all relevant studies regardless of language or publication status.
Electronic searches We searched The Cochrane Central Register of Controlled Trials (CENTRAL), on The Cochrane Library (issue 4, 2005) using the search term: 'suxiao jiuxin wan'.
The following electronic databases were also searched using the same term: 1. MEDLINE (1995 to 2005); 2. EMBASE (1995 to 2005); 3. CBM (Chinese biomedical database, 1995 to 2005); 4. Chinese Cochrane Centre Controlled Trials Register (to 2005).
We also searched databases of ongoing trials: Current Controlled Trials (www.controlled‐trials.com) The National Research Register (www.update‐software.com/National/nrr‐frame.html)
Handsearches We handsearched a number of Chinese traditional medicine journals. These are listed in Table 2. We attempted to identify additional studies by searching the reference lists of relevant trials and reviews identified. Authors of identified studies were contacted.
2. Handsearched traditonal Chinese medicine journals.
| Journals |
| Acta Chinese Medicine and Pharmacology Beijing Journal of Medicine Beijing Journal of Traditional Chinese Medicine China Journal of Chinese Materia Medica China Journal of Basic Medicine in Traditional Chinese Medicine Chinese Journal of Integrated Traditional and Western Medicine Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care Chinese Journal of Traditional Medical Science and Technology Chinese Journal of Traditional & Western Medicine Chinese Medicine Chinese Traditional Patent Medicine Chinese Traditional Patent Medicine Research Chinese Traditional and Herbal Drags Chinese Pharmaceutical Abstracts Clinical Journal of Anhui Traditional Chinese Medicine Critical Care Forum on Traditional Chinese Medicine Fujian Journal of Medicine Fujian Journal of Traditional Chinese Medicine Guang Ming Zhong Yi Journal of Traditional Chinese Medicine Gansu Journal of Medicine Gansu Journal of Traditional Chinese Medicine Guangxi Journal of Medicine Guangxi Journal of Traditional Chinese Medicine Gangdong Journal of Medicine Guangdong Journal of Traditional Chinese Medicine Hebei Integrated Traditional and Western Medicine Hebei Journal of Medicine Hebei Journal of Traditional Chinese Medicine Heilongjang Journal of Medicine Heilongjang Journal of Traditional Chinese Medicine Henan Journal of Medicine Henan Journal of Traditional Chinese Medicine and Pharmacy Henan Journal of Traditional Chinese Medicine Hubei Journal of Traditional Chinese Medicine Hunan Journal of Medicine Hunan Journal of Traditional Chinese Medicine Information on Traditional Chinese Medicine Jiangxi Journal of Medicine Jiangxi Journal of Traditional Chinese Medicine Jiangsu Journal of Medicine Jiangshu Journal of Traditional Chinese Medicine Jilin Journal of Medicine Jilin Journal of Traditional Chinese Medicine Journal of Anhui College of Traditional Chinese Medicine Journal of Beijing University of Traditional Chinese Medicine Journal of Chengdu University of Traditional Chinese Medicine Journal of Chinese Medicinal Materials Journal of Emergency in Traditional Chinese Medicine Journal of Guangzhou University of Traditional Chinese Medicine Journal of HeNnan College of Traditional Chinese Medicine Journal of Integrated Traditional and Western Medicine Journal of Practical Traditional Chinese Medicine Journal of Practical Chinese Traditional Internal Medicine Journal of Sichuan of Traditional Chinese Medicine Journal of Sichuan Medicine Journal of Traditional Chinese Medicine Journal of Emergency Syndromes in Chinese Medicine Liaoning Journal of Traditional Chinese Medicine Modern Journal of Integrated Chinese Traditional and Western Medicine Modern Traditional Chinese Medicine Neimongol Journal of Traditional Chinese Medicine New Jounal of Traditional Chinese Medicine Pharmacology and Clinics of Chinese Materia Medica Research of Traditional Chinese Medicine Sanxi Journal of Medicine Shanxi Journal of Traditional Chinese Medicine Shanxi Journal of Medicine Shanxi Journal of Traditional Chinese Medicine Shandong Journal of Medicine Shandong Journal of Traditional Chinese Medicine Shanghai Journal of Medicine Shanghai Journal of Traditional Chinese Medicine Shenzhen Journal of Integrated Traditional and Western Medicine Tianjin Journal of Medicine Tianjin Journal of Traditional Chinese Medicine Traditional Chinese Medicine Research Xinjiang Journal of Medecine Xinjiang Journal of Traditional Chinese Medicine Yunnan Journal of Medicine Yunnan Journal of Traditional Chinese Medicine Yunnan Journal of Traditional Chinese Medicine and Materia Medica Zhejiang Journal of Traditional Chinese Medicine |
Other search strategies Organisations (including the World Health Organisation), individual researchers working in the field, and medicinal herbs manufacturers (Tianjin Zhongxin Pharmaceuticals Co.Ltd.) were contacted in order to obtain possible additional references, unpublished trials, or ongoing trials, confidential reports and raw data of published trials.
Data collection and analysis
Trial selection The titles, abstracts and keywords of every record retrieved were scanned to determine which were possibly relevant to the review. Any record that appeared likely to meet the inclusion criteria was obtained in full text. If there was any doubt regarding eligibility from the information given in the title and abstract, the full article was retrieved for clarification. Differences in opinion between reviewers were resolved by discussion.
Quality assessment of trials The quality of each trial was assessed based largely on the quality criteria specified by Schulz and by Jadad (Jadad 1996; Schulz 1995). In particular, the following factors were studied.
Selection bias: a) was the randomization procedure adequate? b) was the allocation concealment adequate?
Performance bias: were the patients and people administering the treatment blind to the intervention?
Attrition bias: a) were withdrawals and dropouts completely described? b) was analysis by intention to treat?
Detection bias: were outcome assessors blind to the intervention?
Based on these criteria, studies were broadly divided into the following three categories. This classification used as the basis of a sensitivity analysis. Additionally, we explored the influence of individual quality criteria in a sensitivity analysis.
A: all quality criteria met ‐ low risk of bias.
B: one or more of the quality criteria only partly met ‐ moderate risk of bias.
C: one or more criteria not met ‐ high risk of bias.
Each trial was assessed by two reviewers independently (XD, TW). Disagreements were resolved, where necessary, by recourse to a third reviewer (LZ). In cases of disagreement, the rest of the group were consulted and a judgement was made based on consensus.
Data extraction Data concerning details of study population, intervention and outcomes were extracted independently by two reviewers (XD, TW). Differences in data extraction were resolved by consensus, referring back to the original article. When necessary, information was sought from the authors of the primary studies. Disagreement were resolved by discussion and, where necessary, in consultation with a third reviewer (LZ). For binary outcomes, number of events and total number in each group were extracted. For continuous outcomes, mean, standard deviation and sample size of each group were abstracted or imputed.
The data extraction form included the following items:
1. General information: published/unpublished, title, authors, reference/source, contact address, country, urban/rural etc., language of publication, year of publication, duplicate publications, sponsor, and setting. 2. Trial characteristics: design, duration of follow‐up, method of randomisation, allocation concealment, blinding (patients, people administering treatment, outcome assessors). 3. Intervention(s): intervention(s) (dose, route, and timing), comparison intervention(s) (dose, route, and timing), and co‐medication (dose, route, and timing). 4. Patients: exclusion criteria, total number and number in comparison groups, age (adults), baseline characteristics, diagnostic criteria, similarity of groups at baseline (including any co‐morbidity), assessment of compliance, withdrawals/losses to follow‐up (reasons/description), subgroups. 5. Outcomes: outcomes specified above, any other outcomes assessed, other events, length of follow‐up, quality of reporting of outcomes. 6. Results: for outcomes and times of assessment (including a measure of variation), if necessary converted to measures of effect specified below, intention‐to‐treat analysis.
Subgroup analyses We planned to perform subgroup analyses in order to explore effect size differences as follows:
1. Duration of treatment (4 weeks versus > 4 weeks); and 2. Patients with Asian ethnic origin compared with non‐Asians.
Sensitivity analyses We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
1. Repeating the analysis excluding unpublished studies; and 2. Repeating the analysis taking account of study quality, as specified above.
Heterogeneity between trials results was tested using a standard chi‐squared test. The results are reported as risk ratios (RR) with corresponding 95% confidence interval (CI) for dichotomous data using the fixed‐effect model (APT 1994). For continuous data, the difference in means are computed for outcomes measured on the same scale.
Results
Description of studies
A total of 54 studies of suxiao jiuxin wan for angina pectoris were identified by the searches. All were published in Chinese. No unpublished studies or other information was obtained from contact with WHO, individual researchers and herb manufactures. Of the 54 studies, 39 were excluded upon further scrutiny. Details of the excluded studies are shown in the characteristics of excluded studies. Studies excluded for not being randomised controlled trials (Cheng 2005; Feng 2000; Gao 2003; Han 2000; Hu 2000c; Jia 2000; Lai 2003; Li 1996; Li 1998; Li 1999; Li 2000b; Li 2000c; Liang 1995; Liu 1996a; Liu 1996b; Liu 1996c; Lu 2000; Luo 2002; Pu 2000; Wang 1996; Wang 2000b; Wang 2000c; Wei 1995; Yuan 2000; Yuan 2002; Zhang 1997; Zhang 2000b; Zhou 2000a; Zhou 2000b; Zhou 2002; Zhu 2005; Zhuang 1999), not reporting relevant outcomes (Duan 2002; Ma 2004; Wu 2003), other drugs potentially interfering with the outcomes (Cai 2003; Zheng 2003) and study duration < 4 weeks (Guo 1996; Hou 2000).
Included studies Details of the 15 included studies are shown in the characteristics of included studies table. All studies included were of a parallel design, single centre and had a positive control group. For the randomisation procedure units for allocation were all individuals. Trial duration ranged from 4 weeks to 2 years. Trials came only from China and were written in Chinese. Numbers of participants of the individual studies ranged from 48 to 248 with a total of 1776 participants included in this review. Ages of participants ranged from 35 to 85 years old. 664 participants were women.
Interventions in included studies Eleven of the studies used nitroglyerin (also known as xiaoxintong) in the control group (Gao 1996; Hu 2000a; Hu 2000b; Ji 1996; Li 2000a; Liu 2000; Shi 2002; Sun 2002; Tang 2000; Yang 2000; Zhang 2000a). In three studies, Salvia miltiorrhiza (also known as danshen) was used as the control (He 1995; Song 1995; Wang 2000a). Pharmacological studies have shown that danshen can reduce blood viscosity, dilate blood vessels, reduce arterial pressure, improve platelet function, anticoagulate, stabilise cell membrane, maintain cell function and fight infection (Cai 1999). In one study isosorbide dinitrate (Xiaosuanyishanlizhi) was the control (Zhan 2000). Only two studies lasted longer than 4 weeks (Gao 1996; Liu 2000), the remaining 13 studies had a duration of 4 weeks (He 1995; Hu 2000a; Hu 2000b; Ji 1996; Li 2000a; Shi 2002; Song 1995; Sun 2002; Tang 2000; Wang 2000a; Yang 2000; Zhan 2000; Zhang 2000a). All interventions were given orally. The treatment regimen of suxiao jiuxin wan varied in the studies: four pills were used in two studies(He 1995; Song 1995); five pills in eight studies(Li 2000a; Liu 2000; Sun 2002; Shi 2002; Tang 2000; Wang 2000a; Zhan 2000; Zhang 2000a); four to six pills in one study (Hu 2000a); six pills in two studies (Hu 2000b; Ji 1996) and 10 pills in two studies (Gao 1996; Yang 2000). In two studies participants in both groups were given additional treatments (Hu 2000a; Wang 2000a). Outcome measures in included studies None of the studies assessed mortality, severity of angina pectoris, levels of plasma endothelin level and nitric oxide or health‐related quality of life. One study (Hu 2000b) reported frequency of acute attacks of angina. All studies reported on 'symptom improvement'. Thirteen studies (Gao 1996; Hu 2000a; Hu 2000b; Ji 1996; Li 2000a; Liu 2000; Shi 2002; Song 1995; Sun 2002; Tang 2000; Yang 2000; Wang 2000a; Zhang 2000a) reported on ECG improvement. One study (Hu 2000a) reported on the frequency of taking nitroglycerin.One study (Tang 2000) reported changes in blood pressure.
Risk of bias in included studies
All studies were of poor methodological quality, and are at high risk of bias. All studies were described as 'randomised' but none of the studies mentioned allocation concealment. No study mentioned blinding of participants or of outcome assessors. None of the studies provided any data on dropouts. In all studies the characteristics of participants in different treatment groups were similar at baseline (age, sex, race, severity of angina and smoking status).
Effects of interventions
Most of the 15 included trials did not separately report on all 10 outcome measures of interest. Data were only available for the following outcomes: symptom improvement (15 trials), frequency of acute attacks (one trial), changes in blood pressure (one trial) and ECG improvement (13 trials).
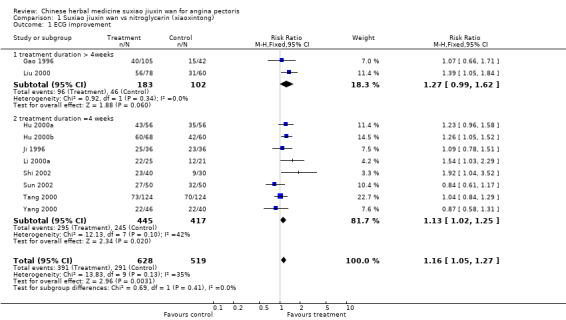
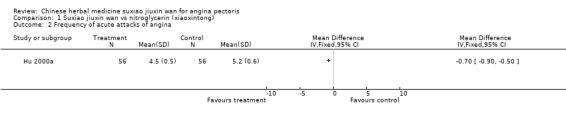
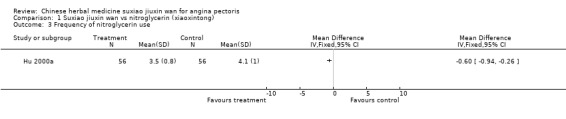
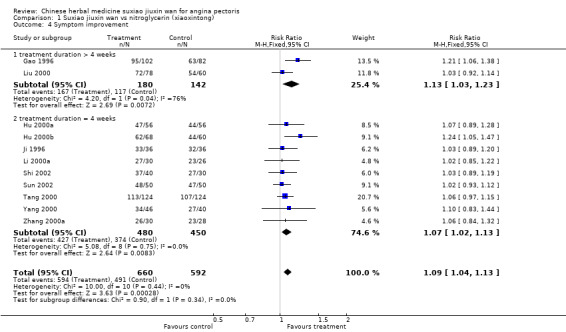
Suxiao jiuxin wan compared to nitroglycerin (xiaoxintong) There was evidence from one trial that suxiao jiuxin wan reduced frequency of acute angina attacks compared to nitroglycerin use (difference in means ‐0.70, 95% CI ‐0.50 to ‐0.90, P < 0.00001). In the one trial with relevant data, there was evidence of a small fall in diastolic blood pressure (difference in means ‐3mmHg, 95% CI ‐5.73 to ‐0.27) but no difference in systolic blood pressure (difference in means 0.7mmHg, 95% CI ‐3.13 to 4.53). Patients in 10 studies taking suxiao jiuxin wan had better ECG results than those taking nitroglycerin (RR 1.16, 95% CI 1.05 to 1.27, P = 0.0003). In 10 trials, patients on suxiao jiuxin wan reported better symptom improvement compared to those on nitroglycerin (RR 1.09, 95% CI 1.04 to 1.13, P = 0.0003). There was evidence from one trial that people receiving suxiao jiuxin wan took fewer supplementary nitroglycerin doses than those receiving nitroglycerin only (difference in means ‐0.60, 95% CI ‐0.94 to ‐0.26, P = 0.0005).
Suxiao jiuxin wan compared to Salvia miltiorrhiza (danshen) Two studies reported improvement in ECG for people taking suxiao jiuxin wan as compared to those taking Salvia miltiorrhiza (danshen) (RR 1.55, 95% CI 1.30 to 1.84, P < 0.0001) There was no evidence of heterogeneity (chi2 = 0.19, df = 1, P = 0.67, I2 = 0%). Patients receiving suxiao jiuxin wan reported more symptom improvement compared to those receiving danshen (RR 1.21, 95% CI 1.11 to1.31, P < 0.0001). There was no evidence of heterogeneity (chi2 = 0.80, df = 2, P = 0.67, I2 = 0%).
Suxiao jiuxin wan compared to isosorbide dinitrate (xiaosuanyishanlizhi) In one study, there was no evidence to show suxiao jiuxin wan improved ECG compared to isosorbide dinitrate (RR 1.34, 95% CI 0.91 to 1.98 ) and no evidence to show suxiao jiuxin wan improved symptoms (RR 1.11, 95% CI 0.86 to 1.43) .
Subgroup analyses The trend toward greater ECG improvement in the suxiao jiuxin wan group compared to nitroglycerin group with duration of treatment of 4 weeks (RR 1.13, 95% CI 1.02 to 1.25) was similar to studies with a treatment duration of more than 4 weeks (RR 1.27, 95% CI 0.99 to 1.62).
There was only very weak evidence of any improvement in symptoms in the suxiao jiuxin wan group compared to the nitroglycerin group with a treatment duration of 4 weeks (RR 1.07, 95% CI 1.02 to 1.13). In those treated for longer than 4 weeks the evidence favoured treatment with suxiao jiuxin wan but there was significant heterogeneity (RR 1.13, 95% CI 1.03 to 1.23, chi2 = 4.20, df = 1, P = 0.04, I2 = 76.2%).
Sensitivity analyses We did not carry out any of the planned sensitivity analyses as no unpublished studies were found and all included studies were of poor methodological quality (graded C ‐ high risk of bias).
Discussion
We found a tendency towards symptom improvement with suxiao jiuxin wan. However, all identified studies were of poor quality and many of the outcome measures that we considered to be important were not assessed. There was a lack of clinically relevant event outcomes and no measure of patient quality of life. In the studies identified, differing treatment regimes were used and outcomes were measured in different ways.
Limitations of the review The conclusions of this review must be considered with great caution. Only a small number of studies were included in this review, and none of these abided by the criteria laid down in the CONSORT statement (CONSORT 2001). The reporting of quality issues in the studies was generally poor. For most of the trials the method of randomisation was not reported clearly, and none of the trials reported blinding of assessors of outcomes. The poor evidence does not allow any conclusion regarding the effectiveness of suxiao jiuxin wan per se and none of the included studies were ideally suited to investigate the effectiveness of suxiao jiuxin wan in treating angina pectoris. While suxiao jiuxin wan is undoubtedly the most widely used treatment for angina pectoris in China, the results of the review suggest that it is not suitable for all situations. We intend to look at this in a future review.
The review included studies only conducted in China. Delivery of treatment and quality control of suxiao jiuxin wan is probably a little difficult in remote areas in China and this method of treating angina pectoris may therefore be problematic.
Studies generally concentrated on measuring angina improvement by ECG ‐ presumably because these measurements can be easily and quickly obtained. However, these values may not reflect long‐term clinical improvement and therefore other indicators should be used, for example, the need for additional nitroglycerin to control symptoms of an attack, frequency of acute angina attacks, levels of plasma endothelin level and nitric oxide or health‐related quality of life. These would give a more accurate picture of any improvement.
There have been concerns about adverse effects of suxiao jiuxin wan. Abdomen discomfort (Ji 1996; Liu 2000), thirst (Song 1995) and reddening of the skin (Ji 1996; Song 1995; Tang 2000) were reported. These symptoms were not serious and could be tolerated by patients. Headache was reported in some of the included studies (Ji 1996; Song 1995; Tang 2000; Zhang 2000a), as was bradycardia (Zhang 2000a). However symptoms of bradycardia and headache were not often severe; symptoms were relieved after a short rest and none of the patients who had these symptoms needed special management.
Authors' conclusions
Implications for practice.
Although trials of suxiao jiuxin wan alone or in combination with other anti‐anginal treatments showed weak evidence of a reduction in symptoms and an improvement in ECG measurements, methodological concerns including concealment of allocation, lack of blinding, lack of statistical power, lack of information on hazards of treatment, and lack of other clinically relevant outcomes, make the role of suxiao jiuxin wan in the management of angina pectoris uncertain.
Implications for research.
More high quality controlled trials are required for assessing the effects of suxiao jiuxin wan in comparison to other drugs. These studies should also address the most effective dosage to be used (under given conditions). Studies should be large and long term, lasting at least 1 year, including participants of all ages. The outcomes studied should not be restricted to symptom improvement and ECG improvement, but should include the other outcome measures specified above, such as mortality and health‐related quality of life. Special attention should be paid to adverse effects and methodological challenges, such as inadequate randomisation, blinding, sample size, need to be tackled.
What's new
| Date | Event | Description |
|---|---|---|
| 23 January 2013 | Review declared as stable | Authors unable to update. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 1, 2008
| Date | Event | Description |
|---|---|---|
| 16 July 2009 | Amended | Order of authors corrected ‐ error due to RevMan conversion. |
| 8 September 2008 | Amended | Converted to new review format. |
| 13 November 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
Authors unable to update.
Acknowledgements
Our thanks to Liu Chang for assisting the author team and the Cochrane Heart Group for advice on the writing of the review.
Data and analyses
Comparison 1. Suxiao jiuxin wan vs nitroglycerin (xiaoxintong).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ECG improvement | 10 | 1147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.05, 1.27] |
| 1.1 treatment duration > 4weeks | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.99, 1.62] |
| 1.2 treatment duration =4 weeks | 8 | 862 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.02, 1.25] |
| 2 Frequency of acute attacks of angina | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Frequency of nitroglycerin use | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Symptom improvement | 11 | 1252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.04, 1.13] |
| 4.1 treatment duration > 4 weeks | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.03, 1.23] |
| 4.2 treatment duration = 4 weeks | 9 | 930 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [1.02, 1.13] |
| 5 Blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 systolic pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 diastolic pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Suxiao jiuxin wan vs nitroglycerin (xiaoxintong), Outcome 1 ECG improvement.
1.2. Analysis.
Comparison 1 Suxiao jiuxin wan vs nitroglycerin (xiaoxintong), Outcome 2 Frequency of acute attacks of angina.
1.3. Analysis.
Comparison 1 Suxiao jiuxin wan vs nitroglycerin (xiaoxintong), Outcome 3 Frequency of nitroglycerin use.
1.4. Analysis.
Comparison 1 Suxiao jiuxin wan vs nitroglycerin (xiaoxintong), Outcome 4 Symptom improvement.
1.5. Analysis.
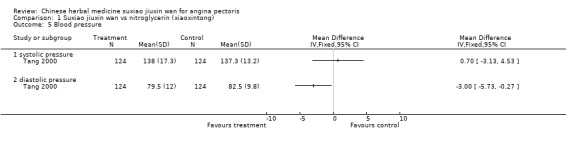
Comparison 1 Suxiao jiuxin wan vs nitroglycerin (xiaoxintong), Outcome 5 Blood pressure.
Comparison 2. Suxiao jiuxin wan vs salvia miltiorrhiza (danshen).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ECG improvement | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2 treatment duration = 4 weeks | 2 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.30, 1.84] |
| 2 Symptom improvement | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2 treatment duration =4 weeks | 3 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.11, 1.31] |
2.1. Analysis.
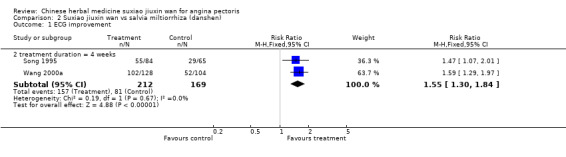
Comparison 2 Suxiao jiuxin wan vs salvia miltiorrhiza (danshen), Outcome 1 ECG improvement.
2.2. Analysis.
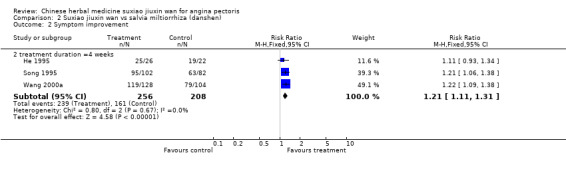
Comparison 2 Suxiao jiuxin wan vs salvia miltiorrhiza (danshen), Outcome 2 Symptom improvement.
Comparison 3. Suxiao jiuxin wan vs isosorbide dinitrate (xiaosuanyishanlizhi).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ECG improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Symptom improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
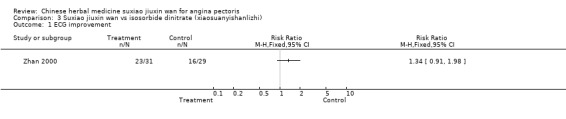
Comparison 3 Suxiao jiuxin wan vs isosorbide dinitrate (xiaosuanyishanlizhi), Outcome 1 ECG improvement.
3.2. Analysis.
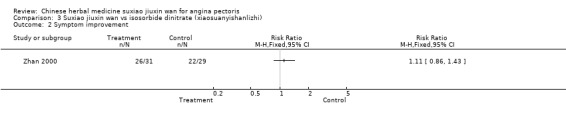
Comparison 3 Suxiao jiuxin wan vs isosorbide dinitrate (xiaosuanyishanlizhi), Outcome 2 Symptom improvement.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gao 1996.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 2 years and 4 months Location: China Hebei | |
| Participants | 147 people (35‐85 years) 82 men and 65 women | |
| Interventions | 1.Suxiao jiuxin wan (10 pills when angina attack) n = 105 2. Nitroglycerin (0.5mg when angina attack) n = 42 | |
| Outcomes | Symptoms improvement, ECG, adverse effects | |
| Notes | ||
He 1995.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Jiangsu | |
| Participants | 48 people (40‐60 years) 33 men and 15 women | |
| Interventions | 1. Suxiao jiuxin wan (4 pills three times a day and 10 pills when angina attack) n = 26 2. Dansheng pill (3 pills three times a day and 0.5 mg nitroglycerin when angina attack) n = 22 | |
| Outcomes | Symptoms improvement, haemodynamics, adverse effects | |
| Notes | ||
Hu 2000a.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Anhui | |
| Participants | 112 people( 41‐76 years) 73 men and 39 women | |
| Interventions | Other treatment: aspirin 75 mg three times a day, vit.C 200mg once a day, vit.E 100mg three times a day, fufang danshengpian 3 pills three times a day. 1. Suxiao jiuxin wan (4‐6 pills three times a day and 10‐15 pills when angina attack) n = 56 2. Xiaoxintong (10 mg ) n = 56 | |
| Outcomes | Symptoms improvement, ECG, adverse effects | |
| Notes | ||
Hu 2000b.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Tianjin | |
| Participants | 128 people( 43‐75 years) 78 men and 50 women | |
| Interventions | 1. Suxiao jiuxin wan (6 pills, three times a day and 10 pills when angina attack) n = 68 2. Xinxiaotong (10 mg) n = 60 | |
| Outcomes | Symptoms improvement, ECG, adverse effects | |
| Notes | ||
Ji 1996.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Guizhou | |
| Participants | 72 people( 45‐79 years) 43 men and 39 women | |
| Interventions | 1. Suxiao jiuxin wan (6 pills three times a day and 10 pills when angina attack) n = 36 2. Nitroglycerin (0.5mg three times a day and 0.5 mg when angina attack) n = 36 | |
| Outcomes | Symptoms improvement, ECG, adverse effects | |
| Notes | ||
Li 2000a.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Anhui | |
| Participants | 56 people( 49‐65 years) 37 men and 19 women | |
| Interventions | 1. Suxiao jiuxin wan (5 pills and nitroglycerin 0.5 mg when angina attack) n = 30 2. Xiaoxintong (10 mg) n = 26 | |
| Outcomes | Symptoms improvement, ECG, adverse effects | |
| Notes | ||
Liu 2000.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 60 days Location: China, JIangxi | |
| Participants | 138 people( 46‐72 years) 92 men and 46 women | |
| Interventions | 1. Suxiao jiuxin wan (5 pills three times a day ) n = 78 2. Xiaoxintong (10 mg three times a day) n = 60 | |
| Outcomes | Symptoms improvement, ECG, adverse effects | |
| Notes | ||
Shi 2002.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Shanghai | |
| Participants | 70 people( 50‐65 years) 37 men and 35 women | |
| Interventions | 1. Suxiao jiuxin wan(5 pills three times a day ) n = 40 2. Xiaoxintong (10 mg three times a day) n = 30 | |
| Outcomes | ECG improvement, symptoms improvement | |
| Notes | ||
Song 1995.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Shandong | |
| Participants | 184 people 133 men and 51 women( 45‐74 years) | |
| Interventions | 1. Suxiao jiuxin wan (5 pills three times a day and 10‐15 pills when angina attack) n = 102 2.Dansheng pills (3 pills three times a day) n = 82 | |
| Outcomes | Frequency of acute attack angina, ECG improvement, BP and HR, symptoms improvement, adverse effects | |
| Notes | ||
Sun 2002.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Shandong | |
| Participants | 100 people (37‐72 years) 62 men and 38 women | |
| Interventions | 1. Suxiao jiuxin wan (5 pills three times a day and 10‐15 pills when angina attack) n = 50 2. Xiaoxintong (10 mg threes a day and 10‐20 mg when angina attack) n = 50 | |
| Outcomes | Symptoms improvement, ECG improvement | |
| Notes | ||
Tang 2000.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Hubei | |
| Participants | 248 people (46‐78 years) 138 men and 110 women | |
| Interventions | 1. Suxiao jiuxin wan (5 pills three times a day and 10 pills when angina attack) n = 124 2. Xiaoxintong (10 mg threes a day and 10 mg when angina attact) n = 124 | |
| Outcomes | Symptoms improvement, ECG improvement, HR, BP and RPP, adverse effects | |
| Notes | ||
Wang 2000a.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, LiaoYang | |
| Participants | 232 people (41‐79 years) 154 men and 78 women | |
| Interventions | Other treatment: isoine 0.2g, threes a day; vit B1,10 mg, three times a day 1. Suxiao jiuxin wan (4 pills three times a day) n = 128 2. Dansheng pills (3 pills three times a day ) n = 104 | |
| Outcomes | Symptoms improvement , ECG improvement, adverse effects | |
| Notes | ||
Yang 2000.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Shandong | |
| Participants | 86 people (38‐69 years) 56 men and 30 women | |
| Interventions | 1. Suxiao jiuxin wan (10 pills three times a day ) n = 46 2. Xiaoxintong (10 mg three times a day ) n = 40 | |
| Outcomes | Symptoms improvement, ECG improvement, adverse effects | |
| Notes | ||
Zhan 2000.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Hubei | |
| Participants | 60 people (43‐69 years) 48 men and 12 women | |
| Interventions | 1. Suxiao jiuxin wan (5 pills three times a day) n = 31 2. Xiaoshuanyishanlizhi (isosorbide dinitrate) (10 mg three times a day) n = 29 | |
| Outcomes | Symptoms improvement, ECG improvement, adverse effects | |
| Notes | ||
Zhang 2000a.
| Methods | Randomised controlled trial, not blinded Comparison: individuals Duration: 4 weeks Location: China, Hubei | |
| Participants | 91 people (46‐64 years) 54 men and 37 women | |
| Interventions | 1. Suxiao jiuxin wan (5 pills three times a day and 10‐15 pills when angina attack) n = 30 2. Xiaoxintong (10‐15mg three times a day ) n = 28 | |
| Outcomes | Symptoms improvement, adverse effects | |
| Notes | ||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cai 2003 | Interfered by other drugs (aspirin) only control group used |
| Cheng 2005 | Not a randomised controlled trial |
| Duan 2002 | Did not present outcomes of interest (ejection fraction, cardiac output, cardiac minute output and haematodynamics) |
| Feng 2000 | Not a randomised controlled trial |
| Gao 2003 | Not a randomised controlled trial |
| Guo 1996 | Insufficient duration (only 2 weeks) |
| Han 2000 | Not a randomised controlled trial |
| Hou 2000 | Insufficient duration (only 10 days) |
| Hu 2000c | Not a randomised controlled trial |
| Jia 2000 | Not a randomised controlled trial |
| Lai 2003 | Not a randomised controlled trial |
| Li 1996 | Not a randomised controlled trial |
| Li 1998 | Not a randomised controlled trial |
| Li 1999 | Not a randomised controlled trial |
| Li 2000b | Not a randomised controlled trial |
| Li 2000c | Not a randomised controlled trial |
| Liang 1995 | Not a randomised controlled trial |
| Liu 1996a | Not a randomised controlled trial |
| Liu 1996b | Not a randomised controlled trial |
| Liu 1996c | Not a randomised controlled trial |
| Lu 2000 | Not a randomised controlled trial |
| Luo 2002 | Not a randomised controlled trial |
| Ma 2004 | have not the result that we wanted |
| Pu 2000 | Not a randomised controlled trial |
| Wang 1996 | Not a randomised controlled trial |
| Wang 2000b | Not a randomised controlled trial |
| Wang 2000c | Not a randomised controlled trial |
| Wei 1995 | Not a randomised controlled trial |
| Wu 2003 | have not the result that we wanted |
| Yuan 2000 | Not a randomised controlled trial |
| Yuan 2002 | Not a randomised controlled trial |
| Zhang 1997 | Not a randomised controlled trial |
| Zhang 2000b | Not a randomised controlled trial |
| Zheng 2003 | Interfered by other drugs (drugs for diabetes and hypertension, the study didn't report the name of the drugs) |
| Zhou 2000a | Not a randomised controlled trial |
| Zhou 2000b | Not a randomised controlled trial |
| Zhou 2002 | Not a randomised controlled trial |
| Zhu 2005 | Not a randomised controlled trial |
| Zhuang 1999 | Not a randomised controlled trial |
Contributions of authors
Xin Duan, Taixiang Wu, Likun Zhou: conceived the review, prepared and designed the protocol, coordinated the review process, developed and ran search strategy, screened results, organised retrieval of papers, extracted data, helped interpret data and provided a methodological, policy and clinical perspective on the data, participated in writing the review. Guanjian Liu: appraised papers, extracted and analysed data. Jieqi Qiao, Qin Wang, Liu Chang, Jiafu Wei, Juan Ni, Jie Zheng, Xiaoyan Chen: searched for trials.
Sources of support
Internal sources
Chinese Cochrane Centre, West China Hospital of Sichuan University, China.
External sources
Chinese Medical Board of New York (CMB), USA.
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Gao 1996 {published data only}
- Gao YC, He SZ, Liu AX, Yin DF, Chen CH, Gao RX. Therapeutic effect of sudden acesodyne obseveration of Suxiao Jiuxin Wan for CHD angina pectoris. Journal of Emergency Syndromes in Chinese Medicine 1996;5(2):74‐5. [Google Scholar]
He 1995 {published data only}
- He GH, Zhu LP. Clinical obseveration of Suxiao Jiuxin Wan for coronary heart disease and angina pectoris. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care 1995;2(5):214. [Google Scholar]
Hu 2000a {published data only}
- Hu J, Pu Y. Therapy effect observation of Suxiao Jiuxin Wan for angina pectoris. Tianjin Journal of Medicine 2000, (Suppl):19‐21. [Google Scholar]
Hu 2000b {published data only}
- Hu M. Clinical observation of Suxiao Jiuxin Wan for CHD angina pectoris 68 cases. Tianjin Journal of Medicine 2000, (Suppl):36‐7. [Google Scholar]
Ji 1996 {published data only}
- Ji K. Suxiao Jiuxin Wan for CHD angina pectoris 36 cases. Journal of Emergency in Traditional Chinese Medicine 1996;5(3):118. [Google Scholar]
Li 2000a {published data only}
- Li S. Analysis of therapeutical effect of Suxiao JIuxin Wan for CHD angina pectoris 30 cases. Tianjin Journal of Medicine 2000, (Suppl):33‐4. [Google Scholar]
Liu 2000 {published data only}
- Liu X. Observation on the therapeutic effect of Suxiao Jiuxin Wan for CHD angina pectoris 78 cases. Tianjin Journal of Medicine 2000, (Suppl):31‐2. [Google Scholar]
Shi 2002 {published data only}
- Shi H. Treatment of 40 cases of coronary heart disease and angina pectoris by Suxiao Jiuxin Wan. Chinese Traditional Patent Medicine 2002;24:852‐4. [Google Scholar]
Song 1995 {published data only}
- Song ZJ, Ren ZG. Observing recent curative effect of Suxiao Jiuxin Wan for coronary heart disease and angina pectoris. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care 1995;2(2):83. [Google Scholar]
Sun 2002 {published data only}
- Sun B. Comparing the clinical effect of Suxiao Jiuxin Wan with Xiaoxintong. Chinese journal of natural Medicine 2002;4:185‐6. [Google Scholar]
Tang 2000 {published data only}
- Tang G. Clinical observation of Suxiao Jiuxin Wan for CHD angina pectoris 124 cases. Tianjin Journal of Medicine 2000, (Suppl):40‐1. [Google Scholar]
Wang 2000a {published data only}
- Wang L, Jiang X. Clinical research of Suxiao Jiuxin Wan for angina pectoris. Tianjin Journal of Medicine 2000, (Suppl):17. [Google Scholar]
Yang 2000 {published data only}
- Yang G, Zhou J. Suxiao Jiuxin Wan combined Xiaoxintong for CHD angina pectoris. Tianjin Journal of Medicine 2000, (Suppl):48. [Google Scholar]
Zhan 2000 {published data only}
- Zhan Y. Observation of therapeutic effects of Suxiao Jiuxin Wan for CHD andina pectoris of elder people. Tianjin Journal of Medicine 2000, (Suppl):46‐7. [Google Scholar]
Zhang 2000a {published data only}
- Zhang J, Wu J, Zheng X, Zhen R. Suxiao Jiuxin Wan combined with Xiaoxintong for CHD Angina pectoris. Tianjin Journal of Medicine 2000, (Suppl):34‐35. [Google Scholar]
References to studies excluded from this review
Cai 2003 {published data only}
- Cai H, Tan F. Treatment of 50 cases of coronary heart disease and angina pectoris by Suxiao Jiuxin Wan. Modern Medicine and Sanitary Modern Traditional Chinese Medicine 2003;19:1163‐4. [Google Scholar]
Cheng 2005 {published data only}
- Cheng X, Li F, Li X. Clinical observation of Suxiao Jiuxin Wan and nitroglycerine for acute angina pectoris. Applied Journal of General Practice 2005;3:354. [Google Scholar]
Duan 2002 {published data only}
- Duan K, Zhang Z, Yang X. Clinical observation on 40 cases of CHD and angina pectoris treated by Suxiao Jiuxin Wan. Tianjin Journal of Traditional Chinese Medicine 2002;19(1):20‐1. [Google Scholar]
Feng 2000 {published data only}
- Feng L, Han T, Zhou YL. The conclusion of the clinic effect for Suxiao Jiuxin Wan for CHD and angina pectoris. Journal of Emergency Syndromes in Chinese Medicine 2000;9(1):4‐6. [Google Scholar]
Gao 2003 {published data only}
- Gao J. Observation of 50 cases of coronary heart disease and angina pectoris by Suxiao Jiuxin Wan. Chinese General Practice 2003;16:250‐1. [Google Scholar]
Guo 1996 {published data only}
- Guo YY, Gao ZZ. Clinical observation of Suxiao JIuxin Wan for coronary heart disease and angina pectoris. Journal of Emergency Syndromes in Chinese Medicine 1996;5(3):115. [Google Scholar]
Han 2000 {published data only}
- Han T, Den LJ, Feng L. Clinical therapeutic effect and mechanism research of Suxiao JiuXin Wan for CHD angina pectoris. Journal of Traditional Chinese Medicine 2002;41(12):733‐42. [Google Scholar]
Hou 2000 {published data only}
- Hou Y. Observation of the clinical therapeutic effect of Suxiao Jiuxin Wan for CHD angina pectoris 84 cases. Tianjin Journal of Medicine 2000, (Suppl):42‐3. [Google Scholar]
Hu 2000c {published data only}
- Hu B, Feng N. Clinical observation of Suxiao Jiuxin Wan for attacks of angina pectoris. Tianjin Journal of Medicine 2000, (Suppl):55‐6. [Google Scholar]
Jia 2000 {published data only}
- Jia Y. Analysing the therapeutic effect of Suxiao Jiuxin Wan for CHD angina pectoris. Tianjin Journal of Medicine 2000, (Suppl):26‐8. [Google Scholar]
Lai 2003 {published data only}
- Lai Z, Lai C, Fan W. Evaluation of the effect of two different kinds of traditional Chinese patent medicine on unstable angina pectoris. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine 2003;13:27‐8. [Google Scholar]
Li 1996 {published data only}
- Li F, Li X, Wang C, Zhang Q, Wang H. Clinical research of Suxiao Jiuxin wan for CHD. Journal of Emergency in Traditional Chinese Medicine 1996;5(3):113‐4. [Google Scholar]
Li 1998 {published data only}
- Li F, Ma J, Zhang Z, Sun Y. Clinical compared observation of Fufang Dangseng Diwan and Suxiao Jiuxin Wan for CHD. Chinese Traditional Patent Material 1998;20(3):29‐30. [Google Scholar]
Li 1999 {published data only}
- Li S, Liu S. Clinical analysis of western medicine combined with Chinese medicine for CHD angina pectoris. Sichuan Journal of Medicine 1999;20(2):134‐5. [Google Scholar]
Li 2000b {published data only}
- Li S. Observation of the therapeutic effect of Suxiao Jiuxin Wan for CHD 56 cases. Tianjin Journal of Medicine 2000, (Suppl):48‐9. [Google Scholar]
Li 2000c {published data only}
- Li Y. Observation of Suxiao Jiuxin Wan for CHD angina pectoris. Tianjin Journal of Medicine 2000, (Suppl):47. [Google Scholar]
Liang 1995 {published data only}
- Liang Y, Chen L, Zhao J, Den W, Li W, Jiang L, et al. Analysis of Suxiao Jiuxin Wan for CHD angina pectoris 55 cases. The Practical Journal of Integrating Chinese with Modern Medicine 1995;8(1):31‐2. [Google Scholar]
Liu 1996a {published data only}
- Liu J. Observation of therapeutic effects of Suxiao Jiuxin Wan for CHD. The Practical Journal of Intergrating Chinese with Modern Medicine 1996;9(9):476. [Google Scholar]
Liu 1996b {published data only}
- Liu Z, Liu Y, Guo D, Wang S, Shao L. Clinical observation of Suxiao Jiuxin Wan for CHD Angina pectoris 385 cases. Journal of Emergency Syndromes in Chinese Medicine 1996;5(3):116‐7. [Google Scholar]
Liu 1996c {published data only}
- Liu Z, Liu Y, Zhou D, Wang S, Shao L. Clinical observation of Suxiao Jiuxin Wan for CHD angina pectoris 385 cases. Journal of Emergency in Traditional Chinese Medicine 1996;5(3):116‐7. [Google Scholar]
Lu 2000 {published data only}
- Lu W. Suxiao Jiuxin Wan for angina pectoris 406 cases. Tianjin Journal of Medicine 2000, (Suppl):15‐6. [Google Scholar]
Luo 2002 {published data only}
- Luo Y. Report of Suxiao Jiuxin Wan for angina pectoris 31 cases. Journal of Chinese Country Medicine and Medical Science 2002;9(2):10‐1. [Google Scholar]
Ma 2004 {published data only}
- Ma X, Yang Q. 83 cases of clinical observations of Suxiao Jiuxin Wan for coronary heart disease of angina pectoris. Modern Medicine and Sanitary 2004;20:2211‐2. [Google Scholar]
Pu 2000 {published data only}
- Pu Y, Hu J. Clinical conclusion of Suxiao Jiuxin Wan for CHD. Tianjin Journal of Medicine 2000, (Suppl):23‐5. [Google Scholar]
Wang 1996 {published data only}
- Wang D, Chen J. Comparing the effect of Sexiang Baoxin Wan to Suxiao Jiuxin Wan for CHD angina pectoris. Shanghai Medical Science 1996, (12):25‐6. [Google Scholar]
Wang 2000b {published data only}
- Wang Y, Yang X. Observation of the therapeutic effect of Suxiao Jiuxin Wan for CHD angina pectoris 120 cases. Tianjin Journal of Medicine 2000, (Suppl):35‐6. [Google Scholar]
Wang 2000c {published data only}
- Wang L. Clinical conclusion of Suxiao Jiuxin Wan for CHD angina pectoris. Tianjin Journal of Medicine 2000, (Suppl):39‐40. [Google Scholar]
Wei 1995 {published data only}
- Wei J, Tang Y. Comparing research on the clinical effect of Suxiao Jiuxin Wan for ischemic heart disease. Journal of Emergency Syndromes in Chinese Medicine 1995, (4):159‐160,164. [Google Scholar]
Wu 2003 {published data only}
- Wu TP. Clinical observation of Suxiao Jiuxin Wan and Numanxinkang for coronary heart disease, angina pectoris. Modern Medicine and Sanitary 2003;19:274‐5. [Google Scholar]
Yuan 2000 {published data only}
- Yuan J, Guo Q, Yuan H. Analysis of the therapeutic effect of Suxiao Jiuxin Wan for CHD. Tianjian Journal of Medicine 2000, (Suppl):37‐9. [Google Scholar]
Yuan 2002 {published data only}
- Yuan G. Curative effect observions on Suxiao jiuxin wan compared to Xiao xin tong for Angina pectoris. Medical Journal of Healing of Heart and Vessel 2002;9(9):71‐2. [Google Scholar]
Zhang 1997 {published data only}
- Zhang W, Sun B. Clinical controlled observation on the method of Senzhongbuqi for treating angina pectoris. Journal of Emergency Syndromes in Traditional Chinese Medicine 1997;6(6):248‐50. [Google Scholar]
Zhang 2000b {published data only}
- Zhang J, Wu J, Zheng S. Suxiao Jiuxin wan combined with Xiaoxintong for CHD angina pectoris 33 cases. Tianjin Journal of Medicine 2000, (Suppl):34‐5. [Google Scholar]
Zheng 2003 {published data only}
- Zheng J. Observation on the clinical effects of capsules of Sunaoxintong and Suxiao Jiuxin Wan for senile coronary heart disease angina pectoris. Chinese Journal of Primary Medicine and Pharmacy 2003;10:1066‐7. [Google Scholar]
Zhou 2000a {published data only}
- Zhou Z. Clinical research of Suxiao Jiuxin Wan for angina pectoris. Tianjin Journal of Medicine 2000, (Suppl):10‐1. [Google Scholar]
Zhou 2000b {published data only}
- Zhou L. Clinical observation on Suxiao Jiuxin Wan for CHD angina pectoris. Tianjin Journal of Medicine 2000, (Suppl):25‐6. [Google Scholar]
Zhou 2002 {published data only}
- Zhou D. Report of Suxiao Jiuxin Wan for angina pectoris 37 cases. Acta Academiea Medicinea Jiangxi 2002;42(2):134. [Google Scholar]
Zhu 2005 {published data only}
- Zhu D, Xia R. Suxiao Jiuxin Wan and Jihuaye for coronary heart disease angina pectoris. Henan Traditional Chinese Medicine 2005;25:76‐7. [Google Scholar]
Zhuang 1999 {published data only}
- Zhuang Z, Wang Y, Zhang Q, Yao G, Feng G. Observation on the therapeutic effect of Suxiao Jiuxin Wan for non‐syndrome myocardial ischeamia of angina. Modern Journal of Integrated Chinese Traditional and Western Medicine 1999;8(11):1781‐2. [Google Scholar]
Additional references
ACC/AHA 1999
- Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM. ACC/AHA/ACP‐ASIM guidelines for the management of patients with chronic stable angina: Executive summary and recommendations: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina). Circulation 1999;99(21):2829‐48. [DOI] [PubMed] [Google Scholar]
Akhras 1991
- Akhras F, Jackson G. Efficacy of nifedipine and isosorbide mononitrate in combination with atenolol in stable angina. Lancet 1991;338(8774):1036‐9. [DOI] [PubMed] [Google Scholar]
Alderman 1990
- Alderman EL, Bourassa MG, Cohen LS, Davis KB, Kaiser GG, Killip T, et al. Ten year follow up of survival and myocardial infarction in the randomised coronary artery surgery study. Circulation 1990;82(5):1629‐46. [DOI] [PubMed] [Google Scholar]
APT 1994
- Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy ‐ 1: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81‐106. [PMC free article] [PubMed] [Google Scholar]
Asirvatham 1998
- Asirvatham S, Sebastian C, Thadani U. Choosing the most appropriate treatment for stable angina safety: safety consideration. Drug Safety 1998;19(1):23‐44. [DOI] [PubMed] [Google Scholar]
Braunwald 1992
- Braunwald E (Editor). Heart disease : a textbook of cardiovascular medicine. 4th Edition. Philadelphia, Pa ; London: W.B. Saunders, 1992. [Google Scholar]
Cai 1999
- Cai YM, Ren YR, Wang L, Zhang GT. Zhui Xin Zhong Yao Yao Li Yu Ling Chuang Ying Yong. Beijing: Hua Xia Chu Ban She, 1999. [Google Scholar]
Chen 2000
- Chen HZ. Atherosclerosis and coronary atherosclerotic heart disease. In: Chen HZ editor(s). Internal Medicine. Fourth. Beijing: People's Health Publication House, 2000. [Google Scholar]
Cleland 1996
- Cleland JGF. Can improved quality of care reduce the costs of managing angina pectoris. European Heart Journal 1996;17(Suppl A):29‐40. [DOI] [PubMed] [Google Scholar]
CONSORT 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357:1191‐4. [PubMed] [Google Scholar]
Fox 1996
- Fox KM, Mulcahy D, Findlay I, Ford I, Dargie HJ. The Total Ischaemic Burden European Trial (TIBET). Effects of atenolol, nifedipine SR and their combination on the exercise test and the total ischaemic burden in 608 patients with stable angina. The TIBET Study Group. European Heart Journal 1996;17(1):96‐103. [DOI] [PubMed] [Google Scholar]
Fulder 1996
- Fulder S. The handbook of alternative and complementary medicine. 3rd Edition. Oxford: Oxford University Press, 1996. [Google Scholar]
Gibbons 1999
- Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM. Guidelines for the management of patients with chronic stable angina. Journal of the American College of Cardiology 1999;33(7):2092‐197. [DOI] [PubMed] [Google Scholar]
Hilton 1991
- Hilton TC, Chaitman BR. The prognosis in stable and unstable angina. Cardiology Clinics 1991;9(1):27‐38. [PubMed] [Google Scholar]
Jackson 2001
- Jackson G. Combination therapy in angina: A review of combined haemodynamic and treatment and the role for combined haemodynamic and cardiac metabolic agents. International Journal of Clinical Practice 2001;55(4):256‐61. [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Liang 1999
- Liang Q. Pharmacological action and new progress in clinical appliance of Suxiao Jiuxin Wan. Fujian Journal of Traditional Chinese Medicine 1999;30:43‐4. [Google Scholar]
Liu 2002
- Liu JP, McIntosh H, Lin H. Chinese medicinal herbs for chronic hepatitis B. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD001940] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2003
- Liu Y, Zhang B, Hu L. Pharmacological research of Borneolum syntheticum. Tianjin Journal of Traditional Chinese Medicine 2003;20:85‐7. [Google Scholar]
PTCL 2002
- Physical & Theoretical Chemistry Laboratory Oxford University. Safety data for borneol. http://physchem.ox.ac.uk/MSDS/BO/borneol.html 2002.
Savonitto 1996
- Savonitto S, Ardissiono D, Egstrup K, Rasmussen K, Bae EA, Omland T, et al. Combination therapy with metoprolol and nifedipine versus monotherapy in patients with stable angina pectoris. Results of the International Multicenter Angina Exercise (IMAGE) Study. Journal of the American College of Cardiology 1996;27(2):311‐6. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects. JAMA 1995;273(5):408‐412. [DOI] [PubMed] [Google Scholar]
Thadani 1999
- Thadani U. Treatment of stable angina. Current Opinion in Cardiology 1999;14(4):349‐58. [DOI] [PubMed] [Google Scholar]
Yusuf 1994
- S. Yusuf S, Zucker D, Passamani E, Peduzzi P, Takaro T, Fisher LD. Effect of coronary artery bypass graft surgery on survival: overview of 10‐year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563‐70. [DOI] [PubMed] [Google Scholar]


